Abstract
Background
The role of hepatic ATP-binding cassette transporter 1 (ABCA1) in maintaining plasma high density lipoprotein cholesterol (HDL-C) levels is well established, but its role in reverse cholesterol transport (RCT) is unclear. Probucol is a compound that reduces HDL-C levels but also reduces atherosclerosis in animal models and xanthomas in humans. The aim of the present study was to test the hypothesis that probucol inhibits hepatic ABCA1 activity, thereby reducing HDL-C levels but promoting RCT from macrophages.
Methods and Results
Wild-type (WT) C57BL/6 mice and scavenger receptor class B type I (SR-BI) knockout mice were fed a chow diet containing 0.5% probucol or normal chow for 2 weeks. In WT mice, probucol, despite decreasing HDL-C by >80%, effectively maintained macrophage RCT. In SR-BI knockout mice, probucol also substantially reduced HDL-C but significantly increased macrophage RCT. Furthermore, probucol significantly enhanced the excretion of HDL-derived cholesterol into feces in both WT and SR-BI knockout mice. Probucol inhibited ABCA1-dependent cholesterol efflux from mouse primary hepatocytes, and this effect was shown to be responsible for the effect of probucol on increasing the fecal excretion of HDL-derived cholesterol in vivo.
Conclusions
We demonstrate that pharmacological inhibition of hepatic ABCA1 activity with probucol reduced HDL-C levels but promoted RCT through diversion of HDL-derived cholesterol from efflux back into plasma instead to excretion in the bile. These results explain the beneficial effects of probucol on atherosclerosis and xanthomas despite its HDL-lowering effects and suggest that inactivation of hepatic ABCA1 leads to increased RCT despite reducing plasma HDL-C levels.
Keywords: atherosclerosis, cholesterol, HDL, lipids, lipoproteins, pharmacology
Probucol is a small molecule with antioxidant properties that decreases plasma levels of low-density lipoprotein cholesterol and high-density lipoprotein cholesterol (HDL-C) in animal models and in humans.1 Interestingly, despite its effects in reducing HDL-C, probucol reduces atherosclerosis in Watanabe heritable hyperlipidemic rabbits2 and scavenger receptor class B type I (SR-BI)/apolipoprotein (apo) E double-knockout mice3 and is widely believed to induce regression of xanthomas in humans,4 with the suggestion of a positive correlation between the decrease in HDL-C and the rate of xanthoma regression.5 The molecular mechanisms by which probucol decreases HDL-C yet reduces atherosclerosis and promotes xanthoma regression remain unknown.
Reverse cholesterol transport (RCT) is the physiological process by which excess cholesterol in peripheral tissues is effluxed by cells to acceptors in interstitial fluid, ultimately returned by HDL directly or indirectly to the liver, and excreted in the bile and finally the feces.6 Reverse cholesterol transport, at least from macrophages, is believed to be protective against atherosclerosis. Several lines of evidence indicate that HDL-C levels are not always reflective of the rate of macrophage RCT; eg, hepatic overexpression of SR-BI reduces plasma HDL-C levels but increases the rate of macrophage RCT,7 thereby reducing atherosclerosis.8,9
We hypothesized that the mechanism by which probucol reduces HDL-C levels results, seemingly paradoxically, in an increase in the rate of macrophage RCT. We carried out experiments in mice using a validated assay of macrophage RCT.6 In this report, we show that probucol promotes macrophages-to-feces RCT, as well as the biliary and fecal excretion of HDL-derived cholesterol, and that SR-BI is not required for this effect. Moreover, we demonstrate that probucol inhibits ATP-binding cassette transporter 1 (ABCA1)– dependent cholesterol efflux from hepatocytes, explaining both the decrease in HDL-C and the increase in macrophage RCT and HDL-derived cholesterol excretion. These findings illuminate an important biological property of probucol, helping to explain its seemingly paradoxical effects on HDL metabolism; in addition, they point to hepatic ABCA1 as a critical modulator of not only HDL-C levels but also the biliary disposal of HDL-derived cholesterol and of RCT.
Methods
Materials
Dulbecco modified Eagle medium and PBS were purchased from Invitrogen (Carlsbad, CA). [1,2-3H]cholesterol, [1,2-3H]cholesteryl oleate (3H-CE-HDL), and [1,2-3H]cholesteryl hexadecyl ether (3H-CEt) were purchased from Perkin-Elmer Life Science (Boston, MA). Other reagents without specification in this article were purchased from Fisher Scientific (Pittsburg, PA). Probucol was synthesized by Fluka and obtained from Sigma (St Louis, MO). The liver X receptor agonist GW3965 was obtained from GlaxoSmithKline and was a kind gift of Dr Colin MacPhee.
Mice and Diets
Wild-type C57BL/6, DBA/J, SR-BI knockout (KO), and ABCA1 KO mice were obtained from The Jackson Laboratory, and breeders bred them in house for the present studies. Experiments were performed in wild-type, SR-BI KO, and ABCA1 KO mice. Mice were fed a standard chow diet or a diet with 0.5% probucol purchased from TestDiet (Mod Laboratory Diet; 1811955). In all studies, fasting plasma was obtained by retro-orbital bleeding while the mice were under isoflurane anesthesia. Animal experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Macrophage Reverse Cholesterol Transport Study
Experiments were performed in wild-type mice and SR-BI KO mice. For each experiment, mice were fed a normal chow diet or 0.5% probucol–containing diet for 2 weeks. On day 15, RCT studies were performed as previously described.7,10–12 In brief, 3H-cholesterol–labeled and acetylated low-density lipoprotein cholesterol–loaded J774 cells (typically 4.5×106 cells containing 7.5×106 counts per minute in 0.5 mL minimum essential media) were injected intraperitoneally. Blood was collected at 6, 24, and 48 hours, and plasmas were used for liquid scintillation counting. Feces were collected continuously from 0 to 48 hours and stored at 4°C before extraction of cholesterol and bile acid.
High-Density Lipoprotein Turnover Study
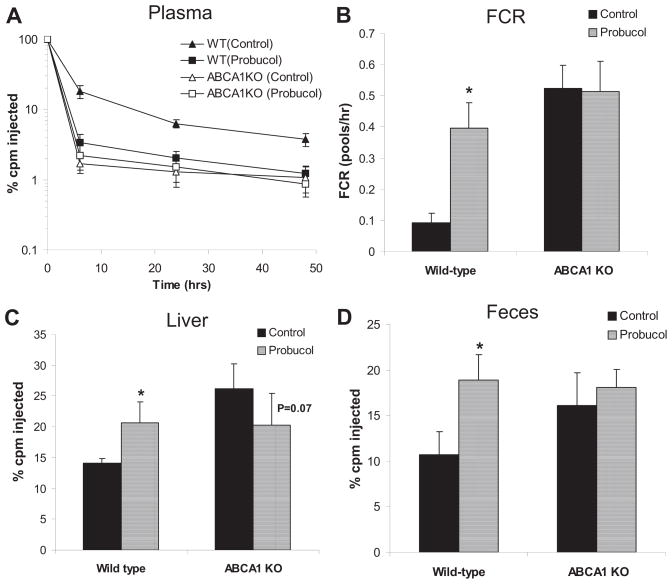
Human HDL was isolated from human plasma by differential ultracentrifugation; HDL (1.063<d<1.21) was labeled with 3H-CE-HDL, 3H-CEt, and 125I-N-methyl tyramine cellobiose (125I-NMTC) according to the methods described previously with slight modifications.13,14 Experiments were performed in wild-type mice, SR-BI KO mice, and ABCA1 KO mice. After probucol treatment for 2 weeks, labeled HDL was intravenously injected via the tail vein under anesthesia. Blood was collected at 2 minutes and 1, 3, 6, 9, 24, and 48 hours, and liver and feces were collected at 48 hours. The fractional catabolic rate (FCR) was assessed with a multicompartmental model and the WinSAAM program as described previously.13,14
RNA Quantification and Western Blotting
Total RNA was isolated from mouse primary hepatocytes with the EZ1 RNA Tissue Mini Kit (Qiagen). cDNA was produced from total RNA via reverse transcription with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantification of mRNA expression with Taqman assay systems was performed by the ABI 7300 Real Time PCR System (Applied Biosystems). All reagents necessary for running a TaqMan RT-PCR assay, including primers and probes, were purchased from Applied Biosystems and used according to the manufacturer’s instructions. Immunoblotting was performed as described previously.15 Liver membrane fractions (40 μg) were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Antibodies and dilutions were as follows: mouse anti-ABCA1 (Abcam ab18180, 1:5000), mouse anti–β-actin (Abcam ab8226, 1:5000), and horseradish peroxidase–conjugated goat anti-mouse IgG (Jackson 111-035-003, 1:4000).
In Vitro Cholesterol Efflux From Mouse Primary Hepatocytes
Primary hepatocytes obtained from wild-type or ABCA1 KO mice were prepared as described.16,17 Hepatocytes were seeded on collagen-coated 6-well plates (Becton Dickinson Labware) at a density of 6×105 cells per well in Dulbecco modified Eagle medium supplemented with 10% FBS. After 6 hours, cells were washed with PBS and labeled by incubation in Dulbecco modified Eagle medium supplemented with 1% FBS containing [3H]cholesterol (2 μCi/mL) for 24 hours. After the labeling period, cells were washed by PBS and incubated with Dulbecco modified Eagle medium containing 0.2% (wt/vol) BSA (Sigma) in the presence of liver X receptor-α/β agonist (GW3965) for 18 hours. The cells were washed with PBS, and cholesterol efflux was initiated by the addition of Dulbecco modified Eagle medium–0.2% BSA with 10 μg/mL human apoAI in the presence or absence of probucol. After 24 hours of incubation, aliquots of the medium were filtered by MultiScreen-HV filter plates (Millipore) to remove the floating cells, and the supernatants were counted in a liquid scintillation counter. The cells were extracted with 2-propanol, evaporated under nitrogen gas, resuspended in toluene, and counted in a liquid scintillation counter. Cholesterol efflux was expressed as percentage of total tritium radioactivity present in the cells at baseline that appeared in the medium.
Statistical Analyses
For the HDL turnover studies, the WinSAAM program was used to fit observed plasma tracer data to a model using a weighted least-squares approach to determine the best fit. All FCR values are presented as mean±SD. A 2-tailed Student t test was used to compare FCRs from the 2 comparator groups to test for statistical significance. For the macrophage RCT studies, appearance of tracer in plasma was analyzed by repeated measures ANOVA, and a Bonferroni correction was applied to correct for multiple comparisons.
Results
Probucol Promotes Macrophage Reverse Cholesterol Transport in Scavenger Receptor Class B Type I Knockout Mice
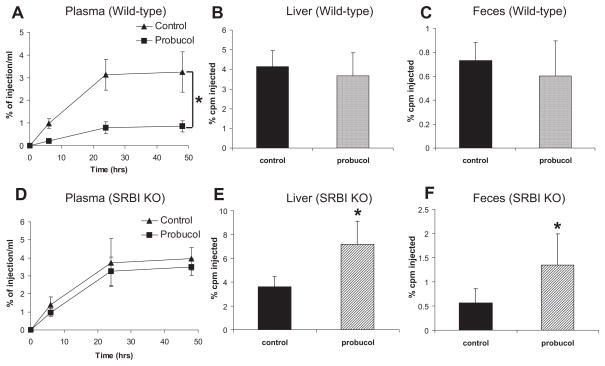
To investigate the effect of probucol on macrophage RCT, wild-type mice were fed a normal chow diet or a chow diet containing 0.5% probucol for 2 weeks, and diets were continued as the macrophage RCT study was performed. As shown in the Table, probucol treatment decreased HDL-C by 84% in wild-type C57BL6/SW129 mice. After injection of the 3H-cholesterol–labeled J774 cells into the peritoneal cavity of wild-type mice, plasma 3H-cholesterol levels were significantly lower across all time points in the probucol-treated group (Figure 1A). However, despite the markedly reduced plasma HDL-C levels, there was no difference in the liver or fecal excretion of the 3H-tracer by the 48-hour time period (Figure 1 through 1C). Thus, in wild-type mice, despite inducing a marked decrease in HDL-C levels, probucol treatment resulted in no decrease in macrophage-to-feces RCT.
Table.
Effects of Probucol Treatment of Mice on Plasma Total and High-Density Lipoprotein Cholesterol Levels
| Mice | Diet | Total Cholesterol, mg/100 mL Plasma | HDL Cholesterol, mg/100 mL Plasma |
|---|---|---|---|
| C57BL6/SW129 | Control | 83.5±6.0 | 70.7±5.2 |
| Probucol | 14.7±3.1 | 11.2±2.8 | |
| SR-BI KO | Control | 213.3±15.8 | 167.8±8.6 |
| Probucol | 72.2±12.2 | 62.8±11.5 | |
| DBA/J | Control | 69.0±6.0 | 47.0±4.4 |
| Probucol | 11.0±2.4 | 5.3±1.0 | |
| ABCA1 KO | Control | 9.4±2.2 | 1.2±0.4 |
| Probucol | 8.0±2.0 | 1.5±0.5 |
HDL indicates high-density lipoprotein; SR-BI, scavenger receptor class B type I; KO, knockout; and ABCAI, ATP-binding cassette transporter 1. Values are mean±SD. In each group, n=6 samples were determined. See Methods for details on diets and lipid analysis.
Figure 1.
Effects of probucol on reverse cholesterol transport (RCT) in mice. Mice (n=8 in each group) were fed the experimental diets for 2 weeks, and RCT studies were performed as described in Methods on C57BL6/SW129 (A through C) and scavenger receptor class B type I knockout (SR-BI KO; D through F) mice. A and D, 3H-cholesterol in plasma after macrophage injection. B and E, 3H-cholesterol in liver. C and F, 3H-cholesterol in feces. cpm Indicates counts per minute. Significant difference, *P<0.05 (vs control).
The SR-BI is a cell surface HDL receptor that mediates selective uptake of cholesterol from HDL.18 The SR-BI knockout mice have elevated HDL-C levels,19 impaired macrophage RCT,7 and increased atherosclerosis and cardiovascular death.20,21 Probucol has been shown to markedly reduce HDL-C levels22 and to reduce atherosclerosis and mortality in SR-BI KO mice.3 To test whether probucol accelerates macrophage RCT in the absence of SR-BI, we performed a macrophage RCT study in probucol-treated SR-BI knockout mice. Probucol treatment for 2 weeks resulted in a 63% reduction in HDL-C, as shown in the Table. Despite the reduction in plasma HDL-C levels with probucol treatment, plasma counts of macrophage-derived 3H-cholesterol were not significantly different between probucol-treated and control mice (Figure 1D). Interestingly, probucol treatment significantly increased the amount of macrophage-derived 3H-cholesterol tracer in the liver and feces of SR-BI KO mice (Figure 1E and 1F), consistent with substantial promotion of macrophage RCT by probucol.
Probucol Promotes the Fecal Excretion of High-Density Lipoprotein–Derived Cholesterol
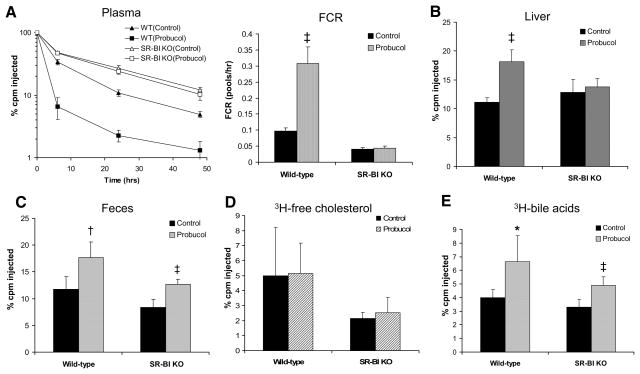
To investigate the effect of probucol on the turnover and fecal excretion of HDL-derived cholesterol, we injected HDL labeled with 3H-CE-HDL into probucol-treated and control wild-type mice. Plasma 3H-CE-HDL decreased much more rapidly in probucol-fed mice than in control mice (Figure 2A, left), consistent with a significantly faster FCR (Figure 2A, right). Furthermore, probucol treatment significantly increased labeled sterol tracer in the liver and feces (Figure 2B and 2C), indicating that probucol enhances the excretion of HDL-derived cholesterol into the bile and feces. Interestingly, fecal excretion of 3H-bile acid, but not of 3H-cholesterol, was increased significantly in the probucol-treated group (Figure 2D and 2E), consistent with bile acids as the predominant form by which the HDL-derived cholesterol is excreted after promotion by probucol.
Figure 2.
Effects of probucol on 3H-cholesteryl oleate high-density lipoprotein (3H-CEs-HDL) clearance in mice. Mice (n=6 in each group) were fed the experimental diets for 2 weeks, and HDL turnover studies were performed as described in Methods on C57BL6/SW129 (wild-type [WT]) and scavenger receptor class B type I knockout (SR-BI KO) mice. A, Change in 3H-CEs-HDL in plasma and plasma fractional catabolic rate (FCR). B, 3H-cholesterol in the liver. C, 3H-sterol in feces. D, 3H-free cholesterol in feces. E, 3H-bile acid in feces. cpm Indicates counts per minute. Significant difference, *P<0.05 (vs control).
We performed a similar study in probucol-treated SR-BI KO mice. Despite the substantial reduction in plasma HDL-C levels on probucol treatment, we found that probucol had no effect on the HDL-CE FCR (Figure 2A), nor did it increase hepatic 3H-cholesterol counts (Figure 2B). However, unexpectedly, probucol treatment substantially increased the fecal excretion of HDL-derived 3H-sterol in SR-BI KO mice (Figure 2C). As in wild-type mice, this was due to an increase in excretion of 3H-bile acids but not 3H-cholesterol (Figure 2D and 2E). Thus, consistent with the macrophage RCT results, probucol markedly promotes the fecal excretion of HDL-derived cholesterol in mice lacking SR-BI despite the fact that it does not accelerate the clearance from plasma of HDL-CE in these mice.
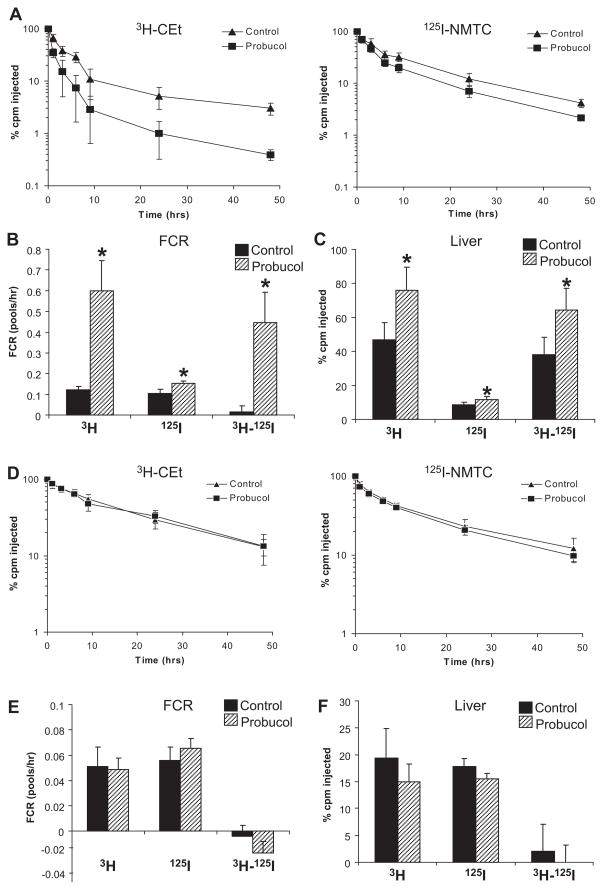
We used as trapped ligands nonhydrolyzable 3H-CEt– and 125I-NMTC–labeled HDL for in vivo HDL turnover studies to better determine the sites of tissue uptake. As expected, in wild-type mice, the plasma FCR for 3H-CEt was higher than for 125I-NMTC, and selective CE uptake by the liver was much higher in the probucol-treated mice (Figure 3A through 3C). In contrast, in SR-BI KO mice, we found no effect of probucol on the FCR of either 3H-CEt-HDL or 125I-NMTC-HDL mice or on selective CE uptake by the liver (Figure 3D and 3E).
Figure 3.
Effects of probucol on plasma decay kinetics of 3H-cholesteryl hexadecyl ether (3H-CEt)/125I-N-methyl tyramine cellobiose (125I-NMTC) high-density lipoprotein (HDL) in mice. Mice (n=6 in each group) were fed the experimental diets for 2 weeks, and HDL turnover studies were performed as described in Methods on C57BL6/SW129 (A through C) and scavenger receptor class B type I knockout (D through F) mice. A and D, 3H-CEt and 125I-NMTC levels in plasma after injection. B and E, The CE-selective plasma fractional catabolic rate (FCR). C and F, The CE-selective uptake in the liver. cpm Indicates counts per minute. Significant differences, *P<0.05 (vs control).
Overall, these results indicate that probucol accelerates the plasma clearance of HDL-CE in wild-type mice through increasing the activity of hepatic SR-BI but that probucol also reduces HDL-C levels, promotes the biliary excretion of HDL-derived cholesterol, and accelerates macrophage RCT through an SR-BI–independent mechanism, accounting for its striking effects in SR-BI KO mice.
Probucol Inhibits ATP-Binding Cassette Transporter 1 Activity in Hepatocytes
In the above experiment, the HDL-derived cholesteryl oleate is able to be hydrolyzed after uptake by the liver and, once the tracer is converted to 3H-unesterified cholesterol, has a number of potential fates, including resecretion back into the plasma via the hepatic ABCA1 transporter. Indeed, hepatic ABCA1 activity is a major determinant of plasma HDL-C levels.23 Probucol has been shown to inhibit ABCA1-dependent cholesterol efflux from several cell types in vitro.24–26 We hypothesized that probucol reduces ABCA1 activity in the hepatocyte, leading to reduced efflux of the HDL-derived cholesterol to plasma and thus increased probability of biliary excretion.
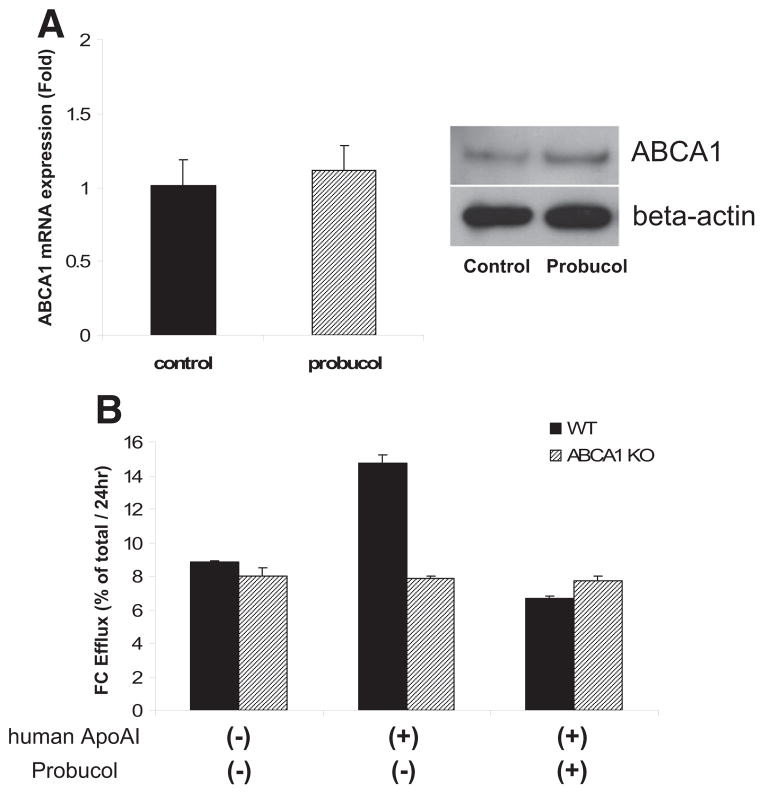
We isolated primary hepatocytes from wild-type mice and incubated cells with or without probucol for 24 hours. Probucol had no significant effect on ABCA1 mRNA or total protein by Western blot (Figure 4A). However, treatment with probucol completely abolished the ability of lipid-free apoA-I to increase cholesterol efflux from primary hepatocytes, similar to the effect seen in ABCA1-deficient primary hepatocytes (Figure 4B). This result is consistent with the concept that probucol markedly reduces hepatocyte ABCA1 activity.
Figure 4.
Effects of probucol on ATP-binding cassette transporter 1 (ABCA1) expression in mouse hepatocytes. A, The mRNA (left) and protein (right) expression of hepatic ABCA1 (n=3 in each group) B, The probucol effect on apolipoprotein AI–dependent free cholesterol (FC) efflux from wild-type (WT) or ABCA1-deficient (ABCA1 KO) primary hepatocytes (n=3 in each group).
If the ability of probucol to promote the excretion of HDL-derived cholesterol is dependent on suppression of hepatic ABCA1 activity, then ABCA1 KO mice should mimic the effects of probucol on the disposal of HDL-derived cholesterol, and probucol should have no effect in ABCA1 KO mice. We therefore performed studies of the metabolism of 3H-CE-HDL in control and probucol-treated ABCA1 KO mice compared with wild-type mice. As expected, the catabolism of 3H-CE-HDL in control diet–fed ABCA1 KO mice was faster than that of control diet–fed wild-type mice (Figure 5A and B). At 48 hours, the counts in the liver were higher in the ABCA1 KO mice than in the wild-type mice (Figure 5C), and the fecal excretion of total 3H-tracer was significantly higher in the ABCA1 KO versus wild-type mice (Figure 5D). These results are consistent with a key role of hepatic ABCA1 in the disposition of HDL-derived cholesterol that is taken up by the liver; in its absence, more HDL-derived cholesterol is excreted into the bile and feces. This pattern is similar to the effect of probucol treatment in wild-type mice. On the other hand, probucol treatment had no effect on the turnover rate of the 3H-CE-HDL in ABCA1 KO mice (Figure 5A and 5B). Likewise, although probucol treatment increased liver counts and fecal excretion of total 3H-tracer in wild-type mice, it had no effect on these parameters in ABCA1 KO mice (Figure 5C and 5D). These results indicate that the ability of probucol to promote the excretion of HDL-derived cholesterol is ABCA1 dependent and are consistent with the hypothesis that probucol reduces HDL-C levels and increases cholesterol excretion into feces by inhibiting hepatic ABCA1-dependent cholesterol efflux from the liver to plasma.
Figure 5.
Effects of probucol on 3H-cholesteryl oleate high-density lipoprotein (3H-CEs-HDL) clearance in ATP-binding cassette transporter 1 (ABCA1) knockout (KO) mice. Mice (n=6 in each group) were fed the experimental diets for 2 weeks, and HDL turnover studies were performed as described in Methods on DBA/J (wild-type [WT]) and ABCA1 KO mice. A, Change in 3H-CEs-HDL in plasma. B, Fractional catabolic rate (FCR) of 3H-CEs-HDL. C, 3H-cholesterol in the liver. D, 3H-sterol in feces. cpm Indicates counts per minute. Significant differences indicated with *P<0.05 (versus control).
Discussion
The mechanisms by which probucol reduces HDL-C levels are poorly understood. Given its beneficial effects on atherosclerosis in mice and xanthomas in humans, we hypothesized that it may promote RCT. Here, we show that probucol increases macrophage-to-feces RCT by promoting the fecal excretion of HDL-derived cholesterol. We further show that this effect is due to the inhibition of hepatic ABCA1 activity. These effects of probucol are also demonstrated in SR-BI knockout mice, in which probucol is known to reduce atherosclerosis and death caused by cardiovascular disease3 despite markedly reducing plasma HDL-C levels. These results have implications for our understanding of the effects of probucol on RCT and on the role of hepatic ABCA1 in the disposal of HDL-derived cholesterol and in integrated RCT.
There are 3 key steps in RCT: cholesterol efflux from macrophages to plasma HDL acceptors, HDL-C uptake from plasma to the liver, and HDL-derived cholesterol excretion from the liver to bile. Given the fact that probucol reduces plasma HDL-C levels, we speculated that the second or third step is increased in probucol-treated animals. To test these steps, we performed 3H-CE-HDL (hydrolysable CE) and 3H-CEt-HDL (nonhydrolyzable CEt) turnover studies in wild-type and SR-BI KO mice. Rinninger et al27 showed that probucol enhanced selective uptake of HDL-CE in vitro by an SR-BI–dependent mechanism, and Hirano et al28 showed that probucol may stabilize SR-BI protein in certain settings. Indeed, we found that probucol accelerated the FCR of both 3H-CE-HDL and 3H-CEt-HDL and increased hepatic selective uptake of HDL-CE and HDL-CEt in wild-type mice, clearly establishing the role of increased hepatic SR-BI activity in mediating the effect of probucol on the hepatic uptake of HDL-CE. In addition, probucol substantially increased the fecal excretion of HDL-derived cholesterol in wild-type mice.
However, the profound HDL-lowering effects of probucol in SR-BI KO mice indicate that probucol has other non–SR-BI–dependent effects on HDL metabolism. We isolated the SR-BI–independent effects of probucol on HDL metabolism and RCT by using SR-BI knockout mice. In contrast to wild-type mice, probucol failed to accelerate the FCR of HDL-CE and HDL-CEt and did not increase the rate of hepatic selective uptake of HDL-CE in SR-BI KO mice, establishing that hepatic SR-BI activity is required for this effect of probucol on HDL metabolism. However, probucol significantly enhanced the fecal excretion of HDL-derived cholesteryl even in SR-BI–deficient mice, indicating that this effect of probucol is SR-BI independent. This unexpected result indicates that probucol stimulates excretion of HDL-derived cholesterol from the liver into the bile by an SR-BI–independent mechanism. Indeed, this effect likely accounts for the overall increased macrophage RCT in SR-BI KO mice on probucol treatment.
We reasoned that HDL-derived CE, once hydrolyzed in the liver, is a substrate for efflux back into the plasma via hepatic ABCA1. Previous reports have shown that probucol has an inhibitory effect on ABCA1-dependent cholesterol efflux from fibroblasts,26 macrophages,24,26 and HepG2 cells25 in vitro. We hypothesized that probucol inhibits ABCA1-dependent cholesterol efflux from hepatocytes in vivo. To investigate the possibility, we first analyzed the ABCA1 expression in mouse primary hepatocytes and found that probucol had no effect on ABCA1 mRNA but increased its protein level, consistent with previous reports.25,26 We then showed that probucol treatment of primary hepatocytes abolished ABCA1-mediated efflux to lipid-free apoA-I. We showed that ABCA1 KO mice behave very similarly to probucol-treated wild-type mice with regard to increased fecal excretion of HDL-derived cholesterol. Finally, we showed that there was no effect of probucol on the turnover of HDL-CE in ABCA1 KO mice and that probucol treatment of ABCA1 KO mice had no further effect on fecal excretion of HDL-derived cholesterol. Our results indicate that hepatic ABCA1 protein is required for probucol to increase fecal excretion of HDL-derived cholesterol and that the effects of probucol on HDL metabolism and RCT can be explained by inhibition of hepatic ABCA1. This would explain the profound effect of probucol on reducing HDL-C levels, increasing hepatic disposal of HDL-derived cholesterol, and increasing integrated macrophage RCT.
The molecular mechanism by which probucol inhibits hepatic ABCA1 activity remains to be determined. Favari et al24 have shown that probucol inhibits ABCA1 translocation to the plasma membrane in J774 cells, and Wu et al26 have shown that probucol inactivates ABCA1 in the plasma membrane of human fibroblasts with regard to its function in mediating binding of and lipid release by apolipoproteins. Further studies in hepatocytes are required to elucidate the mechanism by which probucol inhibits hepatocyte ABCA1 activity.
Given that probucol has been shown to inhibit ABCA1-mediated cholesterol efflux from macrophages in vitro, it is reasonable to question the effect of probucol on the ABCA1-mediated efflux of the injected macrophages. However, we do not know whether necessary inhibitory concentrations were achieved in the peritoneal fluid of the treated mice in our RCT studies and suspect that they may not have been. The injected J774 macrophages had no prior exposure to probucol and were simply injected into the peritoneal cavity, where they were exposed for a relatively short period of time to whatever probucol concentration had been achieved. In contrast, probucol is lipophilic and is metabolized by the liver and excreted in the bile; thus, this protocol achieved high exposure of the hepatocytes to probucol for a sustained period of time. Thus, we believe that the hepatocytes were exposed to a substantially greater concentration of probucol for a longer period of time, resulting in greater relative inhibition of endogenous hepatocyte ABCA1 compared with injected J774 macrophage ABCA1.
Conclusions
Our results indicate that probucol promotes macrophage RCT in vivo by inhibiting hepatic ABCA1 activity, thus reducing the efflux of HDL-derived cholesterol from hepatocytes back into the plasma and diverting this cholesterol to bile acid synthesis and biliary excretion. Probucol also accelerates plasma HDL-CE clearance via the SR-BI pathway, but this effect is not essential to promote in vivo RCT because the effect of probucol on the biliary disposal of HDL-derived cholesterol and on integrated RCT is apparent even in SR-BI KO mice. This study helps to explain the beneficial effects of probucol on atherosclerosis and xanthomas despite the reduction in HDL-C levels and suggests that inhibition of hepatic ABCA1, although expected to lead to a decrease in HDL-C levels, may promote RCT and be atheroprotective.
CLINICAL PERSPECTIVE.
Plasma levels of high-density lipoprotein cholesterol (HDL-C) do not always reflect the dynamic process of reverse cholesterol transport (RCT) from macrophage to bile and feces and the risk of atherosclerosis. For example, mice lacking the hepatic HDL receptor scavenger receptor class B type I have markedly elevated HDL-C levels but impaired RCT and increased atherosclerosis. The ATP-binding cassette transporter 1 (ABCA1) is expressed in the liver, and by exporting cholesterol out of the liver to the HDL protein, apolipoprotein A-I plays a critical role in maintaining plasma HDL-C levels. However, the relationship of hepatic ABCA1 to RCT and atherosclerosis remains poorly understood. Because hepatic ABCA1 pumps cholesterol from the liver into the blood instead of the bile, it might reduce the rate at which the liver excretes HDL-derived cholesterol. Probucol is a drug that reduces HDL-C levels but also, paradoxically, reduces atherosclerosis and xanthomas. We tested the hypothesis that probucol inhibits hepatic ABCA1 activity, thereby reducing HDL-C levels but promoting RCT from macrophages. In studies in mice lacking the hepatic HDL receptor scavenger receptor class B type I, probucol substantially reduced HDL-C but significantly increased macrophage RCT. Furthermore, probucol significantly enhanced the excretion of HDL-derived cholesterol into the feces. Probucol markedly inhibited ABCA1-dependent cholesterol efflux from mouse primary hepatocytes, and this effect was shown to be responsible for the effect of probucol on increasing the fecal excretion of HDL-derived cholesterol in vivo. These results provide an explanation for the beneficial effects of probucol on atherosclerosis despite its HDL-lowering effects and suggest that inactivation of hepatic ABCA1 leads to increased RCT despite reducing plasma HDL-C levels.
Acknowledgments
We thank Dawn Marchadier, Debra Cromley, Aisha Wilson, Edwige Edouard, Mao-Sen Sun, and Michelle Joshi for their excellent technical assistance; Dr John Millar for helping to design, implement, and analyze the HDL turnover studies; Dr Kathleen Propert for assistance with the statistical analysis; and Dr George Rothblat for helpful discussions.
Source of Funding
This project was supported by grant P50 HL70128 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Footnotes
Disclosures
Dr Yamamoto is currently an employee of Shionogi & Co, Toyonaka, Japan. Dr Rader is a founder of Vascular Strategies, which provides research support services related to lipoprotein metabolism, cholesterol efflux, and RCT. The other authors report no conflicts.
References
- 1.Kesaniemi YA, Grundy SM. Influence of probucol on cholesterol and lipoprotein metabolism in man. J Lipid Res. 1984;25:780–790. [PubMed] [Google Scholar]
- 2.Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun A, Zhang S, Miettinen HE, Ebrahim S, Holm TM, Vasile E, Post MJ, Yoerger DM, Picard MH, Krieger JL, Andrews NC, Simons M, Krieger M. Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor SR-BI/apolipoprotein E double knockout mouse. Proc Natl Acad Sci U S A. 2003;100:7283–7288. doi: 10.1073/pnas.1237725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B. Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol. 1986;57:29H–35H. doi: 10.1016/0002-9149(86)90434-0. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzawa Y, Yamashita S, Funahashi T, Yamamoto A, Tarui S. Selective reduction of cholesterol in HDL2 fraction by probucol in familial hypercholesterolemia and hyperHDL2 cholesterolemia with abnormal cholesteryl ester transfer. Am J Cardiol. 1988;62:66B–72B. doi: 10.1016/s0002-9149(88)80055-9. [DOI] [PubMed] [Google Scholar]
- 6.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. Role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2008;50(suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- 9.Ueda Y, Gong E, Royer L, Cooper PN, Francone OL, Rubin EM. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J Biol Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 11.Tanigawa H, Billheimer JT, Tohyama J, Zhang Y, Rothblat G, Rader DJ. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116:1267–1273. doi: 10.1161/CIRCULATIONAHA.107.704254. [DOI] [PubMed] [Google Scholar]
- 12.Alexander ET, Weibel GL, Joshi MR, Vedhachalam C, de la Llera-Moya M, Rothblat GH, Phillips MC, Rader DJ. Macrophage reverse cholesterol transport in mice expressing ApoA-I Milano. Arterioscler Thromb Vasc Biol. 2009;29:1496–1501. doi: 10.1161/ATVBAHA.109.191379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tietge UJF, Maugeais C, Cain W, Grass D, Glick J, De Beer F, Rader DJ. Overexpression of secretory phospholipase A2 causes rapid catabolism and altered tissue uptake of HDL cholesteryl ester and apolipoprotein A-I. J Biol Chem. 2000;275:10077–10084. doi: 10.1074/jbc.275.14.10077. [DOI] [PubMed] [Google Scholar]
- 14.Maugeais C, Tietge UJ, Broedl UC, Marchadier D, Cain W, McCoy MG, Lund-Katz S, Glick JM, Rader DJ. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 2003;108:2121–2126. doi: 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- 15.Lagor WR, Heller R, de Groh ED, Ness GC. Functional analysis of the hepatic HMG-CoA reductase promoter by in vivo electroporation. Exp Biol Med. 2007;232:353–361. [PubMed] [Google Scholar]
- 16.Goncalves LA, Vigario AM, Penha-Goncalves C. Improved isolation of murine hepatocytes for in vitro malaria liver stage studies. Malar J. 2007;6:169. doi: 10.1186/1475-2875-6-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Millar JS, Brownell N, Briand F, Rader DJ. Modulation of HDL metabolism by the niacin receptor GPR109A in mouse hepatocytes. Biochem Pharmacol. 2010;80:1450–1457. doi: 10.1016/j.bcp.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acton S, Rigotti A, Landschultz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:460–461. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 19.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipo-protein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest. 2001;108:1717–1722. doi: 10.1172/JCI13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 25.Tsujita M, Wu CA, Abe-Dohmae S, Usui S, Okazaki M, Yokoyama S. On the hepatic mechanism of HDL assembly by the ABCA1/apoA-I pathway. J Lipid Res. 2005;46:154–162. doi: 10.1194/jlr.M400402-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Wu CA, Tsujita M, Hayashi M, Yokoyama S. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem. 2004;279:30168–30174. doi: 10.1074/jbc.M403765200. [DOI] [PubMed] [Google Scholar]
- 27.Rinninger F, Wang N, Ramakrishnan R, Jiang XC, Tall AR. Probucol enhances selective uptake of HDL-associated cholesteryl esters in vitro by a scavenger receptor B-I-dependent mechanism. Arterioscler Thromb Vasc Biol. 1999;19:1325–1332. doi: 10.1161/01.atv.19.5.1325. [DOI] [PubMed] [Google Scholar]
- 28.Hirano K, Ikegami C, Tsujii K, Zhang Z, Matsuura F, Nakagawa-Toyama Y, Koseki M, Masuda D, Maruyama T, Shimomura I, Ueda Y, Yamashita S. Probucol enhances the expression of human hepatic scavenger receptor class B type I, possibly through a species-specific mechanism. Arterioscler Thromb Vasc Biol. 2005;25:2422–2427. doi: 10.1161/01.ATV.0000185834.98941.3d. [DOI] [PubMed] [Google Scholar]