Background: Lung function is dependent upon the regulation of tissue and alveolar lipids.
Results: Activation of SREBP in alveolar cells caused neutral lipid accumulation, inflammation, and tissue remodeling.
Conclusion: The accumulation of neutral lipids in the lung caused inflammation consistent with findings in lipid storage disorders.
Significance: Pulmonary lipotoxicity may contribute to lung dysfunction associated with diabetes, obesity, and metabolic disorders.
Keywords: Inflammation, Insulin, Lipids, Lipid Synthesis, Lung, Insulin-induced Gene, Lipid Storage, Lung Inflammation
Abstract
Pulmonary inflammation is associated with altered lipid synthesis and clearance related to diabetes, obesity, and various inherited metabolic disorders. In many tissues, lipogenesis is regulated at the transcriptional level by the activity of sterol-response element-binding proteins (SREBP). The role of SREBP activation in the regulation of lipid metabolism in the lung was assessed in mice in which both Insig1 and Insig2 genes, encoding proteins that bind and inhibit SREBPs in the endoplasmic reticulum, were deleted in alveolar type 2 cells. Although deletion of either Insig1 or Insig2 did not alter SREBP activity or lipid homeostasis, deletion of both genes (Insig1/2Δ/Δ mice) activated SREBP1, causing marked accumulation of lipids that consisted primarily of cholesterol esters and triglycerides in type 2 epithelial cells and alveolar macrophages. Neutral lipids accumulated in type 2 cells in association with the increase in mRNAs regulating fatty acid, cholesterol synthesis, and inflammation. Although bronchoalveolar lavage fluid phosphatidylcholine was modestly decreased, lung phospholipid content and lung function were maintained. Insig1/2Δ/Δ mice developed lung inflammation and airspace abnormalities associated with the accumulation of lipids in alveolar type 2 cells, alveolar macrophages, and within alveolar spaces. Deletion of Insig1/2 activated SREBP-enhancing lipogenesis in respiratory epithelial cells resulting in lipotoxicity-related lung inflammation and tissue remodeling.
Introduction
Precise regulation of alveolar lipid homeostasis is required for lung function. Lack of surfactant phospholipids and proteins causes respiratory distress syndrome in neonates and participates in the pathogenesis of acute lung injury in adults. The fibrosing sequellae that often accompany these syndromes can cause chronic respiratory insufficiency. Lack of pulmonary surfactant, related to mutations in genes critical for surfactant synthesis and activity, causes both acute respiratory failure and life-threatening chronic lung disorders, including pulmonary fibrosis (1–4). Conversely, excess accumulation of surfactant lipids occurs in the lungs of patients with pulmonary alveolar proteinosis, a disorder caused by defects in GM-CSF signaling that impair clearance of surfactant by alveolar macrophages (5). Lipid storage diseases are often accompanied by chronic inflammation and fibrosing alveolitis (6, 7).
The abundance and composition of lung lipids are precisely regulated. Pulmonary surfactant is enriched in phosphatidylcholine (PC)3 and phosphatidylglycerol that play important roles in reduction of surface tension. Surfactant PC is relatively enriched in saturated species. Although cholesterol is present in varying quantities in mammalian surfactants, excess cholesterol impairs the surface tension reducing properties of surfactants (8). Triacylglycerols, an important storage form of lung lipids, are normally present in small amounts in alveolar tissues and are thought to serve as substrates for phospholipid synthesis by alveolar type 2 cells during development (9). The role of neutral lipid pools in surfactant lipid homeostasis has not been clearly defined in the adult lung. Surfactant lipid composition and abundance are influenced by lung injury, likely mediated by intracellular and extracellular processes that control synthesis and catabolism of lipids present in pulmonary surfactant (10). Thus, a complex regulatory system has evolved to precisely control lung lipid content and composition in the alveolus.
Lung lipid homeostasis is regulated by multitiered processes influencing 1) the uptake of lipids from the systemic circulation, 2) synthesis and storage of lipids by epithelial and stromal compartments in the alveolus, and 3) secretion, reuptake, reutilization, and catabolism of surfactant lipids, which together precisely control intracellular and extracellular surfactant pools before and after birth. As in other tissues, lipid homeostasis in the lung is influenced, in part, by sterol-regulatory element-binding proteins (SREBPs). SREBP1c expression increases before birth in association with increased lipogenesis in type 2 epithelial cells (11). Deletion of the Scap gene (encoding the SREBP cleavage-activating protein) inhibited SREBP activity in type 2 cells and enhanced neutral lipid accumulation in lung fibroblasts in the fetal and postnatal mouse lung (12), demonstrating important roles for the SREBP pathway in lung lipid homeostasis.
SREBP is activated at the post-transcriptional level by SCAP. SCAP serves as a lipid sensor that in the absence of sterols/phospholipids transports SREBPs from the endoplasmic reticulum to the Golgi where S1P and S2P proteases release a transcriptionally active N-terminal fragment of SREBP. Conversely, SREBP activity is inhibited by insulin-induced gene proteins 1 and 2 that anchor the SCAP-SREBP complex to the endoplasmic reticulum membrane in a lipid-dependent manner. Insig1 and Insig2 share structural similarities and have partially redundant functions (13). Although germ line deletion of Insig1 in the mouse caused death at birth, germ line deletion of Insig2 did not influence survival. Deletion of both Insig genes in hepatic tissues increased transcription of SREBP target genes causing accumulation of cholesterol and triglycerides (13). There is increasing evidence that the accumulation of lipid droplets in various tissues during substrate excess causes tissue inflammation and is inhibited in part by the recruitment and activation of tissue macrophages (14).
This study was designed to further define the roles of SREBP and insulin-induced genes in the regulation of lung lipid homeostasis and to test whether enhanced lipogenesis influenced surfactant homeostasis, lung lipid content, or lung inflammation. Deletion of Insig1 and -2 induced SREBP1 in alveolar type 2 cells, causing neutral lipid accumulation in type II cells and in the alveoli. The accumulation of lung lipids caused inflammation and airspace remodeling with pathological findings similar to those associated with lipid storage disorders, diabetes mellitus, and obesity.
EXPERIMENTAL PROCEDURES
Transgenic Animals
Insig1flox/flox and Insig2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). In Insig1flox/flox mice, loxP sites flank exon 1 of the Insig1 gene. The Insig2 gene is disrupted by replacement of exons II and III with a neo cassette eliminating the first 123 of 225 amino acids (13). Mice were mated with SFTPC-rtTAWT/Tg/(tetO)7CMV-CreWT/Tg to generate the following: 1) single transgenic Insig2−/− mice; 2) triple transgenic SFTPC-rtTAWT/Tg/(tetO)7CMV-CreWT/Tg/Insig1flox/flox, in which Insig1 was deleted from the respiratory epithelium in utero when dams were exposed to doxycycline from E6.5 to E12.5, herein termed Insig1Δ/Δ, which were compared with littermates lacking either SFTPC-rtTA or (tetO)7CMV-Cre; and 3) quadruple transgenic SFTPC-rtTAWT/Tg/(tetO)7CMV-CreWT/Tg/Insig1flox/flox/Insig2−/− mice. Doxycycline-exposed SFTPC-rtTAWT/Tg/(tetO)7CMV-CreWT/Tg/Insig1flox/flox/Insig2−/− mice are termed Insig1/2Δ/Δ mice throughout. In Insig1/2Δ/Δ mice exposed to doxycycline in utero, Insig1 was conditionally deleted in the Insig2−/− background; Insig1/2Δ/Δ mice were compared with Insig1flox/flox/Insig2−/− mouse littermates lacking either SFTPC-rtTA or (tetO)7CMV-Cre. With this system, recombination of the loxP flanked allele is typically obtained in most alveolar type 2 cells (15). Genotypes were identified by PCR from genomic tail DNA as described previously (13, 16).
Animal Husbandry and Doxycycline Administration
Mice were maintained in a pathogen-free environment in accordance with protocols approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Research Foundation. Gestation was dated by detection of the vaginal plug, and dams bearing pups carrying the Insig1flox allele were maintained on doxycycline in food (625 mg/kg dry weight; Harlan Teklad, Madison, WI) from embryonic days 6.5 to 12.5 to delete Insig1 in progenitor cells that form the peripheral lung and to minimize effects of Cre-recombinase (Cre) or reverse tetracycline transactivator that can occur later in gestation. Mice were killed at 2, 6, and 8 months of age for study.
Preparation of Tissue for Morphologic Analysis
Mice were killed by overdose of anesthetic and exsanguinated by sectioning the abdominal aorta. The anterior thoracic wall was removed. The trachea was cannulated, and the lungs were inflated at a pressure of 25 cm of H2O with 4% paraformaldehyde in phosphate-buffered saline for 1 min. The trachea was ligated, and the heart and lungs were removed and immersed in the same fixative at 4 °C for 18 h. After three rinses in cold PBS, the right lung and the upper half of the left lung were dehydrated in graded ethanol and embedded in paraffin; the lower half of the left lung was immersed in 30% sucrose in PBS for 24 h, immersed in a 2:1 mixture of 30% sucrose and OCT sectioning medium (Sakura, Torrance, CA) for another 24 h, and then embedded in OCT and stored at −80 °C. Five-μm-thick sections of paraffin-embedded tissue were prepared for Movat's pentachrome or hematoxylin and eosin stains (Poly Scientific, Bay Shore, NY) and immunohistochemistry studies. Eight-μm-thick frozen sections were prepared for Oil Red O staining, Nile Red staining, and immunofluorescence studies.
Whole Lung Protein Extraction, Immunoblotting
The left atrium was cut open, and the lungs were rinsed with 10 ml of PBS injected through the right ventricle and snap-frozen in liquid nitrogen. The lungs were homogenized in 50 mm Tris, pH 7.4, with 150 mm NaCl, 2 mm EDTA, 25 mm NaF, 25 mm β-glycerophosphate, 0.1 mm sodium vanadate, 0.2% Triton X-100, 0.3% Nonidet P-40, and 0.1% protease inhibitor mixture (P8340, Sigma) and centrifuged at 13,000 rpm for 15 min. The supernatant was stored at −20 °C. Proteins (10 μg) were separated by SDS-PAGE on polyacrylamide gels and transferred onto 0.2-μm nitrocellulose membranes (Bio-Rad). Membranes were probed with antibodies targeting SREBP1 (sc-8984, Santa Cruz Biotechnology, Santa Cruz, CA), fatty-acid synthase (FASN) (BD Transduction Laboratories), and GAPDH (A300-641A, Bethyl Laboratories, Montgomery, TX) as a loading control. Band density was analyzed using ImageJ (National Institutes of Health, Bethesda).
Oil Red O Staining
Frozen lung sections or BALF cells were fixed in 4% paraformaldehyde for 15 min and rinsed three times in distilled water. Slides were immersed for 2 min in absolute propylene glycol and incubated for 1 h (BALF cells) or overnight (lung sections) at room temperature in Oil Red O solution (Poly Scientific). Slides were then immersed in 85% propylene glycol, rinsed twice in distilled water, and counterstained with Harris' hematoxylin.
pro-SP-C Immunofluorescence and Nile Red Staining
To identify lipid-laden lung cells, frozen lung sections were rehydrated in PBS, blocked with 4% normal goat serum, and stained with an anti-pro-SP-C antibody (WRAB-SPC, Seven Hills Bioreagents, Cincinnati, OH) followed by an Alexa Fluor 488-conjugated goat anti-rabbit antibody (Invitrogen). Sections were then counterstained for 5 min with 2.5 mg/ml Nile Red in 75% aqueous glycerol and coverslipped. Nile Red fluorescence was analyzed using a 535–580 nm excitation filter and a 591–667 emission filter. With this filter set, fluorescence emitted by Nile Red predominantly reflects neutral lipids over phospholipids. The number of Nile Red-positive cells relative to the number of pro-SP-C-positive cells in five microscopic fields at ×10 magnification was determined.
Electron Microscopy
Lungs from 8-week-old mice were fixed in 2% glutaraldehyde and 2% paraformaldehyde in 0.1 m sodium cacodylate buffer and 0.1% calcium chloride, pH 7.3. Tissue was postfixed in 1% osmium tetroxide, dehydrated, and embedded in epoxy resin (EMbed 812, Electron Microscopy Sciences, Fort Washington, PA). Ultrathin sections were viewed in a Hitachi H7600 transmission electron microscope, and digitized images were collected with an AMT Advantage Plus 2k × 2k digital camera (Advanced Microscopy Techniques, Danvers, MA).
BALF
Mice were anesthetized; the trachea was cannulated, and the lungs were lavaged twice with 1 ml of PBS, pH 7.4. The total number of cells was determined using trypan blue exclusion, and 10,000 cells were plated onto glass slides using a Cytospin centrifuge (Thermo, Waltham, MA) and stained with hematoxylin and eosin for differential counting or kept at −20 °C for Oil Red O staining and immunostaining. In other experiments, BALF cells were lysed, and total RNA was extracted.
Isolation and Sorting of Alveolar Type 2 Cells
Mouse alveolar type 2 cells were isolated as described previously (17). Briefly, the lungs were perfused to remove blood, inflated with Dispase (Stem Cell Technologies, British Columbia, Canada) for 45 min, and trimmed manually. The resulting suspension was filtered on 100-, 40-, and 20-μm filters and incubated for 2 h on 100-mm cultures dishes coated with anti-CD45 and anti-CD16/32 antibodies to eliminate leukocytes. Alveolar type 2 cells were recovered from the supernatant by centrifugation. Total alveolar type 2 cells from 2-month-old animals were used for mRNA microarray and RT-PCR.
Type 2 cells were purified from 6-month-old Insig1/2Δ/Δ mice and subjected to flow cytometry and sorting using the lipid probe Nile Red with an excitation wavelength of 488 nm. Phospholipid-rich cells were sorted using a 660/20 nm emission filter. Neutral lipid-rich cells were sorted using a 545/35-nm emission filter (18). Neutral lipid-positive cells were gated based on side scatter and intensity of Nile Red fluorescence. Cells were analyzed and sorted by a FACSAria II cell sorter (BD Biosciences).
Cytokine and Chemokine Assays
Lung homogenates were centrifuged at 2,000 × g for 10 min, and supernatants were used for cytokine protein analyses. IL-6, IL-1β, TNF-α, keratinocyte chemoattractant, and MIP2 were analyzed using immunoassays (MILLIPLEXTM Map, Millipore, Billerica, MA).
RNA Extraction, RT-PCR Analysis
RNA was extracted using RNeasy reagents (Qiagen, Valencia, CA). Potentially contaminating genomic DNA was removed by exposure to recombinant DNase I (Roche Applied Science). RNAs were retrotranscribed to complementary DNAs using Moloney murine leukemia virus reverse transcriptase (Invitrogen), according to the manufacturer's instructions, and analyzed by RT-PCR using TaqMan reagents (Applied Biosystems, Foster City, CA) or iQ SYBR Green Supermix (Bio-Rad). Gene expression was reported relative to that of 18 S RNA as an internal control. Primer sequences and thermal cycling conditions are listed in supplemental Table 1. IL-1B, IL-12B, and TNF-α mRNA was assessed by RT-PCR using StepOne Plus Time PCR (Applied Biosystems) and normalized to 18 S RNA using RNA isolated from alveolar macrophages isolated from control and Insig1/2-deleted mice, n = 8 per group.
RNA Microarray Analysis in Alveolar Type 2 Cells
For microarray analysis, 700 ng of alveolar type 2 cell RNA from Insig1/2Δ/Δ (n = 3) and Insig1flox/flox/Insig2−/− (n = 3) mice were hybridized to the murine genome 430 2.0 array (Affymetrix, Santa Clara, CA). The RNA quality and quantity assessment, probe preparation, labeling, hybridization, and image scan were carried out in the Cincinnati Children's Hospital Medical Center Affymetrix Core using standard procedures. Data were analyzed using BRB Array Tools software package (linus.nci.nih.gov). Differentially expressed mRNAs were identified using a random variance t test, which permits sharing information among genes about within-class variation without assuming that all genes have the same variance (19). mRNAs were considered differentially expressed with a p value of ≤0.05 and a fold change of ≥1.5. Affymetrix “Present Call” in at least two of three replicates was set as a prerequisite for gene selection.
Differentially expressed genes were subjected to gene ontology, promoter analysis, and pathway analysis. Gene Ontology analysis was performed using the publicly available web-based tool David (data base for annotation, visualization, and integrated discovery) (21). Over-represented transcription factor-binding site analyses were performed using 2 kb of promoter sequences upstream of transcription start sites of differentially expressed genes (Genomatix). Gene ontology categories were considered to be significant when a Fisher's exact test p value was ≤0.01 and gene hits were ≥10. Over-represented functions, diseases, known pathways, as well as potential protein/protein or protein/DNA interactions were identified using ingenuity pathway analysis (Ingenuity). Customized gene networks were constructed by integrating microarray results, literature mining, and experimental observations. Changes in mRNA expression were compared with those obtained in the lungs of Scap-deleted animals (12). Differentially expressed genes in the Insig deletion array were compared with published gene expression data in the L2L data base.
Lipid Mass Spectrometry Analysis
To assess PC synthesis, turnover, and secretion, animals were injected intraperitoneally 3 h before sacrifice with 6.7 μmol of [methyl-9-2H]choline chloride (CDN Isotopes, Pointe-Claire, Canada). Mice were anesthetized; the trachea was cannulated, and the lungs were lavaged five times with 1 ml of PBS. Lavaged lungs were homogenized in 2 ml of PBS. For lamellar body isolation, lungs were homogenized in 2 ml of 1.2 m sucrose, and the lamellar body fraction was isolated by sucrose gradient density centrifugation as described previously (20). Lipids were extracted with chloroform and methanol, as described previously (21), from lung homogenate, lamellar bodies, and BALF and from isolated alveolar type 2 cells in an independent experiment. Dimyristoylphosphatidylcholine (PC 14:0/14:0, 15 nmol) and a deuterated triglyceride (glyceryl tri(octadecanoate-18,18,18-d3), CDN Isotopes, 10 nmol) were added as internal standards. The dried lipid extract was divided in two portions for separate electrospray ionization mass spectrometry (ESI-MS) analysis of phospholipids and neutral lipids. PC was analyzed by ESI-MS as described previously (22), employing precursor ion scans of the m/z 184 phosphocholine headgroup for endogenous PC species and of the corresponding m/z 193 for newly synthesized PC species. A neutral lipid fraction was isolated as the flow-through from 100 mg of aminopropyl (NH2) BondElut columns (Agilent Technologies UK Ltd.) after application of the second portion of the lipid extract dissolved in chloroform. The neutral lipid fraction was dissolved in 250 μl of 50:50 (v/v) chloroform/methanol solution containing 1% NH4OH (0.880) and introduced into the mass spectrometer by direct infusion at 5 μl/min. Neutral lipids were quantified from the ESI+ scan with a series of fatty acid neutral loss scans for 14:0, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3, 20:4, 22:5, and 22:6 used to identify the dominant fatty acid profiles of triacylglycerol, diacylglycerol, monoacylglycerol, and cholesterol ester species. Specific precursor scans at m/z 369+ were used to confirm the identity of cholesterol ester species, whereas neutral loss of m/z 35 fragments identified diacylglycerol species.
Measurement of Lung Mechanics
Lung mechanics were studied in anesthetized and tracheostomized mice using the FlexiVent System (SCIREQ Scientific Respiratory Equipment, Montreal, Quebec, Canada), as described previously (23). Dynamic resistance, elastance, and compliance of the airways were measured as well as tissue damping, tissue compliance, and tissue hysteresivity.
Statistical Analysis
Data were expressed as means ± S.D. Student's t test was used for comparisons of continuous variables. Association between categorical variables was tested using the χ2 test. A p value <0.05 was considered statistically significant.
RESULTS
Germ Line Deletion of Insig2 or Conditional Deletion of Insig1 Alone Did Not Activate SREBP Signaling in the Lung
Insig1+/+ and Insig2+/− mice were bred to generate littermate Insig2+/+ and Insig2−/− mice. As reported previously (13), Insig2−/− mice were born at the expected Mendelian ratio from heterozygous parents. Deletion of Insig2 had no effect on the levels of the 72-kDa activated SREBP1 (aSREBP1) in the lung and did not change mRNAs of known SREBP target genes, including Fasn, Scd1, Ldlr, and Hmgcs1; compensatory increases in Insig1 mRNA were not observed in the lungs of Insig2−/− mice (data not shown). At 2 and 6 months of age, lungs of Insig2−/− mice were morphologically indistinguishable from the lungs of Insig2+/+ mice by light microscopy and Oil Red O staining. BALF cell counts were similar in both groups of mice, and there were no changes in Oil Red O-stained alveolar macrophages in Insig2−/− and Insig2+/+ mice (13.5 ± 3.5 versus 11.0 ± 0.6%, p = 0.59) at 2 months of age; at 6 months of age, percent of Oil Red O-stained macrophages in BALF was 22.3 ± 3.8 and 19.7 ± 4.7%, respectively (p = 0.51). These data indicate that SREBP signaling was not activated in lungs of Insig2−/− mice, suggesting either that Insig2 has no detectable function in the lung or that Insig1 fully compensates for the lack of Insig2.
Mice in which Insig1 was conditionally deleted with the hSFTPC-rtTA/(otet)Cre system in alveolar type 2 cells (Fig. 1A) were compared with Insig1flox/flox mice. Again, deletion of the single Insig1 gene in the respiratory epithelium did not cause detectable changes in lung by histology and Oil Red O staining (data not shown). Altogether, these data indicate that deletion of Insig1 or Insig2 alone did not activate SREBP, suggesting that Insig1 or Insig2 can fully compensate for each other.
FIGURE 1.
Respiratory epithelium-specific deletion of Insig1 in Insig2−/−mice increased SREBP. A, schematic of Insig1 deletion strategy in the alveolar epithelium is shown. In utero treatment with doxycycline induced Cre-mediated deletion of exon 1 of the Insig1 gene in SFTPC-rtTAWT/Tg/(tetO)7CMV-CreWT/Tg/Insig1flox/flox mice, termed Insig1Δ/Δ. These mice were bred into Insig2−/− mice. Mice lacking either SFTPC-rtTA or (tetO)7CMV-Cre are termed Insig1flox/flox. B, aSREBP1 (72 kDa) was ∼2-fold higher in the lungs of Insig1/2Δ/Δ mice compared with Insig1flox/flox/Insig2−/− mice. A representative Western blot is shown. GAPDH was used as an internal control, n = 4 in each group, mean ± S.E., *, p < 0.05. DOX, doxycycline; rtTA, reverse tetracycline transactivator; CRE, Cre-recombinase.
Deletion of Both Insig1- and Insig2-induced SREBP Activity and Caused Lipid Accumulation in Alveolar Type 2 Cells
Insig1/2Δ/Δ mice were produced in which Insig1 was deleted in the alveolar epithelium in addition to germ line deletion of Insig2. Insig1 mRNA was reduced by 72% in purified alveolar type 2 cells from Insig1/2Δ/Δ mice compared with Insig1flox/flox/Insig2−/− mice. Western blot analysis demonstrated that activated SREBP1 levels were increased 2.2-fold, p = 0.021 (Fig. 1B). At 2 months of age, lung morphogenesis was generally preserved in Insig1/2Δ/Δ mice; however, a subset of alveolar type 2 cells were hypertrophic and contained large cytoplasmic inclusions containing neutral lipids as demonstrated by Oil Red O staining (Fig. 2A). The cells were stained with Nile Red, at a fluorescence consistent with neutral lipids, and by pro-SP-C antibody, an alveolar type 2 cell-specific marker (Fig. 2B). At 2 months of age, 30.9 ± 2.6% of alveolar type 2 cells contained cytoplasmic neutral lipid inclusions in the lungs of Insig1/2Δ/Δ mice, compared with 1.75 ± 1.75% in Insig1flox/flox/Insig2−/− mice (p = 0.049). At 6 months of age, 30.4 ± 4% of alveolar type 2 cells contained neutral lipid inclusions in Insig1/2Δ/Δ mice versus 3.04 ± 3.04% in Insig1flox/flox/Insig2−/− mice (p = 0.034) (Fig. 2C).
FIGURE 2.
Cytoplasmic accumulation of neutral lipids in alveolar type 2 cells of Insig1/2Δ/Δ mice. A, alveolar cells from Insig1/2Δ/Δ mice were enlarged and contained optically empty vacuoles after staining with pentachrome. Vacuoles contained neutral lipids as shown by Oil Red O staining. Photomicrographs are representative of n = 5. Insets show higher magnification. B, lungs were double-stained with a pro-SP-C antibody, a marker of alveolar type 2 cells (green color), and Nile Red (red color). C, alveolar type 2 cells were stained with Nile Red and counted in Insig1flox/flox/Insig2−/− mice (open bars) and Insig1/2Δ/Δ mice (closed bars) at 2 and 6 months of age and expressed as a percentage, n = 3, *, p < 0.05.
Ultrastructural Alterations of Alveolar Type 2 Cells in Insig1/2Δ/Δ Mice
Although ultrastructural abnormalities were not seen in Insig1Δ/Δ or Insig1flox/flox/Insig2−/− mice as compared with wild type mice, extensive ultrastructural changes were observed in alveolar type 2 cells in lungs from Insig1/2Δ/Δ mice (Fig. 3A) wherein large electron lucent lipid droplets were observed (Fig. 3, B and C). Normal, but smaller, lamellar bodies were present in the cytoplasm of lipid-laden cells. Type 2 epithelial cells with normal morphology were also observed, perhaps indicating incomplete deletion of Insig1 in some cells. The morphology of pulmonary lipofibroblasts located in alveolar septa of Insig1/2Δ/Δ mice was unaltered (data not shown), and the ultrastructure of tubular myelin and other forms of secreted surfactant was not changed (data not shown) in Insig1/2Δ/Δ mice, consistent with the preservation of lung function.
FIGURE 3.
Ultrastructural changes in alveolar type 2 cells from Insig1/2Δ/Δ mice. A, ultrastructure of alveolar type 2 cells from Insig1flox/flox/Insig2−/− was not altered. In contrast, large electron lucent lipid droplets were present in the cytoplasm of a subset of Insig1/2Δ/Δ alveolar type 2 cells (B and C). Some lipid droplets lacked limiting membranes (arrow); others contained membranous phospholipid material (black arrowhead). Small lamellar bodies were also present in the cytoplasm of Insig1Δ/Δ alveolar type 2 cells (white arrowhead).
Accumulation of Triacylglycerols and Cholesterol Esters in Lungs of Insig1/2Δ/Δ Mice
Although there was no difference in total PC concentration in either lung homogenate or isolated type 2 alveolar cells (Fig. 4, A and B), marked increases in cholesterol esters and triacylglycerols were observed in both fractions for Insig1/2Δ/Δ mice, consistent with the intracellular accumulation of Oil Red O-stained material seen in alveolar type 2 cells of these mice. By contrast, neither neutral lipid was increased in purified lamellar bodies, confirming minimal contamination of this subcellular preparation with lipid vesicles (Fig. 4C). Although triacylglycerol concentration was not elevated in BALF from Insig1/2Δ/Δ mice, cholesterol esters were significantly increased (Fig. 4D). Molecular species analysis demonstrated that BALF cholesterol ester composition was very different from that in serum, confirming a local lung synthetic origin in both Insig1/2Δ/Δ (Fig. 4E) and Insig1flox/flox/Insig2−/− mice (results not shown). The presence of elevated cholesterol ester in BALF combined with its absence in lamellar bodies suggests that, at least in Insig1/2Δ/Δ mice, it may either be secreted independently from lamellar bodies or be subject to considerably decreased intra-alveolar catabolism and turnover.
FIGURE 4.
Concentrations of PC, triacylglycerol (TAG), and cholesterol ester (CE). Lipid concentrations are shown for lavaged lungs (A), isolated alveolar type 2 cells (B), isolated lamellar body preparations (C), and BALF from Insig1flox/flox/Insig2−/− (open bars) and Insig1/2Δ/Δ mice (closed bars) (D) *, p < 0.05. n = 6. E, precursor scan of m/z 369 shows that BALF and serum BALF cholesterol ester compositions were distinct, indicating that BALF cholesterol ester was not blood-derived. F demonstrates that decreased PC16:0/16:0 was the major contributor to the lower total BALF PC concentration in Insig1/2Δ/Δ mice, n = 6, p < 0.05.
Synthesis de Novo and Secretion of Phosphatidylcholine in Insig1/2Δ/Δ Mice
Lipid mass spectrometry analysis revealed significant quantitative and qualitative changes in lung phospholipid contents in Insig1/2Δ/Δ mice. Most strikingly, a 35% (p = 0.002) decrease in total PC was observed in BALF (Fig. 4D), in contrast to the unchanged total PC content of lung homogenate (Fig. 4A) and alveolar type 2 cells (Fig. 4B).
In addition to the reduction of total PC content in BALF, the relative proportions of individual PC molecular species in all analyzed tissue fractions were significantly altered in Insig1/2Δ/Δ mice. In particular, the fractional concentration (% total PC) of the predominant PC16:0/16:0 was consistently but modestly decreased in lung (31.9 ± 5.9 versus 38.1 ± 2.2, p < 0.05), lamellar bodies (35.7 ± 2.5 versus 44.8 ± 2.2, p < 0.001), and BALF (42.2 ± 5.2 versus 49.7 ± 2.0, p < 0.005), whereas that of second predominant PC16:0/16:1 was increased in both lamellar body (28.4 ± 1.8 versus 23.5 ± 1.8, p < 0.001) and BALF PC (29.2 ± 2.1 versus 22.2 ± 1.6, p < 0.001) in Insig1/2Δ/Δ mice. Expressed in concentration terms, the 45.3% decreased content of PC16:0/16:0 (Fig. 4F) contributed most significantly to the lower total BALF PC concentration.
The fractional incorporation of [methyl-9-2H]choline (expressed as % total labeled + unlabeled PC) was unaltered in lung tissue, lamellar body, and alveolar type 2 cells but increased in BALF PC from Insig1/2Δ/Δ mice (Fig. 5A). As lamellar body and BALF PC have a precursor/product relationship, we used fractional incorporation of [methyl-9-2H]choline in lamellar body PC to calculate the apparent amount of PC secreted into the lung airspace over the 3-h incubation with label. Although the absolute values of this calculation need to be regarded with caution, as an accurate assessment of secretion would have required incorporation data from multiple time points, the apparent rate of secretion of lamellar body PC was unaltered in Insig1/2Δ/Δ compared with Insig1flox/flox/Insig2−/− mice (Fig. 5B).
FIGURE 5.
Lipid concentrations and incorporation of [methyl-9-2H]choline and secretion of BALF PC. A, fractional incorporation of [methyl-9-2H]choline into lavaged lungs, purified lamellar bodies, and isolated alveolar type 2 cells was unaltered in Insig1/2Δ/Δ mice (closed bars) compared with Insig1flox/flox/Insig2−/− (open bars) but was significantly increased in BALF PC. B, correcting the incorporation of [methyl-9-2H]choline in BALF PC for the enrichment of stable isotope label in the substrate lamellar body pool suggested the rate of PC secretion into BALF was unaltered in Insig1/2Δ/Δ mice, n = 7, *, p < 0.05. C, comparison of the molecular species compositions of [methyl-9-2H]choline incorporation into BALF PC (closed bars) with endogenous PC compositions (open bars) demonstrated that there was an initial synthesis of unsaturated species with fatty acyl groups containing 18 or more carbon atoms (Unsat PC) for both Insig1/2Δ/Δ and Insig1flox/flox/Insig2−/− mice, with increased acyl remodeling to PC16:0/16:1 compared with PC16:0/16:0 in the Insig1/2Δ/Δ mice.
In the lungs, synthesis of PC is achieved by a combination of biosynthesis de novo following the Kennedy pathway, with the enzyme choline phosphotransferase (Chpt1) catalyzing the incorporation of CDP-choline with diacylglycerol or by acyl remodeling of unsaturated PC species by two concerted reactions as follows: deacylation of unsaturated PC by a phospholipase A2, followed by reacylation of the resultant 1-palmitoyl-2-lysophosphatidylcholine (lyso-PC) with palmitoyl-CoA by lysophosphatidylcholine (lyso-PC) acyltransferase 1 (Lpcat1) (24). In addition, the final composition of secreted surfactant PC is also modulated by the ABCA3-dependent selection of PC species into lamellar bodies (25), and also by the specificity of the ABCA1-dependent basolateral secretion of unsaturated PC species from type II alveolar epithelial cells (26). Consequently, the pattern of incorporation of [methyl-9-2H]choline into lung PC molecular species will reflect the net sum of all these activities. This pattern was essentially identical to the endogenous PC compositions of whole lung, alveolar type II cells, and lamellar bodies, suggesting that newly synthesized PC in all three compartments was at equilibrium composition with endogenous PC by 3 h (27). By contrast, the composition of newly synthesized PC secreted into BALF was, for both Insig1flox/flox/Insig2−/− and Insig1/2Δ/Δ mice, enriched in unsaturated PC species containing 18 carbon atoms or greater, compared with the total BALF PC composition (Fig. 5C). A proportion of newly synthesized surfactant PC is secreted before the processes of acyl remodeling and completion of species selection, suggesting a similar initial elevated fractional synthesis of long chain unsaturated PC species in both groups of mice. The major difference caused by deletion of Insig1/2 was the increased fractional content and label incorporation of PC16:0/16:1 in BALF PC from Insig1/2Δ/Δ mice at the expense of decreased content and incorporation into PC16:0/16:0. As lamellar bodies can select and package PC16:0/16:1 equally well as PC16:0/16:0 (24), this contrasting specificity suggests that the altered PC composition in Insig1/2Δ/Δ mice was possibly due to altered specificity of acyl remodeling related to increased substrate CoA16:1 as a consequence of increased expression of Scd1 (Fig. 7B). Altered activity of the acyl remodeling pathway would be expected to alter the kinetics of lyso-PC labeling, but neither the concentration nor the [methyl-9-2H]choline labeling of lyso-PC in lung tissue were altered by Insig deletion (data not shown). Although BALF PC16:0/16:0 was decreased, lung mechanics were similar in 6-month-old Insig1/2Δ/Δ mice and Insig1flox/flox/Insig2−/− mice (data not shown), indicating maintenance of surfactant activity.
FIGURE 7.
Quantitation of mRNAs in distinct alveolar type 2 cell subpopulations. A, alveolar type 2 cells of Insig1/2Δ/Δ mice were sorted according to their neutral lipid content as determined by Nile Red staining. Phospholipid-rich cells were detected using a 660/20-nm emission filter, neutral lipid-rich cells using a 545/30-nm emission filter. Neutral lipid negative (NL−) and neutral lipid positive (NL+) cell populations were sorted. Gene expression was determined by RT-PCR in Insig1flox/flox/Insig2−/− alveolar type 2 cells (empty bars), in NL− (gray bars), and NL+ (filled bars) cells from Insig1/2Δ/Δ mice. B, Insig1 mRNA was strongly reduced in NL+ cells but not in NL− cells. C, genes related to lipid biosynthesis and surfactant metabolism were induced in NL+ but not in NL− cells from Insig1/2Δ/Δ mice. D, mRNAs related to lipid transport, catabolism, and leukocyte recruitment were induced in NL− cells. Figure represents n = 5, $, p < 0.05 versus Insig1flox/flox/Insig2−/− (empty) alveolar type 2 cells and NL− cells (gray); *, p < 0.05 versus Insig1flox/flox/Insig2−/− and NL+ alveolar type 2 cells.
RNA Microarray Analysis Revealed Activation of Fatty Acid and Cholesterol Biosynthetic Pathways in Insig1/2Δ/Δ Alveolar Type 2 Cells
RNA microarray analysis of purified alveolar type 2 cells from 8-week-old Insig1/2Δ/Δ mice has been submitted to Gene Expression Omnibus, accession number GSE 31797. We identified 650 mRNAs that were significantly altered (p < 0.05 and a fold change of >1.5) in comparison with Insig1flox/flox/Insig2−/− mice. Of these, 181 mRNAs were significantly decreased and 469 induced (Fig. 6A). Of the 469 induced mRNAs, 99 were related to lipid metabolism. mRNAs encoding proteins regulating fatty acid synthesis, acyl chain elongation, and cholesterol biosynthesis were increased in alveolar type 2 cells from the Insig1/2Δ/Δ mice. Approximately 100 mRNAs related to inflammatory processes were increased in the Insig1/2Δ/Δ mice (Fig. 10 and supplemental Table 2). Decreased expression of Insig1 and increased expression of Hmgcs1, Fasn, and Stard4 were verified by RT-PCR (Fig. 6B). Consistent with RNA microarray data, Western blot analyses (Fig. 6C) indicated that FASN was significantly increased in Insig1/2Δ/Δ lungs (9.5-fold increase, p = 0.0004). Although LPCAT1 activity was not measured, LPCAT1 mRNA was not altered (p = 0.38), consistent with preservation of acyl chain remodeling seen in the analysis of lung lipids.
FIGURE 6.
RNA microarray data from alveolar type 2 cells isolated from Insig1/2Δ/Δ mice. A, two-dimensional hierarchical clustering identified 650 mRNAs that were significantly altered in type 2 cells isolated from Insig1/2Δ/Δ compared with Insig1flox/flox/Insig2−/− mice. The intensity in the red and green color ranges indicates increased versus decreased mRNAs, respectively. Each row represents a single mRNA, and each column represents a biological replicate. B, RT-PCR analysis confirmed RNA microarray data demonstrating decreased expression of Insig1 and increased expression of Hmgcs1, Fasn, and Stard4 in Insig1/2Δ/Δ in cells from Insig1/2Δ/Δ mice (filled bars) compared with control (open bars), n = 3, *, p < 0.05. C, Western blot analysis confirmed increased expression of FASN in lung homogenates from the Insig1/2Δ/Δ mice, n = 4. GAPDH was used as a loading control.
FIGURE 10.
mRNAs induced in type 2 cells from Insig1/2Δ/Δ mice were functionally enriched in the lipid metabolism and immune/inflammatory response. The regulatory relationships linking the two categories of genes were identified through literature mining using Ingenuity knowledge base. Red and green nodes represent the genes induced or repressed in Insig1/2Δ/Δ versus control, respectively. Triangular nodes are genes induced in Insig1/2Δ/Δ and repressed in ScapΔ/Δ mice (22). Color range indicates the relative fold change (the darker the color, the larger the fold change).
According to Gene Ontology classification, the biological processes most induced by deletion of Insig1/2 in type 2 cells were related to “immunity and defense,” “lipid, fatty acid, and steroid metabolism,” “macrophage-mediated immunity,” “cytokine- and chemokine-mediated signaling pathway,” “T-cell mediated immunity,” and “cholesterol metabolism.”
mRNA expression profiles from Insig1/2Δ/Δ mice were compared with those in which SREBP was inhibited by deletion of Scap in type 2 cells (12). We identified 30 mRNAs that were both induced by Insig deletion and decreased by Scap deletion (supplemental Table 2). These genes were highly enriched in pathways regulating lung “lipid metabolism,” e.g. fatty acid biosynthesis, metabolism, steroid synthesis, and liver X receptor/RXR activation, many of which are regulated by SREBP in liver or lung, consistent with the important role of the SREBP pathways in lipid metabolism. A number of genes in this list, including Abca1, Fasn, Fdft1, Hmgcr, Lpin1, Rora, Srebf1, and Steap2, are known to be associated with dyslipidemia, a disorder of high cholesterol and high blood triglycerides and hypercholesterolemia.
Gene Expression Profile of Alveolar Type 2 Cell Subpopulations
Because ultrastructural studies identified subsets of cells that either were lipid-laden or normal in appearance, subpopulations of alveolar type 2 cells were sorted according to their neutral lipid content (Fig. 7A). Insig1/2Δ/Δ neutral-lipid positive cells (NL+) lacked Insig1 mRNA, whereas neutral lipid-negative (NL−) cells expressed Insig1 at levels similar to Insig1flox/flox/Insig2−/− alveolar type 2 cells (Fig. 7B), indicating that Insig1 was not deleted in the NL− cells. Consistent with SREBP activation in NL+ cells, levels of mRNAs implicated in neutral lipid, phospholipid, and surfactant apoprotein synthesis were considerably increased in NL+ cells compared with either NL− cells or normal alveolar type 2 cells from Insig1flox/flox/Insig2−/− mice (Fig. 7C). A number of mRNAs were induced in NL− alveolar type 2 cells in the Insig1/2Δ/Δ mice, perhaps indicating an adaptive response to the induction of lipid synthesis caused by deletion of Insigs. These mRNAs included Fabp4 (a lipid transporter), Lipa (a lipid catabolism enzyme), and pro-inflammatory molecules Cxcl3, Ccl6, and Il1b (Fig. 7D).
Analysis of the promoter regions of the group of genes induced in the NL+ versus NL− cells identified SREBP as the most enriched common regulatory cis-element in the NL+ (Insig1/2Δ/Δ) cells (p = 3.17E-13), whereas the promoters of genes induced in NL− (nondeleted) cells were enriched in common cis-elements for peroxisome proliferator-activated receptor/RXR (p = 5.51E-04) and TR4 (NR2C2, p = 2.73E-05), a member of nuclear receptor subfamily 2 known to inhibit peroxisome proliferator-activated receptor α/RXRα (27). The genes altered in NL+ cells are likely to represent direct effects of insulin-induced gene/SREBP. In contrast, mRNAs induced in NL− type II cells may represent compensatory responses regulated in part via peroxisome proliferator-activated receptor/RXR signaling.
Differentially expressed genes identified after Insig1/2 deletion were similar to those in lungs of lysosomal acid lipase (Lipa) knock-out mice (28). LIPA hydrolyzes triglycerides and cholesterol esters in lysosomes. Phenotypic changes in Insig1/2Δ/Δ mice share similarities with the Lipa−/− mice, including accumulation of triglycerides and cholesterol esters in type II cells, progressive inflammation, foamy macrophages, lung remodeling, and epithelial hyperplasia (29). In humans, mutation of LIPA causes Wolman disease a cholesterol ester storage disease in which cholesterol esters and triglycerides accumulate in most tissues (30). In this study, Lipa mRNA was significantly reduced in NL+ and induced in NL− cells, correlating with Insig1 mRNA levels in the two subpopulations of type II cells. We speculate that reduction of Lipa mRNA contributes to the accumulation of cholesterol esters and triglycerides in Insig1/2-deficient type II cells. The expression of mRNAs encoding a number of lipid transport proteins was increased in Insig1Δ/Δ/Insig2−/− alveolar type 2 cells, including Abca3, Abcg1, Abca1, Stard4, and Stard2 (supplemental Table 2A) (31–33).
Inflammation in Lungs of Insig1/2Δ/Δ Mice
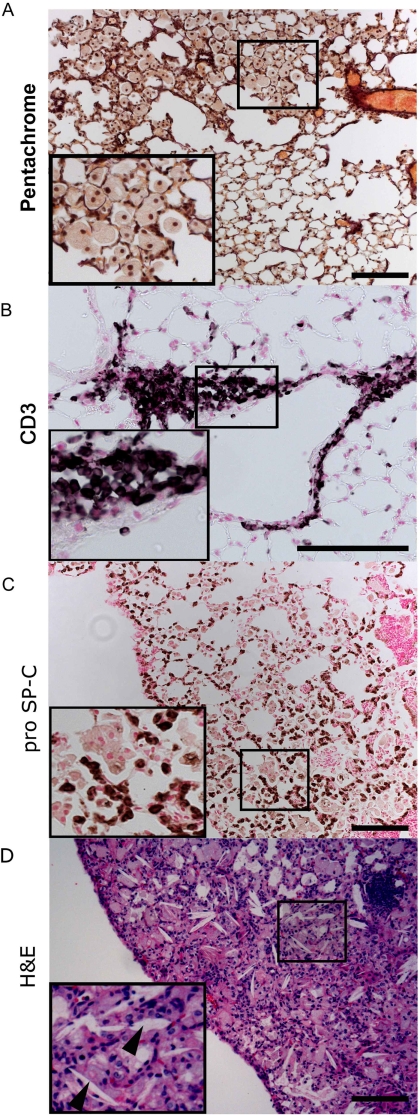
Although inflammatory infiltrates were not observed in lung sections of 2-month-old Insig-deficient mice, mRNA levels of many inflammatory response genes were moderately induced in type II epithelial cells isolated from the mice (Fig. 6 and supplemental Table 2). RNAs associated with the IL-12 signaling pathway were modestly but significantly induced, including Infg, Il-12b, Irf8, Stat4, and Stat5a mRNAs. Increased expression of mRNAs encoding a number of cytokines, chemokines, and associated receptors and transcriptional regulators was observed, consistent with a proinflammatory environment caused by deletion of Insig1/2. At 6 months of age, focal inflammatory lesions were evident in the lungs of Insig1/2Δ/Δ but not in Insig1flox/flox/Insig2−/− mice. Accumulation of foamy and multinucleated macrophages was observed in the alveoli and interstitium in 67% of Insig1/2Δ/Δ mice (p = 0.016, χ2 test, see Fig. 8A). Lymphocyte infiltration was shown by CD3 immunostaining in 40% of Insig1/2Δ/Δ mice (p = 0.078, χ2 test, see Fig. 8B). Moreover, inflammatory changes were accompanied by alveolar type 2 cell hyperplasia in Insig1/2Δ/Δ mice, as shown by pro-SP-C immunostaining (Fig. 8C). Extensive pulmonary pathology was observed in older Insig1/2-deficient mice (8–9 months), and alveoli were filled with lipids, foamy macrophages, and cholesterol crystals (Fig. 8D).
FIGURE 8.
Inflammation and alveolar remodeling in Insig1/2Δ/Δ mice. The lungs of Insig1/2Δ/Δ mice are shown at 6 and 8 months of age. Focal accumulation of enlarged foamy multinuclear alveolar macrophages are shown by Movat's pentachrome stain (A), and peribronchial infiltrates include T lymphocytes demonstrated by CD3 immunostaining (B). C, inflammation was accompanied by alveolar type 2 cell hyperplasia shown by pro-SP-C immunostaining. Lungs of Insig1flox/flox/Insig2−/− mice were normal (data not shown). Immunostaining shown with nickel-diaminobenzidine appears brown; nuclei were counterstained with nuclear Fast Red. D, photomicrographs are representative of n = 9 mice of each genotype. At 10 months of age, extensive lesions were observed in the lungs of Insig1Δ/Δ/Insig2−/− mice, with prominent foamy macrophage infiltration, and cholesterol clefts (arrowhead). Bars, 100 μm. Insets show higher magnification.
Numbers of BALF cells were markedly increased in Insig1/2Δ/Δ compared with Insig1flox/flox/Insig2−/− mice at 2 and 6 months of age (Fig. 9A and data not shown). BALF cells were predominantly (99%) monocytes/macrophages in both genotypes. At 2 and 6 months of age, lipid-laden macrophages as shown by Oil Red O staining were increased (Fig. 9B). The atypical alveolar macrophages stained for anti-arginase1, a marker of alternative macrophage activation, in Insig1/2Δ/Δ mice (14.8 ± 4%) compared with controls (3 ± 1.9%, p = 0.028, Fig. 9C), and expressed high levels of Arg1 mRNA but low levels of Nos2 mRNA (Fig. 9D). BALF PGE2, IL4, IL10, IL13, and IFN-γ levels were similar in both groups of mice (data not shown). Concentrations of cytokines and chemokines associated with inflammation in diabetes and obesity were assessed in lung homogenates at 8 weeks of age (Fig. 9F). IL-6 and keratinocyte chemoattractant concentrations were significantly increased in the Insig1/- deleted mice, consistent with the inflammation seen histologically. Likewise, IL-1B and IL-12B mRNAs in alveolar macrophages isolated from BALF were significantly increased at this age (Fig. 9G).
FIGURE 9.
Accumulation of lipid-laden alveolar macrophages and inflammatory mediators in Insig1/2Δ/Δ mice. A, BALF cell counts; B, percentage of BALF cells stained with Oil Red O in 2- and 6-month-old Insig1flox/flox/Insig2−/− (white) and Insig1/2Δ/Δ mice (black). C, percentage of BALF cells stained with an antibody against arginase-1 (ARG1). D, expression of mRNAs for Arg1 and Nos2 in BALF cells from 2-month-old Insig1flox/flox/Insig2−/− and Insig1/2Δ/Δ mice. E, PGE2 in BALF; F, IL-6, IL-1β, TNF-α, keratinocyte chemoattractant, and MIP2 in lung homogenates of 2-month-old Insig1flox/flox/Insig2−/− and Insig1/2Δ/Δ mice, n = 6; *, p < 0.05. mRNA was isolated from alveolar macrophages isolated after lung lavage from 8-week-old control (n = 10) and Insig1/2Δ/Δ mice, n = 5. G, IL-1b and IL-12b mRNAs were measured by RT-PCR and were normalized to 18 S RNA levels. Bars represent means ± S.D., and differences were assessed by two-tailed Student t test.
DISCUSSION
Conditional deletion of Insig1 in type 2 alveolar epithelial cells in combination with germ line deletion of Insig2 1) caused constitutive activated SREBP activity in type II alveolar epithelial cells, 2) resulted in the accumulation of neutral lipids in type II epithelial cells, alveolar macrophages, and alveoli, and 3) caused chronic pulmonary inflammation and alveolar remodeling. Despite dramatic changes in lipid homeostasis, surfactant function was preserved in Insig1/2Δ/Δ mice, supporting the presence of compensatory mechanisms that preserve pulmonary surfactant despite activation of SREBP and marked accumulation of neutral lipids. The present findings demonstrate an important role for Insig1/2 and SREBP activation in the regulation of lipid synthesis in alveolar type II cells. The disruption of alveolar lipid homeostasis caused by deletion of Insig1/2 and activation of SREBP1 resulted in progressive lung inflammation and alveolar remodeling related to lipotoxicity.
The finding that conditional deletion of either Insig1 in alveolar cells in the lung or germ line deletion of Insig2 alone was not sufficient to significantly alter lipid homeostasis in the lung supports concepts regarding the substantially redundant functions of Insig1 and Insig2 genes previously described in liver metabolism (13). In alveolar type 2 cells bearing deletions of both Insig genes, increased nuclear SREBP1 was associated with increased expression of known SREBP target mRNAs such as Hmgcs1, Hmgcr, Ldlr, Acaca, and Fasn (34). Despite SREBP activation and abnormal lipid accumulation, surfactant function was maintained. The expression of mRNAs encoding a number of lipid transport proteins was increased in Insig1Δ/Δ/Insig2−/− alveolar type 2 cells, including Abca3, Abcg1, Abca1, Stard4, and Stard2 (supplemental Table 2) (31–33, 35), which may represent a direct response of SREBP activation to handle the increased lipid accumulation. The mRNAs regulating lipid biosynthetic pathways activated by Insig deletion in the lung were similar to those induced by Insig deletion in the liver; nevertheless, quantitative differences were evident between the two organs. In the liver, Insig deletion led to moderate increases in mRNAs coding enzymes involved in cholesterol synthesis (Hmgcs1, 1.2-fold; Hmgcr, 2-fold; Ldlr, 1.2-fold), whereas mRNAs coding enzymes involved in fatty acid synthesis were markedly increased (Acaca, 2.7-fold; Fasn, 4.5-fold). A distinct expression profile was observed after Insig1/2 deletion in the lung, as mRNAs encoding protein in both cholesterol and phospholipid biosynthetic pathways were similarly but modestly induced. These results show that transcriptional responses to SREBPs follow organ-specific patterns. Fig. 10 provides a schematic of a proposed gene network representing changes in mRNAs regulating lipid homeostasis and immune response that were influenced by Insig1/2 deletion in alveolar type II cells.
Although Insig1 mRNA was reduced by 70% in type 2 cells in Insig1/2-deficient mice, heterogeneity in type 2 cell morphology and the cell sorting studies indicated that Insig1 was incompletely targeted in a subset of type 2 cells. Neutral lipids did not accumulate in the nontargeted cells as indicated by cell sorting with Nile Red. In Insig1/2-deleted cells, genes regulating the lipid synthetic pathways (e.g. Acaca, Fasn, Scd1, Elolv1, Hmgcr, and Ldlr) were substantially increased; likewise, in Insig1/2-deficient cells, mRNAs associated with phospholipid synthesis and surfactant proteins were modestly increased. Conversely, the mRNAs in nontargeted type 2 cells were generally unchanged, although Fabp4, Lipa, Cxcl3, Il1b, and Ccl6 were induced in the nontargeted cells, indicating a potential compensatory response by type 2 cells with remaining Insig1 activity. The induction of surfactant proteins in the Insig1/2-deficient cells is consistent with their regulation by SREBP (12) and may indicate a cell autonomous response to SREBP activation. Likewise, induction of Foxa2 and Cebpa (Fig. 7), genes known to regulate surfactant lipid and protein synthesis in the developing lung, indicates a potential compensatory response by Insig-deleted alveolar type 2 cells. Thus, findings from the mRNA microarray of the total lungs from the Insig1/2-targeted mice are likely to represent the integration of direct and indirect compensatory responses in targeted and nontargeted cells, as well as by other cells within the lung following activation of SREBP. The interactions of targeted and nontargeted type 2 cells may serve to maintain surfactant function despite significant neutral lipid accumulation in the model.
The activation of lipogenic pathways in the lungs of Insig1/2Δ/Δ mice resulted in the accumulation of neutral lipid species shown morphologically by Oil Red O and Nile Red staining and by mass spectrometry. Both triglycerides and cholesterol ester were increased in lung tissue but not in lamellar bodies isolated by differential centrifugation from the Insig-deleted mice. Combined with the unaltered triacylglycerol profile in BALF, these results support the presence of distinct pathways organizing neutral lipid storage and lamellar body formation in alveolar type 2 cells. Furthermore, the increased cholesterol ester concentration in BALF but not in lamellar bodies (Fig. 4D) also suggests that neutral lipids in BALF have a different intracellular origin in type 2 alveolar cells other than from surfactant lamellar bodies. The accumulation of triglycerides in the lungs of Insig-deficient mice was not associated with increases in the expression levels of Dgat1 or Dgat2 in alveolar type 2 cells, the enzymes mediating triglyceride synthesis.
In sharp contrast to mice in which other members of the transcriptional network controlling alveolar type 2 cell surfactant lipid homeostasis, e.g. Foxa2, Nfatc3, Cebpa, and Nkx2–1, were deleted or mutated in the respiratory epithelium (36–39), deletion of Insig genes and activation of SREBP did not impair lung formation and maturation. Surfactant function was maintained despite increased SREBP activity and its activation of nonsurfactant lipogenic pathways. The distinct gene expression profiles seen in the subpopulation of deleted and nondeleted alveolar type 2 cells in Insig1Δ/Δ mice supports metabolic compensation within and between type 2 cells. Increased numbers and associated lipid catabolic activity of alveolar macrophages in the Insig-deficient mice likely contribute to the increased lung lipid clearance in Insig1/2Δ/Δ mice, consistent with the important role of alveolar macrophages in lung lipid homeostasis. The unaltered surfactant PC content in lungs of Insig1/2Δ/Δ mice was consistent with preserved expression of proteins known to be essential for lamellar body formation and secretion, for example Sftpb or Abca3, the latter being induced by deletion of Insigs.
The focal accumulation of giant foamy multinucleated macrophages in the alveolar spaces and airspace remodeling was a striking feature of older Insig1/2Δ/Δ mice. This phenomenon is also observed in other models of lung lipid overload, as seen in mice bearing deletion of the Sftpd (40) or Abcg1 (41) genes and in mice with mutations of the Hps1 or Ap3 genes that model the Hermanski-Pudlak syndrome in humans (42).
Lipid accumulation and pulmonary infiltration with lipid-laden macrophages increased with age in the Insig-deleted mice. In Insig1/2Δ/Δ mice, alveolar macrophages had morphological and functional characteristics consistent with macrophage activation. IL-6 and keratinocyte chemoattractant levels were increased in lung tissue, consistent with the mRNA microarray data from alveolar type II cells isolated from the Insig1/2Δ/Δ mice. Pathway enrichment analysis supports the concept that macrophages were activated via an IL-12/STAT4/IFNG signaling cascade. Supporting this concept, expression of IL-12B and IL-1B mRNAs was increased in alveolar macrophages isolated from the Insig1/2-deleted mice. mRNAs encoding proteins in the IL-12/STAT4-dependent signaling pathways were significantly induced in Insig1/2Δ/Δ mice, including Il12b, Il18, Stat4, Stat5a, Ifng, Ccr5, Irf1, and Selp. In contrast, expression of Th2-type cytokines IL-4 and IL-13, indicators of Th2 activation, was not changed. Although mechanisms by which both epithelial and macrophage activation and focal alveolar remodeling occurs in this study are unclear, there is increasing evidence that excess lipid accumulation in tissues recruits and activates macrophages.
The present findings are also consistent with the studies in Abcg1 gene-deleted mice in which cholesterol and phospholipids accumulate in both type II cells and alveolar macrophages causing inflammation. Changes in expression of pro-inflammatory cytokines and chemokine-associated pathways in lungs from Abcg1−/− mice are similar to those induced by deletion of Insig1/2, including increased mRNAs encoding Ccl 5–9,17,22, Cxdl 3,5, and chemokine receptors Ccr 5–7, Infg, and Tgfβ1. In vitro loading of human monocyte-derived macrophages with cholesterol directly induces the expression of IL-1β (43). Thus, in the present model, excessive synthesis of cholesterol and other neutral lipids in the type II epithelial cells caused lipid accumulation in both epithelial cells and alveolar macrophages. Although lipid synthetic pathways were induced in Insig1/2-deleted alveolar type II cells, Cxcl3, Ccl6, and Il-1β mRNAs were induced in the nondeleted cells and in alveolar macrophages (Fig. 7), indicating that both alveolar macrophages and epithelial cells likely contribute to the inflammation and remodeling seen in the Insig1/2-deleted mice. Fig. 10 provides a proposed gene network linking changes in lipogenesis and lung inflammation observed in the Insig1/2-deleted mice.
Pulmonary inflammation and lung dysfunction is associated with diabetes mellitus, obesity, and in lipid storage disorders (14). Likewise, lung dysfunction, inflammation, and remodeling are associated with lipid droplet accumulation in lungs of diabetic Zucker rats (44). Taken together, the accumulation of neutral lipids related to enhanced lung lipid synthesis or content may represent a form of pulmonary lipotoxicity similar to that associated with obesity and diabetes (45–46).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL085610 (to J. A. W.) and HL105433 (to Y. X.).

This article contains supplemental Tables 1–3.
- PC
- phosphatidylcholine
- SREBP
- sterol-regulatory element-binding protein
- SCAP
- SREBP cleavage-activating protein
- BALF
- bronchoalveolar lavage fluid
- SP-C
- surfactant protein C
- RXR
- retinoid X receptor.
REFERENCES
- 1. Whitsett J. A., Wert S. E., Weaver T. E. (2010) Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu. Rev. Med. 61, 105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Moorsel C. H., van Oosterhout M. F., Barlo N. P., de Jong P. A., van der Vis J. J., Ruven H. J., van Es H. W., van den Bosch J. M., Grutters J. C. (2010) Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am. J. Respir. Crit. Care. Med. 182, 1419–1425 [DOI] [PubMed] [Google Scholar]
- 3. Bullard J. E., Wert S. E., Whitsett J. A., Dean M., Nogee L. M. (2005) ABCA3 mutations associated with pediatric interstitial lung disease. Am. J. Respir. Crit. Care. Med. 172, 1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selman M., Lin H. M., Montaño M., Jenkins A. L., Estrada A., Lin Z., Wang G., DiAngelo S. L., Guo X., Umstead T. M., Lang C. M., Pardo A., Phelps D. S., Floros J. (2003) Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum. Genet. 113, 542–550 [DOI] [PubMed] [Google Scholar]
- 5. Trapnell B. C., Whitsett J. A., Nakata K. (2003) Pulmonary alveolar proteinosis. N. Engl. J. Med. 349, 2527–2539 [DOI] [PubMed] [Google Scholar]
- 6. Nakatani Y., Nakamura N., Sano J., Inayama Y., Kawano N., Yamanaka S., Miyagi Y., Nagashima Y., Ohbayashi C., Mizushima M., Manabe T., Kuroda M., Yokoi T., Matsubara O. (2000) Interstitial pneumonia in Hermansky-Pudlak syndrome. Significance of florid foamy swelling/degeneration (giant lamellar body degeneration) of type-2 pneumocytes. Virchows Arch. 437, 304–313 [DOI] [PubMed] [Google Scholar]
- 7. Minai O. A., Sullivan E. J., Stoller J. K. (2000) Pulmonary involvement in Niemann-Pick disease. Case report and literature review. Respir. Med. 94, 1241–1251 [DOI] [PubMed] [Google Scholar]
- 8. Leonenko Z., Gill S., Baoukina S., Monticelli L., Doehner J., Gunasekara L., Felderer F., Rodenstein M., Eng L. M., Amrein M. (2007) An elevated level of cholesterol impairs self-assembly of pulmonary surfactant into a functional film. Biophys. J. 93, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torday J., Hua J., Slavin R. (1995) Metabolism and fate of neutral lipids of fetal lung fibroblast origin. Biochim. Biophys. Acta 1254, 198–206 [DOI] [PubMed] [Google Scholar]
- 10. Gregory T. J., Longmore W. J., Moxley M. A., Whitsett J. A., Reed C. R., Fowler A. A., 3rd, Hudson L. D., Maunder R. J., Crim C., Hyers T. M. (1991) Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J. Clin. Invest. 88, 1976–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang F., Pan T., Nielsen L. D., Mason R. J. (2004) Lipogenesis in fetal rat lung. importance of C/EBPα, SREBP-1c, and stearoyl-CoA desaturase. Am. J. Respir. Cell Mol. Biol. 30, 174–183 [DOI] [PubMed] [Google Scholar]
- 12. Besnard V., Wert S. E., Stahlman M. T., Postle A. D., Xu Y., Ikegami M., Whitsett J. A. (2009) Deletion of Scap in alveolar type II cells influences lung lipid homeostasis and identifies a compensatory role for pulmonary lipofibroblasts. J. Biol. Chem. 284, 4018–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engelking L. J., Liang G., Hammer R. E., Takaishi K., Kuriyama H., Evers B. M., Li W. P., Horton J. D., Goldstein J. L., Brown M. S. (2005) Schoenheimer effect explained. Feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J. Clin. Invest. 115, 2489–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lumeng C. N., Saltiel A. R. (2011) Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perl A. K., Wert S. E., Loudy D. E., Shan Z., Blair P. A., Whitsett J. A. (2005) Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am. J. Respir. Cell Mol. Biol. 33, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perl A. K., Tichelaar J. W., Whitsett J. A. (2002) Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 11, 21–29 [DOI] [PubMed] [Google Scholar]
- 17. Rice W. R., Conkright J. J., Na C. L., Ikegami M., Shannon J. M., Weaver T. E. (2002) Maintenance of the mouse type II cell phenotype in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L256–L264 [DOI] [PubMed] [Google Scholar]
- 18. Greenspan P., Fowler S. D. (1985) Spectrofluorometric studies of the lipid probe, Nile Red. J. Lipid Res. 26, 781–789 [PubMed] [Google Scholar]
- 19. Wright G. W., Simon R. M. (2003) A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics 19, 2448–2455 [DOI] [PubMed] [Google Scholar]
- 20. Besnard V., Matsuzaki Y., Clark J., Xu Y., Wert S. E., Ikegami M., Stahlman M. T., Weaver T. E., Hunt A. N., Postle A. D., Whitsett J. A. (2010) Conditional deletion of Abca3 in alveolar type II cells alters surfactant homeostasis in newborn and adult mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L646–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 22. Postle A. D., Gonzales L. W., Bernhard W., Clark G. T., Godinez M. H., Godinez R. I., Ballard P. L. (2006) Lipidomics of cellular and secreted phospholipids from differentiated human fetal type II alveolar epithelial cells. J. Lipid Res. 47, 1322–1331 [DOI] [PubMed] [Google Scholar]
- 23. Ikegami M., Falcone A., Whitsett J. A. (2008) STAT-3 regulates surfactant phospholipid homeostasis in normal lung and during endotoxin-mediated lung injury. J. Appl. Physiol. 104, 1753–1760 [DOI] [PubMed] [Google Scholar]
- 24. Bridges J. P., Ikegami M., Brilli L. L., Chen X., Mason R. J., Shannon J. M. (2010) LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. J. Clin. Invest. 120, 1736–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitsett J. A. (2010) Review. The intersection of surfactant homeostasis and innate host defense of the lung: lessons from newborn infants. Innate Immun. 16, 138–142 [DOI] [PubMed] [Google Scholar]
- 26. Agassandian M., Mathur S. N., Zhou J., Field F. J., Mallampalli R. K. (2004) Oxysterols trigger ABCA1-mediated basolateral surfactant efflux. Am. J. Respir. Cell. Mol. Biol. 31, 227–233 [DOI] [PubMed] [Google Scholar]
- 27. Yan Z. H., Karam W. G., Staudinger J. L., Medvedev A., Ghanayem B. I., Jetten A. M. (1998) Regulation of peroxisome proliferator-activated receptor α-induced transactivation by the nuclear orphan receptor TAK1/TR4. J. Biol. Chem. 273, 10948–10957 [DOI] [PubMed] [Google Scholar]
- 28. Lian X., Yan C., Qin Y., Knox L., Li T., Du H. (2005) Neutral lipids and peroxisome proliferator-activated receptor-γ control pulmonary gene expression and inflammation-triggered pathogenesis in lysosomal acid lipase knockout mice. Am. J. Pathol. 167, 813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lian X., Yan C., Yang L., Xu Y., Du H. (2004) Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L801–L807 [DOI] [PubMed] [Google Scholar]
- 30. Du H., Sheriff S., Bezerra J., Leonova T., Grabowski G. A. (1998) Molecular and enzymatic analyses of lysosomal acid lipase in cholesteryl ester storage disease. Mol. Genet. Metab. 64, 126–134 [DOI] [PubMed] [Google Scholar]
- 31. Wong J., Quinn C. M., Brown A. J. (2006) SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR. Biochem. J. 400, 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soccio R. E., Adams R. M., Romanowski M. J., Sehayek E., Burley S. K., Breslow J. L. (2002) The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc. Natl. Acad. Sci. U.S.A. 99, 6943–6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ecker J., Langmann T., Moehle C., Schmitz G. (2007) Isomer-specific effects of conjugated linoleic acid on macrophage ABCG1 transcription by a SREBP-1c-dependent mechanism. Biochem. Biophys. Res. Commun. 352, 805–811 [DOI] [PubMed] [Google Scholar]
- 34. Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. (2003) Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. U.S.A. 100, 12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Besnard V., Xu Y., Whitsett J. A. (2007) Sterol-response element-binding protein and thyroid transcription factor-1 (Nkx2.1) regulate Abca3 gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1395–L1405 [DOI] [PubMed] [Google Scholar]
- 36. Wan H., Xu Y., Ikegami M., Stahlman M. T., Kaestner K. H., Ang S. L., Whitsett J. A. (2004) Foxa2 is required for transition to air breathing at birth. Proc. Natl. Acad. Sci. U.S.A. 101, 14449–14454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martis P. C., Whitsett J. A., Xu Y., Perl A. K., Wan H., Ikegami M. (2006) C/EBPα is required for lung maturation at birth. Development 133, 1155–1164 [DOI] [PubMed] [Google Scholar]
- 38. DeFelice M., Silberschmidt D., DiLauro R., Xu Y., Wert S. E., Weaver T. E., Bachurski C. J., Clark J. C., Whitsett J. A. (2003) TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J. Biol. Chem. 278, 35574–35583 [DOI] [PubMed] [Google Scholar]
- 39. Davé V., Childs T., Xu Y., Ikegami M., Besnard V., Maeda Y., Wert S. E., Neilson J. R., Crabtree G. R., Whitsett J. A. (2006) Calcineurin/Nfat signaling is required for perinatal lung maturation and function. J. Clin. Invest. 116, 2597–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wert S. E., Yoshida M., LeVine A. M., Ikegami M., Jones T., Ross G. F., Fisher J. H., Korfhagen T. R., Whitsett J. A. (2000) Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc. Natl. Acad. Sci. U.S.A. 97, 5972–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baldán A., Gomes A. V., Ping P., Edwards P. A. (2008) Loss of ABCG1 results in chronic pulmonary inflammation. J. Immunol. 180, 3560–3568 [DOI] [PubMed] [Google Scholar]
- 42. Lyerla T. A., Rusiniak M. E., Borchers M., Jahreis G., Tan J., Ohtake P., Novak E. K., Swank R. T. (2003) Aberrant lung structure, composition, and function in as murine model of Hermansky-Pudlak syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L643–L653 [DOI] [PubMed] [Google Scholar]
- 43. Feng Y., Schreiner G. F., Chakravarty S., Liu D. Y., Joly A. H. (2001) Inhibition of the mitogen-activated protein kinase, p38α, prevents proinflammatory cytokine induction by human adherent mononuclear leukocytes in response to lipid loading. Atherosclerosis 158, 331–338 [DOI] [PubMed] [Google Scholar]
- 44. Foster D. J., Ravikumar P., Bellotto D. J., Unger R. H., Hsia C. C. (2010) Fatty diabetic lung. Altered alveolar structure and surfactant protein expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L392–L403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kodolova I. M., Lysenko L. V., Saltykov B. B. (1982) Changes in the lungs in diabetes mellitus. Arkh. Patol. 44, 35–40 [PubMed] [Google Scholar]
- 46. Reinilä A., Koivisto V. A., Akerblom H. K. (1977) Lipids in the pulmonary artery and the lungs of severely diabetic rats. A histochemical and chemical study. Diabetologia 13, 305–310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.