Abstract
Human frataxin (FXN) has been intensively studied since the discovery that the FXN gene is associated with the neurodegenerative disease Friedreich’s ataxia. Human FXN is a component of the NFS1-ISD11-ISCU2-FXN (SDUF) core Fe-S assembly complex and activates the cysteine desulfurase and Fe-S cluster biosynthesis reactions. In contrast, the Escherichia coli FXN homolog CyaY inhibits Fe-S cluster biosynthesis. To resolve this discrepancy, enzyme kinetic experiments were performed for the human and E. coli systems in which analogous cysteine desulfurase, Fe-S assembly scaffold, and frataxin components were interchanged. Surprisingly, our results reveal that activation or inhibition by the frataxin homolog is determined by which cysteine desulfurase is present and not by the identity of the frataxin homolog. These data are consistent with a model in which the frataxin-less Fe-S assembly complex exists as a mixture of functional and nonfunctional states, which are stabilized by binding of frataxin homologs. Intriguingly, this appears to be an unusual example in which modifications to an enzyme during evolution inverts or reverses the mode of control imparted by a regulatory molecule.
Keywords: Friedreich’s ataxia, frataxin, enzyme kinetics, regulator, Fe-S cluster
Iron-sulfur (Fe-S) clusters are protein cofactors that are essential for most living organisms. Fe-S clusters primarily function in electron transfer, but also participate in substrate binding and activation, protein stabilization, regulation of gene expression or enzyme activity, radical generation, and as a sulfur donor (1–3). These biological functions require the Fe-S cluster to vary from solvent accessible to completely buried, and to be accommodated in a diverse set of protein scaffolds (4). Despite this diversity, conserved metallochaperone proteins recognize and insert Fe-S clusters into apo Fe-S proteins through mechanisms that remain poorly understood. This assembly system must also control the reactivity of the building blocks for Fe-S clusters, ferrous iron and sulfide, to reduce toxicity and cell death. In humans, ferrous iron is prone to undergo Fenton chemistry: reaction with hydrogen peroxide to generate highly reactive hydroxyl radicals that damage proteins, lipids, and DNA, whereas sulfide is more toxic than cyanide, is a potent inhibitor of cytochrome c oxidase, and targets olfactory nerves, the eyes, and the brain (5). Defects in these metallochaperone systems have profound effects on iron metabolism, and result in mitochondrial dysfunction and human disease (6–8).
In prokaryotes, three different Fe-S assembly systems have been characterized: the nitrogen fixation (NIF), iron-sulfur cluster assembly (ISC), and mobilization of sulfur (SUF) pathways (9–13). Escherichia coli contains both ISC and SUF systems and appears to use the ISC pathway under ‘normal’ growth conditions and the SUF system under conditions of iron limitation or oxidative stress (14). The ISC operon is controlled by IscR, a Fe-S cluster binding transcriptional repressor, and contains genes for a cysteine desulfurase (IscS), scaffold protein (IscU), ferredoxin (Fdx), IscA, molecular chaperones (hscA and hscB), and IscX (also known as yfhj and ORF3). The scaffold protein IscU (in this manuscript the E. coli homolog is referred to as IscUec, or Uec) is the central protein in Fe-S cluster biosynthesis and is responsible for the assembly of [2Fe-2S] and [4Fe-4S] clusters (15). IscS (E. coli homolog referred to as IscSec or Sec) catalyzes the PLP-dependent breakdown of cysteine to alanine and provides the inorganic sulfur for Fe-S clusters (16, 17). Fdx likely provides electrons for cluster assembly and IscA may be an iron donor or alternate scaffold (18–20). The function of IscX is unknown, but it appears to compete with CyaY for binding to IscS (21–23). Once the cluster is formed, the chaperones hscA and hscB facilitate the transfer of intact Fe-S clusters to apo target proteins (24–26). A homolog of human frataxin (E. coli homolog referred to as CyaY or as Cec in protein complexes in this manuscript) is not part of the ISC operon, but has been shown to bind to the IscSec-IscUec (SecUec) complex (21, 27, 28) and inhibit Fe-S cluster assembly (29).
A similar ISC Fe-S cluster assembly pathway functions in the matrix space of eukaryotic mitochondria (8, 30). The Fe-S assembly proteins are encoded by nuclear DNA and often contain a targeting sequence that is cleaved upon import into mitochondria. For the human pathway, the cysteine desulfurase NFS1 (59% identical to IscSec), which forms a eukaryotic-specific functional complex with ISD11 (31–33), the scaffold protein ISCU2 (also known as Isu2; 70% identical to IscUec), and FXN (20% identical to CyaY) form a NFS1-ISD11-ISCU2-FXN (SDUF) complex (34, 35) that is analogous to the IscSec-IscUec-CyaY (SecUecCec) complex. Recent in vitro studies indicate that FXN is an allosteric activator of Fe-S cluster assembly that increases the catalytic efficiency (kcat/KM) for the cysteine desulfurase and the rate of Fe-S cluster biosynthesis (34). Intriguingly, in vitro data indicate E. coli CyaY and human FXN have opposing regulator functions in Fe-S cluster biosynthesis (29, 34). Different physiological functions may also be supported by different in vivo phenotypes for E. coli and mouse model systems lacking frataxin (36, 37). However, both CyaY and FXN can at least partially complement strains of yeast lacking the frataxin homolog Yfh1 (38, 39), which implies similar functions.
To resolve this discrepancy and better understand the role of frataxin in Fe-S cluster biosynthesis, enzyme kinetic experiments were performed for the human and E. coli systems in which analogous components were interchanged. Surprisingly, our results reveal that the cysteine desulfurase component, IscSec/NFS1-ISD11, rather than CyaY/FXN or IscUec/ISCU2 dictates the functional differences in how the frataxin homologs modulate Fe-S cluster assembly.
Experimental Procedures
Preparation of human recombinant proteins
Human NFS1 (Δ1–55) and ISD11 were coexpressed in Escherichia coli strain BL21(DE3) as previously described (34, 40). Cells were lysed by French press in 50 mM Tris pH 8.0, 500 mM NaCl, and 5 mM imidiazole. The NFS1-ISD11 (SD) complex was purified as previously described (34) except that a cation exchange column was added between the Ni-NTA and S300 gel filtration chromatography steps. The yellow SD fractions from the Ni-NTA column were combined with 100 μM pyridoxal 5′-phosphate (PLP), 5 mM dithiothreitol (DTT), and 2 mM ethylenediaminetetracetic acid (EDTA), diluted 3-fold with 50 mM Tris pH 8.0, loaded onto a cation exchange column (16/14, POROS 50HS), and eluted with a linear gradient from 0–1000 mM NaCl. Human ISCU2 (Δ1–35) was purified as previously described (34) except the fractions for the cation exchange column were eluted with a linear gradient from 0 – 1000 mM NaCl in 50 mM Tris, pH 7.5 buffer and a S100 rather than a S300 gel filtration column was used. Human FXN (Δ1–55) was purified as previously described (34) except a S100 rather than a S300 column was used. The Bradford method (41) or extinction coefficients of 42670, 8250, and 26030 M−1cm−1 at 280 nm (42) were used to estimate the protein concentration of SD, ISCU2, and FXN, respectively.
Preparation of E. coli recombinant proteins
A plasmid containing the IscSec gene (29) was transformed into BL21(DE3) E. coli cells and grown at 37 °C. Protein expression was induced with 0.5 mM IPTG when the OD600 was ~0.6. Cells were harvested 5 hours later and lysed by sonication in 50 mM Tris pH 8.0, 500 mM NaCl, 5 mM imidiazole, and 100 μM PLP. After centrifugation, the supernatant was loaded on a Ni-NTA column (16/13, GBiosciences) and eluted with a linear gradient from 5 to 250 mM imidazole. Yellow fractions were concentrated and further purified on a Sephacryl S300 column (26/60, GE Healthcare) equilibrated in 50 mM HEPES pH 8.0, 250 mM NaCl. Plasmids that contained either IscUec or CyaY genes (29) were transformed into BL21(DE3) cells and grown at 37 °C. Protein expression was induced with 0.5 mM IPTG when the OD600 was ~0.6. Cells were harvested 3.5 hours later and lysed by sonication in 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, and 1 mM DTT. The supernatant was loaded onto a GST column and eluted with a linear gradient from 0–7 mM glutathione. The fractions that bound the column were concentrated and dialyzed into 50 mM Tris pH 8.0, 0.5 mM EDTA, and 1 mM DTT and then incubated at room temperature overnight with TEV protease. The tag cleavage product was loaded onto the GST column and the flow through was further purified on a S100 gel filtration column equilibrated in 50 mM HEPES pH 7.5, 150 mM NaCl. The Bradford method (41) or extinction coefficients of 41370, 11460, and 28990 M−1cm−1 at 280 nm (42) were used to estimate the protein concentration of IscSec, IscUec, and CyaY, respectively.
Titrations to favor protein complex formation
Cysteine desulfurase assays were used to determine reaction stoichiometries to favor protein complexes for different combinations of human and E. coli proteins. Cysteine desulfurase assays (see below for details) were initiated with 100 μM L-cysteine and included 0.5 μM of the cysteine desulfurase (SD or IscSec) and increasing amounts of the scaffold protein (ISCU2 or IscUec). Once the saturating number of equivalents of the scaffold protein were determined (Table 1), additional titrations were performed with increasing amounts of frataxin (FXN or CyaY).
Table 1.
Protein complexes and reaction stoichiometries.
| Name | Components | Protein ratios |
|---|---|---|
| SDU | human NFS1/ISD11 and ISCU2 | 1:3 |
| SDUec | human NFS1/ISD11; E. coli IscUec | 1:3 |
| SDUF | human NFS1/ISD11, ISCU2, and FXN | 1:3:3 |
| SDUecF | human NFS1/ISD11; E. coli IscUec; human FXN | 1:3:200 |
| SDUCec | human NFS1/ISD11 and ISCU2; E. coli CyaY | 1:3:80 |
| SDUecCec | human NFS1/ISD11; E. coli IscUec and CyaY | 1:3:30 |
| SecUec | E. coli IscS and IscUec | 1:5 |
| SecU | E. coli IscS; human ISCU2 | 1:5 |
| SecUecCec | E. coli IscS, IscUec, and CyaY | 1:5:10 |
| SecUCec | E. coli IscS; human ISCU2; E. coli CyaY | 1:5:10 |
| SecUecF | E. coli IscS and IscUec; human FXN | 1:5:15 |
| SecUF | E. coli IscS; human ISCU2 and FXN | 1:5:15 |
Cysteine desulfurase activity measurements
Assay mixtures (800 μL) contained 0.5 μM cysteine desulfurase (SD or IscSec), 10 μM PLP, 2 mM DTT, 5 μM Fe(NH4)2(SO4)2, and increasing or saturating amounts (Table 1) of the scaffold protein (ISCU2 or IscUec) and frataxin (FXN or CyaY) in 50 mM Tris pH 8.0, and 250 mM NaCl (34, 40, 43). Samples were incubated in an anaerobic glovebox (10~14 °C) for 30 minutes. The cysteine desulfurase reactions were initiated by addition of L-cysteine (0.01 to 1 mM) at 37 °C and quenched after 10 minutes by the addition of 100 μl of 20 mM N,N-dimethyl-p-phenylenediamine in 7.2 N HCl and 100 μl of 30 mM FeCl3 in 1.2 N HCl, which also initiated the conversion of sulfide to methylene blue. After a twenty minute incubation at 37 °C, the absorption at 670 nm due to methylene blue formation was measured and compared with a Na2S standard curve to quantitate sulfide production. Cysteine desulfurase reactions were also performed for mixed species Fe-S assembly complexes with 0 to 80 equivalents of Fe(NH4)2(SO4)2 and 100 μM L-cysteine. Units are defined as μmol sulfide/μmol cysteine desulfurase per minute at 37 °C. Rates were fit to the Michaelis-Menten equation using KaleidaGraph (Synergy Software).
Fe-S Cluster Formation
Assay mixtures contained 8 μM of the cysteine desulfurase (SD or IscSec), 5 mM DTT, 200 μM Fe(NH4)2(SO4)2, 100 μM L-cysteine, 50 mM Tris pH 8.0, and 250 mM NaCl in a total volume of 0.2 mL. In addition, the number of equivalents (relative to the cysteine desulfurase) for the scaffold protein (ISCU2 or IscUec) and frataxin (FXN or CyaY) required to saturate the activity (above; Table 1) were included in the assay mixture. The scaffold protein was incubated with 5 mM DTT in 50 mM Tris pH 8, 250 mM NaCl in an anaerobic glovebox for 30 minutes prior to mixing with the remaining assay components in an anaerobic cuvette and then incubated at 10 °C for 30 minutes in the cuvette holder. The reaction was initiated by injecting L-cysteine to a final concentration of 100 μM with a gas-tight syringe. Fe-S cluster formation was monitored at 456 nm at 10 °C and then the first 3000 seconds were fit as first-order kinetics using KaleidaGraph. The rate was converted to the activity using an extinction coefficient of 5.8 mM−1cm−1 at 456 nm for [2Fe-2S] cluster absorbance (15). Units are defined as the μmol of [2Fe-2S] cluster / μmol cysteine desulfurase per minute at 10 °C.
RESULTS
Ratio of protein complex components for activity measurements
The objectives of this study were to provide insight into functional differences in frataxin-mediated regulation of Fe-S cluster biosynthesis for prokaryotes and eukaryotes by measuring cysteine desulfurase and Fe-S formation activities of mixed species protein complexes. The sulfur generated by the cysteine desulfurase reaction can be directly measured or can be used as a component of the Fe-S formation reaction. Titrations were performed to determine the relative ratios of the different components for maximum activity. Previous experiments revealed that adding 3 equiv of ISCU2 minimized the cysteine desulfurase activity of the NFS1-ISD11 (SD) complex (Table 1), likely through formation of a NFS1-ISD11-ISCU2 (SDU) species (34). Likewise, adding 3 equiv of FXN to SDU maximized the cysteine desulfurase activity by forming a NFS1-ISD11-ISCU2-FXN (SDUF) complex (34). A similar strategy was used here to determine and then compensate for weaker binding of E. coli proteins to the human assembly complex and human proteins to the E. coli assembly complex.
First, protein reaction ratios were determined for the human SD complexes by measuring cysteine desulfurase activities with increasing amounts of the scaffold protein, either ISCU2 or IscUec, and frataxin, either FXN or CyaY. The cysteine desulfurase activity of SD decreased with addition of either ISCU2 or IscUec and minimized after the addition of ~2 equiv/SD (Figure 1A). Three equiv of ISCU2 or IscUec were therefore included with SD for kinetic experiments of the SDU or NFS1-ISD11-IscUec (SDUec) complexes (Table 1). The binding of frataxin homologs to the SDU complex increased the cysteine desulfurase activity that maximized after the addition of either 3 equiv of FXN or 80 equiv of CyaY (Figure 1B). Similarly, the binding of frataxin homologs to the SDUec complex increased the cysteine desulfurase activity that maximized after either 200 equiv of FXN or 30 equiv of CyaY (Figure 1C).
Figure 1.
Determination of protein ratios that favor complex formation. Cysteine desulfurase activities were measured for (A) the human SD complex with increasing amounts of ISCU2 or IscUec, and for (B) the SDU and (C) SDUec complexes with increasing amounts of FXN or CyaY. (D) The cysteine desulfurase activity was measured for E. coli IscS with increasing amounts of ISCU2 or IscUec.
Second, protein reaction ratios were determined for IscSec using similar titrations. The cysteine desulfurase activity of IscSec slightly decreased with addition of IscUec and slightly increased with addition of ISCU2 (Figure 1D). This perturbation in IscSec activity maximized after the addition of 3–5 equiv of IscUec or ISCU2. To favor complex formation, five equiv of IscUec or ISCU2 were therefore added to IscSec (Table 1) for all kinetic experiments of IscSec-IscUec (SecUec) and IscSec-ISCU2 (SecU) complexes, respectively. Titrations of up to 50 equiv of CyaY or FXN did not significantly change the cysteine desulfurase activity of either the SecUec or SecU complex (data not shown). Therefore, protein complex stoichiometries of 1:5:10 were used for the SecUecCec and SecUCec complexes based on the number of equivalents of CyaY previously determined to inhibit Fe-S cluster formation (29). Similarly, protein complex stoichiometries of 1:5:15 were arbitrarily chosen for the Michaelis-Menten kinetics (below) of the SecUecF and SecUF complexes (Table 1).
IscUec and CyaY stimulate the cysteine desulfurase activity of the human SD complex
Saturating amounts of ISCU2 or IscUec and FXN or CyaY determined above were added to the SD complex and the rates of the cysteine desulfurase reaction were measured as a function of the L-cysteine concentration. The human SD complex exhibited a kcat/KM of 660 M−1s−1 for the cysteine desulfurase reaction (Table 2), which is slightly higher (primarily due to a lower KM for L-cysteine) than previously reported values of ~100 M−1s−1 (34, 40). The addition of ISCU2 or IscUec and formation of the SDU or SDUec complex slightly decreased the kcat (Figure 2 and Table 2). The binding of FXN to form the SDUF and SDUecF complexes stimulated the kcat for the cysteine desulfurase reaction by ~7-fold relative to the SDU and SDUec complexes (Figure 2). Similarly, CyaY binding and formation of the SDUCec and SDUecCec complexes resulted in a 4–6 fold increase in the kcat relative to SDU and SDUec (Figure 2). These experiments show that IscUec can functionally substitute for ISCU2 and that CyaY can replace FXN as an activator of the cysteine desulfurase reaction for the human Fe-S assembly complex.
Table 2.
Kinetic data for Fe-S assembly complexes.
| Name | kcat (min−1) | KM (mM) | kcat/KM (M−1sec−1) | Fe-S cluster assembly activity (min−1) |
|---|---|---|---|---|
| SD | 1.5 ± 0.1 | 0.038 ± 0.012 | 660 ± 200 | |
| SDU | 1.0 ± 0.1 | 0.028 ± 0.008 | 600 ± 180 | 0.26 ± 0.22 |
| SDUF | 6.7 ± 0.4 | 0.011 ± 0.004 | 9700 ± 3900 | |
| SDUF + Fe | 15.4 ± 1.1 | 0.015 ± 0.006 | 16800 ± 6300 | 30.39 ± 1.18 |
| SDUCec | 5.7 ± 0.4 | 0.027 ± 0.01 | 3500 ± 1300 | |
| SDUCec + Fe | 6.7 ± 0.2 | 0.016 ± 0.003 | 7100 ± 1400 | 3.94 ± 0.48 |
| SDUec | 0.59 ± 0.02 | 0.018 ± 0.003 | 550 ± 120 | 1.30 ± 0.32 |
| SDUecF | 4.2 ± 0.1 | 0.009 ± 0.002 | 7900 ± 2000 | |
| SDUecF + Fe | 6.1 ± 0.3 | 0.012 ± 0.003 | 8300 ± 2300 | 25.54 ± 1.17 |
| SDUecCec | 2.1 ± 0.1 | 0.012 ± 0.003 | 2900 ± 840 | |
| SDUecCec + Fe | 1.9 ± 0.1 | 0.011 ± 0.002 | 2800 ± 480 | 1.21 ± 0.19 |
| Sec | 7.5 ± 0.1 | 0.017 ± 0.002 | 7300 ± 860 | |
| SecUec | 4.7 ± 0.3 | 0.019 ± 0.006 | 4100 ± 1400 | 12.23 ± 0.59 |
| SecUecCec | 5.8 ± 0.2 | 0.025 ± 0.003 | 3800 ± 440 | |
| SecUecCec + Fe | 4.2 ± 0.1 | 0.020 ± 0.004 | 3500 ± 680 | 0.79 ± 1.76 |
| SecUecF | 5.2 ± 0.1 | 0.017 ± 0.002 | 5100 ± 870 | |
| SecUecF + Fe | 4.5 ± 0.2 | 0.024 ± 0.004 | 3200 ± 940 | 4.56 ± 0.40 |
| SecU | 6.5 ± 0.2 | 0.019 ± 0.003 | 5800 ± 1000 | 14.63 ± 0.95 |
| SecUCec | 7.4 ± 0.1 | 0.016 ± 0.002 | 7600 ± 900 | |
| SecUCec + Fe | 7.3 ± 0.3 | 0.017 ± 0.004 | 7100 ± 1600 | 0.88 ± 0.64 |
| SecUF | 7.6 ± 0.3 | 0.020 ± 0.006 | 6300 ± 1600 | |
| SecUF + Fe | 6.9 ± 0.1 | 0.016 ± 0.002 | 7200 ± 890 | 3.62 ± 0.24 |
Figure 2.
Stimulation of cysteine desulfurase reaction by addition of frataxin homologs. The kcat for the cysteine desulfurase reaction was plotted for complexes of human SD with the scaffold protein (ISCU2 or IscUec) and frataxin (FXN or CyaY).
Iron-dependent stimulation of cysteine desulfurase reaction for SD complexes
Cysteine desulfurase activities were determined for SD complexes as a function of added iron. From 0 to 80 equiv of Fe(II) were added to the SDUF, SDUCec, SDUecF, and SDUecCec complexes and the cysteine desulfurase activity was measured (Figure 3A). The activity maximized after about 1, 1, and 5 equiv for the SDUF, SDUCec, and SDUecF complexes, respectively. In contrast, the SDUecCec complex did not exhibit a significant Fe-based activation. In addition, the SD complexes were incubated with 10 equiv of ferrous iron and the Michaelis-Menten parameters for the cysteine desulfurase activity were determined (Table 2). Ferrous iron had a large effect on the kcat of the SDUF complex, as previously reported (34), a moderate effect on the SDUCec and SDUecF complexes, and no effect on the SDUecCec complex (Figure 3B).
Figure 3.
Iron-dependent perturbation of cysteine desulfurase activity for SD complexes. (A) Cysteine desulfurase activity plotted as a function of added ferrous iron. (B) Plot of kcat values for the cysteine desulfurase reaction of SD complexes with (black) and without (gray) 10 equiv of ferrous iron.
CyaY can substitute for FXN in Fe-S cluster synthesis by human SD complexes
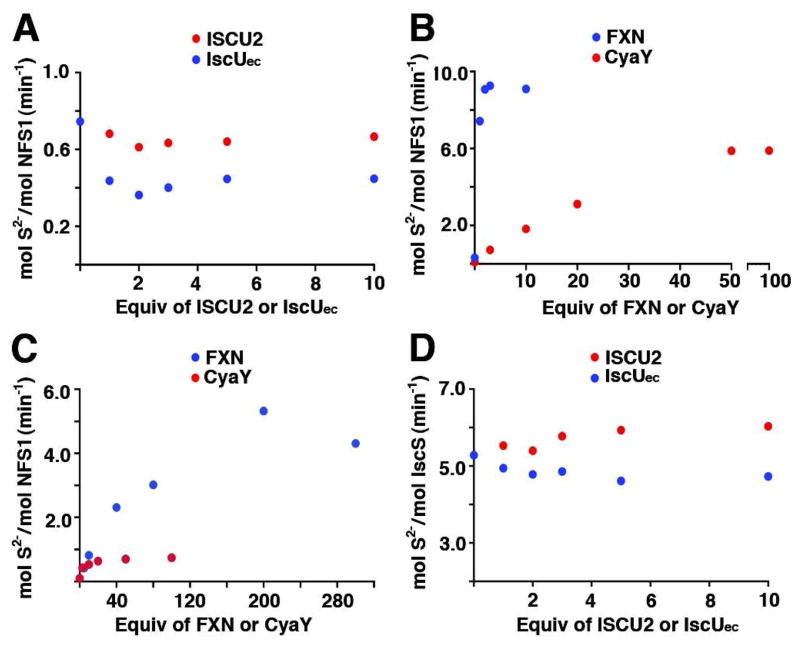
The rate of Fe-S cluster assembly for the SDU, SDUec, SDUF, SDUCec, SDUecF, and SDUecCec complexes were determined by monitoring the increase in absorbance at 456 nm (15) at 10 °C and converting this change in absorbance to an activity measurement (μmol [2Fe-2S] / μmol of complex per minute). In the absence of FXN or CyaY, the SDU and SDUec complexes exhibited very low or no activity and were similar to a control reaction in which sulfide was substituted for cysteine (Table 2 and Figure 4). The addition of FXN greatly stimulated the Fe-S assembly activity of SDU and SDUec. In contrast, addition of CyaY resulted in a very modest or no increase in Fe-S assembly activity of the SDU and SDUec complexes, respectively. A CyaY-based stimulation was more readily observed when the Fe-S cluster assembly assays were repeated at room temperature (Figure 4A inset). Thus, E. coli CyaY can substitute for human FXN in the human Fe-S assembly complex and strongly stimulate the cysteine desulfurase activity. CyaY also weakly stimulates the Fe-S cluster formation reaction.
Figure 4.
Stimulation of Fe-S cluster assembly activity by frataxin homologs. (A) The Fe-S assembly activities were measured at 10 °C for the Na2S control and SDU, SDUCec, and SDUF complexes. The inset shows the activities of the SDU, SDUCec, and Na2S control at 25 °C. (B) The Fe-S assembly activities were measured at 10 °C for the Na2S control and SDUec, SDUecCec, and SDUecF complexes.
CyaY and FXN have minor effects on the cysteine desulfurase activity of IscSec complexes
Purified recombinant IscSec exhibited a kcat of 7.5 min−1 (Figure 5A and Table 2), which is similar to the previously reported value of 8.5 min−1 (44). Addition of IscUec diminished the kcat for the cysteine desulfurase reaction by a factor of 1.6, whereas a more moderate decrease in kcat was observed upon ISCU2 addition (Figure 5A). Previously, IscUec was shown to either have no effect on the activity of IscSec (45) or stimulate the activity 6-fold (46) using different experimental conditions and assays. The kcat for SecUec and SecU were increased slightly upon addition of either CyaY or FXN (Figure 5A and Table 2). The IscSec cysteine desulfurase activity was slightly decreased by added ferrous iron, consistent with a previous report (47), that appeared to occur within the first equiv of added iron for the SecUecF and SecUecCec complexes and 10 equiv of iron for the SecUF complex (Figure 5B). Steady state kinetics also revealed the kcat slightly decreased upon addition of 10 equiv of ferrous iron (Figure 5A). The slight decrease in cysteine desulfurase activity upon addition of iron (Figure 5) is opposite of the general Fe-based activation observed for SD complexes (Figure 3). Overall, these results indicate that binding of frataxin homologs and ferrous iron have minor effects on the cysteine desulfurase activity of IscS complexes.
Figure 5.
Cysteine desulfurase activities for IscS complexes with and without iron. (A) The kcat for the cysteine desulfurase was plotted for complexes of E. coli IscS (Sec) without (gray or colored) and with (black) 10 equiv of ferrous iron. (B) The cysteine desulfurase activities for IscS complexes were measured with 100 μM L-cysteine and increasing amounts of ferrous iron.
CyaY and FXN inhibit Fe-S assembly by IscSec complexes
The Fe-S cluster assembly rate for the SecUec and SecU complexes were similar to each other (Figure 6) and significantly higher than analogous SDUec and SDU complexes with NFS1-ISD11 (Figure 4). The addition of 10 equiv of CyaY to the SecUec and SecU complexes completely inhibited Fe-S cluster formation (Figure 6A and 6B), consistent with previous results (29). At 2 equiv, CyaY is a slightly more effective inhibitor of the native SecUec complex than the mixed species SecU complex. The addition of FXN to the SecUec and SecU complexes (Figure 6C and 6D) also lowered the rate and possibly the amount of cluster bound; however, FXN did not completely eliminate Fe-S cluster formation. Thus, bacterial IscS assembly complexes are inhibited in Fe-S cluster formation by the inclusion of either frataxin ortholog.
Figure 6.
Frataxin-based inhibition of Fe-S cluster formation for IscS complexes. The rate of Fe-S cluster formation were measured for the SecUec complex with increasing amounts of (A) CyaY and (C) FXN, and for the SecU complex with increasing amounts of (B) CyaY and (D) FXN.
DISCUSSION
The physiological function of frataxin has been intensively investigated since it was associated with the neurodegenerative disease Friedreich’s ataxia (FRDA; reviewed in (48, 49)). A decrease in FXN levels in yeast and mouse model systems results in a phenotype that includes loss of Fe-S cluster enzyme activity, accumulation of iron in mitochondria, and susceptibility to oxidative stress (50–52). In addition, FXN levels are correlated with the age of disease onset for FRDA patients (53, 54), and FXN missense mutations were identified that show FRDA-like phenotypes in mouse (52) and yeast (55) model systems. A role for frataxin as an Fe chaperone and/or donor in Fe-S cluster biosynthesis is often cited due to the frataxin depletion phenotype (50–52), the ability of frataxin to bind Fe (56–63), and the acceleration of Fe-S cluster formation by Fe-bound FXN in vitro (58). Recent in vitro data as well as data presented here reveals that human FXN binds to the SDU complex and activates both the cysteine desulfurase and Fe-S formation reactions (34). Moreover, assays using FXN missense mutations exhibit defects in binding and activation that appear to correlate with the severity of clinical progression for FRDA patients (64, 65). The physiological function of CyaY is less clear. In contrast to FXN in eukaryotes, deletion or overexpression of CyaY does not affect cellular growth, iron content, and survival after exposure to H2O2 (36, 66). Part of the complication for these types of studies is the presence of both the ISC and SUF Fe-S assembly systems in E. coli. If the physiological role of CyaY were to inhibit Fe-S cluster formation by the ISC system, then one would expect overexpression of CyaY in the absence of the SUF system to give a loss of Fe-S cluster phenotype. To the best of our knowledge this experiment has not been reported. Unfortunately, the complicated phenotype for human FXN depletion, the lack of a phenotype for CyaY depletion, and the opposing in vitro functions in Fe-S cluster assembly have contributed to the general confusion over the physiological role of this protein.
Here we used a function replacement approach to understand the factors that mediate the dramatically different in vitro responses to frataxin homolog binding. More specifically, in vitro cysteine desulfurase and Fe-S cluster assembly activities were evaluated for protein complexes in which components from the recombinant human Fe-S assembly system: the NFS1-ISD11 cysteine desulfurase complex, the scaffold protein ISCU2, and FXN, and the recombinant E. coli system composed of the IscS cysteine desulfurase, the scaffold protein IscUec, and CyaY, were interchanged. Surprisingly, our results reveal that activation or inhibition by the frataxin homolog is not controlled by the frataxin homolog but rather dictated by the cysteine desulfurase.
Interestingly, even though IscSec and NFS1 are almost 60% identical, IscSec exhibited a kcat/KM for the cysteine desulfurase activity that is 10–70 fold higher than that of the SD complex and was largely unchanged by the addition of IscUec and CyaY (34, 40). In contrast, the low activity of the SD complex could be rescued by the addition of ISCU2 and FXN; the SDUF complex had a kcat/KM that is about double that of IscSec alone or as a SecUec or SecUecCec complex. The SecUec and SDU complexes also exhibit striking differences in their ability to facilitate Fe-S cluster formation. The SecUec complex is highly active in the Fe-S cluster assembly assay, whereas the SDU complex exhibits little ability to mediate this reaction in the absence of frataxin. These differences were not due to the identity of the scaffold protein: interchanging the IscUec and ISCU2 proteins did not substantially alter the higher rate of the complexes containing Sec or the substantially inhibited rates of complexes containing SD (Figures 4, 6). Moreover, addition of CyaY or FXN to these reactions inhibited Fe-S assembly by Sec-containing complexes and activated Fe-S assembly by SD-containing complexes (Figures 4, 6), albeit inhibition of the E. coli complex by CyaY was more efficient than by FXN and activation of the human complex by FXN was more efficient than by CyaY.
Our data therefore suggest that intrinsic features of IscSec and human SD cysteine desulfurase components control both the basal activity as well as the effect of binding the frataxin homologs CyaY and FXN. In both cases, binding of the effector molecule switches the activity, either from “off” to “on” or from “on” to “off”. In this sense, NFS1 behaves less like its ortholog IscS and more like its paralogs, such as SufS, which are characterized by modest activity that can be increased or activated by accessory proteins (67–69). Evolutionary innovations in cysteine desulfurases from eukaryotes that control the basal activity and intrinsic features of frataxin homolog regulation are not known; binding of ISD11 and conserved amino acid changes in eukaryotes near the active site could play important roles in this process. Precise molecular details that regulate these features are currently under investigation.
Our data are consistent with FXN and CyaY binding playing a role in modulating Fe-S cluster formation by trapping an “on” conformation of SDU or inducing an “off” conformation of SecUec (Figure 7). This model is consistent with the fact that SDU forms a stable complex with FXN (34, 35) and SecUec forms a stable complex with CyaY (21, 27, 28). The fact that FXN and CyaY can functionally replace each other in vitro, albeit at lowered efficiencies, is consistent with the formation of a complex with a common structure and with the ability of both of these proteins to suppress defects caused by deletion of YFH1, which encodes the S. cerevisiae frataxin homolog (38, 39). These modifications to the cysteine desulfurase may function as part of a protein-level regulatory mechanism. Future experiments will be aimed at providing additional mechanistic insight into the differences between the human SD complex and IscSec and how these differences control Fe-S cluster assembly.
Figure 7.
Working model for frataxin regulation of Fe-S cluster biosynthesis. In eukaryotes, a pre-equilibrium model is proposed in the absence of FXN in which a cysteine desulfurase and Fe-S cluster assembly deficient form is favored over a functional form of the SDU complex. FXN binding stabilizes the functional form and promotes sulfur transfer from NFS1 to ISCU2 and Fe-S cluster synthesis activities. In contrast, the prokaryotic SecUec complex that lacks CyaY exhibits cysteine desulfurase and Fe-S assembly activities. CyaY binding may induce a conformational change in the SecUec complex that does not significantly affect the cysteine desulfurase activity, but abolishes Fe-S cluster synthesis. The “ON” and “OFF” labels indicate the Fe-S assembly activity of the respective complexes, the blue arrows from the PLP to the mobile Cys loop indicate the presence of cysteine desulfurase activity, and the blue arrows from the Cys loop to the scaffold protein (shown in green) indicate interprotein sulfur transfer. The displayed model represents half of the expected dimeric Fe-S assembly complexes.
Acknowledgments
We thank Christopher D. Putnam for helpful discussion and suggestions.
Abbreviations
- Cec
E. coli frataxin homolog CyaY
- DTT
dithiothreitol
- EDTA
ethylenediaminetetracetic acid
- FRDA
Friedreich’s ataxia
- FXN
human frataxin
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- ISC
iron-sulfur cluster assembly pathway
- IscSec
the E. coli cysteine desulfurase IscS
- IscUec
the E. coli scaffold protein IscU
- NIF
nitrogen fixation Fe-S cluster assembly pathway
- PLP
pyridoxal-5′-phosphate
- SD
protein complex composed of human NFS1 and ISD11
- SDU
protein complex composed of human NFS1, ISD11, and ISCU2
- SDUec
protein complex composed of human NFS1 and ISD11 with E. coli IscU
- SDUF
protein complex composed of human NFS1, ISD11, ISCU2, and FXN
- SDUecF
protein complex composed of human NFS1, ISD11, and FXN with E. coli Iscu2
- SDUCec
protein complex composed of human NFS1, ISD11, and ISCU2 with E. coli CyaY
- SDUecCec
protein complex composed of human NFS1 and ISD11 with E. coli IscU and CyaY
- Sec
E. coli IscS
- SecUec
protein complex composed of E. coli IscS and IscU
- SecU
protein complex composed of E. coli IscS with human ISCU2
- SecUecCec
protein complex composed of E. coli IscS, IscU, and CyaY
- SecUCec
protein complex composed of E. coli IscS and CyaY with human ISCU2
- SecUecF
protein complex composed of E. coli IscS and IscU and human frataxin
- SecUF
protein complex composed of E. coli IscS with human ISCU2 and frataxin
- SUF
sulfur mobilization Fe-S cluster assembly pathway
- Tris
Tris(hydroxymethyl)aminomethane
- Uec
E. coli IscU
Footnotes
Start-up funds from Texas A&M University are gratefully acknowledged. This project was supported in part by grants A-1647 from the Robert A. Welch foundation (DPB), 1R01GM096100 from the National Institute of Health (DPB), and U117584256 from the MRC (AP).
References
- 1.Johnson DC, Dean DR, Smith AD, Johnson M. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 2.Brzóska K, Meczyńska S, Kruszewski M. Iron-sulfur cluster proteins: electron transfer and beyond. Acta Biochim Pol. 2006;53:685–691. [PubMed] [Google Scholar]
- 3.Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- 4.Meyer J. Iron-sulfur protein folds, iron-sulfur chemistry, and evolution. J Biol Inorg Chem. 2008;13:157–170. doi: 10.1007/s00775-007-0318-7. [DOI] [PubMed] [Google Scholar]
- 5.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Sheftel AD, Lill R. The power plant of the cell is also a smithy: the emerging role of mitochondria in cellular iron homeostasis. Ann Med. 2009;41:82–99. doi: 10.1080/07853890802322229. [DOI] [PubMed] [Google Scholar]
- 7.Ye H, Rouault TA. Human iron-sulfur cluster assembly, cellular iron homeostasis and disease. Biochemistry. 2010;49:4945–4956. doi: 10.1021/bi1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouault TA, Tong WH. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson MR, Cash VL, Weiss MC, Laird NF, Newton WE, Dean DR. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol Gen Genet. 1989;219:49–57. doi: 10.1007/BF00261156. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, Nakamura M. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of FeS clusters in Escherichia coli. J Biochem. 1999;126:917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- 13.Fontecave M, Choudens SOd, Py B, Barras F. Mechanisms of iron-sulfur cluster assembly: the SUF machinery. J Biol Inorg Chem. 2005;10:713–721. doi: 10.1007/s00775-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 14.Outten FW, Djaman O, Storz G. A suf operon requirement for FeS cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 15.Agar J, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson M. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- 16.Zheng L, White RH, Cash VL, Jack RF, Dean DR. Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc Natl Acad Sci USA. 1993;90:2754–2758. doi: 10.1073/pnas.90.7.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz C, Djaman O, Imlay JA, Kiley PJ. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc Natl Acad Sci USA. 2000;97:9009–9014. doi: 10.1073/pnas.160261497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding H, Clark RJ. Characterization of iron binding in IscA, an ancient iron-sulphur cluster assembly protein. Biochem J. 2004;379:433–440. doi: 10.1042/BJ20031702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs C, Agar J, Smith AD, Frazzon J, Dean DR, Huynh BH, Johnson M. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry. 2001;40:14069–14080. doi: 10.1021/bi015656z. [DOI] [PubMed] [Google Scholar]
- 20.Ollagnier de Choudens S, Mattioli T, Takahashi Y, Fontecave M. Iron-sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J Biol Chem. 2001;276:22604–22607. doi: 10.1074/jbc.M102902200. [DOI] [PubMed] [Google Scholar]
- 21.Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, Trempe JF, Matte A, Armengod ME, Cygler M. Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 2010;8:e1000354. doi: 10.1371/journal.pbio.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastore C, Adinolfi S, Huynen MA, Rybin V, Martin SR, Mayer M, Bukau B, Pastore A. YfhJ, a molecular adaptor in iron-sulfur cluster formation or a frataxin-like protein? Structure. 2006;14:857–867. doi: 10.1016/j.str.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Shimomura Y, Takahashi Y, Kakuta Y, Fukuyama K. Crystal structure of Escherichia coli YfhJ protein, a member of the ISC machinery involved in assembly of iron-sulfur clusters. Proteins. 2005;60:566–569. doi: 10.1002/prot.20481. [DOI] [PubMed] [Google Scholar]
- 24.Hoff KG, Silberg JJ, Vickery L. Interaction of the iron-sulfur cluster assembly protein IscU with the Hsc66/Hsc20 molecular chaperone system of Escherichia coli. Proc Natl Acad Sci USA. 2000;97:7790–7795. doi: 10.1073/pnas.130201997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandramouli K, Johnson M. HscA and HscB stimulate [2Fe-2S] cluster transfer from IscU to apoferredoxin in an ATP-dependent reaction. Biochemistry. 2006;45:11087–11095. doi: 10.1021/bi061237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickery L, Cupp-Vickery J. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit Rev Biochem Mol Biol. 2007;42:95–111. doi: 10.1080/10409230701322298. [DOI] [PubMed] [Google Scholar]
- 27.Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, Martin SR, Svergun DI, Pastore A. Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat Commun. 2010;1:95. doi: 10.1038/ncomms1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layer G, Ollagnier de Choudens S, Sanakis Y, Fontecave M. Iron-sulfur cluster biosynthesis: characterization of Escherichia coli CYaY as an iron donor for the assembly of [2Fe-2S] clusters in the scaffold IscU. J Biol Chem. 2006;281:16256–16263. doi: 10.1074/jbc.M513569200. [DOI] [PubMed] [Google Scholar]
- 29.Adinolfi S, Iannuzzi C, Prischi F, Pastore C, Iametti S, Martin S, Bonomi F, Pastore A. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat Struct Mol Biol. 2009;16:390–396. doi: 10.1038/nsmb.1579. [DOI] [PubMed] [Google Scholar]
- 30.Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 31.Adam AC, Bornhövd C, Prokisch H, Neupert W, Hell K. The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. Embo J. 2006;25:174–183. doi: 10.1038/sj.emboj.7600905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, Ghosh M, Tong WH, Rouault TA. Human ISD11 is essential for both iron-sulfur cluster assembly and maintenance of normal cellular iron homeostasis. Hum Mol Genet. 2009;18:3014–3025. doi: 10.1093/hmg/ddp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedemann N, Urzica E, Guiard B, Müller H, Lohaus C, Meyer HE, Ryan MT, Meisinger C, Muhlenhoff U, Lill R, Pfanner N. Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins. Embo J. 2006;25:184–195. doi: 10.1038/sj.emboj.7600906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai CL, Barondeau DP. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry. 2010;49:9132–9139. doi: 10.1021/bi1013062. [DOI] [PubMed] [Google Scholar]
- 35.Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donzé M, Reutenauer L, Puccio H. Mammalian Frataxin: An Essential Function for Cellular Viability through an Interaction with a Preformed ISCU/NFS1/ISD11 Iron-Sulfur Assembly Complex. PLoS ONE. 2011;6:e16199. doi: 10.1371/journal.pone.0016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D, Ohshima K, Jiralerspong S, Bojanowski M, Pandolfo M. Knock-out of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS Lett. 1999;456:13–16. doi: 10.1016/s0014-5793(99)00896-0. [DOI] [PubMed] [Google Scholar]
- 37.Cossee M, Puccio H, Gansmuller A, Koutnikova H, Dierich A, LeMeur M, Fischbeck K, Dollé P, Koenig M. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum Mol Genet. 2000;9:1219–1226. doi: 10.1093/hmg/9.8.1219. [DOI] [PubMed] [Google Scholar]
- 38.Bedekovics Gajdos, Kispal G, Isaya G. Partial conservation of functions between eukaryotic frataxin and the Escherichia coli frataxin homolog CyaY. FEMS Yeast Res. 2007;7:1276–1284. doi: 10.1111/j.1567-1364.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 39.Cavadini P, Gellera C, Patel PI, Isaya G. Human frataxin maintains mitochondrial iron homeostasis in Saccharomyces cerevisiae. Hum Mol Genet. 2000;9:2523–2530. doi: 10.1093/hmg/9.17.2523. [DOI] [PubMed] [Google Scholar]
- 40.Marelja Z, Stöcklein W, Nimtz M, Leimkühler S. A novel role for human Nfs1 in the cytoplasm: Nfs1 acts as a sulfur donor for MOCS3, a protein involved in molybdenum cofactor biosynthesis. J Biol Chem. 2008;283:25178–25185. doi: 10.1074/jbc.M804064200. [DOI] [PubMed] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 43.Siegel LM. A direct microdetermination for sulfide. Anal Biochem. 1965;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- 44.Urbina HD, Silberg JJ, Hoff KG, Vickery L. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J Biol Chem. 2001;276:44521–44526. doi: 10.1074/jbc.M106907200. [DOI] [PubMed] [Google Scholar]
- 45.Iannuzzi C, Adinolfi S, Howes BD, Garcia-Serres R, Clemancey M, Latour JM, Smulevich G, Pastore A. The Role of CyaY in Iron Sulfur Cluster Assembly on the E. coli IscU Scaffold Protein. PLoS ONE. 2011;6:e21992. doi: 10.1371/journal.pone.0021992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato SI, Mihara H, Kurihara T, Takahashi Y, Tokumoto U, Yoshimura T, Esaki N. Cys-328 of IscS and Cys-63 of IscU are the sites of disulfide bridge formation in a covalently bound IscS/IscU complex: implications for the mechanism of iron-sulfur cluster assembly. Proc Natl Acad Sci USA. 2002;99:5948–5952. doi: 10.1073/pnas.082123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu G, Li P, Wu X. Regulation of Escherichia coli IscS desulfurase activity by ferrous iron and cysteine. Biochem Biophys Res Commun. 2008;374:399–404. doi: 10.1016/j.bbrc.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 48.Schmucker S, Puccio H. Understanding the molecular mechanisms of Friedreich Ataxia to develop therapeutic approaches. Hum Mol Genet. 2010;19:R103–R110. doi: 10.1093/hmg/ddq165. [DOI] [PubMed] [Google Scholar]
- 49.Santos R, Lefevre S, Sliwa D, Seguin A, Camadro JM, Lesuisse E. Friedreich’s Ataxia: Molecular Mechanisms, Redox Considerations and Therapeutic Opportunities. Antioxid Redox Signal. 2010;13:651–690. doi: 10.1089/ars.2009.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhlenhoff U, Richhardt N, Ristow M, Kispal G, Lill R. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum Mol Genet. 2002;11:2025–2036. doi: 10.1093/hmg/11.17.2025. [DOI] [PubMed] [Google Scholar]
- 51.Stehling O, Elsässer HP, Brückel B, Muhlenhoff U, Lill R. Iron-sulfur protein maturation in human cells: evidence for a function of frataxin. Hum Mol Genet. 2004;13:3007–3015. doi: 10.1093/hmg/ddh324. [DOI] [PubMed] [Google Scholar]
- 52.Calmels N, Schmucker S, Wattenhofer-Donze M, Martelli A, Vaucamps N, Reutenauer L, Messaddeq N, Bouton C, Koenig M, Puccio H. The first cellular models based on frataxin missense mutations that reproduce spontaneously the defects associated with Friedreich ataxia. PLoS ONE. 2009;4:e6379. doi: 10.1371/journal.pone.0006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, Brice A, Koenig M. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335:1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 54.Filla A, De Michele G, Cavalcanti F, Pianese L, Monticelli A, Campanella G, Cocozza S. The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet. 1996;59:554–560. [PMC free article] [PubMed] [Google Scholar]
- 55.Leidgens S, De Smet S, Foury F. Frataxin interacts with Isu1 through a conserved tryptophan in its beta-sheet. Hum Mol Genet. 2010;19:276–286. doi: 10.1093/hmg/ddp495. [DOI] [PubMed] [Google Scholar]
- 56.Cavadini P, O’Neill HA, Benada O, Isaya G. Assembly and iron-binding properties of human frataxin, the protein deficient in Friedreich ataxia. Hum Mol Genet. 2002;11:217–227. doi: 10.1093/hmg/11.3.217. [DOI] [PubMed] [Google Scholar]
- 57.Adinolfi S, Trifuoggi M, Politou A, Martin SR, Pastore A. A structural approach to understanding the iron-binding properties of phylogenetically different frataxins. Hum Mol Genet. 2002;11:1865–1877. doi: 10.1093/hmg/11.16.1865. [DOI] [PubMed] [Google Scholar]
- 58.Yoon T, Cowan JA. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. J Am Chem Soc. 2003;125:6078–6084. doi: 10.1021/ja027967i. [DOI] [PubMed] [Google Scholar]
- 59.Cook JD, Bencze KZ, Jankovic AD, Crater AK, Busch CN, Bradley PB, Stemmler AJ, Spaller MR, Stemmler TL. Monomeric yeast frataxin is an iron-binding protein. Biochemistry. 2006;45:7767–7777. doi: 10.1021/bi060424r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bencze KZ, Yoon T, Millán-Pacheco C, Bradley PB, Pastor N, Cowan JA, Stemmler TL. Human frataxin: iron and ferrochelatase binding surface. Chem Commun (Camb) 2007:1798–1800. doi: 10.1039/b703195e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J, Dizin E, Cowan JA. Mapping iron binding sites on human frataxin: implications for cluster assembly on the ISU Fe-S cluster scaffold protein. J Biol Inorg Chem. 2008;13:825–836. doi: 10.1007/s00775-008-0369-4. [DOI] [PubMed] [Google Scholar]
- 62.Correia A, Pastore C, Adinolfi S, Pastore A, Gomes C. Dynamics, stability and iron-binding activity of frataxin clinical mutants. FEBS J. 2008;275:3680–3690. doi: 10.1111/j.1742-4658.2008.06512.x. [DOI] [PubMed] [Google Scholar]
- 63.Kondapalli K, Kok N, Dancis A, Stemmler TL. Drosophila Frataxin: An Iron Chaperone during Cellular Fe-S Cluster Bioassembly. Biochemistry. 2008:6917–6927. doi: 10.1021/bi800366d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai CL, Bridwell-Rabb J, Barondeau DP. Friedreich’s Ataxia Variants I154F and W155R Diminish Frataxin-Based Activation of the Iron-Sulfur Cluster Assembly Complex. Biochemistry. 2011:6478–6487. doi: 10.1021/bi200666h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bridwell-Rabb J, Winn AM, Barondeau DP. Structure-function analysis of Friedreich’s ataxia mutants reveals determinants for frataxin binding and activation of the Fe-S assembly complex. Biochemistry. 2011;50:7265–7274. doi: 10.1021/bi200895k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vivas E, Skovran E, Downs DM. Salmonella enterica strains lacking the frataxin homolog CyaY show defects in Fe-S cluster metabolism in vivo. J Bacteriol. 2006;188:1175–1179. doi: 10.1128/JB.188.3.1175-1179.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tirupati B, Vey JL, Drennan CL, Bollinger JM. Kinetic and structural characterization of Slr0077/SufS, the essential cysteine desulfurase from Synechocystis sp. PCC 6803. Biochemistry. 2004;43:12210–12219. doi: 10.1021/bi0491447. [DOI] [PubMed] [Google Scholar]
- 68.Loiseau L, Ollagnier de Choudens S, Nachin L, Fontecave M, Barras F. Biogenesis of Fe-S cluster by the bacterial Suf system: SufS and SufE form a new type of cysteine desulfurase. J Biol Chem. 2003;278:38352–38359. doi: 10.1074/jbc.M305953200. [DOI] [PubMed] [Google Scholar]
- 69.Outten FW, Wood MJ, Munoz FM, Storz G. The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in Escherichia coli. J Biol Chem. 2003;278:45713–45719. doi: 10.1074/jbc.M308004200. [DOI] [PubMed] [Google Scholar]