Background: A disordered immune regulation contributes to chronic inflammatory skin diseases.
Results: Human keratinocytes show a restricted ability to up-regulate SOCS3.
Conclusion: Keratinocytes miss an important negative feedback mechanism.
Significance: The failure of keratinocytes to up-regulate SOCS3 efficiently may contribute to ongoing proinflammatory epithelial responses that impact on leukocyte infiltration and thus to the maintenance of chronic skin inflammation.
Keywords: Cytokine Action, Keratinocytes, Macrophages, Signaling, STAT3, IL-27, SOCS3, STAT1, Chronic Inflammatory Skin Diseases, Eczema
Abstract
Suppressor of cytokine signaling (SOCS)3 belongs to a family of proteins that are known to exert important functions as inducible feedback inhibitors and are crucial for the balance of immune responses. There is evidence for a deregulated immune response in chronic inflammatory skin diseases. Thus, it was the aim of this study to investigate the regulation of SOCS proteins involved in intracellular signaling pathways occurring during inflammatory skin diseases and analyze their impact on the course of inflammatory responses. Because we and others have previously described that the cytokine IL-27 has an important impact on the chronic manifestation of inflammatory skin diseases, we focused here on the signaling induced by IL-27 in human primary keratinocytes compared with autologous blood-derived macrophages. Here, we demonstrate that SOCS3 is critically involved in regulating the cell-specific response to IL-27. SOCS3 was found to be significantly up-regulated by IL-27 in macrophages but not in keratinocytes. Other STAT3-activating cytokines investigated, including IL-6, IL-22, and oncostatin M, also failed to up-regulate SOCS3 in keratinocytes. Lack of SOCS3 up-regulation in skin epithelial cells was accompanied by prolonged STAT1 and STAT3 phosphorylation and enhanced CXCL10 production upon IL-27 stimulation compared with macrophages. Overexpression of SOCS3 in keratinocytes significantly diminished this enhanced CXCL10 production in response to IL-27. We conclude from our data that keratinocytes have a cell type-specific impaired capacity to up-regulate SOCS3 which may crucially determine the course of chronic inflammatory skin diseases.
Introduction
Inflammatory skin diseases such as chronic eczema or psoriasis are characterized by an ongoing proinflammatory type 1 deviated immune response and a disordered regulation of the immune response. A plethora of different mediators are involved in this process, and their complex interactions are still incompletely understood.
A recently described important player in chronic inflammatory skin diseases is the cytokine IL-27 (1–3). IL-27 belongs to the IL-12 family of cytokines and is a heterodimeric cytokine composed of the subunits p28 and Epstein-Barr virus-induced gene 3 (4). The unique IL-27 receptor α/WSX-1 and the common gp130 chain build up the functional IL-27 receptor that is widely expressed on cells of the immune system, including antigen-presenting cells and keratinocytes. Downstream of the IL-27 receptor both STAT1 and STAT3 are activated and coordinate the pleiotropic effects of IL-27 on a variety of different cell types of the innate and adaptive immune systems (2, 5–7). Initially, IL-27 was identified to act as a Th1 polarizing mediator (6, 8), a well known function of IL-12 family members. By acting directly on naive T cells it sensitizes CD4+ T cells to respond to IL-12 stimulation during early immune responses. Furthermore, IL-27 promotes a proinflammatory type 1 response by priming human primary keratinocytes, macrophages or inflammatory dendritic epidermal cells for IL-6, TNFα, CXCL10, and IL-23 production, respectively. IL-27 is therefore significantly involved in initiating and driving the chronic phase of inflammatory skin diseases (1–3, 7).
Interestingly, as shown by Owaki et al. (9) for CD4+ T cells, the STAT3-responsive molecule SOCS33 is also induced by IL-27. SOCS3 is a member of the SOCS family of intracellular proteins that, as inducible feedback inhibitors, are crucial for regulating signaling responses (10). SOCS3 has been described to inhibit STAT3 signaling by binding directly to the gp130 receptor chain and thus preventing JAK phosphorylation (11, 12). It was also shown that SOCS3 directly inhibits JAK2 activity (13). Of note, SOCS3 is not a common STAT3 inhibitor but acts in a receptor-specific manner, in particular for gp130. In this context it has for example been shown that SOCS3 shapes the specificity of STAT1/STAT3 activation in IL-6 but not IL-10 signaling (14–16).
The present study was designed to examine cell type-dependent differences in IL-27 signaling with the aim to identify mechanisms which contribute to skin inflammation becoming chronic. Using keratinocytes and autologous macrophages, we found increased SOCS3 expression upon IL-27 stimulation in macrophages but not in keratinocytes. Consequently, the restricted ability of skin epithelial cells to up-regulate SOCS3 leads to a prolonged IL-27 activity with increased CXCL10 secretion in this cell type. Manipulation of SOCS3 expression in keratinocytes could therefore be an interesting approach to limit epidermal inflammation.
EXPERIMENTAL PROCEDURES
Cytokines and Reagents
IL-27 and IFNγ were used as purified recombinant human preparations and were purchased from R&D Systems. Actinomycin D and dimethyl sulfoxide were from Sigma-Aldrich.
Cell Isolation and Culture of Human Macrophages and Human Primary Keratinocytes
Peripheral blood mononuclear cells from healthy donors were separated by Ficoll-Hypaque density gradient centrifugation and resuspended in Iscove's (Biochrom, Berlin, Germany) medium supplemented with 4% AB serum (IAB medium). Monocytes were enriched by plastic adherence: 1 × 108 peripheral blood mononuclear cells were plated in Petri dishes (Heraeus, Hannover, Germany) or in 80-cm2 culture flasks (NuclonTM; Nunc GmbH & Co. AG, Wiesbaden). After 1 h (37 °C, 5% CO2), nonadherent cells were carefully removed by several washes with prewarmed phosphate-buffered saline (PBS; Pan, Aidenbach, Germany), and thereafter the adherent cell population was detached using a cell scraper after culture dishes were incubated on ice for 10 min. Detached cells were washed and resuspended in IAB medium and then allowed to rest in round bottom 96-well microtiter plates (Nunc) for 2 days before they were used for further experiments.
Primary cultures of normal human keratinocytes were prepared from foreskin (17) or from epidermal stem cells of the hair follicle of anagen head hairs (18). Cells were cultured in keratinocyte medium (Keratinocyte Growth Medium 2 kit; PromoCell), which was changed every second day. When cells reached 70–80% confluence they were used for further experiments or passaged. Before stimulation, hydrocortisone and EGF were omitted from the medium.
This study was approved by the medical ethical committee of the Hannover Medical School and was conducted according to the Declaration of Helsinki Principles.
Flow Cytometric Analysis of Intracellular Molecules
Prior to flow cytometric analysis keratinocytes were detached by using 0.025% EDTA (10 min; PAN Biotech) and HyQTase (10 min; Perbio, Bonn, Germany). For intracellular staining macrophages and keratinocytes were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences). Phycoerythrin-labeled mAbs (mouse anti-human) or nonconjugated CXCL10 antibody used were from R&D Systems. A rabbit anti-human antibody was used for SOCS3 detection (Abcam). As secondary antibody goat anti-mouse or goat anti-rabbit IgG F(ab)′2 fragment-allophycocyanin (BD Biosciences) was used, respectively. Stained cells were measured by flow cytometry (FACSCalibur) and analyzed using Cell QuestProTM software (BD Biosciences). Control staining was performed with matched isotypes used in the same final concentration as corresponding staining antibodies (phycoerythrin-conjugated mouse IgG1 (Sigma), unlabeled IgG1, and unlabeled rabbit anti-human antibody (R&D Systems)).
mRNA Isolation and Quantitative RT-PCR
RNA was isolated using the High Pure RNA Isolation kit (Roche Molecular Biochemicals). Reverse transcription was performed using the QuantiTect Reverse Transcription kit (Qiagen). The quantitative RT-PCR was performed on a LightCycler (Roche Molecular Biochemicals) using the QuantiTect SYBR Green PCR kit (Qiagen). The primers 5′-AgACTTCgATTCgggACCA and 5′-AACTTgCTgTgggTgACCA (TIB MOLBIOL, Berlin, Germany) were used for SOCS3, and for GAPDH we used QuantiTect Primers (Qiagen). For quantitative analysis targets were quantified using the Relative Quantification software (Roche Molecular Biochemicals) as described previously (19).
CXCL10 ELISA
Cell-free supernatants were used for CXCL10 ELISA. The ELISA kit was purchased from R&D Systems.
Phospho-STAT and SOCS3 ELISA
A total of 1 × 105 keratinocytes or 1 × 106 macrophages were homogenized in M-Per Mammalian Extraction Reagent (Thermo Fisher Scientific) containing protease and phosphatase inhibitor (complete mini and PhosSTOP; Roche Molecular Biochemicals). After cell lyses, extracts were centrifuged at 3000 × g for 10 min (4 °C). Supernatants were collected and used for pSTAT1 and pSTAT3 ELISA (Cell Signaling, New England BioLabs) or SOCS3 ELISA (Uscn Life Science Inc., Biozol, Eching, Germany) respectively. All ELISAs were performed according to the manufacturer's instructions.
Western Blotting
Cell extracts were obtained as described for phospho-STAT and SOCS3 ELISA. After centrifugation (3000 × g for 10 min at 48 ºC), samples were separated on a 4–15% Tris-HCl gel (Ready Gel; Bio-Rad) by using SDS-PAGE running conditions (Tris/glycine/SDS buffer; Bio-Rad). The semidry system was used to transfer proteins to a nitrocellulose membrane (25 min at 18 V, room temperature). Membranes were incubated in blocking buffer composed of TBS (Bio-Rad), 0.1% Tween 20, and 5% nonfat milk powder (2 h at room temperature; Roth GmbH, Karlsruhe, Germany). The first antibody incubation was performed in TBS/0.1% Tween 20 containing 5% BSA (overnight at 4 °C; Sigma-Aldrich). Rabbit anti-STAT1 and rabbit anti-STAT3, respectively, were used to detect total STAT1 and STAT3, followed by a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody. β-Actin was detected using a mouse anti-β-actin antibody and a horseradish peroxidase-conjugated goat anti-mouse antibody as secondary antibody. All antibodies were purchased from Cell Signaling. Second antibody incubation was performed in blocking buffer (see above) for 1 h at room temperature. Detection was performed using chemiluminescence for which the membrane was incubated for 5 min in SuperSignal West Pico substrate (Thermo Fisher Scientific) according to the manufacturer's instructions. Subsequently, the signal was detected using the MultiImage Light Cabinet and the Alpha DigDoc 1000 Software (Alpha Innotech Corporation, Biozym, Hess Oldendorf, Germany).
Overexpression of SOCS3 in Keratinocytes
Cells were grown in a 12-well plate until they reached 80–90% confluence. Then they were transfected using FuGENEHD (Roche Molecular Biochemicals) transfection reagent and 2 μg of a plasmid encoding for human eGFP-SOCS3 fusion (20). Transfection efficacy was between 8 and 20%.
Statistical Analysis
Data were either analyzed using the t test (columns: mean values ± S.E. are depicted), paired t test (individual values are depicted), or Wilcoxon Signed Rank test according to the distribution of the data. The software used to perform the statistical analyses was GraphPad Prism for Macintosh version 5.0a.
RESULTS
SOCS3 Is Regulated Differentially in Macrophages and Keratinocytes
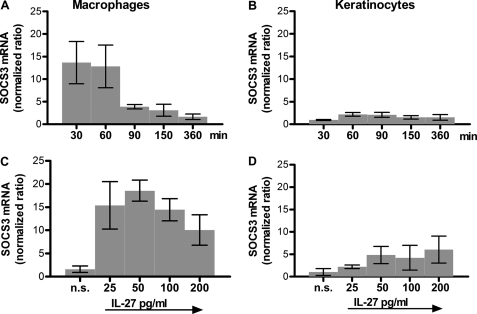
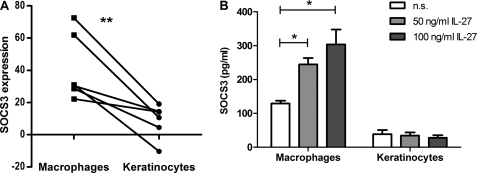
SOCS3 has been described to be induced upon IL-27 stimulation in naive CD4+ T cell (9). Our own previous data based on microarray analyses (InfHuman) (21) and CXCL10 protein secretion showed that both cell types, keratinocytes as well as human macrophages, clearly respond to IL-27 (2, 3). To determine whether SOCS3 is involved in the regulation of IL-27 signaling in human macrophages and keratinocytes, we investigated SOCS3 mRNA expression profiles by quantitative real-time PCR and protein expression by FACS analysis. We performed time kinetic experiments and examined dose-dependent responses by analyzing mRNA levels. As shown in Fig. 1, SOCS3 was rapidly up-regulated in macrophages with maximal induction after 30–60 min. In contrast to the 10–20-fold induction of SOCS3 mRNA observed in human macrophages (Fig. 1A), keratinocytes only showed timely delayed (e.g. after 60–90 min), minor inducibility of SOCS3 mRNA after stimulation with IL-27 (Fig. 1B). A clear dose-dependent response, analyzed 60 min after stimulation, was observed in macrophages as depicted in Fig. 1C. Cells responded with an increase in SOCS3 mRNA expression upon stimulation with IL-27 up to 50 ng/ml; higher doses of IL-27 were less effective in inducing SOCS3 mRNA levels. Keratinocytes showed a modest increase in SOCS3 mRNA with increasing doses of IL-27 (Fig. 1D). Thus, in macrophages we found an up to 18-fold induction of SOCS3 mRNA following IL-27 stimulation whereas in keratinocytes the maximal expression observed was a 6-fold induction. These results were confirmed on the protein level by intracellular FACS analysis (Fig. 2A) and ELISA (Fig. 2B). Using autologous keratinocytes and macrophages, the latter showed a significant increase in SOCS3 protein compared with keratinocytes (Fig. 2A). Absolute protein levels of SOCS3 are depicted in Fig. 2B and were in line with the results obtained by FACS. Interestingly, we observed even lower basal levels of SOCS3 in keratinocytes than in macrophages. Of note, the first increase of SOCS3 was detectable 5 min after IL-27 stimulation on the mRNA level and after 15 min on the protein level. Furthermore, other STAT3-activating cytokines such as IL-6, IL-22, oncostatin M, or IL-31 also failed to up-regulate SOCS3 in keratinocytes (data not shown), which points to a general cell-specific impairment of SOCS3 induction in skin epithelial cells.
FIGURE 1.
IL-27 induces higher levels of SOCS3 mRNA in macrophages than in keratinocytes. Macrophages (A) and human primary keratinocytes (B) were stimulated for the indicated time periods with IL-27 (50 ng/ml) or for 60 min with the indicated concentrations of IL-27 (C and D). Quantitative RT-PCR was performed for SOCS3. n = 6. Error bars, S.D.
FIGURE 2.
SOCS3 protein is highly inducible in stimulated macrophages compared with autologous keratinocytes. A, autologous macrophages and keratinocytes were stimulated for 4 h with 50 ng/ml IL-27 before intracellular FACS staining was performed using rabbit anti-hSOCS3 AB. The percentage of IL-27-induced SOCS3 increase with regard to percentage positive cells (relative to the nonstimulated value of each independent experiment) is depicted. n = 6. **, p < 0.02. B, macrophages and keratinocytes were stimulated for 4 h as indicated. Cell extracts were taken and analyzed for SOCS3 by ELISA. As control, the same cell extracts were used to perform β-actin Western blotting. n = 4. *, p < 0.05; n.s., not significant; error bars, S.D.
IL-27-induced Pattern of STAT1 and STAT3 Phosphorylation Differs between Keratinocytes and Macrophages
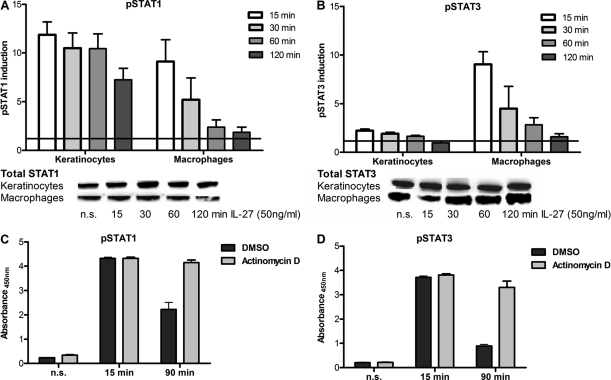
IL-27 induces the phosphorylation of STAT1 and STAT3 in both human monocytes and keratinocytes (2, 5). To investigate whether SOCS3 influences IL-27 signaling by blocking STAT-dependent signaling cascades we performed pSTAT1 and pSTAT3 ELISAs using cell extracts. We focused on the differences in intensity and duration of STAT1 and STAT3 tyrosine phosphorylation between both cell types (Fig. 3). 15 min after stimulation we found for both cell types equal amounts of pSTAT1 that were up to 10-fold higher than basal levels. By contrast, both cell types showed a profoundly different response with regard to STAT3 activation. First, the level of pSTAT3 was significantly higher in stimulated macrophages than in stimulated keratinocytes (Fig. 3, A and B). Second, keratinocytes that only showed a 2-fold increase in pSTAT3 after stimulation were found to express approximately 3-fold higher basal level of pSTAT3 than macrophages (compare also Fig. 4C). The levels of total STAT1 and STAT3 were checked by Western blotting and showed a uniform expression for the analyzed time period. Of note, the pSTAT1/3 response pattern was not restricted to proliferating keratinocytes because highly confluent, nonproliferating cells showed the same STAT-activating responses upon IL-27 stimulation (data not shown). Interestingly, the phosphorylation state of both STAT proteins was found to be considerably prolonged in keratinocytes compared with macrophages. These results indicate that SOCS3 may be less efficient in counterregulating STAT pathway activation in epithelial cells.
FIGURE 3.
Keratinocytes show reduced pSTAT3 induction and prolonged phosphorylation of STAT1/3 upon IL-27 stimulation. Cells were stimulated for the indicated time periods with 50 ng/ml IL-27 before cell extracts were prepared for pSTAT1 (A and C) or pSTAT3 (B and D) analysis by ELISA. A and B, induction levels were calculated by referring values of stimulated cells to those of nonstimulated cells for each independent experiment. The respective total levels of STAT1 and STAT3 are shown exemplarily by Western blotting. C and D, macrophages were preincubated with actinomycin D (5 μg/ml) or corresponding amounts of dimethyl sulfoxide (DMSO) for 30 min before subsequent IL-27 stimulation. n = 4. Error bars, S.D.
FIGURE 4.
Keratinocytes but not macrophages are able to respond to repetitive IL-27 stimulation. As depicted in A, cells were stimulated for 1 h with IL-27 (50 ng/ml) before medium was changed. After another 3 h, cells were stimulated again with IL-27 (50 ng/ml) (repetitive stimulation) and incubated for another 30 min before protein extracts were taken for pSTAT1 (B and D) and pSTAT3 (C and E) ELISA. D and E, macrophages were preincubated with actinomycin D (5 μg/ml) or corresponding amounts of dimethyl sulfoxide (DMSO) for 30 min before subsequent IL-27 stimulation. F and G, autologous human primary keratinocytes and macrophages were stimulated as described above. After restimulation, cells were incubated for another 20 h before supernatants were taken for CXCL10 ELISA. n = 3 (B and C), n = 4 (D–G). *, p < 0.05; n.s., not significant; error bars, S.D.
Regulation of IL-27-induced STAT1/3 Phosphorylation Duration Requires Transcription
SOCS3 is known to act as a feedback inhibitor and was shown to be responsible for the abrogation of IL-6 signaling (22). However, constitutively expressed phosphatases such as SHP2 may be involved in the observed limitation of STAT1/3 phosphorylation patterns in IL-27 signaling. To show that IL-27 induces a negative feedback loop that requires the transcription of de novo induced protein we blocked transcription by actinomycin D treatment. If only constitutively expressed phosphatases were involved in the regulation of IL-27-induced STAT1/3 phosphorylation, the actinomycin D treatment would have no or only minor effects on the phosphorylation pattern. However, as depicted in Fig. 3, C and D, treatment of macrophages with actinomycin D clearly prolonged the phosphorylation compared with dimethyl sulfoxide-treated controls. Taken together, we conclude that the regulation of IL-27-induced STAT1/3 phosphorylation duration requires transcription and that this regulation is not solely dependent on constitutively expressed phosphatases.
SOCS3 Leads to Desensitization toward IL-27 Stimulation in Macrophages but Not in Keratinocytes
To test whether SOCS3 is able to block IL-27 signaling, we performed restimulation experiments. Keratinocytes and macrophages were first stimulated with IL-27 for 1 h. Medium was than changed, and after another 3 h cells were stimulated again with IL-27 (Fig. 4A). Because SOCS3 up-regulation was observed in macrophages, but not in keratinocytes (Fig. 1), we hypothesized that only macrophages would become unresponsive to secondary IL-27 stimulation. Cell extracts were prepared for pSTAT1 and pSTAT3 determinations after a 15-min incubation, and supernatants were taken 20 h after addition of the second IL-27 stimulation. As shown in Fig. 4, B and C, keratinocytes were able to respond repeatedly to IL-27 with activation of STAT1. STAT3 activation in restimulated keratinocytes was not higher than in nontreated cells. By contrast, macrophages completely failed to respond to a secondary IL-27 stimulation. We confirmed that the desensitization of macrophages to IL-27 requires transcriptional activity because culture in the presence of actinomycin D abolished the desensitization (Fig. 4, D and E).
In a continuative approach we analyzed a STAT1 downstream molecule to understand the functional consequences of different IL-27 signaling in the investigated cell types. CXCL10, which has previously been shown to be inducible by IL-27 in keratinocytes and monocytes (2, 7), was analyzed in supernatants of stimulated cells by ELISA. As shown in Fig. 4, F and G, CXCL10 production paralleled results obtained by pSTAT ELISA. Keratinocytes were able to respond repeatedly to IL-27 stimulation, resulting in even increasing amounts of CXCL10 whereas macrophages failed to do so.
Overexpression of SOCS3 Results in Decreased Expression of CXCL10 in Keratinocytes
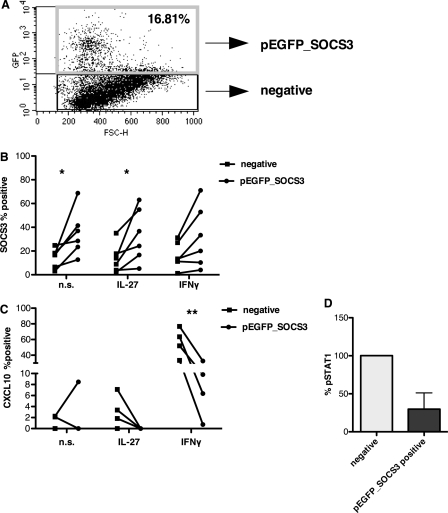
To prove that SOCS3 is responsible for the differential production of CXCL10 in keratinocytes and macrophages, we transfected keratinocytes with a plasmid encoding for a GFP-SOCS3 fusion protein. Transfected cells were identified by their subsequent eGFP expression. This population was compared with the eGFP negative population by FACS analysis as a built-in internal control (Fig. 5A). Transfected keratinocytes were analyzed by FACS for their yield of SOCS3 and CXCL10 protein. As depicted in Fig. 5B, eGFP-positive cells expressed significantly more SOCS3 than the negative ones. In line with an increase of SOCS3, CXCL10 production was significantly reduced in these cells after stimulation (Fig. 5C). STAT1 expression as detected by pSTAT1 ELISA was markedly reduced in eGFP-positive keratinocytes overexpressing SOCS3 (Fig. 5D). Interestingly, overexpression of SOCS3 affected not only IL-27 but also IFNγ-induced CXCL10. Taken together, these results confirm a role of SOCS3 in regulating CXCL10 production.
FIGURE 5.
Overexpression of eGFP-SOCS3 in keratinocytes results in a decrease of STAT1 activation and CXCL10 production. A–C, eGFP-SOCS3-transfected cells were analyzed by FACS. eGFP-positive cells were considered as successfully transfected (pEGFP_SOCS3-positive) (A). This population was gated and compared with the eGFP-negative cells for the expression of SOCS3 (B) and CXCL10 (C) (50 ng of IL-27, 20 ng of IFNγ; n = 6). *, p < 0.05; **, p < 0.02. D, eGFP-positive or -negative fractions were sorted by FACS. Protein extracts were taken from both sorted fractions and analyzed for their amount of activated STAT1 by ELISA (50 ng/ml IL-27, 15 min).
DISCUSSION
Here, we show for the first time that skin epithelial cells have a restricted ability to up-regulate SOCS3. We demonstrated that SOCS3 regulates the intensity and duration of IL-27 signaling in human macrophages and keratinocytes. This regulation occurs in macrophages in the form of a well known negative feedback-loop. IL-27 directly induces SOCS3 in this cell type. By contrast, this feedback mechanism is less evident in keratinocytes resulting in prolonged tyrosine phosphorylation of STAT1 and STAT3 as well as enhanced production of proinflammatory mediators including CXCL10. In the case of keratinocytes, IL-27 failed to up-regulate SOCS3, which might be due to a reduced sensitivity to gp130-induced STAT3 activation. This is in line with our findings of higher basal levels of pSTAT3 but not of pSTAT1 in keratinocytes compared with macrophages. Apart from a reduced sensitivity to gp130-induced STAT3 activation, epigenetic alterations in the SOCS3 gene might be responsible for the restricted SOCS3 expression. It has been described that SOCS3 is regulated by promoter-associated histone modifications as well as by DNA methylation of the SOCS promoter region (23–25). So far, we have checked the SOCS3 gene at two different sites for CpG island methylation status: in the promoter region (Hs_SOCS3_01_PM PyroMark CpG Assay; Qiagen) and at 18 CpG sites in intron 1 (the analyzed region extended over the region +647 to +768 of the SOCS3 gene) but have not found a difference between macrophages and keratinocytes (data not shown).
Interestingly, we observed high basal levels of activated STAT3 in keratinocytes which may be due to the reduced basal levels of SOCS3 in these cells compared with macrophages. Because keratinocytes are proliferative active cells, the higher basal levels of pSTAT3 may be necessary for their cell division, and increased SOCS3 activity could therefore interfere with the repair of skin defects. Thus, an epigenetic modification of the SOCS3 gene in keratinocytes could be hypothesized because it may ensure essential functions of the epidermal compartment.
As described by Pflanz et al. (5) IL-27-induced STAT3 activation is attributed to gp130 whereas STAT1 activation is mediated mainly by WSX-1 (26). Interestingly, our data support that SOCS3 targets the signaling downstream of both IL-27 receptor subunits gp130 and WSX-1. SOCS3 has been described to inhibit receptor-specific STAT3 signaling in human and murine macrophages (14, 16, 27). gp130 signaling is selectively affected by SOCS3 because IL-6 (via gp130)- but not IL-10 (via IL-10 receptor)-induced STAT3 activation is blocked by SOCS3. As shown by Nicholson et al. (12), SOCS3 can interact directly with gp130 by binding on its phosphotyrosine 759 motif and therefore compete with SHP2/JAK. Moreover, Song and Shuai (28) demonstrated that SOCS3 can also inhibit STAT1 tyrosine phosphorylation and its nuclear translocation. This finding is also applicable for IL-27-induced SOCS3 as presented in this study. A major role of SOCS1 (the common regulator of STAT1 activation) (29) in the inhibition of IL-27-activated STAT1 seems unlikely in our experimental setup. This is because first, real-time PCR showed low expression of SOCS1 compared with SOCS3 with crossing points for SOCS1 above cycle 30 (keratinocytes) and 34 (macrophages), respectively (supplemental Fig. S1). Second, manipulation of SOCS3 expression (as shown in Fig. 5) clearly pointed to its functional role in STAT1 pathway regulation in our experimental approach. Furthermore, by actinomycin D treatment of macrophages we excluded receptor internalization or phosphatase activity as important regulatory mechanisms in this context.
IL-27-induced STAT1 activation appears to have an important role in regulating the course of proinflammatory responses. SOCS3 was rapidly up-regulated by IL-27 in macrophages, however, only responded once to IL-27 stimulation, whereas keratinocytes in which IL-27 failed to increase SOCS3 reacted repeatedly with proinflammatory mediator production. We conclude from our data that SOCS3 plays an important role in preventing macrophages from prolonged proinflammatory responses. By contrast, this feedback regulation is not mounted efficiently in epithelial skin cells. To confirm this we overexpressed SOCS3 in keratinocytes which results in an inhibition of STAT1 and STAT3 activation. Interestingly, overexpression of SOCS3 not only reduced IL-27 but also IFNγ-induced CXCL10 production. Thus, the impaired ability of keratinocytes to up-regulate SOCS3 affects their response to STAT1-activating molecules and results in a prolonged, unleashed production of proinflammatory mediators such as CXCL10. CXCL10 is one of the main chemokines attracting CXCR3+ IFNγ-producing T cells into the skin. The infiltration of CXCR3+ Th1 cells characterizes chronic inflammatory diseases such as psoriasis, cutaneous lupus erythematosus, and chronic states of eczematous conditions.
Translating these novel findings to clinical relevance, we conclude that the dysfunctional SOCS3 expression in keratinocytes contributes to the chronification of inflammatory Th1/STAT1-dominated epidermal responses. Recently, we reported that IL-27 was found to be expressed in the chronic (Th1-dominated) but not in the acute phase of eczema (Th2-dominated) (2). In addition, Shibata et al. (1) found an infiltration of IL-27-secreting cells in psoriatic skin lesions. In both cases it may act as a strong proinflammatory mediator able to maintain Th1 cell response in the epidermal compartment. Interestingly, SOCS3 is reported to be mainly expressed under Th2 conditions. Horiuchi et al. (30) described that SOCS3 was found to be expressed in lesions of patients suffering from severe atopic dermatitis (Th2-dominated) but not in lesions from patients with the Th1/Th17-dominated disorder psoriasis. In addition, Ekelund et al. (31) linked the SOCS3 gene to atopic dermatitis, and Seki et al. (32) reported a strong correlation between SOCS3 expression and the pathology of Th2-mediated asthma and atopic dermatitis. These data imply that regulation of SOCS3 expression could be a therapeutic target for Th1-mediated inflammatory skin diseases.
Finally, our data imply that the functional outcome of IL-27 signaling is highly regulated by SOCS3. Cell-dependent differences of macrophages and keratinocytes in response to these mediators can be assigned to a different expression pattern of SOCS3 in both cell types. In addition, previous findings point to a relative lack of SOCS3 expression in Th1-mediated diseases. In the case of chronic eczema this is accompanied by an enhanced expression of IL-27 which results in an abundant release of CXCL10 by keratinocytes. Therefore, we hypothesize that the failure to up-regulate SOCS3 expression in keratinocytes efficiently contributes to the maintenance of ongoing proinflammatory responses in the skin and probably other epithelial interfaces.
Supplementary Material
Acknowledgments
We thank Ilona Klug and Christina Hartmann for technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB566, A6, GRK1441, and a research grant from the Royal Society.

This article contains supplemental Fig. S1.
- SOCS3
- suppressor of cytokine signaling 3.
REFERENCES
- 1. Shibata S., Tada Y., Kanda N., Nashiro K., Kamata M., Karakawa M., Miyagaki T., Kai H., Saeki H., Shirakata Y., Watanabe S., Tamaki K., Sato S. (2010) Possible roles of IL-27 in the pathogenesis of psoriasis. J. Invest. Dermatol. 130, 1034–1039 [DOI] [PubMed] [Google Scholar]
- 2. Wittmann M., Zeitvogel J., Wang D., Werfel T. (2009) IL-27 is expressed in chronic human eczematous skin lesions and stimulates human keratinocytes. J. Allergy Clin. Immunol. 124, 81–89 [DOI] [PubMed] [Google Scholar]
- 3. Zeitvogel J., Werfel T., Wittmann M. (2009) IL-27 acts as a priming signal for IL-23 production on APC. Poster Sessions. Eur. J. Immunol. 39, S209 [Google Scholar]
- 4. Pflanz S., Timans J. C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., Blumenschein W. M., Mattson J. D., Wagner J. L., To W., Zurawski S., McClanahan T. K., Gorman D. M., Bazan J. F., de Waal Malefyt R., Rennick D., Kastelein R. A. (2002) IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16, 779–790 [DOI] [PubMed] [Google Scholar]
- 5. Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J. F., Phillips J. H., McClanahan T. K., de Waal Malefyt R., Kastelein R. A. (2004) WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231 [DOI] [PubMed] [Google Scholar]
- 6. Hibbert L., Pflanz S., De Waal Malefyt R., Kastelein R. A. (2003) IL-27 and IFN-α signal via Stat1 and Stat3 and induce T-Bet and IL-12Rβ2 in naive T cells. J. Interferon Cytokine Res. 23, 513–522 [DOI] [PubMed] [Google Scholar]
- 7. Kalliolias G. D., Ivashkiv L. B. (2008) IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J. Immunol. 180, 6325–6333 [DOI] [PubMed] [Google Scholar]
- 8. Pflanz S., Kurth I., Grötzinger J., Heinrich P. C., Müller-Newen G. (2000) Two different epitopes of the signal transducer gp130 sequentially cooperate on IL-6-induced receptor activation. J. Immunol. 165, 7042–7049 [DOI] [PubMed] [Google Scholar]
- 9. Owaki T., Asakawa M., Kamiya S., Takeda K., Fukai F., Mizuguchi J., Yoshimoto T. (2006) IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J. Immunol. 176, 2773–2780 [DOI] [PubMed] [Google Scholar]
- 10. Dalpke A., Heeg K., Bartz H., Baetz A. (2008) Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology 213, 225–235 [DOI] [PubMed] [Google Scholar]
- 11. Schmitz J., Weissenbach M., Haan S., Heinrich P. C., Schaper F. (2000) SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J. Biol. Chem. 275, 12848–12856 [DOI] [PubMed] [Google Scholar]
- 12. Nicholson S. E., De Souza D., Fabri L. J., Corbin J., Willson T. A., Zhang J. G., Silva A., Asimakis M., Farley A., Nash A. D., Metcalf D., Hilton D. J., Nicola N. A., Baca M. (2000) Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc. Natl. Acad. Sci. U.S.A. 97, 6493–6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masuhara M., Sakamoto H., Matsumoto A., Suzuki R., Yasukawa H., Mitsui K., Wakioka T., Tanimura S., Sasaki A., Misawa H., Yokouchi M., Ohtsubo M., Yoshimura A. (1997) Cloning and characterization of novel CIS family genes. Biochem. Biophys. Res. Commun. 239, 439–446 [DOI] [PubMed] [Google Scholar]
- 14. Lang R., Pauleau A. L., Parganas E., Takahashi Y., Mages J., Ihle J. N., Rutschman R., Murray P. J. (2003) SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 4, 546–550 [DOI] [PubMed] [Google Scholar]
- 15. Croker B. A., Krebs D. L., Zhang J. G., Wormald S., Willson T. A., Stanley E. G., Robb L., Greenhalgh C. J., Förster I., Clausen B. E., Nicola N. A., Metcalf D., Hilton D. J., Roberts A. W., Alexander W. S. (2003) SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 4, 540–545 [DOI] [PubMed] [Google Scholar]
- 16. Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., Hirano T., Chien K. R., Yoshimura A. (2003) IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 4, 551–556 [DOI] [PubMed] [Google Scholar]
- 17. Wittmann M., Purwar R., Hartmann C., Gutzmer R., Werfel T. (2005) Human keratinocytes respond to interleukin-18: implication for the course of chronic inflammatory skin diseases. J. Invest. Dermatol. 124, 1225–1233 [DOI] [PubMed] [Google Scholar]
- 18. Wang D., Drenker M., Eiz-Vesper B., Werfel T., Wittmann M. (2008) Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis Rheum. 58, 3205–3215 [DOI] [PubMed] [Google Scholar]
- 19. Purwar R., Werfel T., Wittmann M. (2006) IL-13-stimulated human keratinocytes preferentially attract CD4+CCR4+ T cells: possible role in atopic dermatitis. J. Invest. Dermatol. 126, 1043–1051 [DOI] [PubMed] [Google Scholar]
- 20. Baetz A., Koelsche C., Strebovsky J., Heeg K., Dalpke A. H. (2008) Identification of a nuclear localization signal in suppressor of cytokine signaling 1. FASEB J. 22, 4296–4305 [DOI] [PubMed] [Google Scholar]
- 21. Purwar R., Wittmann M., Zwirner J., Oppermann M., Kracht M., Dittrich-Breiholz O., Gutzmer R., Werfel T. (2006) Induction of C3 and CCL2 by C3a in keratinocytes: a novel autocrine amplification loop of inflammatory skin reactions. J. Immunol. 177, 4444–4450 [DOI] [PubMed] [Google Scholar]
- 22. Fischer P., Lehmann U., Sobota R. M., Schmitz J., Niemand C., Linnemann S., Haan S., Behrmann I., Yoshimura A., Johnston J. A., Müller-Newen G., Heinrich P. C., Schaper F. (2004) The role of the inhibitors of interleukin-6 signal transduction SHP2 and SOCS3 for desensitization of interleukin-6 signalling. Biochem. J. 378, 449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teofili L., Martini M., Cenci T., Guidi F., Torti L., Giona F., Foà R., Leone G., Larocca L. M. (2008) Epigenetic alteration of SOCS family members is a possible pathogenetic mechanism in JAK2 wild type myeloproliferative diseases. Int. J. Cancer 123, 1586–1592 [DOI] [PubMed] [Google Scholar]
- 24. Pierconti F., Martini M., Pinto F., Cenci T., Capodimonti S., Calarco A., Bassi P. F., Larocca L. M. (2011) Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate 71, 318–325 [DOI] [PubMed] [Google Scholar]
- 25. Xiong H., Du W., Zhang Y. J., Hong J., Su W. Y., Tang J. T., Wang Y. C., Lu R., Fang J. Y. (2012) Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol. Carcinog. 51, 174–184 [DOI] [PubMed] [Google Scholar]
- 26. Takeda A., Hamano S., Yamanaka A., Hanada T., Ishibashi T., Mak T. W., Yoshimura A., Yoshida H. (2003) Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J. Immunol. 170, 4886–4890 [DOI] [PubMed] [Google Scholar]
- 27. Niemand C., Nimmesgern A., Haan S., Fischer P., Schaper F., Rossaint R., Heinrich P. C., Müller-Newen G. (2003) Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J. Immunol. 170, 3263–3272 [DOI] [PubMed] [Google Scholar]
- 28. Song M. M., Shuai K. (1998) The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 273, 35056–35062 [DOI] [PubMed] [Google Scholar]
- 29. Yoshimura A., Naka T., Kubo M. (2007) SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7, 454–465 [DOI] [PubMed] [Google Scholar]
- 30. Horiuchi Y., Bae S. J., Katayama I. (2006) Overexpression of the suppressor of cytokine signalling 3 (SOCS3) in severe atopic dermatitis. Clin. Exp. Dermatol. 31, 100–104 [DOI] [PubMed] [Google Scholar]
- 31. Ekelund E., Saaf A., Tengvall-Linder M., Melen E., Link J., Barker J., Reynolds N. J., Meggitt S. J., Kere J., Wahlgren C. F., Pershagen G., Wickman M., Nordenskjold M., Kockum I., Bradley M. (2006) Elevated expression and genetic association links the SOCS3 gene to atopic dermatitis. Am. J. Hum. Genet. 78, 1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seki Y., Inoue H., Nagata N., Hayashi K., Fukuyama S., Matsumoto K., Komine O., Hamano S., Himeno K., Inagaki-Ohara K., Cacalano N., O'Garra A., Oshida T., Saito H., Johnston J. A., Yoshimura A., Kubo M. (2003) SOCS-3 regulates onset and maintenance of TH2-mediated allergic responses. Nat. Med. 9, 1047–1054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.