Abstract
Thiopurines are effective immunosuppressants and anticancer agents, but intracellular accumulation of their active metabolites (6-thioguanine nucleotides, 6-TGNs) causes dose-limiting hematopoietic toxicity. Thiopurine S-methyltransferase (TPMT) deficiency is known to exacerbate thiopurine toxicity. However, many patients are highly sensitive to thiopurines for unknown reasons. We show that Mrp4 is abundant in myeloid progenitors and tested the role of the multidrug-resistance protein 4 (Mrp4), an ATP binding cassette (ATP) transporter of monophosphorylated nucleosides, in this unexplained thiopurine sensitivity. Mrp4-deficient mice experienced Mrp4 gene dosage–dependent toxicity caused by accumulation of 6-TGNs in their myelopoietic cells. Therefore, Mrp4 protects against thiopurine-induced hematopoietic toxicity by actively exporting thiopurine nucleotides. We then identified a single-nucleotide polymorphism (SNP) in human MRP4 (rs3765534) that dramatically reduces MRP4 function by impairing its cell membrane localization. This SNP is common (>18%) in the Japanese population and indicates that the increased sensitivity of some Japanese patients to thiopurines may reflect the greater frequency of this MRP4 SNP.
INTRODUCTION
Thiopurines (azathioprine, 6-mercaptopurine [6-MP], and thioguanine) are effective immunosuppressants and anticancer agents (1), but cause acute gastrointestinal and hematopoietic toxicity . The intracellular accumulation of their active metabolites, 6-thioguanine nucleotides (6-TGNs) is associated with hematologic toxicity. This toxicity is exacerbated in patients who carry at least one thiopurine S-methyltransferase (TPMT) defective allele (1,2). For largely unknown reasons, a subset of other patients who have not inherited TPMT deficiency also experience severe thiopurine-induced myelosuppression (2-4). Factors known to affect intracellular thiopurine concentration do not appear to be implicated in these cases (2,3). We and others have shown in cell line models that overexpression of multidrug-resistance protein 4 (MRP4) enhances egress of monophosphorylated forms of nucleoside drugs (5-7). However, it is unknown if MRP4 is expressed in thiopurine sensitive hematopoietic cells and if it protects these cells by limiting their accumulation of 6-TGNs.
The MRP (ABCC) gene family is highly polymorphic and MRP4 is among the most polymorphic (8) and over 20 missense genetic variants have been identified in the NCBI database (http:www.ncbi.nlm.nuh.gov/SNP) and the Pharmacogenetics Research Network (http:www.PharmGKB.org). Because patients who are not TPMT-deficient experience severe thiopurine-induced myelosuppression, including many Japanese patients (3), we hypothesized that MRP4 may provide an explanation for this unexplained thiopurine sensitivity. Our studies determined that one MRP4 missense mutation is prevalent in Japanese (>18%) and that this allele is functionally impaired. Collectively, our studies demonstrate that, absence of Mrp4 in a murine model causes thiopurine hematopoietic toxicity and our in vitro studies suggest that some variant Human MRP4s could be a locus accounting for enhanced thiopurine sensitivity among susceptible populations.
METHODS
Chemicals
Mercaptopurine, 6-mercaptopurine riboside, and 6-methylmercaptopurine riboside and vincristine were purchased from Sigma.
This study and all methods described were approved by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee. The Mrp4−/− mice were previously described (9).
In vitro myeloid progenitor assays
Myeloid progenitor assays were performed as described (21).
TPMT activity
Blood was collected into tubes containing sodium heparin. Erythrocyte lysates were prepared and analyzed for TPMT activity as described (10).
De novo purine synthesis assay
The rate of de novo purine synthesis in bone marrow cells was determined as described (10).
Analysis of 6-MP metabolites
The levels of 6-MP and 6-TGNs were measured by high-pressure liquid chromatography as described (11,13).
Histologic evaluation
Femurs, fixed in formalin, were incubated overnight in decalcifier, embedded in paraffin, sectioned (4 μm) and stained with hematoxylin and eosin.
Immunophenotyping
Immunophenotyping studies were performed as described (14).
Peripheral blood hemoglobin analysis
Hemoglobin concentration in the peripheral blood was measured on a Hemavet 3700 hematology analyzer (CDC Technologies, Oxford, CT).
Patient samples
DNA variation panels were obtained from the Coriell Repository (http://locus.umdnj.edu/nigms/cells/humdiv.html) (Coriell Institute for Medical Research, Camden, New Jersey).
Genotyping of MRP4 G2269A by direct sequencing
MRP4 exon 18 encompassing the 2269 SNP was amplified from genomic DNA by use of forward 5′- TCCAGTGGCTGATTTTCTGA- 3′ and reverse 5′- GAGTGTAAACTGCGGTGGT-3′ primers under the following conditions: 95°C for 5 min followed by 32 cycles, each at 95°C for 30 s, 59°C for 40 s, and 72°C for 40 s, and a final extension at 72°C for 7 min. Sequencing was carried out on an ABI Prism 3700 Automated Sequencer (Applied Biosystems, Foster City, California). Sequences were assembled with the Polyphred package (University of Washington, Seattle).
Cell surface biotinylation
Cells were treated with a membrane-impermeable biotinylating agent (Sulfo-NHS-biotin, Pierce) and washed with glycine to remove unbound labeling reagent. The cells were lysed in RIPA containing protease inhibitors. After centrifugation, the lysate was added to monomeric streptavidin agarose beads (Invitrogen). Beads were washed with lysis buffer, and the biotinylated proteins were released by incubation with Laemmli buffer.
MRP4 gene expression
Microarray data was extracted from a previous publication (12). Bone-marrow cells were collected 20 hrs after the start of 6-MP therapy from 8 patients as described (13) with acute lymphoblastic leukemia and total RNA was processed and hybridized to the HG-U133A GeneChip oligonucleotide microarray (Affymetrix Inc.; see manufacturer’s manual for detailed protocol). Default settings of Microarray Suite software version 5.0 (Affymetrix Inc.) were used to calculate scaled gene expression values which are highly correlated with real-time PCR values. The probe set for MRP4 was 203196_at.
RESULTS & DISCUSSION
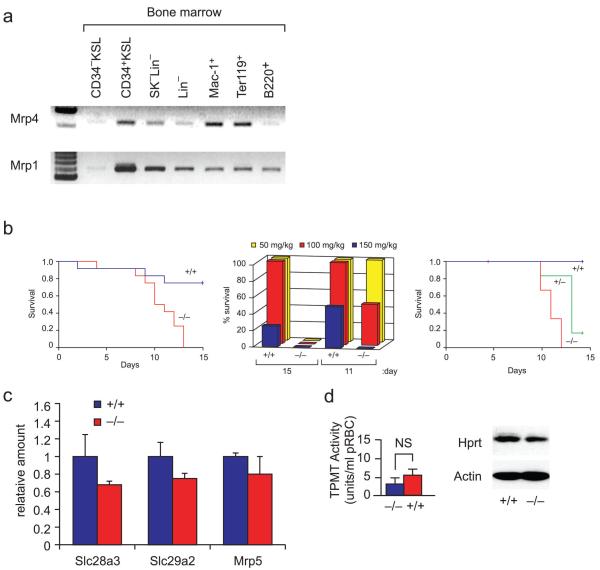
Bone marrow cells of Mrp4 wild-type mice were isolated and RNA prepared from different lineages as described (see supplement Fig. 1a). After Gapdh normalization Mrp4 RNA was highly expressed in monocyte/macrophage and erythroid progenitors (Fig. 1a and supplemental Fig. 1a), which are disproportionately affected by thiopurine toxicity (15), whereas another ABC transporter, Mrp1, was expressed in all cell lineages.
Fig.1.
Mrp4 is variably expressed in bone marrow cell lineages, and its absence sensitizes cells to 6-mercaptopurine. (a) RT-PCR analysis of Mrp4 mRNA expression in bone marrow cell lineages. Mrp4 cDNA aliquots were normalized to GAPDH expression (Supplementary Fig. 1a) as determined by real-time PCR. KSL, cKit+Sca+Lin−; lineage negative; Mac-1+, monocyte/macrophage; Ter119+, erythroid precursor; B220+, B-cell precursor. (b) Survival of Mrp4−/− (red), Mrp4+/+ (blue) littermates that received daily intraperitoneal injections of 6-MP (50 mg/kg 100 mg/kg 150mg/kg; n = 5 each) (left panel). Mrp4−/− mice succumbed to the lethal effects of 6-MP earlier and at lower doses than did Mrp4+/+ mice (middle panel) survival of Mrp4−/− (red), Mrp4+/− (green) and Mrp4+/+ (blue) littermates that received daily intraperitoneal injections of 6-MP (100 mg/kg n=5 each). One mouse died shortly after injection and was censored from analysis on the basis of two prior studies in which no early deaths occurred (total 18 mice per genotype) (right panel). (c) Expression of the nucleoside transporter genes SLC28a3, SLC29a2, and Mrp5 in Mrp4−/− and Mrp4+/+ bone marrow. (d) TPMT activity (left) and Hprt expression assessed by immunoblot analysis (right) in peripheral red blood cells of Mrp4−/− (blue) and Mrp4+/+ (red) mice. NS, not significant.
The 6-MP sensitivity of Mrp4+/+ and Mrp4−/− mice was tested by intraperitoneal injections with 50, 100, or 150 mg/kg of 6-MP daily for 15 days and survival monitored. All Mrp4−/− mice died by Day 13 regardless of 6-MP dose (Fig. 1b, left panel), whereas more than 75% of the wild-type mice survived at Day 15 (P 0.001, log-rank test), including most of those that received 150 mg/kg 6-MP (Fig. 1b, middle panel). Neither age nor sex was associated with 6-MP sensitivity. The number of Mrp4 alleles (gene dosage effect) affected 6-MP toxicity. The Mrp4+/− mice show that a single Mrp4 allele was protective because the duration of survival was greater than mice lacking Mrp4 (Fig.1b, right panel). The enhanced 6-MP sensitivity could not be explained by increased expression of the purine nucleoside uptake transporters Slc28a3, Slc29a2 (16), or reduced expression of Mrp5 (which is capable of 6-MP nucleotide efflux) (11,17) (Fig. 1c) because the expression levels of these genes (P > 0.05) in Mrp4−/− vs. Mrp4+/+ bone marrow was no different. Although Slc29a1 transports mercaptopurine ribosides (17) we could not detect it in bone marrow cells. TPMT activity (responsible for methylation of 6-MP) and expression of hypoxanthine phosphoribosyl transferase (Hprt, the major enzyme bioactivating 6-MP) were also comparable in Mrp4−/−and Mrp4+/+ bone marrow cells (Fig. 1d).
Yeast ABCC family members impact transport of purine biosynthetic intermediates (18). Therefore we assessed if Mrp4 absence affected the mean rate of de novo purine biosynthesis in bone marrow cells. Among Mrp4+/+, Mrp4+/−, and Mrp4−/− genotypes the rate of synthesis was 3.1+/− 1.4, 3.0 +/− 1.0 and 4.0+/−1.0 pmol of newly synthesized purine / nmol unlabelled purine per hr respectively, which indicates Mrp4 absence did not enhance 6-MP sensitivity by reducing de novo purine biosynthesis.
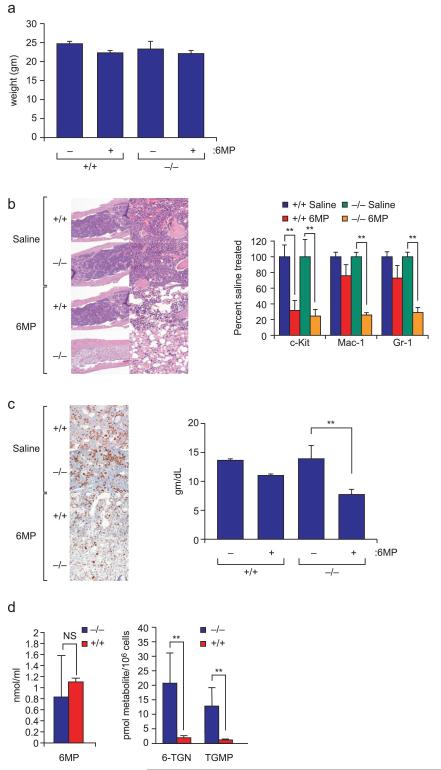
To determine the physical basis of the 6-MP toxicity (15) observed in vivo we evaluated Mrp4+/+ and Mrp4−/− mice after 5 daily doses (6-MP toxicity typically occurs at day 10 in Mrp4−/− mice) of 100mg/kg 6-MP (Fig.2). Although Mrp4 is expressed in the gastrointestinal tract, weight loss on Day 6 was nearly identical in 6-MP treated Mrp4−/− and Mrp4+/+ animals (Fig. 2a). However, on Day 6, bone marrow cellularity and cell number were dramatically reduced in 6-MP–treated Mrp4−/− mice (Fig. 2b, left panel). The reduction in nucleated hematopoietic cells was dependent upon Mrp4 gene dosage as Mrp4+/− bone marrow had more nucleated cells compared to Mrp4−/− (supplemental Fig. 1b). In a parallel experiment, we isolated bone marrow cells to immunophenotypically test progenitor cells for lineage-specific hematopoietic toxicity. After 5 days of 6-MP treatment (100 mg/kg daily), the granulocyte (Gr-1) and monocyte-macrophage (Mac-1) progenitors were reduced 71% and 74%, respectively, in Mrp4−/− animals as compared to untreated controls but were reduced less than 20% in Mrp4+/+ animals (Fig. 2b, right panel). 6-MP toxicity toward progenitor cells (identified by c-kit positive cells) was almost identical and consistent with suggestions that 6-MP is toxic to hematopoietic progenitors (15). Equivalent toxicity between Mrp4−/− and Mrp4+/+ progenitors might be expected because progenitor cells (CD34−KSL) have low levels of Mrp4 (see Fig. 1a). To ensure that Mrp4−/− mice were not inherently more susceptible to hematopoietic toxicity, we compared the effect of 25 and 50 mg/kg of etoposide (not an Mrp4 substrate). The myelotoxic effects of etoposide were indistinguishable in Mrp4−/−and Mrp4+/+ mice (data not shown).
Fig.2.
The hematopoietic toxicity of 6-MP is enhanced in Mrp4−/− mice. (a) weight loss is similar between Mrp4−/− and Mrp4+/+ animals after 5 consecutive days of 100 mg/kg 6-MP (n=5) bars indicate one standard deviation. (b) Hematoxylin and eosin–stained bone marrow from Mrp4−/− and Mrp4+/+ mice after daily treatment with saline or 100 mg/kg 6-MP for 6 days. (left panel) Proportions of early (c-Kit), monocyte-macrophage (Mac-1), and granulocyte (Gr-1) progenitors determined by FACS analysis in bone marrow from the same mice. **, P<0.05 (right panel). (c) Comparison of erythropoietic progenitors by immunohistochemical detection of the erythroid transcription factor GATA-1 (left panel). Blood hemoglobin concentration was significantly reduced in 6-MP–treated Mrp4−/− mice (**, P<0.05) (right panel). (d) Although plasma concentration of 6-MP did not differ in Mrp4−/− and Mrp4+/+ mice 22 h after intraperitoneal injection of 100 mg/kg 6-MP (left panel), bone marrow cellular concentration of the 6-MP nucleotide metabolites 6-TGN and 6-thioguanine monophosphate (6-TGMP) was significantly higher in Mrp4−/− than in Mrp4+/+ mice (right panel) (**, P <0.05). NS, not significant.
Cytotoxicity of 6-MP towards erythroid progenitors was greater in Mrp4−/− mice as revealed by markedly fewer GATA-1positive cells in Mrp4−/− than in Mrp4+/+ bone marrow (Fig. 2c, left panel). Moreover, erythroid progenitor reduction in 6-MP treated mice was paralleled by a 50% reduction in blood hemoglobin concentration (Fig. 2c, right panel). These results are consistent with the anemia and dramatically reduced erythrocytes observed in patients experiencing thiopurine toxicity (20). This demonstrates that the 6-MP sensitivity of erythroid progenitors (designated Terll9, see Fig. 1a) is related to the absence of Mrp4 function.
Thiopurine bone marrow cytotoxicity is dependent on the cellular concentration of 6-TGNs (19,21,23). We measured thiopurine nucleotide concentration in the bone marrow cells of mice 22 hours after a single 100-mg/kg dose of 6-MP. Although plasma 6-MP concentration did not differ significantly in Mrp4+/+ and Mrp4−/− mice (Fig. 2d, left panel), Mrp4−/− bone marrow cell 6-TGN concentration (20.7 ± 5.2 pmol/106 cells; n=4) was 10 times that observed in Mrp4+/+ bone marrow cells (1.7 ± 0.6 pmol/106 cells; n=4) (Fig. 2d,, right panel). Therefore, Mrp4 limits the bone marrow cell accumulation of thiopurine nucleotides.
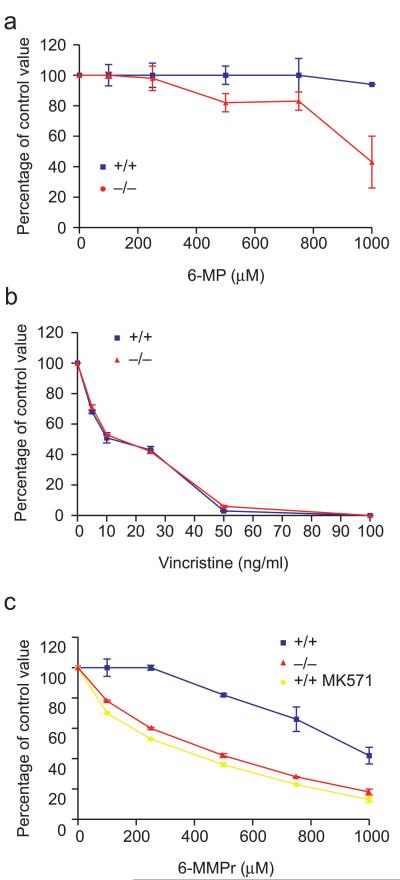
To directly test if Mrp4−/− bone marrow cells were more sensitive to thiopurines, we assayed the formation of myeloid and erythroid colonies in methylcellulose culture from the bone marrow cells of Mrp4−/− and Mrp4+/+ littermates (20) cultured with and without 6-MP (Fig 3a). Growth of either erythroid colonies in the presence of erythropoietin or myeloid colony-forming units was unimpaired by the absence of Mrp4 (not shown). However, addition of 6-MP to the Mrp4−/− hematopoietic cultures reduced both myeloid (Fig. 3a) and erythroid (not shown) colonies. In contrast, colony formation from Mrp4+/+ bone marrow was essentially unaffected by 6-MP (Fig. 3a). Mrp4−/− hematopoietic cells were not inherently more sensitive to cytotoxins as vincristine (which is not an Mrp4 substrate) reduced myeloid progenitor colony formation similarly in Mrp4−/− and Mrp4+/+ bone marrow cells (Fig. 3b).
Fig.3.
Mrp4 absence sensitizes myeloid progenitors to mercaptopurines. (a) Granulocyte-macrophage colony-forming cell (GM-CFC) assay of bone marrow from Mrp4+/+ (blue) and Mrp4−/− (red) mice cultured with increasing concentrations of 6-MP (b) vincristine and (c) 6-MMpr (yellow line indicates Mrp4+/+ cells treated with the Mrp4 inhibitor MK571).
Mrp4 preferentially transports methylated 6-MP nucleotides, therefore to bypass the small reduction in TPMT activity (see Fig 1d), we compared myeloid colony formation from Mrp4−/− and Mrp4+/+ bone marrow cells exposed to various concentrations of 6-methyl mercaptopurine riboside (6-MMPr). The Mrp4−/− cells were 3 times as sensitive to 6-MMPr as Mrp4+/+ cells (IC50, 291 vs. 917 M; P < 0.01, T-test) (Fig. 3c). To test if enhanced thiopurine sensitivity is due to Mrp4 transport we treated Mrp4+/+ cells with the Mrp4 inhibitor MK571. Importantly, when Mrp4 function was blocked with MK571, 6-MMPr toxicity was equivalent in Mrp4+/+ and Mrp4−/− myeloid progenitors. Therefore, loss of Mrp4 function by chemical inhibition or genetic ablation sensitizes myeloid progenitors to thiopurine toxicity.
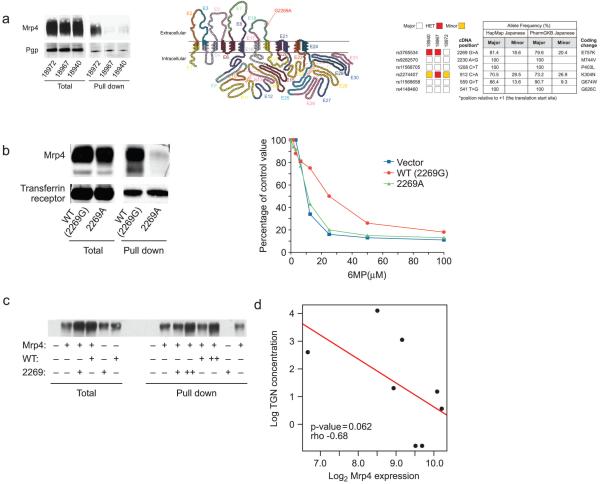
The heightened 6-MP sensitivity of Japanese patients in the absence of TPMT (2,3) remains unexplained and to our knowledge no clinical study has determined if a transporter is responsible. We performed our own genetic and database screens and identified a non-synonymous MRP4 SNP (rs3765534; G2269A nucleotide substitution E857K) that is widespread in the Japanese population (a weighted average of all alleles indicates greater than 18.7% allele frequency) but much less frequent in other populations (see Supplementary Table 1). To test this SNP’s role in MRP4 expression and function, we obtained HapMap lymphocyte cell lines created from Japanese individuals homozygous for wild-type or variant (rs3765534) MRP4 allele (Coriell Repository). These cell lines were screened to have similar growth characteristics because proliferation rate markedly affects thiopurine cell toxicity. An immunoblot of a total cell lysate shows comparable MRP4 expression in these cell lines (Fig.4a). In contrast, when we used surface biotinylation to determine if the surface membrane levels of MRP4 were comparable (labeled “pulldown”), the two cell lines homozygous for variant MRP4 showed markedly less MRP4 membrane localization (Fig. 4a, left panel). Importantly, this SNP is located in the coding region for the fourth extracellular loop (Fig. 4a, middle panel) which is intriguing because other transport proteins with amino acid substitutions in extracellular loops have impaired membrane localization (22).
Fig. 4.
A common Japanese MRP4 SNP reduces cell membrane localization. (a) Expression of MRP4 and Pgp in human lymphocyte cell lines homozygous for the wild-type (18972-2269G) or 2269A variant (18967, 18940) MRP4 alleles. Although protein expression was similar (left; 50 g loaded), surface biotinylation and pull-down demonstrates there was > five times less variant MRP4 (n=3 separate experiments) than wild-type MRP4 (n=3 separate experiments) on the cell surface (right, 300 g total protein) (left panel). Diagram of human MRP4 showing the predicted location of the nonsynonymous SNP (G2269A; E757k) in the fourth extracellular loop. Products of each exon are color-coded. Unexpectedly SIFT (sorting intolerant from tolerant) analysis predicted that the E867K amino acid substitution encoded by the SNP would not affect MRP4 function (middle panel). Six nonsynonymous SNPs in the coding region of MRP4 and their genotypes in the three lymphocyte cell lines. Table (right) shows the frequency of these SNPs in the Japanese population (right panel). (b) Expression of MRP4 and transferrin receptor in HEK293 cells expressing the 2269G or variant (2269A) MRP4 allele. Protein expression was similar (50 μg loaded). However, there was substantially less variant MRP4 (n=3) than wild-type MRP4 (n=3) in the cell membrane (300 μg total protein; mean ± SD, 14.6%±0.8% vs. 3.2%±0.4%; p< 0.0021) (left panel). HEK293 cells expressing the variant MRP4 allele (2269A) were more sensitive to 6-MP (EC50=9.7 M) than those expressing the wild-type allele (EC50=17.3 M; p < 0.05) (right panel). (c) HEK293 cells expressing the 2269G reference MRP4 allele were transfected with either empty plasmid (indicated by “ − ”) or reference MRP4 (indicated by WT) or the variant allele (2269). After labeling the surface with biotin and “pull-down” of membrane proteins the Mrp4 protein was identified by reaction with an anti-MRP4 antibody. (d) bone-marrow leukemia (ALL) cells were collected 20 hrs after the start of 6-MP therapy (1.0gm/m2 infused over 6 hours as previously described in detail (13) from 8 patients with acute lymphoblastic leukemia. TGN nucleotide levels were determined and MRP4 expression was determined by microarray analysis.
We next tested whether the reduced plasma membrane localization of the variant MRP4 affected 6-MP cytotoxicity. Only one variant cell line (NA18967) was tested; the other (NA18940) was excluded because its growth rate was consistently slower than the other two cell lines, preventing comparable measurement of 6-MP toxicity. The 18967 cells (harboring the G2269A substitution) were much more susceptible than the 18972 lymphocytes to 6-MP toxicity (EC50, 28.8 M vs. 99.5 M; P<0.0002), and the cell lines did not differ significantly in expression of the purine nucleoside uptake transporters SLC28a3, SLC29a2, and thiopurine nucleotide efflux transporter, MRP5 (P>0.05) (see supplemental Fig. 1c). These findings reinforce the idea that enhanced thiopurine sensitivity is linked to the reduced surface expression of MRP4.
However, analyzing the MRP4 haplotype of the Japanese HapMap lymphocytes revealed five additional non-synonymous SNPs other than rs3765534 that, although less frequent, might affect MRP4 membrane targeting (Fig. 4a, right panel). Therefore, to investigate the specific effect of the rs3765534 SNP (G2269A nucleotide substitution alone), we engineered it into a reference MRP4 allele and expressed it in HEK293 cell lines. Immunoblot analysis of total lysates revealed comparable levels of expression of the reference allele and variant MRP4 (Fig. 4b, left panel). However, surface biotinylation revealed a 5-fold reduction in cell surface expression of variant MRP4 allele compared to the reference allele (Fig. 4b). The reduced plasma membrane localization of the variant MRP4 was reflected by enhanced 6-MP cytotoxicity: the EC50 was 9.7 M, versus 17.3 M in cells expressing reference MRP4 allele (p < 0.05) and 8.6 M in cells containing empty vector (Fig. 4b, right panel). These studies were extended to show that the cells expressing the variant MRP4 allele were less able to exclude 6-MP metabolites compared to the reference MRP4 allele (not shown).
Transporters can form higher order complexes (dimers and multimers) therefore we tested if the rs3765534 variant MRP4 impairs the co-expression or membrane localization of the reference MRP4 allele. We transfected HEK293 cells stably expressing the reference MRP4 allele with either the reference MRP4 allele or the variant G2269A allele. The variant MRP4 allele had no effect on the amount of MRP4 reference allele localized to the plasma membrane (Fig 4c). This result demonstrates that co-expression of the variant MRP4 allele does not directly impair the membrane localization of the reference MRP4 allele and suggests that the variant MRP4 allele is unlikely to have a dominant negative role and impair function of the MRP4 reference allele.
Extension of these studies showed less TGNs in human leukemic lymphoblasts expressing a high level of MRP4 (Fig. 4d). This finding is consistent with recent studies indicating leukemia cell lines selected for 6-MP resistance overexpress MRP4 and accumulate less 6-MP and its metabolics (23). Thus, variation in the amount of Mrp4 and function in leukemias may be an additional factor to account for reduced therapeutic efficacy of thiopurines.
Our demonstration that MRP4 plays a strong role in protection against 6-MP hematopoietic toxicity reveals a new host factor to account for interindividual variation in thiopurine sensitivity/toxicity. This frequent, less functional, MRP4 allele may account for enhanced thiopurine sensitivity in some Japanese and may prompt the development of clinical studies to test the relationship between MRP4 alleles and thiopurine sensitivity. Moreover, given that the MRP4 gene is highly polymorphic (8) and transports many chemotherapeutic agents (e.g., camptothecins, methotrexate, etc), other MRP4 alleles are likely to contribute to unexplained chemotherapeutic toxicity. Therefore, these findings indicate that the impact of MRP4 variants on hematopoietic toxicity of other chemotherapeutic agents merits investigation as a mechanism that contributes to enhanced cytotoxicity.
Supplementary Material
Supplementary Fig. 1. (a) Gapdh cDNA expression in bone marrow cell lineages separated by fluorescence-activated cell sorting (Fig. 1a) was normalized to GAPDH mRNA expression as determined by RT-PCR (1). (b) Hematoxylin and eosin–stained bone marrow from Mrp4−/− , Mrp4 +/− and Mrp4+/+ mice after daily treatment with saline or 100 mg/kg 6-MP for 6 days. (c) Expression of the nucleoside transporter genes SLC28a3, SLC29a2, and MRP5 in Japanese Hapmap cell lines.
1 Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001 Sep;7(9):1028-34.
Acknowledgments
This work was supported by NIH grants GM60904, ESO58571, GM31304, CA21765, CA23097, the NIH/NIGMS Pharmacogenetics Research Network and Database (U01GM61374, http://pharmgkb.org) under grant U01 GM61393 and by the American Lebanese Syrian Associated Charities (ALSAC), and the Robert Bosch Foundation (MS). We thank Sharon Naron and Angela McArthur for editorial help, Jacqueline Mital for manuscript preparation, Dan Pan for expert cell culture, Erick Vasquez for invaluable help and advice on thiopurine nucleotide and de novo purine biosynthesis analysis, and Wenjian Yang for data analysis.
Reference List
- 1.Krynetski E, Evans WE. Drug methylation in cancer therapy: lessons from the TPMT polymorphism. Oncogene. 2003;22(47):7403–13. doi: 10.1038/sj.onc.1206944. [DOI] [PubMed] [Google Scholar]
- 2.Lennard L. TPMT in the treatment of Crohn’s disease with azathioprine. Gut. 2002;51(2):143–6. doi: 10.1136/gut.51.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando M, Ando Y, Hasegawa Y, Sekido Y, Shimokata K, Horibe K. Genetic polymorphisms of thiopurine S-methyltransferase and 6-mercaptopurine toxicity in Japanese children with acute lymphoblastic leukaemia. Pharmacogenetics. 2001;11(3):269–73. doi: 10.1097/00008571-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Naughton MA, Battaglia E, O’Brien S, Walport MJ, Botto M. Identification of thiopurine methyltransferase (TPMT) polymorphisms cannot predict myelosuppression in systemic lupus erythematosus patients taking azathioprine. Rheumatology (Oxford) 1999;38(7):640–4. doi: 10.1093/rheumatology/38.7.640. [DOI] [PubMed] [Google Scholar]
- 5.Schuetz JD, Connelly MC, Sun D, et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5(9):1048–51. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 6.Adachi M, Reid G, Schuetz JD. Therapeutic and biological importance of getting nucleotides out of cells: a case for the ABC transporters, MRP4 and 5. Adv Drug Deliv Rev. 2002;54(10):1333–42. doi: 10.1016/s0169-409x(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 7.Wielinga PR, Reid G, Challa EE, et al. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62(6):1321–31. doi: 10.1124/mol.62.6.1321. [DOI] [PubMed] [Google Scholar]
- 8.Saito S, Iida A, Sekine A, et al. Identification of 779 genetic variations in eight genes encoding members of the ATP-binding cassette, subfamily C (ABCC/MRP/CFTR. J Hum Genet. 2002;47(4):147–71. doi: 10.1007/s100380200018. [DOI] [PubMed] [Google Scholar]
- 9.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–21. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartford C, Vasquez E, Schwab M, et al. Differential effects of targeted disruption of thiopurine methyltransferase on mercaptopurine and thioguanine pharmacodynamics. Cancer Res. 2007;67(10):4965–72. doi: 10.1158/0008-5472.CAN-06-3508. [DOI] [PubMed] [Google Scholar]
- 11.Y Su, YY Hon, Y Chu, ME Van de Poll, Relling MV. Assay of 6-mercaptopurine and its metabolites in patient plasma by high-performance liquid chromatography with diode-array detection. J Chromatogr B Biomed Sci Appl. 1999;732(2):459–68. doi: 10.1016/s0378-4347(99)00311-4. [DOI] [PubMed] [Google Scholar]
- 12.Ross ME, Zhou X, Song G, et al. Classification of pediatric acute lymphoblastic leukemia by gene expression profiling. Blood. 2003 Oct 15;102(8):2951–9. doi: 10.1182/blood-2003-01-0338. [DOI] [PubMed] [Google Scholar]
- 13.Zaza G, Cheok M, Yang W, et al. Gene expression and thioguanine nucleotide disposition in acute lymphoblastic leukemia after in vivo mercaptopurine treatment. Blood. 2005 Sept 1;106(5):1778–85. doi: 10.1182/blood-2005-01-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross RJ, Bryson JS, Roszman TL. Immunologic disparity in the hypopituitary dwarf mouse. J Immunol. 1992;148(5):1347–52. [PubMed] [Google Scholar]
- 15.Philips FS, Sternberg SS, Hamilton S, Clarke DA. The toxic effects of 6-mercaptopurine and related compounds. Ann N Y Acad Sci. 1954;60(2):283–96. doi: 10.1111/j.1749-6632.1954.tb40019.x. [DOI] [PubMed] [Google Scholar]
- 16.Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5(1):63–84. doi: 10.2174/1389200043489162. [DOI] [PubMed] [Google Scholar]
- 17.Fotoohi AK, Wrabel A, Moshfegh A, Peterson C, Albertioni F. Molecular mechanisms underlying the enhanced sensitivity of thiopurine-resistant T-lymphoblastic cell lines to methyl mercaptopurineriboside. Biochem Pharmacol. 72(7):816–23. doi: 10.1016/j.bcp.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Iwaki T, Giga-Hama Y, Takegawa K. A survey of all 11 ABC transporters in fission yeast: two novel ABC transporters are required for red pigment accumulation in a Schizosaccharomyces pombe adenine biosynthetic mutant. Microbiology. 2006;152(Pt 8):2309–21. doi: 10.1099/mic.0.28952-0. [DOI] [PubMed] [Google Scholar]
- 19.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–21. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal A, Parrott NR, Riad HN, Augustine T. Azathioprine-induced pure red cell aplasia: case report and review. Transplant Proc. 2004;36(9):2689–91. doi: 10.1016/j.transproceed.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy P, Ross DD, Nakanishi T, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279(23):24218–25. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 22.Hahn MK, Mazei-Robison MS, Blakely RD. Single nucleotide polymorphisms in the human norepinephrine transporter gene affect expression, trafficking, antidepressant interaction, and protein kinase C regulation. Mol Pharmacol. 2005;68(2):457–66. doi: 10.1124/mol.105.011270. [DOI] [PubMed] [Google Scholar]
- 23.Peng XX, Shi Z, Damaraju VL, et al. Up-regulation of MRP4 and down-regulation of influx transporters in human leukemic cells with acquired resistance to 6-mercaptopurine. Leuk Res. 2008;32(5):799–809. doi: 10.1016/j.leukres.2007.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. (a) Gapdh cDNA expression in bone marrow cell lineages separated by fluorescence-activated cell sorting (Fig. 1a) was normalized to GAPDH mRNA expression as determined by RT-PCR (1). (b) Hematoxylin and eosin–stained bone marrow from Mrp4−/− , Mrp4 +/− and Mrp4+/+ mice after daily treatment with saline or 100 mg/kg 6-MP for 6 days. (c) Expression of the nucleoside transporter genes SLC28a3, SLC29a2, and MRP5 in Japanese Hapmap cell lines.
1 Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001 Sep;7(9):1028-34.