Background: Selenomethionine cytotoxicity has not been completely elucidated even though it is often used for phase determination in X-ray crystallography.
Results: Yeast strain genetically modified to increase intracellular thiol compounds showed selenomethionine resistance.
Conclusion: Selenomethionine induces depletion of intracellular thiol compounds in yeast cells.
Significance: This work is the first report of cytotoxicity mechanism of selenomethionine in view point of its metabolite.
Keywords: Metabolic Engineering, Metabolomics, Selenium, Yeast, Yeast Metabolism, Cysteine Synthesis, Selenomethionine, Toxicity
Abstract
Although selenium is an essential element, its excessive uptake is detrimental to living organisms. The significance of selenium for living organisms has been exploited for various purposes. However, the molecular basis of selenium toxicity is not completely understood. Here, we applied a capillary electrophoresis time-of-flight mass spectrometry-based metabolomics approach to analysis of yeast cells treated with selenomethionine. The data indicated that intracellular thiol compounds are significantly decreased, and diselenide and selenosulfide compounds are increased in selenomethionine-treated cells. The growth defect induced by selenomethionine was recovered by extracellular addition of cysteine and by genetic modification of yeast cells that have an additional de novo synthetic pathway for cysteine. Because cysteine is an intermediate of thiol compounds, these results suggested that the loss of a reduced form of thiol compounds due to selenomethionine causes a growth defect of yeast cells.
Introduction
Selenium is a chemical element that is found in group 16 of the periodic table and is chemically related to sulfur. Although this trace element is essential for many organisms, its excessive uptake is detrimental. Selenium is biologically significant due to its presence in selenoproteins, into which a selenocysteine (Sec)2 residue is specifically incorporated at the catalytic center. Most selenoproteins participate in the maintenance of redox homeostasis (1). Sec biosynthesis begins with the transfer of serine to tRNASec by seryl-tRNA synthetase (2–4). In Archaea and Eukarya, O-phosphoseryl-tRNASec kinase phosphorylates the hydroxyl group of serine on seryl-tRNASec (4, 5), and the resulting O-phosphoseryl-tRNASec is subsequently converted to selenocysteinyl-tRNASec by Sec synthetase (6, 7). In contrast, in Bacteria, pyridoxal phosphate-dependent Sec synthetase that is encoded by selA in Escherichia coli directly generates selenocysteinyl-tRNASec from seryl-tRNASec (8). The selenocysteinyl-tRNASec is transferred into the ribosome where Sec is incorporated into nascent polypeptide chains at the position corresponding to the UGA codon of the mRNA of selenoproteins (9, 10). Selenomethionine (SeMet) is another organic selenium compound that can be found in plant materials. Plants have the ability to convert inorganic selenium that is absorbed from the soil into SeMet via a methionine synthetic pathway (11, 12). SeMet can be transferred onto tRNAMet as efficiently as methionine and is incorporated into proteins in response to the AUG codon. However, it has been reported that peptide bond formation of selenomethionyl-tRNAMet is slightly lower than that of methionyl-tRNAMet (13).
Proteins containing selenomethionine instead of methionine are valuable tools in structural biology for phase determination of their crystals by using single-wavelength anomalous dispersion or multi-wavelength anomalous dispersion phasing methods (14, 15). However, the toxic effect of SeMet on the cells has not been completely elucidated, and the problem of poor yields of the SeMet-containing proteins has not yet been overcome. Recent studies using budding yeast Saccharomyces cerevisiae have demonstrated the usefulness of SeMet-resistant strains; sam1Δ sam2Δ (16), cys3Δ (17), and mup1–100 (18). All of these strains have mutations in genes involved in sulfur amino acid metabolism. Malkowski et al. reported the interesting result that a met6Δ sam1Δ sam2Δ triple mutant, in which neither S-adenosylmethionine (AdoMet) nor methionine is generated, showed better growth than a met6Δ single mutant (16). It is therefore believed that SeMet toxicity is due to selenium compounds that are derived from SeMet, rather than due to an increase in the intracellular methionine pool that is induced by blocking of the sulfur metabolic pathway or by substitution of SeMet for methionine residues in endogenous proteins. Therefore, metabolic changes induced by SeMet were of interest for understanding SeMet cytotoxicity.
Here, we employed a metabolomics approach using capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS) in an attempt to investigate the molecular basis of selenium toxicity. We demonstrate a specific decrease in the level of intracellular thiol compounds in SeMet-treated yeast. We further genetically engineered the sulfur metabolic pathway to confer SeMet resistance on yeast cells.
EXPERIMENTAL PROCEDURES
Yeast Strains, Culture Medium, and Genetic Procedures
S. cerevisiae W303-1A (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) was used as the wild-type strain (19). Synthetic complete (SC) medium was prepared as described previously (18). Standard yeast genetic methods were used for the construction of yeast strains (20).
Plasmid Construction
The coding regions of cysE and cysK from E. coli, that encode serine acetyltransferase and O-acetylserine sulfhydrylase, respectively, were amplified using PCR from the genomic DNA of the E. coli DH5α strain that was used as a template using Phusion DNA polymerase (New England Biolabs) and the primers shown in supplemental Table S1. The 842-bp fragment that was amplified using the primers EcCysE-f and EcCysE-r was digested with SacI and SalI and was purified using the QIAquick gel extraction kit (Qiagen). The purified fragment was ligated into the corresponding site of the plasmid YEp351GAPII (21) to generate YEp351GAPII-cysE (PTDH3-cysE, 2 μm, LEU2). The 990-bp fragment that was amplified using the primers EcCysK-f and EcCysK-r was digested with EcoRI and SalI and purified. The purified fragment was ligated into the corresponding site of the plasmid YEp352GAPII (21) to generate YEp352GAPII-cysK (PTDH3-cysK, 2 μm, URA3).
SeMet Resistance Assay
Yeast strains were cultured in liquid SC medium lacking methionine (SC-Met) and the amino acids needed to meet their auxotrophic requirements (drop-out medium) until they reached mid-log phase (A600 = 0.8–1.0). The cells were harvested by centrifugation (1,000 × g, 5 min) and were resuspended in SC-Met to an A600 value of 0.15. The cell suspensions (150 μl) were then inoculated into the same medium (300 μl) containing various concentrations of SeMet in a 2-ml-deep 96-well plate (Greiner Bio-One). After incubation for 24 h at 30 °C, the A values at 595 nm were measured using a microplate reader (Bio-Rad).
Comprehensive Analysis of Yeast Metabolites
Metabolomics analysis was performed as described previously with some modification (22–24). In brief, metabolites were extracted from 10 A600 units of cells that were harvested by filtration through a membrane filter (0.45-μm pore; Millipore). Methionine sulfone and d-camphor-10-sulfonic acid were used as internal cationic and anionic standards, respectively. Lyophilized cell extracts were dissolved in 50 μl of Milli-Q water and were then subjected to CE-TOFMS analysis. All CE-TOFMS experiments were performed at Human Metabolome Technologies Inc. (Tsuruoka, Japan). The peaks detected in CE-TOFMS analysis were assigned based on the m/z values and migration times of standard compounds. Because most selenium compounds are not commercially available, selenium-containing metabolites were identified based on the m/z values of the peaks that showed the characteristic isotopic patterns of selenium.
Quantification of Intracellular Thiol Compounds
Yeast metabolites were extracted from 20 A600 units of cells using the same procedures as described above, and the thiol compounds in the extracts were specifically labeled with 4-fluoro-7-sulfobenzofurazan (SBD-F; Dojindo, Japan) as follows. Lyophilized metabolites were dissolved in 60 μl of Milli-Q water, and an aliquot of each extract (50 μl) was mixed with 10 μl of 15 mm EDTA and 15 μl of 10 mg/ml SBD-F in 0.5 m sodium borate (pH 9.5). After incubation for 1 h at 60 °C, 25 μl of 0.2 n HCl was added to terminate the reaction and prevent further labeling. The resulting SBD-F derivatives were separated by high performance liquid chromatography (HPLC) using a 5C18-PAQ column (250-mm × 4.6-mm inner diameter; Nacalai Tesque, Japan). Elution was performed using two solvents at a flow rate of 1.0 ml/min; solvent A was 0.1 m sodium phosphate (pH 6.0), and solvent B was 20% (v/v) methanol in solvent A. The elution program was set as follows: 0–5 min with isocratic elution of solvent A, 5–20 min with a linear gradient of solvent B from 0% (v/v) to 15% (v/v), 20–25 min with a linear gradient of solvent B from 15% (v/v) to 40% (v/v), 25–40 min with a linear gradient of solvent B from 40% to 55% (v/v). The eluate was monitored by fluorescence (excitation wavelength, 385 nm; emission wavelength, 515 nm). Quantification of thiol compounds was performed using standard curves developed based on the peak areas of authentic compounds (cysteine, homocysteine, GSH, and CoA) labeled with SBD-F. To normalize recovery of the extracted metabolites, methionine sulfone labeled with 4-fluoro-7-nitrobenzofurazan (NBD-F; Dojindo) was used as an internal standard. A 5-μl aliquot of the extract was diluted with 30 μl of Milli-Q water, mixed with 15 μl of 0.5 m sodium borate (pH 8.5), 10 μl of 15 mm EDTA, and 15 μl of 10 mg/ml NBD-F in acetonitrile, followed by incubation for 3 min at 60 °C. The labeling reactions were terminated by the addition of 25 μl of 0.2 n HCl, and 10 μl of the resulting mixtures was subjected to HPLC analysis using an 5C18-ARII column (150-mm × 4.6-mm inner diameter; Nacalai Tesque) equilibrated with solvent C (acetonitrile/75 mm phosphoric acid, 12:88). The elution was performed at a flow rate of 1.2 ml/min with isocratic elution of solvent C for 30 min, a linear gradient of solvent D (acetonitrile/methanol/50 mm potassium dihydrogen phosphate, 20:40:40) from 0% (v/v) to 80% (v/v) for 5 min, and a linear gradient of solvent D from 80% (v/v) to 100% (v/v) for 15 min. The eluate was monitored by fluorescence (excitation wavelength, 465 nm; wavelength emission, 520 nm).
RESULTS
Global Analysis of Metabolites in SeMet-treated Yeast
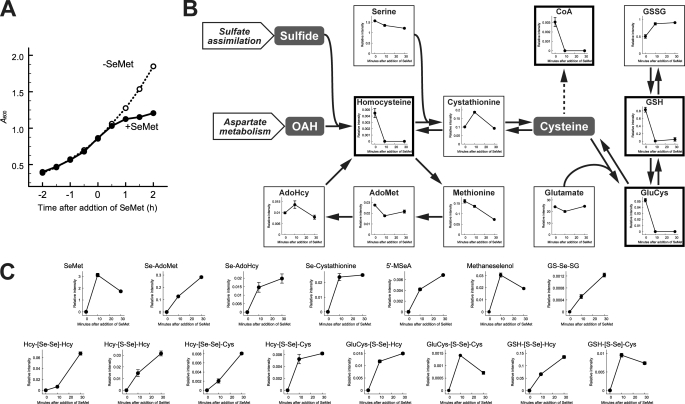
Before extraction of yeast metabolites, we examined the effect of SeMet on yeast cell growth. Although growth retardation was clearly apparent at 1 h after the addition of SeMet in SC-Met, the growth rates of SeMet-treated and untreated cells were almost the same over at least the first 30 min (Fig. 1A). We therefore extracted metabolites from cells treated with SeMet for 0, 10, and 30 min and subjected them to CE-TOFMS analysis. Three independent experiments were carried out, and 221 signals were assigned based on their m/z values and on the migration time of authentic standards (supplemental Table S2). The relative intensities of metabolites in SeMet-treated cells (30 min) were compared with those in the control cells, resulting in identification of 13 candidate metabolites whose levels were significantly increased (6 compounds) or decreased (7 compounds) following exposure to SeMet (Table 1). Although the levels of homoserinelactone, 1-aminocyclopropane-1-carboxylic acid, and urocanic acid were dramatically changed in SeMet-treated cells, the genes involved in the synthesis of these compounds have not yet been identified in S. cerevisiae. Of the other 10 metabolites, 4 metabolites (reduced glutathione (GSH), coenzyme A (CoA), γ-glutamylcysteine (GluCys), and homocysteine), whose levels were decreased by SeMet treatment within 10 min, were sulfur-containing compounds. Because the level of cystathionine, which is an intermediate between homocysteine and GluCys, was not changed by SeMet treatment (Fig. 1B), it appeared that thiol compounds were specifically decreased in SeMet-treated cells. We also investigated the distribution of selenium compounds in SeMet-treated cells. SeMet was detected at 10.82 min in the extracted ion chromatogram at m/z 198.002, which corresponded to the protonated ion of SeMet (supplemental Fig. S1A), and the mass spectrometry of the peak at 10.82 min showed a spectrum that was typical of selenium-containing compounds (supplemental Fig. S1B). Other selenium analogues of sulfur metabolites were also searched for in the same manner. Ultimately, 15 candidate compounds were reproducibly detected in the extracts of cells treated with SeMet for 10 and 30 min from three independent experiments (Fig. 1C). Analogues of endogenous sulfur compounds, Se-adenosylselenomethionine (Se-AdoMet), Se-adenosylselenohomocysteine (Se-AdoHcy), Se-cystathionine, and 5′-methylselenoadenosine (5′-MSeA) were detected within 10 min of SeMet treatment. Although selenol compounds were not detected, we did identify dimeric amino acids that were covalently linked by a diselenide bond between two selenohomocysteine (SeHcy) residues (displayed as Hcy-[Se-Se]-Hcy in Fig. 1C) or between Sec and SeHcy (displayed as Hcy-[Se-Se]-Cys in Fig. 1C). In addition, dimeric compounds formed between thiol and selenol compounds by oxidation were detected (displayed as the linkage -[S-Se]- in Fig. 1C). These results indicate that S. cerevisiae has the ability to convert SeMet into at least Sec. However, selenol compounds including Sec were present as an oxidized form in SeMet-treated cells.
FIGURE 1.
Metabolic profile of SeMet-treated yeast. A, S. cerevisiae W303-1A strain was grown in SC-Met liquid medium at 30 °C until mid-log phase, and then SeMet was added (final concentration, 0.25 mm). The A600 values of the cultures with (solid line) or without (dashed line) SeMet were plotted. B, metabolites extracted from SeMet-treated cells were analyzed using CE-TOFMS. Time-dependent changes in metabolite pools are mapped on the sulfur metabolic pathway. Metabolites whose levels changed significantly (p < 0.05) are indicated in bold frames. C, selenium compounds assigned based on their m/z values and isotopic patters are shown. These graphs are representative results of the assigned peaks (see also supplemental Table S3). The mean values of the relative intensity obtained from three independent experiments were plotted, and the vertical bars indicate S.E. (B and C).
TABLE 1.
Metabolites whose levels were significantly changed by SeMet treatment
The mean values of the ratio (T30/T0) from three independent experiments are presented. The metabolic pathways were assigned according to the KEGG database. CoA, γ-glutamylcysteine, homocysteine, and urocanic acid were not detected in cell extracts after SeMet treatment. All compounds detected in CE-TOFMS analysis are listed in supplemental Table S2.
| Candidate compounds | -Fold | Related metabolic pathway |
|---|---|---|
| Homoserinelactone | 160 | |
| 1-Aminocyclopropane-1-carboxylic acid | 31 | Cysteine and methionine metabolism |
| Propanoate metabolism | ||
| GDP-mannose | 5.6 | Fructose and mannose metabolism |
| N-Glycan biosynthesis | ||
| Amino sugar and nucleotide sugar metabolism | ||
| Ribulose 5-phosphate | 3.6 | Pentose phosphate pathway |
| Pentose and glucuronate interconversions | ||
| Methane metabolism | ||
| Riboflavin metabolism | ||
| Vitamin B6 metabolism | ||
| Glycerophosphocholine | 3.1 | Glycerophospholipid metabolism |
| Ether lipid metabolism | ||
| Trehalose 6-phosphate | 3.1 | Starch and sucrose metabolism |
| Orotidine 5′-monophosphate | 0.21 | Pyrimidine metabolism |
| N-Acetylglucosamine 6-phosphate | 0.10 | Amino sugar and nucleotide sugar metabolism |
| Reduced glutathione | 0.06 | Cysteine and methionine metabolism |
| Glutathione metabolism | ||
| CoA | NDa | Fatty acid metabolism |
| Pantothenate and CoA biosynthesis | ||
| γ-Glutamylcysteine | ND | Glutathione metabolism |
| Homocysteine | ND | Cysteine and methionine metabolism |
| Sulfur metabolism | ||
| Urocanic acid | ND | Histidine metabolism |
a ND, not detected in T30 samples.
Suppression of SeMet Toxicity by Simultaneous Addition of Cysteine
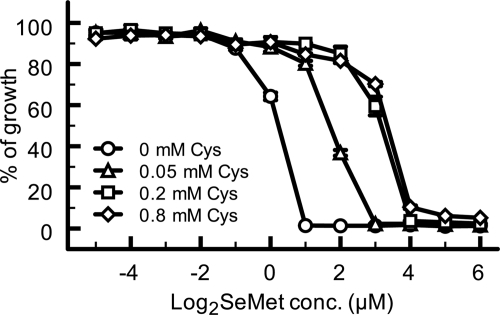
To confirm the hypothesis that SeMet induces the depletion of intracellular thiol compounds, we tested the effect of cysteine addition on the SeMet resistance of S. cerevisiae because cysteine is an intermediate in the synthesis of the thiol compounds whose levels were decreased by SeMet treatment (Fig. 1B). Wild-type S. cerevisiae was grown for 24 h in SC-Met medium containing various concentrations of SeMet and cysteine, and the cell density of the cultures was measured as described under “Experimental Procedures.” The minimum inhibitory concentration of SeMet for cells without cysteine was 1 μm, whereas the values increased as the cysteine concentration was increased, up to 4 μm (Fig. 2). These results strongly suggested that the toxic effect of SeMet on yeast cells was due to the depletion of thiol compounds. However, we previously demonstrated that impairment of the activity of the high affinity methionine permease (Mup1p), by which not only extracellular methionine but also cysteine are incorporated into yeast cells, is responsible for SeMet resistance (18, 25). We therefore further examined the relationship between SeMet resistance and the intracellular level of thiol compounds as follows.
FIGURE 2.
SeMet toxicity is suppressed by cysteine. The growth of wild-type cells at 30 °C in SC-Met medium containing both SeMet and cysteine (0 mm, circles; 0.05 mm, triangles; 0.2 mm, squares; 0.8 mm, diamonds) was monitored. The means of percentage growth normalized to growth without SeMet were plotted. Vertical bars indicate S.E. from triplicate experiments.
Development of Alternative Pathway for de novo Synthesis of Cysteine in Yeast Cells
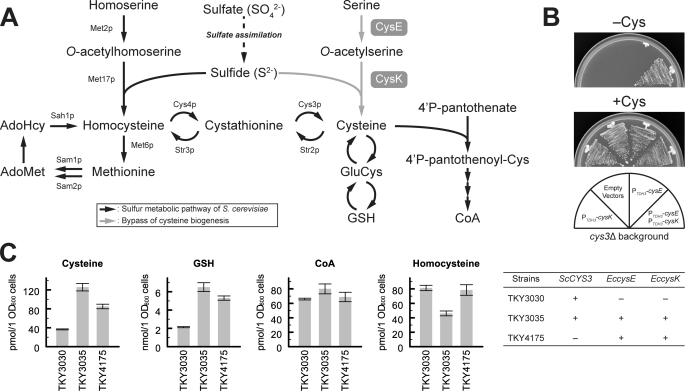
In yeast, sulfide is synthesized though a sulfate assimilation pathway and is incorporated into a four-carbon chain (O-acetylhomoserine) by O-acetylhomoserine sulfhydrylase (encoded by MET17 in S. cerevisiae), yielding homocysteine that is converted into cysteine via a cystathionine intermediate (called the transsulfuration pathway). In contrast, in E. coli and plants, sulfide is incorporated into a three-carbon chain (O-acetylserine) by O-acetylserine sulfhydrylase (encoded by cysK in E. coli), directly yielding cysteine (Fig. 3A) (26). The S. cerevisiae Met17 protein has been reported to be a bifunctional enzyme that also shows O-acetylserine sulfhydrylase activity in vitro (27, 28). This means that the failure of yeast to directly synthesize cysteine is due to the lack of serine acetyltransferase (encoded by cysE in E. coli) in yeast. We therefore attempted to introduce serine acetyltransferase activity into yeast by expression of the E. coli cysE gene in a cys3Δ mutant of S. cerevisiae (TKY417 strain) (18); however, the cysteine auxotrophy of this mutant was not changed by overexpression of the cysE gene (see Fig. 3B). To support the O-acetylserine sulfhydrylase activity of the endogenous Met17 protein, we additionally expressed the E. coli cysK gene in the strain. As shown in Fig. 3B, the cys3Δ mutant that expressed both the cysE and the cysK genes grew on SC medium lacking cysteine, but mutants that expressed only cysE or only cysK showed cysteine auxotrophy. These results indicate that co-expression of E. coli cysE and cysK genes confers an alternative de novo cysteine synthetic pathway on S. cerevisiae cells and that the O-acetylserine sulfhydrylase activity of endogenous Met17p is not sufficient for incorporation of sulfide into the O-acetylserine that is generated by the recombinant serine acetyltransferase in vivo. We further measured the intracellular levels of thiol compounds in TKY3030 (control), TKY3035 (CYS3, PTDH3-cysE, PTDH3-cysK), and TKY4175 cells (cys3Δ, PTDH3-cysE, PTDH3-cysK) as described under “Experimental Procedures” (Fig. 3C). Intracellular levels of cysteine and GSH were predictably increased by overexpression of the cysE and cysK genes, and the levels in TKY3035 cells were higher than those in TKY4175 cells. However, the levels of CoA and homocysteine were not significantly increased in either strain.
FIGURE 3.
Alternative pathway for de novo synthesis of cysteine. A, metabolic pathway of sulfur compounds in S. cerevisiae is shown (black arrows). Endogenous proteins involved in methionine synthesis (Met2p, Met17p, Met6p), the methyl cycle (Sam1p, Sam2p, Sah1p), and the transsulfuration pathway (Cys4p, Cys3p, Str2p, Str3p), are also shown. The de novo synthetic pathway of cysteine that was introduced in this study is indicated (gray arrows). CysE and CysK are E. coli enzymes that display serine acetyltransferase and O-acetylserine sulfhydrylase activities, respectively. B, cysteine auxotrophic mutant (cys3Δ::HIS3MX) was co-transformed with various combinations of multi-copy plasmids that express E. coli cysE (YEp351GAPII-cysE), cysK (YEp352GAPII-cysK), and parental plasmids (empty vectors, YEp351GAPII, and YEp352GAPII). Transformants were grown on an SC-Met/Leu/Ura plate with or without 1 mm cysteine at 30 °C for 2 days. C, yeast strains were grown in SC-Met/Leu/Ura at 30 °C until they reached mid-log phase, and metabolites were then extracted from 20 A600 units of cells. Extracted cysteine, homocysteine, GSH, and CoA were labeled with a fluorogenic reagent (SBD-F) and were quantified. Data represent means ± S.E. (vertical lines) for three experiments.
Effect of Genetic Modification of Cysteine Synthetic Pathway on SeMet Resistance
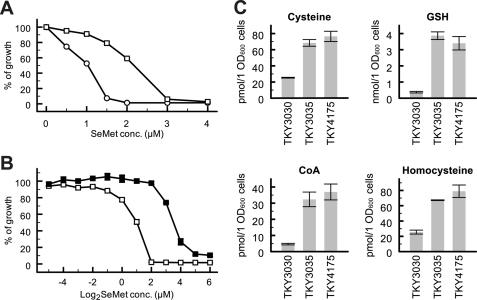
We next tested the SeMet resistance of TKY3035 and TKY4175 strains. As shown in Fig. 4A, TKY3035 cells, in which cysteine was efficiently accumulated (Fig. 3C), showed a significant increase in SeMet resistance compared with TKY3030 cells. Surprisingly, although the intracellular levels of cysteine and GSH in TKY4175 cells were lower than those in TKY3035 cells in the absence of SeMet (Fig. 3C), the SeMet resistance of TKY4175 cells was much higher than that of TKY3035 cells (Fig. 4B). To investigate whether the difference in SeMet resistance depended on the intracellular thiol levels after SeMet treatment, we further measured the levels of cysteine, GSH, CoA, and homocysteine in these strains after treatment with SeMet for 30 min (Fig. 4C). The levels of thiol compounds in TKY3030 cells were reproducibly decreased by SeMet treatment compared with their levels in untreated SeMet cells (Fig. 3C). The levels of the compounds that showed a decrease were partially recovered in TKY3035 and TKY4175 cells, supporting the hypothesis that SeMet induces the depletion of intracellular thiol compounds. Interestingly, there were no significant differences in the intracellular levels of thiol compounds between these strains although TKY4175 cells showed greater resistance than TKY3035 cells.
FIGURE 4.
Effect of metabolic engineering of the cysteine synthetic pathway on SeMet sensitivity. TKY3030 (open circles), TKY3035 (open squares), and TKY4175 (closed squares) were grown at 30 °C for 24 h in SC-Met/Leu/Ura containing various concentrations of SeMet, and cell density was then monitored as in Fig. 2. A, comparison of TKY3030 with TKY3035 at concentrations of SeMet ranging from 0 to 4 μm. B, comparison of TKY3035 with TKY4175 at concentrations of SeMet ranging from 0 to 64 μm. C, intracellular levels of thiol compounds extracted from SeMet-treated cells. SeMet was added to the resting cultures of Fig. 3C to give a final concentration of 0.25 mm followed by incubation for 30 min. Extraction and quantification of thiol compounds were performed using the same procedures described in Fig. 3C. Data represent means ± S.E. (vertical lines) for three experiments.
DISCUSSION
There has been no experimental evidence to explain the molecular mechanism of SeMet toxicity, despite the isolation of SeMet-resistant strains that harbor mutations in the genes responsible for sulfur metabolism (16–18). In the present study, we showed a significant decrease of thiol compounds in SeMet-treated cells. The alteration in the level of intracellular thiol compounds was observed only when SeMet was administered. Although several kinds of selenium compounds besides SeMet are also known to be toxic for living organisms, they may exert their toxicity through distinct mechanism from that shown in this study. Indeed, the inorganic form of selenium, selenite, has been reported to be involved in induction of DNA damage (29). A sensitive method has recently been established to identify selenium compounds from yeast cells treated with SeMet, using Orbitrap mass spectrometry (30). However, metabolic changes in SeMet-treated cells were not clearly addressed in this report. The metabolomics approach we describe in this work demonstrated not only a decrease in thiol compounds, but also an increase in selenium compounds including selenosulfides (e.g. Hcy-[S-Se]-Cys), in SeMet-treated cells, suggesting that a specific depletion of thiol compounds was induced by the selenol compounds that were generated from SeMet. This idea was supported by demonstration of increased SeMet resistance by either the extracellular addition of cysteine or by genetic engineering of the sulfur metabolic pathway.
To increase intracellular cysteine concentration, we overexpressed E. coli cysE and cysK genes in yeast cells. Because TKY3035 cells (CYS3, PTDH3-cysE, PTDH3-cysK) are able to synthesize cysteine via two independent pathways, TKY3035 cells were likely to accumulate cysteine more efficiently than TKY4175 cells (cys3Δ, PTDH3-cysE, PTDH3-cysK), in which cysteine is synthesized only through O-acetylserine (Fig. 3A). The level of cysteine in TKY4175 cells was much higher than that in TKY3030 control cells (Fig. 3C), even though both strains harbored one route for cysteine synthesis. This result suggested that cysteine was generated predominantly in TKY3035 cells via the alternative pathway that was engineered in this study rather than via the endogenous pathway. The overexpression of cysE and cysK genes also increased intracellular GSH, and its level in TKY3035 cells was higher than that in TKY4175 cells (Fig. 3C). On the other hand, the levels of CoA and homocysteine were not increased in these strains. Of note, homocysteine was decreased significantly in TKY3035 cells compared with TKY3030 control cells. It has been reported that the cys3Δ mutant, which is able to convert cysteine to homocysteine via the transsulfuration pathway (see Fig. 3A), efficiently incorporated SeMet from the culture medium into recombinant proteins (maximum 97% occupancy), even in the presence of cysteine, which was included to complement its cysteine auxotrophy (17), indicating a low conversion of cysteine into homocysteine in yeast cells. In addition, sulfide is a common substrate for both the CysK protein (O-acetylserine sulfhydrylase) and the Met17 protein (O-acetylhomoserine sulfhydrylase), and the cysteine generated by the CysK protein using sulfide was accumulated efficiently in the TKY3035 cells as mentioned above. Therefore, the observed decrease in homocysteine in TKY3035 cells might be due to a low level of conversion of cysteine to homocysteine and to depletion of substrate for the generation of homocysteine in these strains. In the case of TKY4175 cells, the homocysteine level was recovered to the wild-type level. This increase was assumed to be caused by inhibition of the transsulfuration pathway from homocysteine to cysteine by CYS3 gene deletion.
Genetic modification of the sulfur metabolic pathway also conferred SeMet resistance on yeast cells (Fig. 4, A and B). However, the enhanced resistance of TKY4175 cells cannot be explained only by an increase in intracellular thiol compounds. By comparison with TKY3035 cells, it is evident that the resistance of TKY4175 cells to SeMet depended on deletion of the CYS3 gene. Bockhorn et al. also demonstrated that of 103 null mutants that showed greater SeMet resistance than wild-type cells, the cys3Δ mutant displayed the highest resistance to SeMet (17). Because it had been reported that oxidative stress is induced by methaneselenol that is generated from SeMet by methionine-γ-lyase (31), they assumed that the high resistance of the cys3Δ mutant to SeMet was due to inhibition of methaneselenol synthesis by Cys3p that shows sequence similarity to mammalian methionine-γ-lyase. In contrast, a second report suggested that methylated selenium compounds are much less toxic than SeMet (32). The results of the metabolomics analysis of the present study demonstrated the presence, not only of methaneselenol, but also of unnatural metabolites formed between thiol and selenol compounds by oxidation, including Hcy-[S-Se]-Cys, GluCys-[S-Se]-Cys, and GSH-[S-Se]-Cys, in SeMet-treated wild-type cells (Fig. 1C). Selenol compounds are structurally similar to their thiol analogues, but the pKa value of selenol (pKa [Se-H] = 5.2) is much lower than that of thiol (pKa [S-H] = 8.3) (33). At physiological pH, selenol groups are mostly deprotonated, and they are susceptible to forming covalent linkages with thiol groups (34, 35). The unnatural compounds observed in CE-TOFMS analysis are likely due to spontaneous formation of selenosulfide bonds between selenol compounds that were generated from SeMet and endogenous thiol compounds. Moreover, generation of Sec from SeMet can be inhibited by deletion of the CYS3 gene. Accordingly, the enhanced SeMet resistance of the TKY4175 strain of this study and that of the cys3Δ mutant reported previously by Bockhorn et al. (17) are most likely to depend on inhibition of Sec synthesis rather than on methaneselenol generated from SeMet.
The necessity of selenium for living organisms is generally considered to be due to the requirement of selenoproteins for Sec at their catalytic center. During selenoprotein synthesis, organisms that can synthesize selenoproteins generate Sec from serine on tRNASec and incorporate it into nascent polypeptide chains in response to the UGA codon. The reason for this costly mechanism, which uses Sec instead of cysteine, is now assumed to be due to the advantage that it confers on the enzymatic reaction of selenoproteins at physiological pH, at which the selenol group shows high nucleophilicity (36, 37). In addition to the above speculation, it seems that the Sec biosynthetic machinery has evolved to trap the toxic amino acid Sec in proteins so that it does not exist as a free selenol compound.
In conclusion, we provided a novel insight into SeMet cytotoxicity. The growth defect of SeMet-treated yeast cells was overcome by the addition of cysteine and by a genetic modification that increased intracellular cysteine. Therefore, the toxic effect of SeMet can be accounted for by the depletion of intracellular thiol compounds.
Supplementary Material
We dedicate this article to Dr. Yoshifumi Jigami (1948–2011) with our gratitude.

This article contains supplemental Fig. S1 and Tables S1–S3.
- Sec
- selenocysteine
- 5′-MseA
- 5′-methylselenoadenosine
- AdoMet
- S-adenosylmethionine
- CE-TOFMS
- capillary electrophoresis time-of-flight mass spectrometry
- GluCys
- γ-glutamylcysteine
- NBD-F
- 4-fluoro-7-nitrobenzofurazan
- SBD-F
- 4-fluoro-7-sulfobenzofurazan
- SC medium
- synthetic complete medium
- Se-AdoHcy
- Se-adenosylselenohomocysteine
- Se-AdoMet
- Se-adenosylselenomethionine
- SeHcy
- selenohomocysteine
- SeMet
- selenomethionine.
REFERENCES
- 1. Pappas A. C., Zoidis E., Surai P. F., Zervas G. (2008) Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 151, 361–372 [DOI] [PubMed] [Google Scholar]
- 2. Hatfield D., Diamond A., Dudock B. (1982) Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc. Natl. Acad. Sci. U.S.A. 79, 6215–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. (1988) Gene for a novel tRNA species that accepts l-serine and cotranslationally inserts selenocysteine. Nature 331, 723–725 [DOI] [PubMed] [Google Scholar]
- 4. Kaiser J. T., Gromadski K., Rother M., Engelhardt H., Rodnina M. V., Wahl M. C. (2005) Structural and functional investigation of a putative archaeal selenocysteine synthase. Biochemistry 44, 13315–13327 [DOI] [PubMed] [Google Scholar]
- 5. Carlson B. A., Xu X. M., Kryukov G. V., Rao M., Berry M. J., Gladyshev V. N., Hatfield D. L. (2004) Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl. Acad. Sci. U.S.A. 101, 12848–12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan J., Palioura S., Salazar J. C., Su D., O'Donoghue P., Hohn M. J., Cardoso A. M., Whitman W. B., Söll D. (2006) RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc. Natl. Acad. Sci. U.S.A. 103, 18923–18927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu X. M., Carlson B. A., Mix H., Zhang Y., Saira K., Glass R. S., Berry M. J., Gladyshev V. N., Hatfield D. L. (2007) Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 5, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leinfelder W., Forchhammer K., Veprek B., Zehelein E., Böck A. (1990) In vitro synthesis of selenocysteinyl-tRNA(UCA) from seryl-tRNA(UCA): involvement and characterization of the selD gene product. Proc. Natl. Acad. Sci. U.S.A. 87, 543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zinoni F., Birkmann A., Stadtman T. C., Böck A. (1986) Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 83, 4650–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. (1986) The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the “termination” codon, TGA. EMBO J. 5, 1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terry N., Zayed A. M., De Souza M. P., Tarun A. S. (2000) Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 401–432 [DOI] [PubMed] [Google Scholar]
- 12. Sors T. G., Ellis D. R., Salt D. E. (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 86, 373–389 [DOI] [PubMed] [Google Scholar]
- 13. Eustice D. C., Kull F. J., Shrift A. (1981) Selenium toxicity: aminoacylation and peptide bond formation with selenomethionine. Plant Physiol. 67, 1054–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hendrickson W. A., Horton J. R., LeMaster D. M. (1990) Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): a vehicle for direct determination of three-dimensional structure. EMBO J. 9, 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rice L. M., Earnest T. N., Brunger A. T. (2000) Single-wavelength anomalous diffraction phasing revisited. Acta Crystallogr. D Biol. Crystallogr. 56, 1413–1420 [DOI] [PubMed] [Google Scholar]
- 16. Malkowski M. G., Quartley E., Friedman A. E., Babulski J., Kon Y., Wolfley J., Said M., Luft J. R., Phizicky E. M., DeTitta G. T., Grayhack E. J. (2007) Blocking S-adenosylmethionine synthesis in yeast allows selenomethionine incorporation and multiwavelength anomalous dispersion phasing. Proc. Natl. Acad. Sci. U.S.A. 104, 6678–6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bockhorn J., Balar B., He D., Seitomer E., Copeland P. R., Kinzy T. G. (2008) Genome-wide screen of Saccharomyces cerevisiae-null allele strains identifies genes involved in selenomethionine resistance. Proc. Natl. Acad. Sci. U.S.A. 105, 17682–17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitajima T., Chiba Y., Jigami Y. (2010) Mutation of high-affinity methionine permease contributes to selenomethionyl protein production in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 76, 6351–6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas B. J., Rothstein R. (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56, 619–630 [DOI] [PubMed] [Google Scholar]
- 20. Sherman F. (1991) Getting started with yeast. Methods Enzymol. 194, 3–21 [DOI] [PubMed] [Google Scholar]
- 21. Abe H., Shimma Y., Jigami Y. (2003) In vitro oligosaccharide synthesis using intact yeast cells that display glycosyltransferases at the cell surface through cell wall-anchored protein Pir. Glycobiology 13, 87–95 [DOI] [PubMed] [Google Scholar]
- 22. Soga T., Heiger D. N. (2000) Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 72, 1236–1241 [DOI] [PubMed] [Google Scholar]
- 23. Soga T., Ueno Y., Naraoka H., Ohashi Y., Tomita M., Nishioka T. (2002) Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 74, 2233–2239 [DOI] [PubMed] [Google Scholar]
- 24. Soga T., Ohashi Y., Ueno Y., Naraoka H., Tomita M., Nishioka T. (2003) Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2, 488–494 [DOI] [PubMed] [Google Scholar]
- 25. Kosugi A., Koizumi Y., Yanagida F., Udaka S. (2001) MUP1, high affinity methionine permease, is involved in cysteine uptake by Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 65, 728–731 [DOI] [PubMed] [Google Scholar]
- 26. Thomas D., Surdin-Kerjan Y. (1997) Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61, 503–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cherest H., Eichler F., Robichon-Szulmajster H. (1969) Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J. Bacteriol. 97, 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamagata S., Takeshima K., Naiki N. (1974) Evidence for the identity of O-acetylserine sulfhydrylase with O-acetylhomoserine sulfhydrylase in yeast. J. Biochem. 75, 1221–1229 [DOI] [PubMed] [Google Scholar]
- 29. Letavayová L., Vlasaková D., Spallholz J. E., Brozmanová J., Chovanec M. (2008) Toxicity and mutagenicity of selenium compounds in Saccharomyces cerevisiae. Mutat. Res. 638, 1–10 [DOI] [PubMed] [Google Scholar]
- 30. Rao Y., McCooeye M., Windust A., Bramanti E., D'Ulivo A., Mester Z. (2010) Mapping of selenium metabolic pathway in yeast by liquid chromatography-Orbitrap mass spectrometry. Anal. Chem. 82, 8121–8130 [DOI] [PubMed] [Google Scholar]
- 31. Palace V. P., Spallholz J. E., Holm J., Wautier K., Evans R. E., Baron C. L. (2004) Metabolism of selenomethionine by rainbow trout (Oncorhynchus mykiss) embryos can generate oxidative stress. Ecotoxicol. Environ. Saf. 58, 17–21 [DOI] [PubMed] [Google Scholar]
- 32. Nakamuro K., Okuno T., Hasegawa T. (2000) Metabolism of selenoamino acids and contribution of selenium methylation to their toxicity. J. Health Sci. 46, 418–421 [Google Scholar]
- 33. Huber R. E., Criddle R. S. (1967) Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch. Biochem. Biophys. 122, 164–173 [DOI] [PubMed] [Google Scholar]
- 34. Pleasants J. C., Guo W., Rabenstein D. L. (1989) A comparative study of the kinetics of selenol diselenide and thiol disulfide exchange reactions. J. Am. Chem. Soc. 111, 6553–6558 [Google Scholar]
- 35. Wessjohann L. A., Schneider A., Abbas M., Brandt W. (2007) Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 388, 997–1006 [DOI] [PubMed] [Google Scholar]
- 36. Kim H. Y., Fomenko D. E., Yoon Y. E., Gladyshev V. N. (2006) Catalytic advantages provided by selenocysteine in methionine-S-sulfoxide reductases. Biochemistry 45, 13697–13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nauser T., Steinmann D., Koppenol W. H. (2012) Why do proteins use selenocysteine instead of cysteine? Amino Acids 42, 39–44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.