Background: Little is known about the properties of CIITA, a transactivator for MHC II activation, in NLR-involved innate immunity.
Results: CIITA mutant can initiate NLR-related signaling to produce IL-33, which further results in a Th2-dominant immune response.
Conclusion: mCIITA modulates Th1/Th2 balance via NOD-like receptor in innate immunity.
Significance: mCIITA might be used to induce transplantation tolerance through alterations of the Th1/Th2 balance via the NLR.
Keywords: Immunosupressor, Immunotherapy, Inflammation, Signal Transduction, Translation Initiation Factors, Helper T Cell, IL-33, MHC Class II Transactivator, NLR-related Signaling, Th1/Th2
Abstract
Dominant-negative mutants of class II transactivator (mCIITAs) with N-terminal depletion have been used to repress the transcription of class II genes in xenotransplantation. Here, we report that mCIITA overexpressing myeloid cell line Ana-1 (Ana-1-mCIITA) derived from a C57BL/6 mouse was able to down-regulate the MHC class II expression and reverse immune responses from Th1 (IL-2+IFN-γ+STAT4+) to Th2 (IL-4+IL-5+IL-10+IL-13+STAT6+) when cocultured with T cells. Mechanism analysis indicated that the mCIITA protein is able to initiate a NOD-like receptor-related signaling pathway via binding of the cytoplasmic Nod2 protein, which was followed by activating RIP2, caspase 1, and IKK-α/β. This ensures the expression of the genes encoding the cytokines IL-33, IL-1β, and TNF-α; however, only the highly expressed IL-33 is responsible for inducing the type 2 response, with a skewed Th2 cytokine secretion (IL-4+IL-5+IL-10+IL-13+IL-2−IFN-γ−), which was completely prevented by the deactivation of the Nod2 gene with siRNA or by the blockage of the IL-33-related signaling using the mAb ST2L against the IL-33 receptor. mCIITA-mediated Th2 conversion was also successfully induced in vivo in a mCIITA-transgenic C57BL/6 mouse model. These results indicate that the Th1/Th2 balance could be regulated by an N terminus-depleted CIITA molecule via NOD-like receptor-related signaling, a property valuable for disease control, especially for inducing transplantation tolerance via the repression of class II expression and the attenuation of a Th1-dominant response.
Introduction
Th1 and Th2 subsets and the relevant immune responses are characterized by their unique patterns of cytokine secretion as originally proposed by Mosmann et al. in 1986 (1). The Th1 and Th2 responses are mediated by IFN-γ and by IL-4/IL-5/IL-13, respectively, and the two T helper subsets show cross-inhibition of lineage differentiation via their subset-specific transcription factors T-bet and GATA3 (2, 3). Although some novel CD4 T subpopulations have been recently identified, such as Th17 (4, 5), Th9 (6, 7), and Tfh (follicular helper T) cells (8), the Th1 and Th2 subpopulations are still considered central CD4 T subsets. Th1 responses are involved in immunity to infectious virus and intracellular bacteria and the graft rejection reaction. Th2 responses mediate antibody production, anaphylaxis, and protection against worm infection (2), although some mechanisms regulating inflammation and type 2-associated asthma have also been attributed to the activation of Th17 (9) and Th9 (10), respectively.
Among the elements determining or initiating the differentiation of Th1 and Th2 subsets, cytokines are of special importance; IL-12/IFN-γ are important for Th1, and IL-4 is important for Th2, with which T-bet and Gata-3 are closely associated, respectively (2, 3). In addition, multiple factors are also involved in Th1/Th2 differentiation, including the nature of antigens, co-stimulatory signals, adhesion molecules, and the approaches and duration of immunization (11–13). For example, IL-33, a newly identified member of the IL-1 family, has been demonstrated to be active in evoking the Th2-dominant immune responses (14, 15). Moreover, in recent years, special attention has been paid to the relationship between innate immunity and Th1/Th2 differentiation. For example, the signals released by TLR4 and TLR2 have been suggested to be capable of dictating Th1 and/or Th2 development via activation of differential cytokine or subsets of dendritic cells (16–18). The expression patterns of Notch ligands on antigen-presenting cells are believed to be connected with Th1/Th2 differentiation (19). Muramyl dipeptide, a Nod2-specific ligand, is also reported capable of priming a potent antigen-specific Th2 immune response in vivo (20).
Class II transactivator (CIITA),4 a protein capable of binding transcriptional factors instead of DNA, is crucial for controlling MHC II expression. The C terminus and middle domains of the CIITA molecule are active in conjugating the MHC enhanceosome, which consists of nuclear factors and the general transcriptional complex. The N terminus of CIITA recruits coactivators to initiate the transcriptional activation of the MHC class II genes (21, 22). A deficiency of CIITA may lead to bare lymphocyte syndrome, a severe immunodeficiency disease (23); in this case, the dominant-negative mutants of CIITA, which are being depleted of N terminus (mCIITA), are no longer able to recruit coactivators to initiate the transcription of the class II genes. Thus, the repressed expression of the class II genes on donor cells is expected, and the utilization of mCIITA-expressing donor cells has been recommended for inducing transplantation tolerance (24, 25).
Moreover, CIITA has been recently categorized as a member of the NOD-like receptor (NLR) family (26, 27). The family contains several subfamilies that detect various pathogen-associated molecular patterns in the cytoplasm. After sensing the pathogen-associated molecular patterns with their C-terminal LRR domains, the NLR molecules undergo oligomerization via their NOD domains to activate the CARD- and/or PYD-containing effector domains for initiating signal transduction (28, 29). Among the NLR members, Nod1 and Nod2 sense cytosolic peptidoglycan meso-DAP and muramyl dipeptide, respectively, to induce NF-κB and MAPK signaling via serine-threonine kinase RIP2/RICK (30, 31). However, in response to other microbial components and endogenous danger signals, a different set of NLRs, including NLRP1 and NLRP3, can activate caspase-1 through the recruitment of the adaptor protein ASC and the formation of the inflammasome, a large protein complex (32–34). This leads to a transformation of proinflammatory cytokine precursors into their mature forms; among these, the maturation of pro-IL-1β to IL-1β is especially notable (32, 33).
However, little is known about the mechanisms by which CIITA functions as an NLR member or whether it can evoke an NLR signaling pathway. To address this, we report here that an N terminal-depleted CIITA mutant molecule (mCIITA) was able to form a complex with the Nod2 protein to initiate NF-κB-mediated signal transduction via activation of an essential adaptor protein RIP2. The binding of mCIITA with Nod2 could also activate cappase-1, probably by initiating the inflammasome-associated pathway. Most interestingly, in contrast to initiating a proinflammatory response, the mCIITA-associated, NLR-related signaling pathways resulted in a selective activation of IL-33, a member of IL-1β family. A high level of IL-33 production led to the conversion of Th1 to Th2 responses, in which the secretion patterns of the associated cytokines and the relevant transcriptional factors T-bet and Gata-3 were completely reversed.
This indicates that a structurally modified CIITA gene has the potential to skew immune responses to a Th2-dominant direction via initiation of NLR-involved signaling and the activation of the IL33 gene, a discovery that may provide useful approaches for immunological diseases intervention.
EXPERIMENTAL PROCEDURES
CIITA Mutant Construction
To construct the N terminal-depleted CIITA mutant, PCR was performed with two pairs of primers by using the cDNA of C57BL/6 mice; the first was used to clone the mCIITA sequence, and the second pair was used to introduce a nuclear localization signal into the N terminus of the mCIITA sequence. The primers were synthesized according to the following sequences: primer 1, sense (5′-GCG GAA GGT CCA TAT CAA GCT TCC AAA ATG GCC AGA GGC TG-3′) and antisense (5′-CGG AAT TCT CAT CTC AGA CTC ATC CTG GCA T-3′); primer 2, sense (5′-AAA GGA TCC ATG CCC AAG AAG AAG CGG AAG GTC CAT ATC AAG C-3′) and antisense (5′-CGG AAT TCT CAT CTC AGA CTG ATC CTG GCA T-3′). The full-length CIITA gene was cloned from C57BL/6 mouse cDNA with the following primers: CIITA primer: sense (5′-CGG GAT CCA TCA CTC TGC TCT CTA AAT CAT GCG-3′) and antisense (5′-CGG AAT TCT CAT CTC AGA CTG ATC CTG GCA T-3′). Compared with the full-length CIITA CDS sequence (1–3237 bp), the sequence of the deleted N terminus is 1–828 bp, leaving a CIITA mutant with a 2409-bp sequence (829–3237 bp). A nuclear location sequence (NLS) was added to the N terminus to facilitate the entrance of the mCIITA molecule into the nucleus. The PCR products of mCIITA and wtCIITA were constructed into the pcDNA3.1+ plasmid according to the instructions provided by Molecular Cloning.
Transfection of mCIITA Gene in Ana-1 and EL4 Cell Lines
Ana-1, a C57BL/6 macrophage cell line, was transfected with pcDNA3.1-mCIITA, pcDNA3.1-CIITA, or pcDNA3.1 using FuGENE HD Transfection Reagent according to the manufacturer's protocol. A C57BL/6 T cell line EL4 was also adopted for mCIITA transfection using an Amaxa nucleofactor system according to the manufacturer's protocols. After transfection, the cells were incubated in complete RPMI 1640 medium at 37 °C for 48 h. G418 (400 μg/ml) was added for 2 weeks to select for positive cells.
Mixed Lymphocyte Reactions (MLRs)
MLR cultures were set up with 3000 radian-irradiated splenocytes (2 × 105/well) from BALB/c mice as stimulators and C57BL/6 CD4+T cells as responder cells at the same dose in 96-well plates for 6 days of culture. The CD4+T cells were purified with a T cell isolation kit (Miltenyi-Biotec, Bergisch Gladbach, Germany). Ana-1 cells, including those transfected with mCIITA (Ana-1-mCIITA), wtCIITA (Ana-1-wtCIITA), or vector (Ana-1-vector), were added into the MLR culture system. Because of their original powerful proliferation activity, all Ana-1 cells were irradiated with 3500 radians of gamma rays before their addition into MLR to guarantee their biological activity by producing cytokines but not allowing for interfering allo-lymphoproliferation from their excessive proliferation. In some experiments, blocking antibodies against OX-40L, ICOSL, 4-1BBL, ST2L, IL-1β, and TNF-α were added into the culture at a final concentration of 15 μg/ml. A total of 1 μCi of [3H]TdR (Shanghai Institute of Atomic Nucleus, Chinese Academy of Sciences, Shanghai, China) was added to each well 16 h before the termination of the cultures. The cells were collected with a semi-autologous harvester (Tomtec, Gaithersburg, MD), and isotope incorporation was assayed with a liquid scintillation counter (Rackbeta, GE Healthcare). Relative responses were calculated as RR % = [(cpm of experimental combination)/(cpm of control)] × 100. The culture supernatants of the MLR were collected for ELISA analysis.
Cell Culture with Transwell System
For separate culture in the Transwell system (0.4 μm, Costar), purified C57BL/6 CD4+T cells (5 × 105/well) plus irradiated BALB/c splenocytes (5 × 105/well) were dispensed into the lower chamber in a 24-well plate, and Ana-1-mCIITA or Ana-1-vector cells (5 × 104/well) were suspended in the upper chamber. The cells in upper and lower chambers were separated by a 0.4 μm membrane but shared the same culture medium. After 6 days of culture, the supernatants were collected for detection of cytokines measurement by ELISA.
Real-time PCR
Total RNA was prepared by TRIzol reagent (Invitrogen) according to the manufacturer's protocol. One microgram of total RNA was subjected to reverse transcription (RT) and PCR amplification using quantitative real-time RT-PCR. A method designated as 2−ΔΔCt was included for data analysis according to the protocol offered by the kit (Takara, Tokyo). Fold changes were calculated using β-actin for normalization of the threshold cycle (Ct). The primers were designed according to the following sequences: IL-4, sense (5′-CTG GGG GGG GAT TTG TTA-3′) and antisense (5′-TCA CTC TCT GTG GTG TTC TTC G-3′); IFN-γ, sense (5′-CAA GTG GCA TAG ATG TGG AAG-3′) and antisense (5′-GTT GCC AAG CCT TAT CGG-3′); IL-10, sense (5′-GGA CTC CAG GAC CTA GAC AGA-3′) and antisense (5′-CAA TGG AAA CAG CTT AAA CAC A-3′; IL-33, sense 5′-GGG AAG AAG GTG ATG GTG AA-3′) and antisense (5′-CCG AAG ACT TTT TGT GAA GG-3′); IL-18, sense (5′-GTG TTC GAG GAT ATG ACT GAT-3′) and antisense (5′-CCA GTC CTC TTA CTT CAC TGT-3′); IL-1, sense (5′-CTC AAC TGT GAA ATG CCA CC-3′) and antisense (5′-GAG TGA TAC TGC CTG CCT GA-3′); TNF-α, sense (5′-TCC GGG CAG GTC TAC TTT-3′) and antisense (5′-GGT CAC TGT CCC AGC ATC T-3′); β-actin, sense (5′-CTG TCC CTG TAT GCC TCT G-3′) and antisense (5′-ATG TCA CGC ACG ATT TCC-3′).
Western Blot Analysis
Total protein extracts were prepared using RIPA buffer (Beyotime, Jiangsu, China) in the presence of proteinase inhibitor mixture (Roche Applied Science). Nuclear and cytoplasmic protein extracts were prepared using a Nuclear and Cytoplasmic Protein Extraction kit (Beyotime). The polyacrylamide gel electrophoresis, tank-based transfer to PVDF membranes (Millipore, Billerica, MA) and immunodetection were performed using standard techniques. Antibodies against the following proteins were used in Western blotting analysis in accordance with the manufacturer's instructions: the C terminus of CIITA (Santa Cruz Biotechnology, Santa Cruz, CA), NF-κB p65 (Santa Cruz), β-actin (Abcam, Cambridge, MA), TATA-binding protein (TBP) (Abcam), phosphorylated-IKK-α/β (pIKKα/β), caspase-1 (Biovision, Palo Alto, CA), Nod1 (Cell Signaling Technology, Beverly, MA), Nod2 (eBioscience, San Diego, CA), and RIP-2 (Cell Signaling Technology).
Enzyme-linked Immunosorbent Assay (ELISA)
The concentration of IL-2, IFN-γ, IL-4, IL-5, IL-10, IL-13, and IL-33 was determined in the culture supernatants using the appropriate ELISA kit (eBioscience).
Flow Cytometric Analysis
The Ana-1-mCIITA cells were suspended in cold phosphate-buffered saline (PBS) supplemented with 0.1% BSA and incubated with phosphatidylethanolamine- or FITC-conjugated anti-I-Ab, -CD86, -CD80, -OX40L, -ICOSL, or -4–1BBL mAbs (BD Biosciences or Biolegend) for 30–45 min at 2–8 °C. Splenocytes of mCIITA-transgenic mice were treated with FITC-conjugated anti-I-A/E antibody (eBioscience) or isotype-matched unrelated antibodies (BD Biosciences), washed with 0.1% BSA-supplemented PBS, and resuspended in 2% paraformaldehyde, and 104 cells were analyzed on a FACSCalibur (BD Pharmingen) (35).
Co-immunoprecipitation
Ana-1-wtCIITA or Ana-1-mCIITA cells were lysed in cell lysis buffer and incubated at 4 °C for 30 min. The cell lysate was clarified by centrifugation, and the supernatant was diluted at 1:5 with co-immunoprecipitation buffer. An aliquot of total lysate protein was incubated with 2 μg of anti-CIITA or anti-Nod2 antibody (eBioscience) at 4 °C for 1 h. Fifty microliters of agarose-conjugated protein A (Invitrogen) was added, and the mixture was incubated for another hour under the same conditions. The protein-antibody-protein A complexes were pulled down by centrifugation and washed with co-immunoprecipitation buffer. The proteins were subjected to analysis by electrophoresis on 10% SDS-PAGE, blotted onto PVDF membranes, probed with an antibody against Nod1 (Cell Signaling Technology, Beverly, MA), Nod2, or CIITA, and detected using an ECL kit (Pierce).
Deactivation of Nod2 or RIP-2 Gene with siRNA
Transfection of Nod2 or RIP-2 siRNAs (Invitrogen) was performed using Lipofectamine 2000 (Invitrogen). In brief, Ana-1-mCIITA cells were plated in a 6-well plate to 40% confluence. For each well, 5 μl of siRNA was added into 250 μl of Opti-MEM medium, and 5 μl of Lipofectamine 2000 was added into 250 μl of Opti-MEM medium. The mixture containing siRNA was added to the cells and incubated for 6 h before replacing the medium. The total RNA and protein were prepared 48 h after transfection and used for real-time PCR or Western blotting analysis, respectively. The following Nod2 and RIP-2 siRNA sequences were used: Nod2, sense (GGA CCU CUU UGA UAC CCA UTT) and antisense (AUG GGU AUC AAA GAG GUC CTT); RIP-2, sense (GCC AUU UGA UAU ACC UCA UTT) and antisense (AUG AGG UAU AUC AAA UGG CTT).
Preparation of mCIITA Transgenic Mice
mCIITA transgenic mice on the C57BL/6 background were prepared as follows; the pcDNA3.1+mCIITA was linearized into two fragments with endonuclease NsiI and BglII (New England Biolabs), and the fragment containing the purified mCIITA gene was microinjected into fertilized oocytes of C57BL/6 mice to produce mCIITA-transgenic mice. Two pairs of primers were designed to characterize the transgenic mice: primer 1 sense (5′-GAT GCG GTT TTG GCA GTA CAT CAA TGG-3′) and antisense (5′-TTC CTG GCT CTT GTT GCT GCC TCT TTC-3′); primer 2 sense (5′-CAG AAG ATG CAG CCA AGG ATC TTC CTG-3′) and antisense (5′-TCA GAA GAT GCA GCC AAG GAT CTT CC-3′).
Statistics
A two-tailed Student's t test was used to determine significance (p < 0.05). The error bars in the figures represent the S.D. of triplicates or quadruplicates for cell cultures.
RESULTS
mCIITA-transfected Ana-1 Cells Induced Conversion of Immune Responses from Th1 to Th2
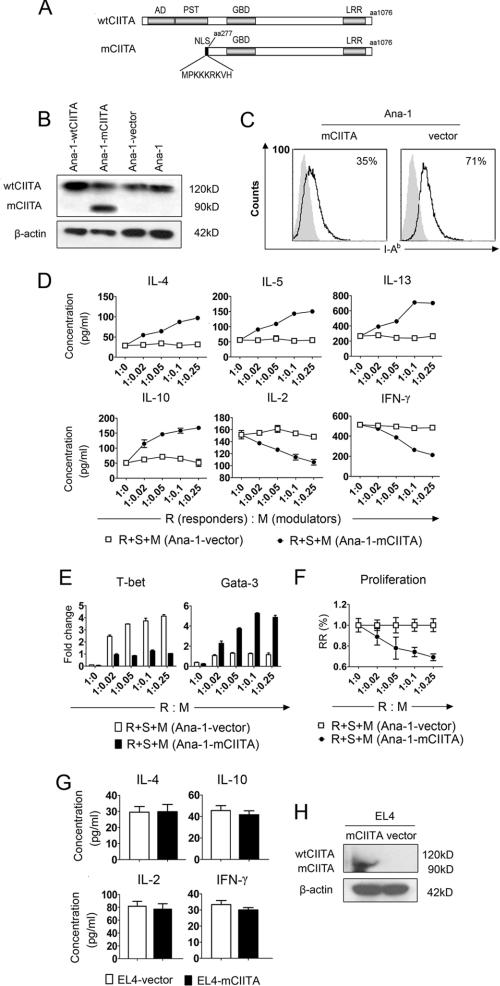
Using a swine epithelial cell line transfected with a dominant-negative mutant gene (mCIITA), we and other laboratories have reported that the mCIITA-mediated repression of class II expression can be used to inhibit xeno-reactions (24, 36). In this study a similar strategy for the construction of mCIITA was performed (Fig. 1A). Because the antibody we used for Western blotting is against the C terminus of the CIITA molecule, both mCIITA (90 kDa) and wtCIITA (120 kDa) can be detected. The appearance of a 90-kDa band in Fig. 1B indicated the successful transfection of mCIITA in Ana-1 cells (Fig. 1B). Additionally, the expression of mouse H-2 I-Ab on the mCIITA-transfected Ana-1 cells (Ana-1-mCIITA) was diminished from 71% (control) to 35%, as indicated in Fig. 1C. Quite unexpectedly, however, the T cell-mediated response pattern was completely shifted from Th1 to Th2 in terms of both the cytokine secretion and the expression intensities of transcription factors when the Ana-1-mCIITA cells, indicated as modulators (M), were added to an MLR system in which the syngeneic responding T cells (R) and the allo-stimulating cells (S) were co-cultured. This led to a phenotypic alteration from IL-2+IFN-γ+IL-4−IL-5−IL-10−IL-13−T-bet+ Gata3− to IL-2−IFN-γ−IL-4+IL-5+IL-10+IL-13+ T-bet−Gata3+ (Fig. 1, D and E). Functionally, the Ana-1-mCIITA cells showed a strong suppression of the Th1-dominant syngeneic MLR in a dose-dependent manner (Fig. 1F). However, there was no effect on the conversion of the Th1 (IL-2, IFN-γ) to the Th2 (IL-4, IL-10) phenotype when EL4-mCIITA was also introduced into the MLR culture (Fig. 1G), although the transfection of mCIITA into the EL4 cells was as successful as that into the Ana-1 cells (Fig. 1H).
FIGURE 1.
A converse pattern of T cell responses from Th1 to Th2 is inducible when co-cultured C57BL/6 myeloid cell line Ana-1 is transfected with mCIITA. A, shown is a schematic diagram of the structures of the mouse mutated CIITA (mCIITA) proteins. Top row, a wild-type CIITA molecule with AD, PST, GBD, and leucine-rich repeat (LRR) domains. Bottom row, shown is an AD/PST-depleted mCIITA molecule of 819 amino acid residues including an additional nine amino acid-containing nuclear location sequence at the N terminus to facilitate nuclear membrane translocation. B, wtCIITA and mCIITA were successfully transfected in Ana-1 cells as indicated by Western blotting. After transfection, the Ana-1 cells were selected by 400 μg/ml G418 for 2 weeks. Both wtCIITA and mCIITA proteins are detectable using mAb against the C terminus of the CIITA molecule: 120 kDa for wtCIITA, 90 kDa for mCIITA. C, mCIITA overexpression reduces the expression level of H-2 class II molecule I-Ab on Ana-1-mCIITA cells, as determined by flow cytometry. D, a typical secretion pattern with the enhanced type 2 cytokines (IL-4, -5, -10, and -13) and the depressed type 1 cytokines (IL-2 and IFN-γ) is revealed by ELISA in supernatants collected from the Ana-1-mCIITA-included MLR cultures at day 6; different amounts of 3500 radian-irradiated Ana-1-mCIITA cells were superimposed as modulators (M) onto the MLR systems that consist of syngeneic C57BL/6 CD4+T cells as responders (R) and allogeneic, irradiated (3000 radian) Balb/c splenocytes as stimulators (S). E, shown is repressed expression of T-bet and increased Gata-3 in the Ana-1-mCIITA cells-superimposed MLR culture, as determined by real-time PCR. F, shown is dose-dependent suppression of the alloantigen-induced lymphoproliferation in the Ana-1-mCIITA-supplemented MLR culture. The MLR was assayed by [3H]TdR uptake and expressed as relative response (RR) of cpm values. G, no effect is shown of mCIITA overexpression on the conversion of secretion levels of Th1-related cytokines (IL-2 and IFN-γ) to Th2-related cytokines (IL-4 and IL-10) when the mCIITA-transfected EL4 cells were added into the syngeneic MLR culture. H, shown is overexpression of mCIITA in the mCIITA-transfected lymphoid EL4 cell line, in which endogenous wtCIITA is not expressed constitutively.
The results strongly suggest that the capability of the mCIITA gene in changing the Th1/Th2 balance is not directly mediated by T lymphocytes. Instead, the transition of Th1 to Th2 may result from the interaction of Ana-1-mCIITA with T cells.
IL-33 Released from Ana-1-mCIITA Cells Is Critical to Differentiation of CD4+T Cells to IL-4/IL-5/IL-13-secreting Th2 Subset
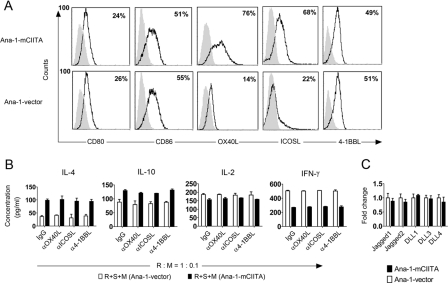
To explore the mechanisms underlying the conversion of Th1 to Th2 response, the expression of membrane molecules involved in T cell differentiation on Ana-1-mCIITA was examined. Enhanced expression of the co-stimulation molecules OX-40L and ICOSL was detected on the Ana-1-mCIITA cells (Fig. 2A). However, functional blockage of these co-stimulation molecules with neutralizing antibodies failed to attenuate the dominant pattern of the Th2-related cytokine secretion (Fig. 2B). This suggests that co-stimulation molecules, at least OX-40L and ICOSL, may not be involved in the Th1/Th2 alteration driven by mCIITA. We also examined the mRNA level of Notch ligands, including Jagged1, Jagged2, DLL1, DLL3, and DLL4 expressed by Ana-1-mCIITA cells by real-time PCR, but no significant differences were detected between Ana-1-mCIITA and Ana-1-vector cells (Fig. 2C). Consistent with this finding, the enhanced pattern of type 2 cytokine expressions was still detectable in the non-contact Transwell system, in which the Ana-1-mCIITA cells and MLR cells were incubated in separated chambers but shared the same culture medium (Fig. 3A). This suggests that soluble factors and not membrane-attached molecules are crucial in the Ana-1-mCIITA-induced alteration of type 1 to type 2 responses.
FIGURE 2.
Membrane-attached molecules OX40L, ICOSL, and Notch ligands are not involved in the mCIITA-initiated Th2 conversion in Ana-1-mCIITA cells. A, among the co-stimulatory molecules we measured, the expressions of OX40L and ICOSL were enhanced by overexpressed mCIITA. The expressions are indicated by the increased percentages of positive cells. B, blockage of OX-40L and ICOSL with mAbs failed to restore the converted pattern back to the type 1 cytokine secretion. Neutralizing mAbs (15 μg/ml) were added to the Ana-1-mCIITA-superimposed MLR as responder:modulator (R:M) = 1:0.1, a reasonable ratio as indicated in Fig. 1E. IgG, and anti-4–1BBL mAb were used as controls. C, no significant differences in the expression of Notch ligands (Jagged1, Jagged2, DLL1, DLL3, and DLL4) were detected between Ana-1-mCIITA and Ana-1-vector cells, which were determined by real-time PCR. S, stimulator.
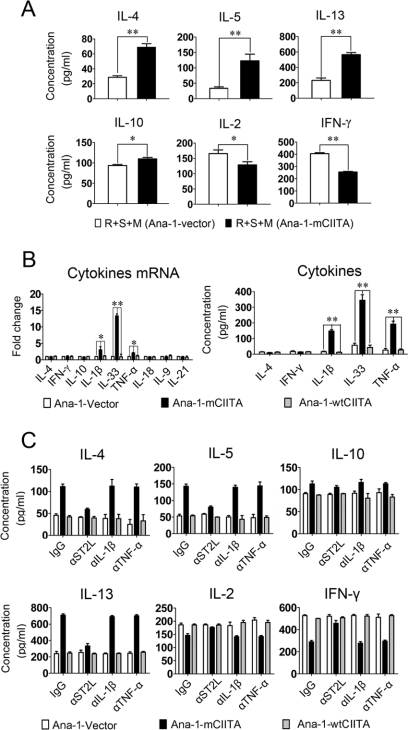
FIGURE 3.
IL-33 released by Ana-1-mCIITA cells is active in the differentiation of CD4+T cells to the IL-4/IL-5/IL-13-secreting Th2 subset. A, enhanced Th2 cytokines reappeared in a non-contact Transwell system, which indicated that soluble factors played an essential role in the Ana-1-mCIITA-induced conversion to Th2 responses. In our Transwell system, purified C57BL/6 CD4+T cells (5 × 105/well) plus irradiated BALB/c splenocytes (5 × 105/well) were dispensed into the lower chamber of 24-well plate, and the irradiated C57BL/6 Ana-1-mCIITA or Ana-1-vector cells (5 × 104/well) were suspended in the upper chamber. The cells in upper and lower chambers were separated by a 0.4 μm membrane but shared the same culture medium. After 6 days of culture, the supernatant was collected for the detection of cytokines by ELISA. B, IL-33 was significantly increased, and IL-33-related IL-1β and TNF-α were also slightly raised in Ana-1-mCIITA cells in comparison with the two control groups Ana-1-wtCIITA and Ana-1-vector, as determined at the mRNA (left) and the protein (right) levels. After 3 days of culture, the Ana-1-mCIITA cells and supernatant were collected for PCR or ELISA analysis, respectively. C, blockade of the IL-33 signaling pathway by a neutralizing mAb against the IL-33 receptor ST2L prevented the differentiation of CD4+T cells to IL-4/IL-5/IL-13-secreting Th2 cells. However, no effects were observed after incubation with the anti-IL-1β and anti-TNF-α mAb. All mAbs (15 μg/ml) were added to the Ana-1-mCIITA-superimposed MLR culture, and the cytokines in supernatants were determined by ELISA.
To identify which components produced by Ana-1-mCIITA are responsible for the mCIITA-related Th2 conversion, factors related to T cell differentiation were analyzed. IL-33 is an IL-1-like cytokine secreted by macrophages. IL-33 is actively involved in the production of Th2-associated cytokines via its heterodimer receptor ST2L and IL-1R accessory protein (IL-1RAP) (14, 15, 37). Indeed, we found that IL-33 was substantially increased at the mRNA level in Ana-1-mCIITA cells, whereas other soluble factors related to proinflammation and differentiation of T subsets remained unchanged, except for a slight increase of IL-1β and TNF-α mRNA. The cytokine secretion phenotypes in the culture supernatants of Ana-1-mCIITA cells were further confirmed at the protein level (Fig. 3B). Anti-ST2L, a neutralizing mAb against the IL-33 receptor, completely prevented CD4+T cells from differentiating into the Th2-like subset, whereas blockage of IL-1β and TNF-α signaling failed to prevent Th2 skewing. As shown in Fig. 3C, the highly expressed IL-4/IL-5/IL-13 and the down-regulated IL-2/IFN-γ, a pattern characteristic for Th2 response, returned to their original levels when the anti-ST2L mAb was introduced. This indicates that IL-33 and its receptor-initiated signal transduction play critical roles in the mCIITA-induced conversion of Th1- to Th2-related immune responses.
IL-33 Gene Activation Is Dependent on NLR-initiated Signal Transduction Pathways
Nod1 and Nod2, two members of the NLR family that are partially homologous with CIITA, are active in innate immunity, especially in the onset of the anti-infection responses through activation of genes encoding proinflammatory molecules, such as IL-1β, IL-18, TNF-α, and IL-6 via NF-κB- and MAPK-involved signaling (28–31). Several pathways have been identified in NLR-related signal transduction including the Nod2-initiated pathway mentioned above and the inflammasome-mediated, NALP3-initiated pathway (32–34). In the Nod2 pathway the activation of a CARD-containing serine/threonine kinase RIP2, or RICK, is crucial for the involvement of NF-κB to convert pro-IL-1β to IL-1β (29, 31).
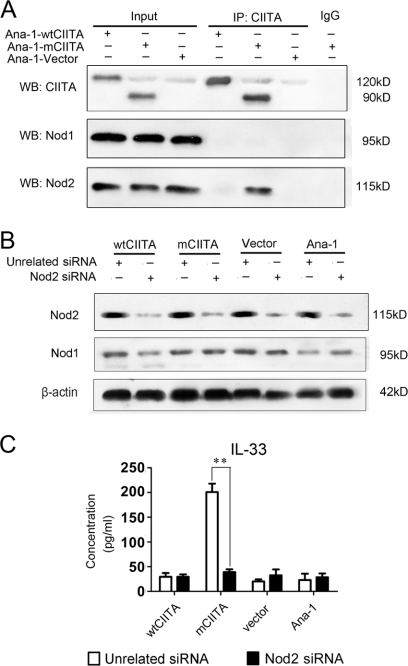
We first explored the relationship of mCIITA with the activation of Nod1/Nod2 in the mCIITA-induced transcription of the IL-33 gene to determine whether the Nod2-initiated signal pathway is provoked. Our co-immunoprecipitation assay indicated that the mCIITA molecule can interact with Nod2, but not with Nod1, in Ana-1-mCIITA cells and that the wtCIITA molecule has no ability to interact with the Nod2 protein (Fig. 4A), suggesting an exclusive involvement of mCIITA in the activation of Nod2 and its related signaling pathway; the counter-co-immunoprecipitation showed a similar result (supplemental Fig. 1). To further explore the relationship of Nod2 and IL-33, Nod2 siRNA was introduced into Ana-1-mCIITA cells. As indicated in Fig. 4B, Nod2 expression was effectively inhibited in all Nod2-siRNA-transfected cell lines, whereas Nod1 expression was undisturbed. More interestingly, the high production of IL-33 in Ana-1-mCIITA cells was completely hindered when a Nod2 siRNA was introduced into the cells (Fig. 4C).
FIGURE 4.
IL-33 gene activation is dependent on the interaction of mCIITA with Nod2. A and B, Nod2 protein, but not Nod1 protein, can be co-precipitated (IP). The results are determined by Western blotting (WB) using anti-Nod1/Nod2 antibodies (indicated as WB: Nod1 and WB:Nod2) after the immune complex was immunoprecipitated by anti-CIITA-C terminus antibody (IP: CIITA). Whole-cell lysates (Input) and irrelevant IgG were used as positive and negative control, respectively. B, Nod2 siRNA can effectively inhibit the expression of Nod2 protein, but not Nod1, in all kinds of Ana-1 cells. Western blotting was performed 48 h after the Ana-1 cells were transfected with Nod2 siRNA. The results are expressed as a representative of three independent experiments. C, Nod2 is involved in the production of IL-33 in Ana-1-mCIITA cells because introduction of Nod2-siRNA repressed the level of IL-33.
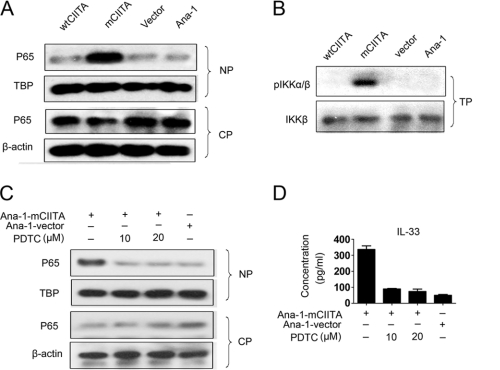
We then explored whether NF-κB activation was driven by overexpressed mCIITA in the Ana-1-mCIITA cells. Our observation indicated that under the influence of mCIITA, greater levels of NF-κB p65 protein (p65) were detectable in the nucleus compared with the cytoplasm, as shown in Fig. 5A; the result suggested that NF-κB was activated and translocated to the nucleus in Ana-1-mCIITA cells. In addition, IKK-α/β, an IκB kinase essential for the phosphorylation of IκB, was also exclusively activated via phosphorylation in the Ana-1-mCIITA cells (Fig. 5B). This phosphorylation led to an ubiquitin-dependent degradation of IκB, allowing for the liberation of the NF-κB heterodimer to pass into the nucleus (38). When pyrrolidine dithiocarbamate, an inhibitor of NF-κB activation, was introduced, the translocation and accumulation of p65 in the nucleus was no longer sustained (Fig. 5C). The depressed expression of IL-33 in pyrrolidine dithiocarbamate-treated Ana-1-mCIITA cells was also demonstrated at the protein levels (Fig. 5D); this result indicates that the elevated IL-33 production induced by mCIITA is tightly connected with the activation of the Nod2-initiated, NF-κB-associated signal transduction pathway.
FIGURE 5.
Activation and translocation of NF-κB are associated with the Nod2-initiated signaling pathway. A, the translocation of activated NF-κB p65 protein to the nucleus was indicated by differential patterns of p65 expression in nuclear proteins (NP) and cytoplasmic proteins (CP). β-Actin and TBP (TATA-binding protein) were used as controls. B, phosphorylation of IKK-α/β (pIKKα/β) was detectable in the total protein (TP) fraction of Ana-1-mCIITA cells by Western blotting. Total IKK-β was used as a control. C and D, when pyrrolidine dithiocarbamate, an inhibitor of NF-κB activation, was introduced, the translocation of p65 in the nucleus (C) and the IL-33 production level in culture supernatants (D) of the Ana-1-mCIITA cells were substantially inhibited. After selection by 400 μg/ml G418 for 2 weeks, the cells were cultured for another 3 days in the presence of pyrrolidine dithiocarbamate at doses of 10–20 μm/ml and assayed for p65 by Western blotting (C) and for IL-33 by ELISA (D).
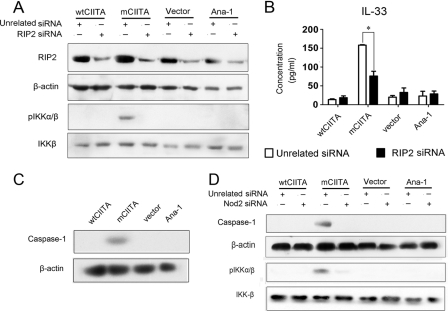
RIP2, another essential component in Nod2-initiated signaling, is a serine/threonine kinase in the CARD domain-containing complex RICK (29). To assay the possible role of RIP2 in NF-κB-associated activation of the IL33 gene, RIP2 siRNA was introduced into Ana-1-mCIITA cells. As indicated in Fig. 6A, the expression of RIP2 was effectively inhibited in all RIP2-siRNA-transfected Ana-1 cells (first row). The phosphorylated IKK-α/β could be detected only in Ana-1-mCIITA cells, as expected. Interestingly, the expression of IKK-α/β phosphorylation was repressed when RIP2-siRNA was introduced in Ana-1-mCIITA cells (Fig. 6A, third row). Moreover, the production of IL-33 was clearly reduced when the expression of the RIP2 protein was interrupted by RIP2 siRNA (Fig. 6B). This suggests that the activation of the IL-33 gene results from the Nod2-initiated signal transduction pathway in which RIP2, IKK-α/β, and NF-κB actively participated.
FIGURE 6.
IL-33 gene activation is dependent on Rip2-involved NLR signal transduction pathways. A and B, RIP2 was involved in NF-κB-mediated activation of IL-33 gene because the IKK-α/β phosphorylation (A) and IL-33 production (B) were reduced by RIP2 siRNA in Ana-1-mCIITA cells as determined by Western blotting and ELISA, respectively. All Ana-1 cells with or without transfection were selected using 400 μg/ml G418 for 2 weeks, then re-transfected with Rip2 siRNA and cultured for another 48 h. β-Actin and total IKK-β were used as controls. C, caspase-1 was also selectively activated in Ana-1-mCIITA as detected by Western blotting of total protein. D, activated caspase-1 and pIKK-α/β protein were detectable only in the Ana-1-mCIITA cells; their activation was synchronously restrained by the introduction of Nod2 siRNA (first and third row), suggesting that Nod2 determines the expression of caspase-1 and pIKK-α/β in Ana-1-mCIITA cells. These cells were selected using 400 μg/ml G418 for 2 weeks, re-transfected with Nod2 siRNA, and cultured for another 48 h. Total protein was collected to determine the expression of caspase-1 and pIKK-α/β expression by Western blotting. IKK-β and β-actin were used as controls.
mCIITA-induced Activation of Caspase-1 Suggests Involvement of Inflammasome in Nod2-initiated Activation of IL-33 Gene
The activation of caspase-1 is important in another caspase-1-containing, inflammasome-mediated pathway during NLR-initiated signal transductions, resulting in the proteolytic conversion of the IL-1β precursor into an active cytokine. Like other members of the caspase family, caspase-1 itself is synthesized as a proenzyme that is cleaved during its activation into large (20 kDa) and small (10 kDa) subunits. We found that the 20-kDa subunit of the activated caspase-1 was detectable in Ana-1-mCIITA but not in Ana-1-wtCIITA or Ana-1-vector cells (Fig. 6C), suggesting that inflammasome-mediated signaling may be involved in the activation of the IL-33 gene, which is driven by mCIITA.
We further explored the relationships among Nod2, NF-κB, and caspase-1 in Nod2-siRNA-transfected Ana-1 cells. Fig. 6D revealed that caspase-1 and IKK-α/β phosphorylation can be interrupted by Nod2 siRNA in the Nod2 siRNA-transfected Ana-1-mCIITA cells, suggesting the connection of Nod2 activation with the two NLR-related signaling pathways: the NF-κB- and the inflammasome-involved pathway. This led to the activation of IL-33 gene, as there was no IL-33 detectable when Nod2 siRNA was transfected in Ana-1-mCIITA cells as previously indicated in Fig. 4C. Therefore, the results we obtained suggest that the CIITA mutant (mCIITA) is able to initiate the transcriptional activation of the IL33 gene via the Nod2-IκB-NF-κB pathway and the Nod2-caspase-1-containing inflammasome pathway.
Pattern Conversion of Type 1-related IFN-γ/STAT4 to Type 2-related IL-4/STAT6 Is Inducible in mCIITA Transgenic C57BL/6 Mice
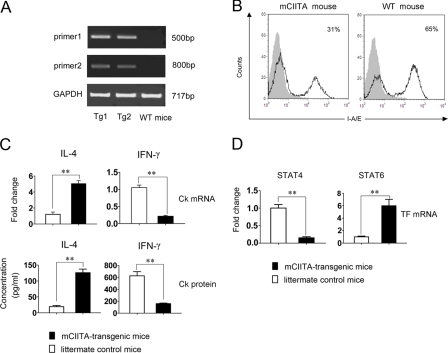
To understand the possible role of the mutated CIITA in vivo, especially whether the mutant is able to convert the Th1-dominant immune response to the Th2-related pattern, mCIITA- transgenic C57BL/6 mice were constructed. PCR analysis of genomic DNA from the resultant adult mice using two pairs of primers for the mutant showed that the introduced mCIITA was detectable in mCIITA-transgenic mice (Fig. 7A), which indicates the success of the mCIITA introduction. The data presented in supplemental Fig. 2 also show that the mCIITA protein was effectively expressed in splenocytes of the mCIITA-transgenic mice. The ability of the N terminus-deleted CIITA mutant to repress the expression of MHC class II was further verified in vivo (Fig. 7B), in which the percentages of H-2 I-A/I-E-positive splenocytes were reduced from 65% (control) to 31% despite the fact that the mice were heterozygous for the mutated CIITA gene.
FIGURE 7.
The conversion of the type 1-related IFN-γ/STAT4 to the type 2-related IL-4/STAT6 is inducible in mCIITA-transgenic C57BL/6 mice. A, detection of the transgenic mCIITA gene in C57BL/6 mice by PCR as indicated as 500- and 800-bp bands is shown. Tg1 and Tg2 represent DNA samples from two individual transgenic mice. Wild-type littermate mice were set as controls (WT mice). B, a depressed percentage of the H-2 I-A/I-E+ splenocytes in the mCIITA mice compared with littermate non-transgenic mice is shown. I-A/I-E expression was determined by flow cytometry. An isotype-matched Ab was used as control. C, increased expression of IL-4 (type 2 cytokine) and repressed expression of IFN-γ (type 1 cytokine) in the mCIITA-transgenic C57BL/6 mice was detectable at the mRNA level by real-time PCR in splenocytes (upper row) and at the protein level by ELISA in the supernatants of cultured splenocytes (bottom row). D, increased STAT6 and decreased STAT4 were also determined by real-time PCR in the splenocytes of mCIITA mice.
After in vivo transduction of mCIITA, the cytokine expression pattern was significantly converted from IL-4loIFN-γhi to IL-4hiIFN-γlo as determined both by real-time PCR for mRNA in splenocytes and by ELISA for cytokines in the supernatants of cultured splenocytes of mCIITA-transgenic mice (Fig. 7C). Synchronously, a conversion of STAT4hiSTAT6lo to STAT4lo STAT6hi appeared in vivo (Fig. 7D). This suggests that the IFN-γ-mediated, Th1-dominant response, a genetically endowed immune reaction pattern typically represented in C57BL/6 mice (39), shifted to an IL-4-mediated, Th2-dominant pattern in response to the introduction of the CIITA mutant into the mouse germ line. More information with regard to the biological changes in the transgenic mice, including the role of IL-33 and the significance of the mCIITA-modified mice in inducing transplantation tolerance, will be reported elsewhere.
DISCUSSION
TLR2, TLR4, and TLR9, three members of the Toll-like receptor family, were recently reported to be active in regulating Th1 or Th2 generation through the alteration of accessory cell phenotypes and/or the activation of differential cytokine-encoding genes (16–18). However, little is known about the roles of the NLRs and the RIG-I-like receptors in Th1/Th2 differentiation. For example, as far we know there is no study dealing with the CIITA functioning as a NLR in inducing effective T cell subsets.
CIITA is a master regulator for the activation of MHC class II gene transcription. When the N terminus of CIITA is depleted to form a mCIITA, the resultant protein is still active in binding the enhanceosome with its C terminus but is no longer able to activate the transcription of class II genes; this suggests that class II expression may not be maintained on mCIITA-transfected cells. This phenomenon has been used to induce transplantation tolerance by attenuating the stimulation intensity of xenogeneic MHC antigens in our laboratory and others (24, 25).
To our surprise, however, in an mCIITA-transfected myeloid cell line, a reduced class II expression and a high level of expression of Th2 cytokines appeared when the cell line was added into the MLR culture with syngeneic T cells as responders. It is notable that the T cells in our experimental system are of C57BL/6 (H-2d) origin, which was originally characteristic of a type 1 immune response with a highly production of IFN-γ and IL-2. Thus, the Th1-related response can be completely converted to a Th2 response just by introducing a mutated CIITA gene, even under heterozygous conditions. As reported here, this was attributable to the depletion of the CARD/PYD domains in the N terminus of the CIITA protein because the protein is still able to function as an NLR member for signal transduction in addition to behaving as a regulator for class II gene transcription.
It is still an open question as to why only the CARD/PYD-depleted mCIITA, instead of wtCIITA, is able to selectively bind and activate Nod2. It is possible that some sequences depleted together with the CARD/PYD domain are capable of inhibiting the binding of wtCIITA with Nod2. This implicates that the Nod2-mediated signaling pathway via mCIITA cannot be initiated under physiological conditions. However, construction and introduction of the mutated CIITA gene may still be valuable for immune intervention to alter the patterns of immune responses for disease control.
At least two groups of signal transduction pathways have been proposed for the NOD-like receptors, including the Nod1/2-initiated pathways and the NALP-initiated pathways (28–34). In some of the latter pathways, the activation of an ASC/caspase-1-containing inflammasome is critical; as a result, both groups of the pathways deal with the NF-κB-dependent activation of cytokines from their precursors, among which IL-1β and IL-18 are especially notable. An elevated level of caspase-1 was also identified when the mCIITA was transfected, suggesting the involvement of both pathways, characterized by RIP2/RICK and caspase-1 activation in the mCIITA-instigated reactions. The mechanism underlying the activation of the inflammasome-associated pathway by mCIITA is not clear, although the possibility of the direct or indirect binding of mCIITA with NALP3/NLRP3 cannot be excluded.
IL-33, a recently identified member of the IL-1 family, has been linked to the induction of Th2-dominant immune responses (14, 15). Administration of exogenous IL-33 in vivo leads to an increase in production of Th2 cytokines, such as IL-4, IL-5, and IL-13 and the enhancement of IgE levels. IL-33 binds to a heterodimeric receptor complex consisting of ST2 and IL-1RAP and induces signal transduction through the TIR domain of IL-1RAP. Binding of IL-33 to ST2L or IL-1RAP activates NF-κB and MAP kinase pathways through recruitment of myeloid differentiation factor 88 (MyD88), IL-1R-associated kinase 1 (IRAK1), and IRAK4 (14, 37). The restricted expression of ST2L on Th2 but not Th1 lymphocytes (15, 40) may provide a mechanism by which IL-33 preferentially induces Th2 responses. Therefore, the activation of the IL33 gene after the Nod2- and inflammasome-related pathways easily accounts for the manner in which the mCIITA protein is able to generate a Th2 cytokine-dominated immune response. However, the mechanism by which the two pathways activate the IL33 gene and whether caspase-1 is involved in the cleavage of IL-33 from its precursor are not clear. Interestingly, IL-33 has been suggested to function as an HMGB1-like alarmin molecule released by necrosis in a TLR/NLR-mediated immune reaction for anti-endogenous danger signals (15, 41). In this connection, it would be interesting to explore whether the N terminal-depleted mCIITA can be regarded as a “damaged” or “cleft” form of an intact natural CIITA protein. In this case the damaged CIITA molecule may be more efficient in passing through the nuclear membrane to behave as a member of the damage-associated molecular pattern (DAMP) family to initiate innate immune responses by mobilization of the alarmin IL-33 via the NLR-related signal pathway; if so, the “mCIITA–IL-33–type 2 response” would provide a feedback mechanism of regulation for inflammations that is mediated both by innate immunity and by Th1-dominant responses.
Supplementary Material
This study was supported by the Natural Science Foundation of China Grants 30530690, 30772018, and 30700766 and the Foundation of Shanghai Sci-Tech Council 07JC14033.

This article contains supplemental Figs. S1 and S2.
- CIITA
- class II transactivator
- mCIITA
- CIITA mutant
- wtCIITA
- wild-type CIITA
- NOD
- nucleotide binding oligomerization domain
- NLR
- NOD-like receptor
- IL-1RAP
- IL-1R accessory protein
- R
- responder
- S
- stimulator
- M
- modulator
- MLR
- mixed lymphocyte reaction
- IKK
- IκB kinase.
REFERENCES
- 1. Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 [PubMed] [Google Scholar]
- 2. Zhu J., Paul W. E. (2008) CD4 T cells. Fates, functions, and faults. Blood 112, 1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou L., Chong M. M., Littman D. R. (2009) Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 [DOI] [PubMed] [Google Scholar]
- 4. Aggarwal S., Ghilardi N., Xie M. H., de Sauvage F. J., Gurney A. L. (2003) Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 [DOI] [PubMed] [Google Scholar]
- 5. Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 [DOI] [PubMed] [Google Scholar]
- 6. Veldhoen M., Uyttenhove C., van Snick J., Helmby H., Westendorf A., Buer J., Martin B., Wilhelm C., Stockinger B. (2008) Transforming growth factor-β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9, 1341–1346 [DOI] [PubMed] [Google Scholar]
- 7. Dardalhon V., Awasthi A., Kwon H., Galileos G., Gao W., Sobel R. A., Mitsdoerffer M., Strom T. B., Elyaman W., Ho I. C., Khoury S., Oukka M., Kuchroo V. K. (2008) IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 9, 1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nurieva R. I., Chung Y., Hwang D., Yang X. O., Kang H. S., Ma L., Wang Y. H., Watowich S. S., Jetten A. M., Tian Q., Dong C. (2008) Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lohr J., Knoechel B., Caretto D., Abbas A. K. (2009) Balance of Th1 and Th17 effector and peripheral regulatory T cells. Microbes Infect. 11, 589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soroosh P., Doherty T. A. (2009) Th9 and allergic disease. Immunology 127, 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paul W. E., Zhu J. (2010) How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 10, 225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. So T., Song J., Sugie K., Altman A., Croft M. (2006) Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc. Natl. Acad. Sci. U.S.A. 103, 3740–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salomon B., Bluestone J. A. (1998) LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J. Immunol. 161, 5138–5142 [PubMed] [Google Scholar]
- 14. Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 [DOI] [PubMed] [Google Scholar]
- 15. Liew F. Y., Pitman N. I., McInnes I. B. (2010) Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 10, 103–110 [DOI] [PubMed] [Google Scholar]
- 16. Netea M. G., Van der Meer J. W., Sutmuller R. P., Adema G. J., Kullberg B. J. (2005) From the Th1/Th2 paradigm towarda Toll-like receptor/T-helper bias. Antimicrob. Agents Chemother. 49, 3991–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bafica A., Scanga C. A., Feng C. G., Leifer C., Cheever A., Sher A. (2005) TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202, 1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imanishi T., Hara H., Suzuki S., Suzuki N., Akira S., Saito T. (2007) Cutting edge: TLR2 directly triggers Th1 effector functions. J. Immunol. 178, 6715–6719 [DOI] [PubMed] [Google Scholar]
- 19. Amsen D., Blander J. M., Lee G. R., Tanigaki K., Honjo T., Flavell R. A. (2004) Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 [DOI] [PubMed] [Google Scholar]
- 20. Magalhaes J. G., Fritz J. H., Le Bourhis L., Sellge G., Travassos L. H., Selvanantham T., Girardin S. E., Gommerman J. L., Philpott D. J. (2008) Nod2-dependent Th2 polarization of antigen-specific immunity. J. Immunol. 181, 7925–7935 [DOI] [PubMed] [Google Scholar]
- 21. Ting J. P., Trowsdale J. (2002) Genetic control of MHC class II expression. Cell 109, S21–S33 [DOI] [PubMed] [Google Scholar]
- 22. LeibundGut-Landmann S., Waldburger J. M., Krawczyk M., Otten L. A., Suter T., Fontana A., Acha-Orbea H., Reith W. (2004) Mini-review. Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 34, 1513–1525 [DOI] [PubMed] [Google Scholar]
- 23. Reith W., Mach B. (2001) The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 19, 331–373 [DOI] [PubMed] [Google Scholar]
- 24. Ou Q., Lin L., Huang L., Chen F., Wu K., Lu P., Zhang J., Chou K. Y. (2004) Persistence of MHC DR nonexpression on swine cells by introduction of a mutated MHC class II transactivator gene. A comparison with the effect induced by antisense RNA. J. Clin. Immunol. 24, 97–106 [DOI] [PubMed] [Google Scholar]
- 25. Yun S., Gustafsson K., Fabre J. W. (1997) Suppression of MHC class II expression by human class II trans-activator constructs lacking the N-terminal domain. Int. Immunol. 9, 1545–1553 [DOI] [PubMed] [Google Scholar]
- 26. Inohara N., Nuñez G. (2003) NODs. Intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3, 371–382 [DOI] [PubMed] [Google Scholar]
- 27. Ting J. P., Lovering R. C., Alnemri E. S., Bertin J., Boss J. M., Davis B. K., Flavell R. A., Girardin S. E., Godzik A., Harton J. A., Hoffman H. M., Hugot J. P., Inohara N., Mackenzie A., Maltais L. J., Nunez G., Ogura Y., Otten L. A., Philpott D., Reed J. C., Reith W., Schreiber S., Steimle V., Ward P. A. (2008) The NLR gene family. A standard nomenclature. Immunity 28, 285–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanneganti T. D., Lamkanfi M., Núñez G. (2007) Intracellular NOD-like receptors in host defense and disease. Immunity 27, 549–559 [DOI] [PubMed] [Google Scholar]
- 29. Rietdijk S. T., Burwell T., Bertin J., Coyle A. J. (2008) Sensing intracellular pathogens-NOD-like receptors. Curr. Opin. Pharmacol. 8, 261–266 [DOI] [PubMed] [Google Scholar]
- 30. Park J. H., Kim Y. G., McDonald C., Kanneganti T. D., Hasegawa M., Body-Malapel M., Inohara N., Núñez G. (2007) RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 31. Hasegawa M., Fujimoto Y., Lucas P. C., Nakano H., Fukase K., Núñez G., Inohara N. (2008) A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J. 27, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martinon F., Burns K., Tschopp J. (2002) The inflammasome. A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
- 33. Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. (2009) The inflammasome. A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanneganti T. D. (2010) Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li K., Xu W., Guo Q., Jiang Z., Wang P., Yue Y., Xiong S. (2009) Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ. Res. 105, 353–364 [DOI] [PubMed] [Google Scholar]
- 36. Yun S., Gustafsson K., Fabre J. W. (1998) Suppression of human anti-porcine T-cell immune responses by major histocompatibility complex class II transactivator constructs lacking the N-terminal domain. Transplantation 66, 103–111 [DOI] [PubMed] [Google Scholar]
- 37. Cherry W. B., Yoon J., Bartemes K. R., Iijima K., Kita H. (2008) A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 121, 1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hatada E. N., Krappmann D., Scheidereit C. (2000) NF-κB and the innate immune response. Curr. Opin. Immunol. 12, 52–58 [DOI] [PubMed] [Google Scholar]
- 39. Watanabe H., Numata K., Ito T., Takagi K., Matsukawa A. (2004) Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22, 460–466 [DOI] [PubMed] [Google Scholar]
- 40. Xu D., Chan W. L., Leung B. P., Huang F., Wheeler R., Piedrafita D., Robinson J. H., Liew F. Y. (1998) Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 187, 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haraldsen G., Balogh J., Pollheimer J., Sponheim J., Küchler A. M. (2009) Interleukin-33-cytokine of dual function or novel alarmin? Trends Immunol. 30, 227–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.