Abstract
BACKGROUND
Recurrent aphthous stomatitis (RAS), commonly referred to as canker sores, is a very common and painful oral mucosal disease. Although the etiology of RAS is not well understood, a number of factors may play a role, including nutritional deficiencies. The objective of this study was to compare dietary vitamin intake in RAS patients to that of a control group.
METHODS
One hundred subjects, who had suffered at least three episodes of minor RAS in the previous 12 months, completed a detailed Diet History Questionnaire designed and validated by the US National Institutes of Health. DietCalc software was used to calculate daily dietary intakes of nine different vitamins in the study subjects. Daily intakes were energy-adjusted and compared to age- and gender-matched nutrient intake data on 9033 subjects from the US National Health and Nutrition Examination Survey.
RESULTS
The study subjects had significantly lower daily intake of vitamin B12 (P < 0.0002) and folate (P < 0.0001) as compared to the controls.
CONCLUSIONS
Our results demonstrate that patients with recurrent aphthous stomatitis are more likely to have lower dietary intakes of vitamin B12 and folate than a control group. These results support and extend previous studies indicating a link between the etiology of RAS and hematological deficiencies of vitamin B12 and folate. These findings suggest that consuming sufficient amounts of these vitamins may be a useful strategy to reduce the number and/or duration of RAS episodes.
Keywords: folate, recurrent aphthous stomatitis, vitamin B12
Introduction
Recurrent aphthous stomatitis (RAS), commonly referred to as canker sores, is the most widespread oral mucosal disease found in humans (1). A study of more than 10 000 young adults from across the world indicated that 38.7% of men and 49.7% of women reported at least two previous episodes of RAS, with 25% of those studied reporting an episode in the past year (2). RAS is most commonly seen in a form called minor RAS, which presents as small oval ulcers with necrotic centers and red margins. These ulcers can be very painful, making eating and practicing proper oral hygiene difficult.
Although the etiology of RAS is not well understood, factors such as genetic predisposition and immune disturbances have been suggested as possible causes (3). For example, Lewkowicz et al. recently reported that CD4(+)CD25(high) T regulatory cells are functionally and quantitatively compromised in patients with RAS (4). It has also been found that RAS more often affects younger people and that their ulcers are associated with stressful periods (5). There is also considerable evidence to suggest that RAS may be caused by one or more haematological deficiencies. Wray et al. conducted two haematological screenings in patients with RAS and found nutritional deficiencies in 14.2% and 17.7% of the samples (6, 7). Other haematological studies on patients with RAS have also found nutritional deficiencies, most commonly low levels of vitamin B12 (8, 9) and folate (10). Replacement therapy with vitamins in such patients results in improvement of their condition and the subjects’ RAS-related symptoms (6, 7, 11, 12).
Data from the third US National Health and Nutrition Examination Survey (NHANES) indicated that teenagers and young adults reported the lowest use of daily multivitamins, before increasing use with age (13). Additionally, young adults consume diets which are less nutritious than those who are older (14, 15). This phenomenon correlates with a high prevalence of RAS in young adults, which lessens as they age (16). Therefore, those age groups consuming diets lowest in vitamins also exhibit a greater frequency of RAS.
However, a direct relationship between dietary intake of vitamins and frequency of RAS episodes has not been well-studied and this constitutes an important gap in knowledge. The specific aim of this study was to compare the average dietary intakes of various nutrients in subjects with RAS to those of a healthy control group. We hypothesized that subjects who suffer from frequent RAS episodes would have lower dietary intake of one or more vitamins when compared to a control group.
Research design and methods
This study was approved by the Institutional Review Board at the University of Connecticut Health Center. 100 subjects, at least 18 years old and with a history of minor RAS, were enrolled in the study after giving informed consent. To be included in the study, the subjects had to have experienced a minimum of three episodes of minor RAS within the past 1 year. Exclusion criteria were as follows: Individuals who suffered from other forms of RAS (major or herpetiform); those who had taken any vitamin supplements in the previous 3 months; patients with a history of any systemic condition associated with oral ulceration, such as Behcet’s syndrome, Sweet’s syndrome, Celiac disease, Crohn’s disease, ulcerative colitis, HIV infection/AIDS, cyclic neutropenia and PFAPA syndrome; patients who were on medications commonly associated with causing oral ulceration, such as methotrexate and chemotherapeutic agents used for cancer; patients who use any medications to treat their RAS episodes, such as topical anti-inflammatory agents, smokers of tobacco products, as well as those who have quit smoking in the previous 30 days, due to an observed inverse relationship between smoking and RAS lesions (17).
All subjects completed a very detailed paper Diet History Questionnaire (DHQ) (18) which includes questions on frequency and amounts of consumption of 124 different types of foods over the preceding 365 days. This questionnaire was designed and validated by the National Institutes of Health (NIH). The DHQ has been shown in validation studies to give nutrient intake estimates that correlate strongly with true intakes when compared to both the Block and Willett food frequency questionnaires (19).
NIH also provides the DietCalc software program which uses food and nutrient information from the USDA’s 1994–1996 Continuing Survey of Food Intake of Individuals (CSF-II) to analyze questionnaire responses. The DietCalc software was used to calculate average daily dietary intakes of the following vitamins in the 100 RAS subjects: vitamin A, vitamin C, vitamin E, thiamin, niacin, riboflavin, vitamin B6, vitamin B12 and folate. These nutrient intakes were energy-adjusted by dividing each subject’s average daily nutrient intake by his/her average daily kilocalorie intake to give an estimate of nutrient density.
Nutrient densities for the RAS subjects were compared to nutrient intake data on 9033 subjects from the National Health and Nutrition Examination Survey (NHANES 2001–2002). The data from the NHANES controls were also energy-adjusted by computing nutrient densities. Age- and gender-matched nutrient densities from the NHANES data were subtracted from the nutrient densities from each of the RAS subjects to calculate nutrient density differences at the subject level. These differences were analyzed with a Wilcoxon signed rank test to determine whether or not they provided significant evidence of a failure to center around zero, i.e., what one would expect if RAS subjects had nutrient intakes comparable to those among age- and gender-matched individuals in the general population.
Results
Our sample of RAS subjects included 100 individuals of whom 63 were female and 37 were male, with a mean age of 38 years. The NHANES control group consisted of 9033 individuals, of whom 4653 were female and 4380 were male. One subject from our RAS sample was removed from the data analysis because the DHQ she filled out was deemed invalid as the responses indicated an extremely low and physiologically unfeasible level of daily caloric intake.
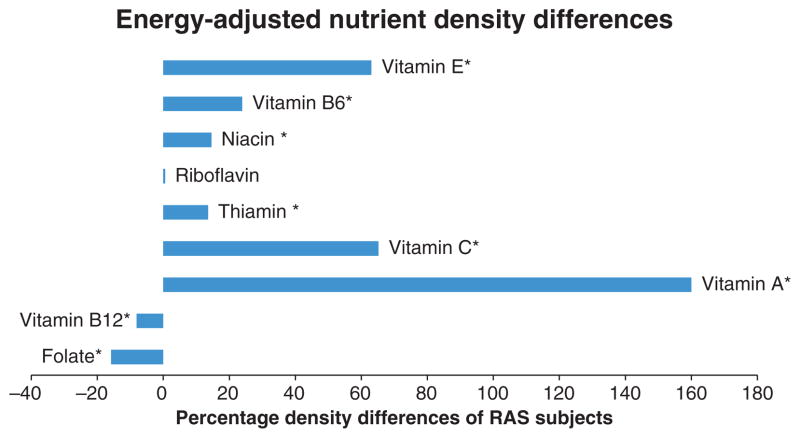
When compared to the NHANES data, calculated dietary intake levels for the RAS subjects in our study were higher for seven of the nine nutrients tested (Fig. 1, Table 1). Folate and vitamin B12, however, were consumed in lower amounts in the RAS subjects as compared to the NHANES data. The mean energy-adjusted vitamin B12 density was 0.0002 mcg/kcal (SD = 0.00075) less in RAS subjects than the NHANES controls (P < 0.0002) and the average energy-adjusted folate density was 0.0403 mcg/kcal (SD = 0.0554) less (P < 0.0001). The median energy-adjusted vitamin B12 density was also 0.0002 mcg/kcal less in RAS subjects than the NHANES controls and the median energy-adjusted folate density was 0.0519 less. When the raw nutrient values were compared, the RAS subjects consumed mean daily levels of vitamin B12 that were lower than those of controls by 0.43 mcg (7% of recommended daily intake). Mean daily levels of folate consumed by RAS subjects were lower than those of controls by 81.3 mcg (20% of recommended daily intake).
Figure 1.
Energy-adjusted nutrient density differences between RAS subjects and NHANES controls as a percentage of mean nutrient densities for NHANES controls. *P < 0.05.
Table 1.
Energy-adjusted nutrient density differences between RAS subjects and age- and sex-matched NHANES controls
| Nutrient | Density difference in mcg/kcal (RAS subjects – NHANES controls) |
|---|---|
| Vitamin A | 0.4444 |
| Vitamin C | 27.6 |
| Thiamin | 0.1 |
| Riboflavin | 0.00562 |
| Niacin | 1.48 |
| Vitamin B6 | 0.2 |
| Vitamin E | 1.94 |
| Folate | −0.0403 |
| Vitamin B12 | −0.0002 |
Discussion
A previous study has shown that NHANES data may systematically underestimate average energy intake (20). As errors in the measurement of energy intake are correlated with errors in measurement of nutrient intakes, nutrient levels were energy-adjusted to compensate for this potential variation (21). Vitamin B12 and folate were the only vitamins tested which were found to be consumed in lower amounts in RAS subjects as compared to NHANES controls. These findings are all the more significant because all other nutrients tested, with the exception of riboflavin, were significantly higher in the RAS group than the NHANES group. One possible explanation may be that the DietCalc software systematically overestimates average nutrient intake and therefore the deficiencies in folate and vitamin B12 consumption may thus be even greater than our results suggest. It is also possible that subjects enrolled in our study consumed a greater amount of the other vitamins tested than those in the NHANES group. However, this is unlikely because taking any supplemental vitamins in the 90 days prior to enrollment was an exclusion criterion. Therefore, our results suggest that patients who suffer from RAS are more likely to have inadequate dietary intakes of vitamin B12 and folate than the general population of the United States.
The precise mechanisms whereby vitamin deficiencies affect RAS are not well understood. The reported prompt response to replacement therapy in some patients suggests a direct action on the oral mucosa (6, 7, 11, 12). However, it has also been postulated that the presence of a deficiency allows the expression of an underlying tendency to ulceration (3). Vitamin B12 acts as a co-enzyme for fat and carbohydrate metabolism, protein synthesis and hematopoiesis. Vitamin B9 (folic acid) is also involved in the formation of co-enzymes for protein synthesis and erythropoiesis. It has been demonstrated recently that changes in the oral mucosa including glossitis and stomatitis may be the only clinical signs of early deficiency of vitamin B12 or folic acid (22).
These results support and extend previous work by Wray and other investigators, indicating a link between the etiology of RAS and deficiencies of vitamin B12 and folate. While previous work has demonstrated that individuals with RAS are more likely to have lower blood levels of vitamin B12 and folate (6–10), our study now finds that RAS patients may actually consume lower levels of these nutrients than the general population. Thus, it may be possible to attribute the lower blood levels of vitamin B12 and folate to inadequate dietary intake as opposed to absorption-related issues. These findings suggest that increasing or supplementing dietary intake of vitamin B12 and folate may be of value in preventing RAS episodes.
Acknowledgments
This research was supported by the Patrick and Catherine Weldon Donaghue Medical Research Foundation and by the (US) National Institute of Dental and Craniofacial Research. The authors thank the following individuals for their assistance in implementing the clinical study: Linda Choquette, Sandra D’Amato-Palumbo, Marie Latortue, Easwar Natarajan, Vijay Parashar, Douglas Peterson, and Harriet Zawistowski. We also gratefully acknowledge the contributions of the subjects who participated in this study.
References
- 1.Ship JA, Chavez EM, Doerr PA, Henson BS, Sarmadi M. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95–112. [PubMed] [Google Scholar]
- 2.Embil JA, Stephens RG, Manuel FR. Prevalence of recurrent herpes labialis and aphthous ulcers among young adults on six continents. Can Med Assoc J. 1975;113:627–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis: a consensus approach. J Am Dent Assoc. 2003;134:200–7. doi: 10.14219/jada.archive.2003.0134. [DOI] [PubMed] [Google Scholar]
- 4.Lewkowicz N, Lewkowicz P, Dzitko K, et al. Dysfunction of CD4 + CD25high T regulatory cells in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2008;37:454–61. doi: 10.1111/j.1600-0714.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 5.Mccullough JM, Abdel-Hafeth S, Scully C. Recurrent aphthous stomatitis revisited; clinical features, associations, and new association with infant feeding practices. J Oral Pathol Med. 2007;36:615–20. doi: 10.1111/j.1600-0714.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 6.Wray D, Ferguson MM, Hutcheon WA, Dagg JH. Nutritional deficiencies in recurrent aphthae. J Oral Pathol. 1978;7:418–23. doi: 10.1111/j.1600-0714.1978.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 7.Wray D, Ferguson MM, Mason DK, Hutcheon AW, Dagg JH. Recurrent aphthae: treatment with vitamin B12, folic acid, and iron. Br Med J. 1975;2:490–3. doi: 10.1136/bmj.2.5969.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weusten BL, van de Wiel A. Aphthous ulcers and vitamin B12 deficiency. Neth J Med. 1998;53:172–5. doi: 10.1016/s0300-2977(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 9.Piskin S, Sayan C, Durukan N, Senol M. Serum iron, ferritin, folic acid, and vitamin B12 levels in recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. 2002;16:66–7. doi: 10.1046/j.1468-3083.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 10.Porter SR, Scully C, Flint S. Hematologic status in recurrent aphthous stomatitis compared with other oral disease. Oral Surg Oral Med Oral Pathol. 1988;66:41–4. doi: 10.1016/0030-4220(88)90064-3. [DOI] [PubMed] [Google Scholar]
- 11.Nolan A, McIntosh WB, Allam BF, Lamey PJ. Recurrent aphthous ulceration: vitamin B1, B2 and B6 status and response to replacement therapy. J Oral Pathol Med. 1991;20:389–91. doi: 10.1111/j.1600-0714.1991.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 12.Porter S, Flint S, Scully C, Keith O. Recurrent aphthous stomatitis: the efficacy of replacement therapy in patients with underlying hematinic deficiencies. Ann Dent. 1992;51:14–6. [PubMed] [Google Scholar]
- 13.Ervin RB, Wright JD, Reed-Gillette D. Prevalence of leading types of dietary supplements used in the Third National Health and Nutrition Examination Survey, 1988–94. Adv Data. 2004;349:1–7. [PubMed] [Google Scholar]
- 14.Drewnowski A, Henderson SA, Driscoll A, Rolls BJ. The Dietary Variety Score: assessing diet quality in healthy young and older adults. J Am Diet Assoc. 1997;97:266–71. doi: 10.1016/s0002-8223(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein MA, Tucker KL, Ryan ND, et al. Higher dietary variety is associated with better nutritional status in frail elderly people. J Am Diet Assoc. 2002;102:1096–104. doi: 10.1016/s0002-8223(02)90246-4. [DOI] [PubMed] [Google Scholar]
- 16.Axell T, Henricsson V. The occurrence of recurrent aphthous ulcers in an adult Swedish population. Acta Odontol Scand. 1985;43:121–5. doi: 10.3109/00016358509046497. [DOI] [PubMed] [Google Scholar]
- 17.Atkin PA, Xu X, Thornhill MH. Minor recurrent aphthous stomatitis and smoking: an epidemiological study measuring plasma cotinine. Oral Dis. 2002;8:173–6. doi: 10.1034/j.1601-0825.2002.01826.x. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. [accessed on 12 August, 2009];Diet History Questionnaire (Internet Document) Available at: http://riskfactor.cancer.gov/DHQ.
- 19.Subar AF, Thompson FE, Kipnis V, et al. Comparative Validation of the Block, Willett, and National Cancer Institute Food Frequency Questionnaires. Am J Epidemiol. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 20.Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65:1203s–9s. doi: 10.1093/ajcn/65.4.1203S. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220s–8s. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 22.Bjorkegren K, Svardsudd K. Reported symptoms and clinical findings in relation to serum cobalamin, folate, methylmalonic acid and total homocysteine among elderly Swedes: a population-based study. J Intern Med. 2003;254:343–52. doi: 10.1046/j.1365-2796.2003.01199.x. [DOI] [PubMed] [Google Scholar]