Background: The function of HtrA proteases in bacterial infections is widely unknown.
Results: Secreted HtrA from various bacterial pathogens exhibits a conserved specificity for cleavage of E-cadherin.
Conclusion: HtrA-mediated E-cadherin cleavage is a prevalent novel mechanism in bacterial pathogenesis.
Significance: HtrA activity plays a direct role in the pathogenesis of different bacteria.
Keywords: Bacterial Pathogenesis, Campylobacter, E-cadherin, Helicobacter pylori, Protease, Gram-negative Pathogens, HtrA, Neisseria
Abstract
The periplasmic chaperone and serine protease HtrA is important for bacterial stress responses and protein quality control. Recently, we discovered that HtrA from Helicobacter pylori is secreted and cleaves E-cadherin to disrupt the epithelial barrier, but it remained unknown whether this maybe a general virulence mechanism. Here, we show that important other pathogens including enteropathogenic Escherichia coli, Shigella flexneri, and Campylobacter jejuni, but not Neisseria gonorrhoeae, cleaved E-cadherin on host cells. HtrA deletion in C. jejuni led to severe defects in E-cadherin cleavage, loss of cell adherence, paracellular transmigration, and basolateral invasion. Computational modeling of HtrAs revealed a conserved pocket in the active center exhibiting pronounced proteolytic activity. Differential E-cadherin cleavage was determined by an alanine-to-glutamine exchange in the active center of neisserial HtrA. These data suggest that HtrA-mediated E-cadherin cleavage is a prevalent pathogenic mechanism of multiple Gram-negative bacteria representing an attractive novel target for therapeutic intervention to combat bacterial infections.
Introduction
Intact epithelial cell layers in mammals provide functional barriers protecting from intruding pathogens. Thus, many bacterial pathogens have evolved sophisticated mechanisms to subvert the epithelial integrity and to promote pathogenesis. The cell adhesion protein and tumor suppressor E-cadherin is a key molecule in the establishment of epithelial barrier functions, which tether adjacent cells by homotypic interactions (1). E-cadherin has five extracellular domains, a transmembrane domain, and an intracellular domain. Matrix metalloprotease 3 (MMP3) and MMP7 or a disintegrin and metalloprotease 10 (ADAM10) cleave E-cadherin, generating a soluble fragment that impairs cell adhesions (2, 3); however, the exact cleavage site is not known.
Recently, we identified high temperature requirement A (HtrA)2 from the class-I carcinogen Helicobacter pylori acting as a novel secreted virulence factor that directly cleaves E-cadherin on the surface of human gastric epithelial host cells. HtrA-mediated cleavage of E-cadherin facilitated the loss of the adherence junction complex, leading to the disruption of the epithelial barrier function in response to H. pylori infection (4).
Structure and functions of HtrA proteases have been widely studied in Escherichia coli. Generally, the E. coli HtrA homologues DegP, DegS, and DegQ exhibit dual functions as chaperones and serine proteases in the periplasm. DegP plays a role in cellular unfolded protein stress responses and protein quality control, as well as maturation and delivery of secreted proteins (5). Structurally, the 48-kDa monomeric DegP harbors an N-terminal trypsin-like protease domain followed by one or two PDZ domains (postsynaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (ZO-1)) responsible for substrate recognition and homo-oligomerization into larger complexes (5). The hexameric oligomer DegP represents the proteolytic inactive form that can be converted into active 12- or 24-mers upon substrate binding or via a temperature-dependent switch (6, 7). The first relevant target protein for DegP was identified as the acylated precursor of colicin A lysis protein (8). Discovery of pilin PapA (9) or α-amylase (MalS) (7) followed, indicating a strong implication of the HtrA chaperone activity, which is important for bacterial survival.
Of particular relevance, chaperone-dependent protein control has been also linked to bacterial pathogenicity (10), but the detailed mechanism remained largely unknown. In our present study, we investigated the proteolytic functions on E-cadherin of different HtrAs expressed by the Gram-negative gastrointestinal pathogens H. pylori, enteropathogenic E. coli (EPEC), Shigella flexneri, and Campylobacter jejuni and the urogenital pathogen Neisseria gonorrhoeae.
EXPERIMENTAL PROCEDURES
Bacteria
H. pylori (Hp26695) was cultured on agar plates containing 10% horse serum under microaerophilic conditions at 37 °C for 48 h. N. gonorrhoeae (MS11, N280) was grown on GC agar plates, whereas EPEC (E2348) and S. flexneri (15.4) were grown on LB agar plates. C. jejuni (81–176) htrA mutant was generated by inserting a chloramphenicol cassette and grown on Mueller-Hinton agar plates. Bacteria were harvested in sterile PBS supplemented with 0.1% Triton X-100 and sonicated to prepare bacterial lysates.
HtrA Expression and Purification
Cloning of H. pylori htrA (HpHtrA aa 18–475) was described previously (11). Bacterial htrA (NgHtrA aa 25–499, CjHtrA aa 17–472, EpHtrA aa 27–474, and SfHtrA aa 27–474) and their mutants were amplified from genomic DNA excluding predicted signal peptides (supplemental Table S1). PCR fragments flanked by restriction sites for BamHI/EcoRI (N. gonorrhoeae and EPEC) or BamHI/XmaI (C. jejuni) were cloned into pGEX-6P-1 (GE Healthcare).
In Vitro Cleavage Experiments
For in vitro cleavage studies, 100 ng of recombinant E-cadherin (R&D Systems) was incubated with 200 ng of recombinant bacterial HtrA in 50 mm Hepes (pH 7.4) at 37 °C for 16 h. Where indicated, 150 μm HHI (4) was added.
Infection Experiments
MKN-28 cells were cultured in RPMI 1640 medium (Biochrom) and 10% FCS. INT-407 cells were grown in Eagle's minimum essential medium (Invitrogen) and 10% FCS. Cells were cultured in 12-well plates for invasion and Transwell assays, and to minimize constitutive E-cadherin shedding, RPMI was replaced by fresh medium without FCS prior to infection.
SDS-PAGE and Western Blot
Cells were lysed (4), proteins were separated by SDS-PAGE and tested for E-cadherin using polyclonal antibodies recognizing the extracellular domain of E-cadherin (H-108, Santa Cruz Biotechnology), and whole cell lysates were tested for GAPDH. Bacterial HtrAs were detected by Coomassie Blue R250 staining (Bio-Rad).
Zymography
Bacterial lysates or recombinant HtrA were separated in casein-containing gels under nonreducing conditions. Subsequently, gels were renatured in 2.5% Triton-X-100 and equilibrated in developing buffer (11). Caseinolytic activity was visualized by staining with 0.5% Coomassie Blue R250. To detect oligomers by SDS-PAGE, HtrA was separated as described for zymograms excluding casein.
HtrA Structure Modeling
HtrA protein sequences from H. pylori (gi 345645045), C. jejuni (gi 87249907), EPEC (gi 215485324), S. flexneri (gi 30039963), and N. gonorrhoeae (gi 268598301) were retrieved from PubMed. For structural comparison of the active sites, cavities located next to the catalytic serine residues were extracted using PocketPicker (12) and converted into PoLiMorph graph representation (13). Based on these graphs (pocket frameworks), a comparison of shapes and of the pharmacophoric feature distributions within (please see supplemental Experimental Procedures for edditional information) the binding sites of HtrA species was calculated.
RESULTS
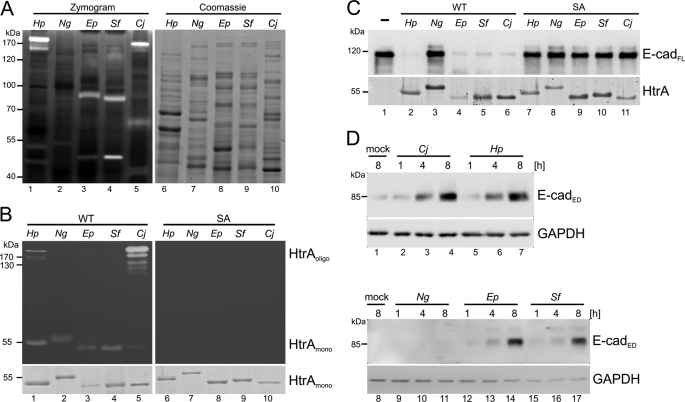
To investigate whether HtrA plays a central role in the pathogenesis of other bacteria, HtrAs of H. pylori, N. gonorrhoeae, EPEC, S. flexneri, and C. jejuni were analyzed by casein zymography (Fig. 1A). Bacterial lysates were separated by SDS-PAGE gels containing casein under nonreducing conditions. After renaturation and Coomassie Blue R250 staining, transparent bands indicate protease activity. As expected, in H. pylori lysates, caseinolytic activities of proteases with molecular masses between 45, 55, and ∼170 kDa were detected; these proteases have been previously identified as self-processed (45 kDa) HtrA, monomeric (55 kDa), and oligomeric HtrA (∼170 kDa) (Fig. 1A, lane 1) (11). Comparable with H. pylori, lysates of C. jejuni contained 170- and ∼60-kDa proteins and only a very little amount of an ∼45-kDa protease (Fig. 1A, lane 5). To identify these proteases, negatively stained proteolytic bands were excised and analyzed by mass spectrometry (supplemental Fig. S1, supplemental Table S2), indicating that the 170-kDa protease represents multimeric HtrA (supplemental Fig. S1, supplemental Table S2). We also identified HtrA in EPEC (Fig. 1A, lane 3) and S. flexneri (Fig. 1A, lane 4) by mass spectrometry analyses (supplemental Fig. S1, supplemental Table S2). Interestingly, EPEC and S. flexneri HtrA appeared mainly as monomers, indicating that multimerization in H. pylori and C. jejuni HtrA might be more stable. Both EPEC and S. flexneri also expressed an ∼80-kDa protease, which could be identified as the ATPase and specificity subunit of ClpA-ClpP ATP-dependent serine protease (supplemental Fig. S1, supplemental Table S2). In comparison with H. pylori, N. gonorrhoeae reproducibly expressed very low caseinolytic protease activities (Fig. 1A, lane 2). Unfortunately, we could not identify the responsible protease, which might be due to small amounts of expressed protein. Equal protein loading was confirmed by Coomassie Blue R250 staining (Fig. 1A, lanes 6–10).
FIGURE 1.
Bacterial HtrA cleaves E-cadherin. A, lysates of H. pylori (Hp), N. gonorrhoeae (Ng), EPEC (Ep), S. flexneri (Sf), and C. jejuni (Cj) were tested for protease activities in casein zymography. Coomassie Blue R250-stained SDS-PAGE gels served as a loading control. B, active (WT) and inactive (SA) recombinant HtrAs were separated by casein zymography. Equal HtrA amounts were demonstrated by Coomassie Blue R250 staining. HtrAoligo, HtrA oligomers; HtrAmono, HtrA monomers. C, in in vitro cleavage assays, recombinant E-cadherin was incubated with active (WT) or inactive HtrA (SA) or left untreated (−). Ectodomain shedding of E-cadherin was detected by the loss of full-length E-cadherin (E-cadFL). HtrAs were shown in SYPRO Ruby-stained gels. D, MKN-28 cells were colonized with C. jejuni, H. pylori, N. gonorrhoeae, EPEC, and S. flexneri for the indicated time periods or left untreated for 8 h (mock). Aliquots of supernatants were analyzed by Western blot for the extracellular domain (E-cadED). GAPDH in whole cell lysates is shown as loading control.
To characterize HtrA activities in more detail, we cloned and purified different recombinant wild-type (WT) HtrAs from H. pylori (HpHtrA), N. gonorrhoeae (NgHtrA), EPEC (EpHtrA), S. flexneri (SfHtrA), and C. jejuni (CjHtrA) and generated corresponding inactive mutants by exchanging serine to alanine (HpHtrA S221A, CjHtrA S225A, NgHtrA S246A, EpHtrA S236A, and SfHtrA S236A) in the active center. Activities of purified recombinant proteases were determined by casein zymography (Fig. 1B) and a protease assay with fluorophore-labeled casein (supplemental Fig. S1B). All tested recombinant HtrAs displayed caseinolytic activities either as a monomer and/or as an oligomer (Fig. 1B, lanes 1–5), whereas the corresponding inactive (SA) HtrA mutants were completely inactive (Fig. 1B, lanes 6–10). The HtrA activity was confirmed by a fluorometric assay using fluorophore-labeled casein to quantify HtrA activities. NgHtrA exhibited similar protease activity as compared with HpHtrA, whereas EpHtrA, SfHtrA, and CjHtrA showed an increased caseinolytic activity revealing differential HtrA activities in Gram-negative bacteria (supplemental Fig. S1B).
We have previously shown that E-cadherin represents an important substrate for HpHtrA allowing paracellular transmigration of H. pylori across the polarized epithelial cell barrier (4). Having established that recombinant HtrAs from various Gram-negative pathogens were indeed proteolytically active, we next tested whether different HtrAs also target E-cadherin as a substrate in vitro or on the cell surface. For this purpose, WT or SA recombinant HtrA proteins were incubated with recombinant E-cadherin in in vitro cleavage experiments followed by Western blot analysis detecting the extracellular domain of E-cadherin. WT HtrAs from H. pylori, EPEC, S. flexneri, and C. jejuni efficiently cleaved E-cadherin as reflected by the disappearance of the full-length molecule (Fig. 1C). Surprisingly, neisserial NgHtrA, which was highly active in casein degradation (Fig. 1B), did not cleave E-cadherin (Fig. 1C, lane 3). As control, E-cadherin was co-incubated with SA variants (Fig. 1C, lanes 7–11). Quantification data indicated that HpHtrA degraded ∼95% E-cadherin, whereas EpHtrA, SfHtrA, and CjHtrA cleaved ∼70–90% E-cadherin (supplemental Fig. S1C, black bars). As expected, E-cadherin cleavage was not observed using the proteolytic inactive HtrAs (Fig. S1C, gray bars).
To demonstrate the physiological relevance of E-cadherin cleavage for Gram-negative gastrointestinal pathogens, we infected MKN-28 cells with different pathogens for 1, 4, or 8 h or left them untreated (Fig. 1D). The pathogen-induced shedding of the extracellular domain of E-cadherin (E-cadED) was detected in the supernatants. Comparable with in vitro E-cadherin cleavage experiments, infection led to strong bacteria-induced E-cadherin shedding induced by H. pylori, C. jejuni, EPEC, and S. flexneri (Fig. 1D, lanes 2–7 and 12–17), but not by N. gonorrhoeae (Fig. 1D, lanes 9–11), although they adhered to and invaded host cells (data not shown). Similar results were observed in infection experiments using other human epithelial cell lines including Caco-2 or INT-407 cells (data not shown).
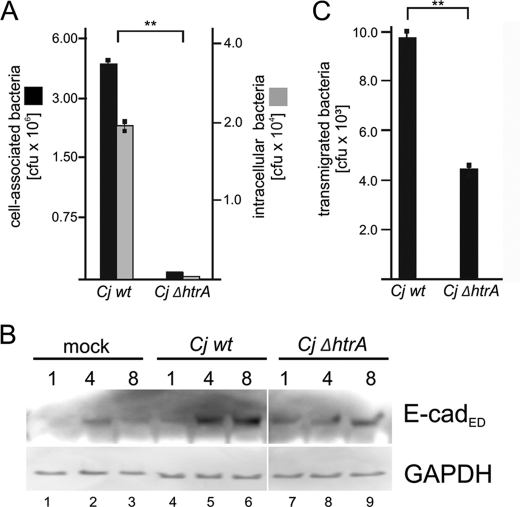
To investigate whether HtrA-mediated E-cadherin cleavage represents a general molecular pathogenicity mechanism, we generated a genomic htrA deletion mutant in C. jejuni (CjΔhtrA). Successful blocking of HtrA expression and secretion was demonstrated by casein zymography (supplemental Fig. S2A) and Western blotting (supplemental Fig. S2B). Importantly, genomic htrA knock-out did not influence survival and growth (supplemental Fig. S2C). We also did not observe an altered bacterial morphology and motility (data not shown). CjΔhtrA exhibited a strong defect in adherence to host cells (Fig. 2A, black bars) and cellular invasion (Fig. 2A, gray bars). In fact, CjΔhtrA did not induce strong E-cadherin shedding as compared with CjWT (Fig. 2B). Consequently, we observed that CjWT efficiently transmigrates across a polarized epithelial monolayer formed by MKN-28 cells (Fig. 2C). Based on these data, we conclude that bacterial HtrAs directly interfere with host cell functions independently from its chaperone activity as a crucial step in its pathogenesis.
FIGURE 2.
HtrA secretion is important for C. jejuni infections. A, INT-407 cells were infected for 6 h with Cjwt and CjΔhtrA and analyzed for adherence (black circles) or invasion (gray circles) at a multiplicity of infection of 100. Asterisks indicate statistical significance (**, p ≤ 0.01). B, to examine E-cadherin cleavage, MKN-28 cells were infected with Cjwt and CjΔhtrA for the indicated time periods or left untreated (mock). Aliquots of supernatants were analyzed for shedded E-cadherin (E-cadED). GAPDH in whole cell lysates was shown as loading control. C, translocating C. jejuni across confluent MKN-28 monolayers were quantified in filter assays. Cjwt and CjΔhtrA were infected with multiplicity of infection 50 for 24 h, transmigrated bacteria across the monolayer were harvested, and CFUs were determined.
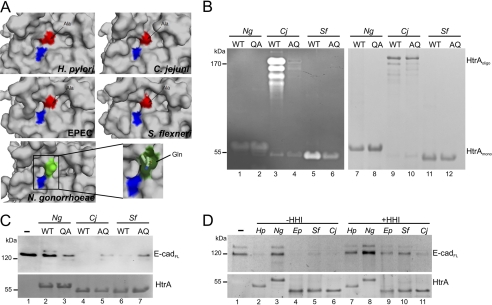
Although active NgHtrA did not target E-cadherin, the above data demonstrate that HtrA-mediated E-cadherin cleavage is not restricted to HpHtrA, but may represent an important and unique mechanism in the pathogenesis of multiple gastrointestinal microbes. Analyzing this important aspect, HtrA protein sequences of selected Gram-negative bacteria were aligned and examined for signal peptides, protease, and PDZ domains (supplemental Fig. S3A). Sequence alignment revealed a slightly higher identity of NgHtrA to the E. coli DegQ homolog (38%) than to DegP (37%) as recently suggested for Neisseria meningitidis (14). Hence, NgHtrA seems to be less related to other HtrAs, whereas HpHtrA and CjHtrA together with EpHtrA and SfHtrA exhibit high pairwise similarity (supplemental Fig. S3B). In particular, our comparative HtrA models suggest high structural conservation of the protease domain. Importantly, NgHtrA differed in the active site pocket by the exchange of an Ala to Gln (A263Q), which crucially protrudes into the pocket, likely interfering with substrate recognition (Fig. 3A). To test whether the A → Q exchange in NgHtrA prevents E-cadherin cleavage, we generated NgHtrA Q263A, and conversely, CjHtrA (A242Q) and SfHtrA (A253Q) mutants. In comparison with WT, NgHtrA QA showed a slight increase in proteolytic activity, whereas A → Q mutations decreased the activity of CjHtrA oligomers and SfHtrA monomers (Fig. 3B, lanes 1–6). This was obviously due to the glutamine in the active center because equal amounts of protease were loaded and did not interfere with oligomer formation of CjHtrA (Fig. 3B, lanes 7–12). Interestingly, in contrast to NgHtrA WT, the NgHtrA QA variant was able to cleave E-cadherin as a substrate, whereas A → Q mutations in CjHtrA and SfHtrA showed a significant decrease in E-cadherin shedding (Fig. 3C). In summary, these data indicate that evolutionary-driven structural differences in the active site pocket of HtrA determine E-cadherin cleavage activity.
FIGURE 3.
Alanine-to-glutamine exchange in active pocket of NgHtrA prevents E-cadherin cleavage. A, the catalytic serine in the active pocket was colored in blue, and the pocket differences between neisserial HtrA and HtrA structures from other species were colored in red (Ala) and green (Gln). B, wild-type (WT) and mutated HtrAs (QA or AQ, respectively) from N. gonorrhoeae, C. jejuni, and S. flexneri were analyzed by zymography to detect proteolytic activity (lanes 1–6) and by nonreducing SDS-PAGE gels to show multimerization (lanes 7–12). HtrAoligo, HtrA oligomers; HtrAmono, HtrA monomers. C, WT and mutated HtrAs were tested in in vitro cleavage experiments using E-cadherin as a substrate. D, samples were co-incubated with HHI. Cleavage was detected by the loss of full-length E-cadherin (E-cadFL). SYPRO Ruby-stained gels served as loading controls.
Small molecule inhibitors binding to the active center of HtrA would represent an attractive strategy in combating microbes. We described the first functional inhibitor HHI targeting HpHtrA (15) to prevent E-cadherin shedding and the paracellular transmigration of H. pylori (4). To test whether HHI also inhibits HtrA from other Gram-negative bacteria, we performed in vitro E-cadherin cleavage experiments (Fig. 3D). Western blots of three independently performed experiments were quantified and expressed as relative intensity as compared with untreated E-cadherin controls (supplemental Fig. S3C). As expected, a strong inhibitory effect of HHI on HpHtrA was observed, whereas inhibition of EpHtrA, SfHtrA, and CjHtrA occurred to lesser extents (Fig. 3B, supplemental Fig. S3C). These data indicate structural differences in the active pockets, which require selective compounds for efficient inhibition.
DISCUSSION
The periplasmic chaperone and serine protease HtrA has been shown to play key roles in protein folding, maturation, and preventing host-induced protein-denaturation and in the bacterial unfolded protein response (5). These data led to the widely accepted opinion that HtrA exhibits a rather indirect effect on infectious diseases (10). Based on current studies, we now expanded this knowledge by unraveling a more direct role of HtrA through its immediate interfering with host cell factors. We identified HpHtrA as a secreted protease that cleaves E-cadherin to disrupt intercellular adhesion of host cells, thus adding a novel aspect to HtrA functions in bacterial pathogenesis (4). Very recently, Chlamydia trachomatis-secreted HtrA was detected in the host cytosol, where it might also manipulate host signaling pathways (16), supporting our findings that HtrA can directly target eukaryotic host cell proteins.
In the present study, we investigated HtrA from N. gonorrhoeae, the etiologic agent inducing gonorrhea, and from the gastrointestinal pathogens H. pylori, C. jejuni, S. flexneri, and EPEC. We found that HtrAs from all tested pathogens were catalytic active and degraded casein as a substrate. Interestingly, NgHtrA did not cleave E-cadherin, which is explainable by the structural differences in the active pocket. NgHtrA was annotated as a serine protease, but obviously revealed a higher identity to the DegQ homolog consistent with HtrA from N. meningitidis (14), suggesting that DegQ and DegP functions are similar, but do not serve as general functional homologs (14). Among other differences, the exchanged Gln-263 crucially protrudes into the pocket of NgHtrA, which might interfere with E-cadherin binding and cleavage. Strikingly, a single Q → A mutation was sufficient to convert NgHtrA into an E-cadherin-cleaving protease. Obviously N. gonorrhoeae does not require E-cadherin processing because it crosses the epithelium through transcytosis (17), whereas other pathogens may benefit from HtrA-mediated E-cadherin shedding.
Adherence junctions are key structures maintaining the epithelial barrier functions, which are frequently disrupted by bacteria crossing via the paracellular route (18, 19). Due to its central function in the architecture of healthy epithelia, E-cadherin represents a highly attractive target for bacteria because it acts as a receptor for Listeria monocytogenes (20) or Streptococcus pneumoniae (21). However, it is unclear whether truncated E-cadherin can function as a receptor for bacteria. Hence, the finding that gastrointestinal pathogens cleave E-cadherin on host cells is exclusively novel and suggests that HtrA-triggered E-cadherin cleavage is a highly conserved and common mechanism in bacterial pathogenesis. It has been described that S. flexneri localizes to adherence junctions (22). Together with the observation that E-cadherin expression appears to be necessary for cell-to-cell spread (23), it is now tempting to investigate whether SfHtrA-mediated E-cadherin cleavage may be involved in S. flexneri intercellular spreading. In fact, HtrA is required for IcsA expression and S. flexneri uptake (24). Although EPEC in turn disrupts adherence junctions via secreted proteins (25) and EpHtrA is important in pili formation or intimin presentation (26, 27), this study is the first report on a functional interaction between host and EpHtrA, which might close the missing link between outer membrane proteins from EPEC and the observed increase of paracellular permeability for bacteria across Caco-2 monolayers (25).
Using HtrA-deficient C. jejuni, we confirmed the in vivo relevance of HtrA-mediated E-cadherin cleavage. CjHtrA induced E-cadherin cleavage on host cells, and we showed an attenuated transmigration of CjΔhtrA. This might be explainable by the finding that CjΔhtrA mutants are less vital (28). However, in our study, CjΔhtrA showed unaltered growth and survival, but had a strong defect in adherence and invasion. Future studies will be necessary to determine whether HtrA processes a bacterial adhesin or whether truncated E-cadherin serves as a receptor in bacterial adherence and internalization. However, deletion of htrA resulted in a drastic loss of bacterial transmigration across a polarized monolayer, a process that has been identified for H. pylori (4).
Due to the functional role of HtrA in infectious diseases, the development of specific and pathogen-selective HtrA inhibitors is desirable. We have developed the inhibitor HHI for HpHtrA (15) that impaired E-cadherin degradation and epithelial barrier (4). On the basis of our data, we concluded that specific inhibition of HtrAs from different pathogens requires highly selective compounds. This appears crucially important because in contrast to unwanted side effects of antibiotics on the microbiota, selective targeting of bacteria would be highly beneficial for patient health.
Supplementary Material
Acknowledgment
We thank Christof Hauck for providing Neisseria gonorrhoeae.
This work was supported by grants from the Paul-Ehrlich Institute (to B. H. and S. W.) and by Science Foundation Ireland Grant UCD 09/IN.1/B2609 (to S. B.).

This article contains supplemental Experimental Procedures, Tables S1 and S2, and Figs. S1–S3.
- HtrA
- high temperature requirement A
- EPEC
- enteropathogenic E. coli
- HHI
- HpHtrA inhibitor
- aa
- amino acids
- E-cad
- E-cadherin
- Ng
- N. gonorrhoeae
- Cj
- C. jejuni
- Ep
- EPEC
- Sf
- S. flexneri
- Hp
- H. pylori
- SA
- inactive serine → alanine HtrA mutant.
REFERENCES
- 1. Niessen C. M., Gottardi C. J. (2008) Molecular components of the adherens junction. Biochim. Biophys. Acta 1778, 562–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maretzky T., Reiss K., Ludwig A., Buchholz J., Scholz F., Proksch E., de Strooper B., Hartmann D., Saftig P. (2005) ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proc. Natl. Acad. Sci. U.S.A. 102, 9182–9187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noë V., Fingleton B., Jacobs K., Crawford H. C., Vermeulen S., Steelant W., Bruyneel E., Matrisian L. M., Mareel M. (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J. Cell Sci. 114, 111–118 [DOI] [PubMed] [Google Scholar]
- 4. Hoy B., Löwer M., Weydig C., Carra G., Tegtmeyer N., Geppert T., Schröder P., Sewald N., Backert S., Schneider G., Wessler S. (2010) Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 11, 798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clausen T., Kaiser M., Huber R., Ehrmann M. (2011) HTRA proteases: regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 12, 152–162 [DOI] [PubMed] [Google Scholar]
- 6. Krojer T., Sawa J., Schäfer E., Saibil H. R., Ehrmann M., Clausen T. (2008) Structural basis for the regulated protease and chaperone function of DegP. Nature 453, 885–890 [DOI] [PubMed] [Google Scholar]
- 7. Spiess C., Beil A., Ehrmann M. (1999) A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97, 339–347 [DOI] [PubMed] [Google Scholar]
- 8. Cavard D., Lazdunski C., Howard S. P. (1989) The acylated precursor form of the colicin A lysis protein is a natural substrate of the DegP protease. J. Bacteriol. 171, 6316–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones C. H., Dexter P., Evans A. K., Liu C., Hultgren S. J., Hruby D. E. (2002) Escherichia coli DegP protease cleaves between paired hydrophobic residues in a natural substrate: the PapA pilin. J. Bacteriol. 184, 5762–5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingmer H., Brøndsted L. (2009) Proteases in bacterial pathogenesis. Res. Microbiol. 160, 704–710 [DOI] [PubMed] [Google Scholar]
- 11. Löwer M., Weydig C., Metzler D., Reuter A., Starzinski-Powitz A., Wessler S., Schneider G. (2008) Prediction of extracellular proteases of the human pathogen Helicobacter pylori reveals proteolytic activity of the Hp1018/19 protein HtrA. PLoS One 3, e3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisel M., Proschak E., Schneider G. (2007) PocketPicker: analysis of ligand binding sites with shape descriptors. Chem. Cent. J. 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reisen F., Weisel M., Kriegl J. M., Schneider G. (2010) Self-organizing fuzzy graphs for structure-based comparison of protein pockets. J. Proteome Res. 9, 6498–6510 [DOI] [PubMed] [Google Scholar]
- 14. Volokhina E. B., Grijpstra J., Stork M., Schilders I., Tommassen J., Bos M. P. (2011) Role of the periplasmic chaperones Skp, SurA, and DegQ in outer membrane protein biogenesis in Neisseria meningitidis. J. Bacteriol. 193, 1612–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Löwer M., Geppert T., Schneider P., Hoy B., Wessler S., Schneider G. (2011) Inhibitors of Helicobacter pylori protease HtrA found by “virtual ligand” screening combat bacterial invasion of epithelia. PLoS One 6, e17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu X., Lei L., Gong S., Chen D., Flores R., Zhong G. (2011) The chlamydial periplasmic stress response serine protease cHtrA is secreted into host cell cytosol. BMC Microbiol. 11, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J., Gray-Owen S. D., Knorre A., Meyer T. F., Dehio C. (1998) Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol. Microbiol. 30, 657–671 [DOI] [PubMed] [Google Scholar]
- 18. Balkovetz D. F., Katz J. (2003) Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 5, 613–619; Correction (2003) Microbes Infect.5, 1415 [DOI] [PubMed] [Google Scholar]
- 19. Wroblewski L. E., Peek R. M., Jr. (2011) Targeted disruption of the epithelial-barrier by Helicobacter pylori. Cell Commun. Signal. 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mengaud J., Ohayon H., Gounon P., Mege R.-M., Cossart P. (1996) E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84, 923–932 [DOI] [PubMed] [Google Scholar]
- 21. Anderton J. M., Rajam G., Romero-Steiner S., Summer S., Kowalczyk A. P., Carlone G. M., Sampson J. S., Ades E. W. (2007) E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42, 225–236 [DOI] [PubMed] [Google Scholar]
- 22. Vasselon T., Mounier J., Hellio R., Sansonetti P. J. (1992) Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect. Immun. 60, 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sansonetti P. J., Mounier J., Prévost M. C., Mège R. M. (1994) Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell 76, 829–839 [DOI] [PubMed] [Google Scholar]
- 24. Purdy G. E., Hong M., Payne S. M. (2002) Shigella flexneri DegP facilitates IcsA surface expression and is required for efficient intercellular spread. Infect. Immun. 70, 6355–6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malladi V., Shankar B., Williams P. H., Balakrishnan A. (2004) Enteropathogenic Escherichia coli outer membrane proteins induce changes in cadherin junctions of Caco-2 cells through activation of PKCα. Microbes Infect. 6, 38–50 [DOI] [PubMed] [Google Scholar]
- 26. Vogt S. L., Nevesinjac A. Z., Humphries R. M., Donnenberg M. S., Armstrong G. D., Raivio T. L. (2010) The Cpx envelope stress response both facilitates and inhibits elaboration of the enteropathogenic Escherichia coli bundle-forming pilus. Mol. Microbiol. 76, 1095–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bodelón G., Marín E., Fernández L. A. (2009) Role of periplasmic chaperones and BamA (YaeT/Omp85) in folding and secretion of intimin from enteropathogenic Escherichia coli strains. J. Bacteriol. 191, 5169–5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brøndsted L., Andersen M. T., Parker M., Jørgensen K., Ingmer H. (2005) The HtrA protease of Campylobacter jejuni is required for heat and oxygen tolerance and for optimal interaction with human epithelial cells. Appl. Environ. Microbiol. 71, 3205–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.