Kaushik et al. EMBO reports, this issue doi:; DOI: 10.1038/embor.2011.260
Macroautophagy, which I will call autophagy, is a critical process that degrades bulk cytoplasm, including organelles, protein oligomers and a range of selective substrates. It has been linked with diverse physiological and disease-associated functions, including the removal of certain bacteria, protein oligomers associated with neurodegenerative diseases and dysfunctional mitochondria [1]. However, the primordial role of autophagy—conserved from yeast to mammals—appears to be its ability to provide nutrients to starving cells by releasing building blocks, such as amino acids and free fatty acids, obtained from macromolecular degradation. In yeast, autophagy deficiency enhances death in starvation conditions [2], and in mice it causes death from starvation in the early neonatal period [3,4]. Two recent articles from the Singh group—one of them in this issue of EMBO reports—also implicate autophagy in central appetite regulation [5,6].
Autophagy seems to decline with age in the liver [7], and it has thus been assumed that autophagy declines with age in all tissues, but this has not been tested rigorously in organs such as the brain. Conversely, specific autophagy upregulation in Caenorhabditis elegans and Drosophila extends lifespan, and drugs that induce autophagy—but also perturb unrelated processes, such as rapamycin—promote longevity in rodents [8].
Autophagy literally means self-eating, and it is therefore interesting to see that this cellular ‘self-eating' has systemic roles in mammalian appetite control. The control of appetite is influenced by central regulators, including various hormones and neurotransmitters, and peripheral regulators, including hormones, glucose and free fatty acids [9]. Autophagy probably has peripheral roles in appetite and energy balance, as it regulates lipolysis and free fatty acid release [10]. Furthermore, Singh and colleagues have recently implicated autophagy in central appetite regulation [5,6].
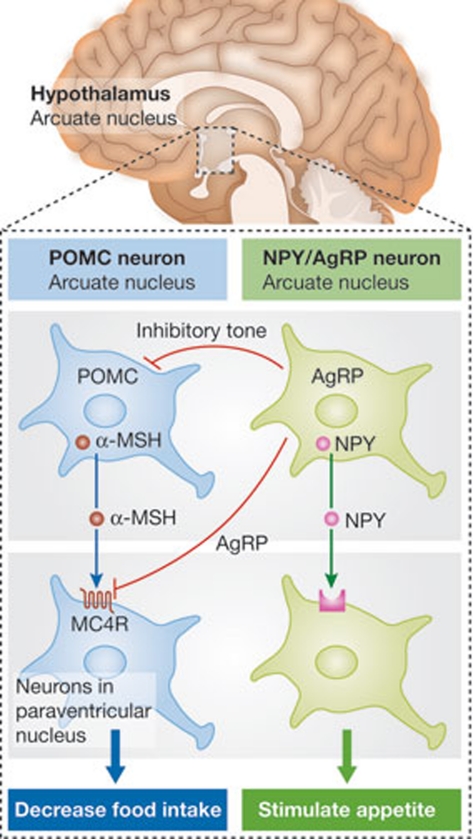
The arcuate nucleus in the hypothalamus has received extensive attention as an integrator and regulator of energy homeostasis and appetite. Through its proximity to the median eminence, which is characterized by an incomplete blood–brain barrier, these neurons rapidly sense metabolic fluctuations in the blood. There are two different neuronal populations in the arcuate nucleus, which appear to have complementary effects on appetite (Fig 1). The proopiomelanocortin (POMC) neurons produce the neuropeptide precursor POMC, which is cleaved to form α-melanocyte stimulating hormone (α-MSH), among several other products. The α-MSH secreted from these neurons activates melanocortin 4 receptors on target neurons in the paraventricular nucleus of the hypothalamus, which ultimately reduce food intake. The second group of neurons contain neuropeptide Y (NPY) and Agouti-related peptide (AgRP). Secreted NPY binds to downstream neuronal receptors and stimulates appetite. AgRP blocks the ability of α-MSH to activate melanocortin 4 receptors [11]. Furthermore, AgRP neurons inhibit POMC neurons [9].
Figure 1.
Schematic diagram illustrating the complementary roles of POMC and NPY/AgRP neurons in appetite control. AgRP, Agouti-related peptide; MC4R, melanocortin 4 receptor; α-MSH, α-melanocyte stimulating hormone; NPY, neuropeptide Y; POMC, proopiomelanocortin.
The first study from Singh's group started by showing that starvation induces autophagy in the hypothalamus [5]. This finding alone merits some comment. Autophagy is frequently assessed by using phosphatidylethanolamine-conjugated Atg8/LC3 (LC3-II), which is specifically associated with autophagosomes and autolysosomes. LC3-II levels on western blot and the number of LC3-positive vesicles strongly correlate with the number of autophagosomes [1]. To assess whether LC3-II formation is altered by a perturbation, its level can be assessed in the presence of lysosomal inhibitors, which inhibit LC3-II degradation by blocking autophagosome–lysosome fusion [12]. Therefore, differences in LC3-II levels in response to a particular perturbation in the presence of lysosomal inhibitors reflect changes in autophagosome synthesis. An earlier study using GFP-LC3 suggested that autophagy was not upregulated in the brains of starved mice, compared with other tissues where this did occur [13]. However, this study only measured steady state levels of autophagosomes and was performed before the need for lysosomal inhibitors was appreciated. Subsequent work has shown rapid flux of autophagosomes to lysosomes in primary neurons, which might confound analyses without lysosomal inhibitors [14]. Thus, the data of the Singh group—showing that autophagy is upregulated in the brain by a range of methods including lysosomal inhibitors [5]—address an important issue in the field and corroborate another recent study that examined this question by using sophisticated imaging methods [15].
“…decreasing autophagy with ageing in POMC neurons could contribute to the metabolic problems associated with age”
Singh and colleagues then analysed mice that have a specific knockout of the autophagy gene Atg7 in AgRP neurons [5]. Although fasting increases AgRP mRNA and protein levels in normal mice, these changes were not seen in the knockout mice. AgRP neurons provide inhibitory signals to POMC neurons, and Kaushik and colleagues found that the AgRP-specific Atg7 knockout mice had higher levels of POMC and α-MSH, compared with the normal mice. This indicated that starvation regulates appetite in a manner that is partly dependent on autophagy. The authors suggested that the peripheral free fatty acids released during starvation induce autophagy by activating AMP-activated protein kinase (AMPK), a known positive regulator of autophagy. This, in turn, enhances degradation of hypothalamic lipids and increases endogenous intracellular free fatty acid concentrations. The increased intracellular free fatty acids upregulate AgRP mRNA and protein expression. As AgRP normally inhibits POMC/α-MSH production in target neurons, a defect in AgRP responses in the autophagy-null AgRP neurons results in higher α-MSH levels, which could account for the decreased mouse bodyweight.
In follow-up work, Singh's group have now studied the effects of inhibiting autophagy in POMC neurons, again using Atg7 deletion [6]. These mice, in contrast to the AgRP autophagy knockouts, are obese. This might be accounted for, in part, by an increase in POMC preprotein levels and its cleavage product adrenocorticotropic hormone in the knockout POMC neurons, which is associated with a failure to generate α-MSH. Interestingly, these POMC autophagy knockout mice have impaired peripheral lipolysis in response to starvation, which the authors suggest might be due to reduced central sympathetic tone to the periphery from the POMC neurons. In addition, POMC-neuron-specific Atg7 knockout mice have impaired glucose tolerance.
This new study raises several interesting issues. How does the autophagy defect in the POMC neurons alter the cleavage pattern of POMC? Is this modulated within the physiological range of autophagy activity fluctuations in response to diet and starvation? Importantly, in vivo, autophagy might fluctuate similarly (or possibly differently) in POMC and AgRP neurons in response to diet and/or starvation. Given the tight interrelation of these neurons, how does this affect their overall response to appetite regulation in wild-type animals?
Finally, the study also shows that hypothalamic autophagosome formation is decreased in older mice. To my knowledge, this is the first such demonstration of this phenomenon in the brain. The older mice phenocopied aspects of the POMC-neuron autophagy null mice—increased hypothalamic POMC preprotein and ACTH and decreased α-MSH, along with similar adiposity and lipolytic defects, compared with young mice. These data are provocative from several perspectives. In the context of metabolism, it is tantalizing to consider that decreasing autophagy with ageing in POMC neurons could contribute to the metabolic problems associated with ageing. Again, this model considers the POMC neurons in isolation, and it would be important to understand how reduced autophagy in aged AgRP neurons counterbalances this situation. In a more general sense, the data strongly support the concept that neuronal autophagy might decline with age.
Autophagy is a major clearance route for many mutant, aggregate-prone intracytoplasmic proteins that cause neurodegenerative disease, such as tau (Alzheimer disease), α-synuclein (Parkinson disease), and huntingtin (Huntington disease), and the risk of these diseases is age-dependent [1]. Thus, it is tempting to suggest that the dramatic age-related risks for these diseases could be largely due to decreased neuronal capacity of degrading these toxic proteins. Neurodegenerative pathology and age-related metabolic abonormalities might be related—some of the metabolic disturbances that occur in humans with age could be due to the accumulation of such toxic proteins. High levels of these proteins are seen in many people who do not have, or who have not yet developed, neurodegenerative diseases, as many of them start to accumulate decades before any sign of disease. These proteins might alter metabolism and appetite either directly by affecting target neurons, or by influencing hormonal and neurotransmitter inputs into such neurons.
References
- Ravikumar B et al. (2010) Physiol Rev 90: 1383–1435 [DOI] [PubMed] [Google Scholar]
- Tsukada M, Ohsumi Y (1993) FEBS Lett 333: 169–174 [DOI] [PubMed] [Google Scholar]
- Kuma A et al. (2004) Nature 432: 1032–1036 [DOI] [PubMed] [Google Scholar]
- Komatsu M et al. (2005) J Cell Biol 169: 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S et al. (2011) Cell Metab 14: 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S et al. (2012) EMBO Rep [Epub ahead of print] January 17 2012. doi:; DOI: 10.1038/embor.2011.260 [DOI] [Google Scholar]
- Cuervo AM et al. (2005) Autophagy 1: 131–140 [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC et al. (2011) Cell 146: 682–695 [DOI] [PubMed] [Google Scholar]
- Garfield AS et al. (2009) Trends Endocrinol Metab 20: 203–215 [DOI] [PubMed] [Google Scholar]
- Singh R et al. (2009) Nature 458: 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BB et al. (2009) J Physiol 587: 5305–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC et al. (2009) Autophagy 5: 585–589 [DOI] [PubMed] [Google Scholar]
- Mizushima N et al. (2004) Mol Biol Cell 15: 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland B et al. (2008) J Neurosci 28: 6926–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M et al. (2010) Autophagy 6: 702–710 [DOI] [PMC free article] [PubMed] [Google Scholar]