Abstract
Inhibition of proliferation by cell-to-cell contact is essential for tissue organization, and its disruption contributes to tumorigenesis. The FERM domain protein Merlin, encoded by the NF2 tumour suppressor gene, is an important mediator of contact inhibition. Merlin was thought to inhibit mitogenic signalling and activate the Hippo pathway by interacting with diverse target-effectors at or near the plasma membrane. However, recent studies highlight that Merlin pleiotropically affects signalling by migrating into the nucleus and inducing a growth-suppressive programme of gene expression through its direct inhibition of the CRL4DCAF1 E3 ubiquitin ligase. In addition, Merlin promotes the establishment of epithelial adhesion and polarity by recruiting Par3 and aPKC to E-cadherin-dependent junctions, and by ensuring the assembly of tight junctions. These recent advances suggest that Merlin acts at the cell cortex and in the nucleus in a similar, albeit antithetic, manner to the oncogene β-catenin.
Keywords: Merlin, type 2 neurofibromatosis, contact inhibition, tumour suppression, Hippo
See Glossary for abbreviations used in this article.
Glossary.
4E-BP1 eukaryotic translation initiation factor 4E-binding protein 1
Akt protein kinase B
AMP adenosine monophosphate
aPKC atypical PKC
AXL AXL receptor tyrosine kinase
Cdc42 cell division control protein 42 homologue
CRL4 cullin-ring E3 ligase 4
CRM1 chromosome region maintenance protein 1
DCAF1 DDB1- and CUL4-associated factor 1
DDB1 DNA damage-binding protein 1
EGFR epidermal growth factor receptor
ErbB2/3 v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2/3
Erbin Erbb2 interacting protein
ERK extracellular-signal-regulated kinase
FAK focal adhesion kinase
FERM 4.1 protein/Ezrin/Radixin/Moesin
HEK-293 human embryonic kidney 293
ICAM intercellular adhesion molecule
IGF1R insulin-like growth factor 1 receptor
Kibra kidney and brain protein
Lats1/2 large tumour suppressor 1/2
MDCK Madine–Darby kidney cancer
MEFs mouse embryonic fibroblasts
Moesin membrane-organizing extension spike protein
MST1/2 macrophage stimulating 1/2
mTORC1/2 mammalian target of rapamycin complex 1/2
MYPT1 myosin phosphatase targeting subunit 1
PAK p21-activated kinase
Pals1 protein associated with Lin-7 1
Par3 partitioning defective 3 homologue
Patj Pals1-associated tight junction protein
PDGFR platelet-derived growth factor receptors
PI3K phosphatidylinositol 3-kinase
PIKE-L phosphoinositide 3-kinase enhancer isoform 1
PIP2 phosphatidylinositol 4,5-bisphosphate
PKA protein kinase A
PKCα protein kinase C alpha type
PP1δ serine/threonine-protein phosphatase PP1-δ catalytic subunit
PTEN phosphatase and tensin homologue
Rac Ras-related C3 botulinum toxin substrate
Ras rat sarcoma
Rich1 RhoGAP interacting with CIP4 homologues 1
Roc1/Rbx1 RING box protein 1
S6K1 S6 kinase 1
TAZ transcriptional coactivator with PDZ-binding motif
TEAD TEA domain family member
TGF-β transforming growth factor-β
TSC1/2 tuberous sclerosis complex 1/2
YAP Yes-associated protein
Introduction
Normal cells cease to proliferate when they come into contact with each other and assemble stable intercellular junctions. Contact inhibition of proliferation is essential for tissue organization and its loss is a characteristic of cancer [1]. Since its discovery as the tumour suppressor that is inactivated in neurofibromatosis type 2 (NF2) almost two decades ago [2,3], the FERM domain protein Merlin has emerged as a major effector of contact inhibition [47]. Furthermore, genetic studies in mice have shown that Merlin has a relatively broad tumour suppressor function (Sidebar A). In addition to NF2, loss of Merlin function contributes to the development of sporadic meningiomas, ependymomas and schwannomas, a significant fraction of malignant pleural mesotheliomas, and a small subset of renal cell carcinomas, melanomas, glioblastomas and colorectal cancers in humans (Sidebar B; [8,14]). As for other oncogenic mutations, it remains unclear why loss of Merlin contributes to tumorigenesis in some but not other tissues (Sidebar C). Cell-type-specificity in the wiring of signalling pathways, previous accumulation of cooperating mutations and/or differences in the stromal microenvironment could all contribute to the tissue specificity of the phenotype.
Sidebar A | Mouse models of NF2.
Nf2-knockout mice succumb in utero, whereas heterozygous Nf2 mutant mice develop multiple malignancies, especially if an allele of p53 is simultaneously inactivated. The tumours arising in Nf2 mutant mice include hepatocellular carcinomas and osteosarcomas among others, but not schwannomas or meningiomas, suggesting that Merlin is a haploinsufficient tumour suppressor in several tissues [117]. Conditional biallelic inactivation of Nf2 in Schwann cells leads to Schwann cell hyperplasia and schwannoma formation [118], mimicking human neurofibromatosis type 2, which results from loss of heterozygosity at the Nf2 locus [8]. Furthermore, biallelic inactivation of Nf2 in arachnoidal cells leads to the formation of meningiomas, which have significant histological similarity to the corresponding human tumours [119]. In a similar fashion, Nf2+/− mice have increased sensitivity to the carcinogenic effect of inhaled asbestos [120], and conditional deletion of Nf2 in mesothelial cells cooperates with loss of Ink4a/Arf and p53 to drive malignant mesothelioma [121]. Biallelic loss of Merlin in the liver was recently shown to result in hepatomegaly and formation of malignant tumours [17,18]. Although the issue has not been completely resolved, it seems that biallelic Nf2 loss in the liver leads to the expansion of a progenitor population able to differentiate at least partly along the ductal lineage [99]. The complete penetrance observed in this model indicates that Merlin is a potent regulator of liver size and tumour suppression, and future studies using this model could provide great insight into the normal biological role of Merlin and its tumour suppressor activity.
Sidebar B | Clinical features of NF2.
Neurofibromatosis type 2 (NF2) is an autosomal dominant genetic disorder with an incidence of approximately 1 in 40,000. It is caused by inactivation of the NF2 gene located on chromosome 22q [2,3]. NF2 patients develop multiple central nervous system (CNS) and peripheral nervous system (PNS) tumours. The locations and types of CNS and PNS tumours seen in these patients are highly specific. Schwannomas arise from Schwann cells that form the myelin sheath surrounding the sensory and motor neurons. The hallmark of NF2 is the development of bilateral vestibular schwannomas (VS), which arise at the vestibular branch of the VIIIth cranial nerve. In addition to VS, most NF2 patients develop schwannomas in other locations, such as other cranial nerves and peripheral nerves, including nerve roots. Most NF2 patients experience progressive hearing loss in adolescence or young adulthood due to bilateral VS. Life-threatening neurological complications occur when these tumours reach a critical size. In addition, schwannomas involving other cranial nerves can impair swallowing, vision and facial function. Other CNS and PNS tumours seen in NF2 patients include meningiomas, which arise from arachnoid cap cells, and ependymomas, which arise from ependymal cells lining the ventricles and central spinal canal. There is a clear association between genotype and phenotype in NF2 patients, with nonsense/frameshift mutations being associated with earlier onset of symptoms, larger tumour burden and shorter life expectancy [122]. Sporadic VS, which occur in non-NF2 patients, consistently lack expression of detectable Merlin [123], and genetic inactivation of the Nf2 gene also occurs in the majority of sporadic meningiomas [124,126], indicating similarities in tumour biology between sporadic and NF2-related VS and meningiomas. Sporadic VS have an incidence of roughly 3,000 per year in the USA, which seems to be rising [127], and sporadic meningiomas are the most common type of brain tumour, accounting for approximately 25% of primary intracranial tumours in the USA [128]. Despite major advances in neuroimaging and neurosurgical techniques over the past decades—including microsurgery and stereotaxis—the neurosurgical management of NF2 patients remains challenging. The identification of effective drugs to treat these patients would also be relevant for large patient populations without NF2, as there is no known effective treatment option for unresectable or progressive sporadic meningiomas.
Although Nf2-knockout embryos deteriorate at an early developmental stage due to defects in extraembryonic structures [15], tissue-specific ablation experiments have provided insight into some of the developmental roles of Merlin. Deletion of Nf2 in the skin causes defects in epithelial adhesion and polarity that disrupt its barrier function [16]. Inactivation of Nf2 in the liver causes a large expansion of progenitor cells, suggesting that Merlin inhibits stem-cell renewal or amplification [17,18]. Intriguingly, deletion of Nf2 in the entire haematopoietic compartment leads to an expansion of progenitor cells that is largely secondary to the expansion of their perivascular niche [19], suggesting that Merlin can regulate stem-cell expansion by a non-cell-autonomous mechanism.
Merlin has significant sequence homology to members of the Ezrin/Radixin/Moesin (ERM) family of proteins, which in their open conformation link various cell-adhesion receptors to the cortical actin cytoskeleton [20]. On this basis, it has been argued that Merlin mediates both contact inhibition and tumour suppression by directly modulating mitogenic signal transduction at or near the plasma membrane [21,22]. Analysis of various cell types indicated that Merlin can potentially affect a variety of mitogenic pathways, such as Rac–PAK signalling [7,23,25], activation of mTORC1 independently of Akt [26,27], the EGFR–Ras–ERK pathway, the PI3K–Akt pathway and FAK–Src signalling [28,31]. In addition, genetic studies in Drosophila and mice showed that Merlin contributes to the activation of the Hippo tumour-suppressor pathway [18,32,33].
Against the backdrop of this rich biology, recent studies have revealed that Merlin can interact with α-catenin and Par3 at nascent adherens junctions [16], as well as with the scaffold and signalling protein Angiomotin at tight junctions [34]. In addition, it has become clear that Merlin translocates to the nucleus to modify gene expression through inhibition of the E3 ubiquitin ligase CRL4DCAF1 [35]. In this review, we discuss these recent results and the models of Merlin function they suggest, in an effort to provide a framework for future studies.
Merlin structure and modifications
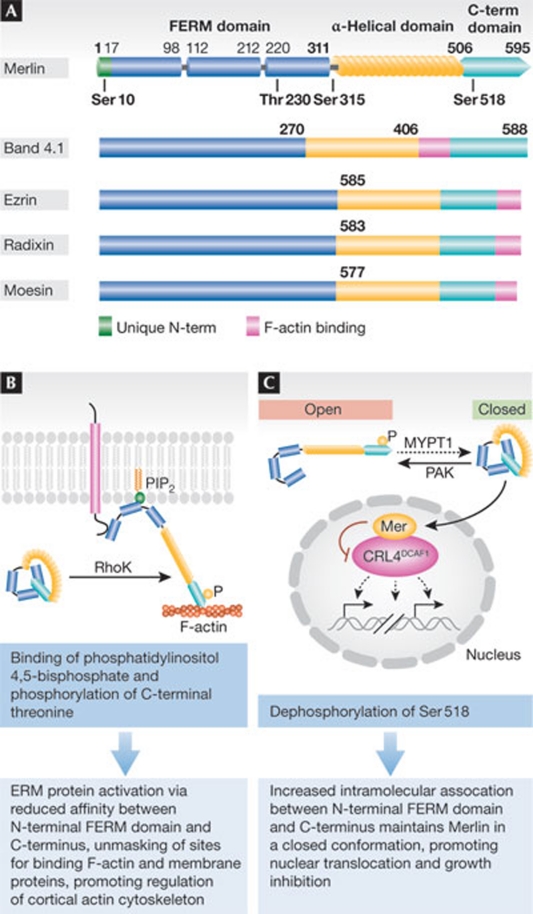
ERM proteins consist of an amino-terminal FERM domain followed by a coiled-coil segment and a hydrophilic tail [20]. Although Merlin has the same domain organization and considerable sequence homology to canonical ERM proteins, it also contains unique sequence motifs [36,37]. In particular, the FERM domain of Merlin contains an evolutionarily conserved Blue box motif (residues 177–183), which is absent in canonical ERM proteins, whereas the carboxy-terminal domain of the protein lacks a canonical actin-binding motif that is present in all ERM proteins. In addition, Merlin has an extended 17 amino-acid-long N-terminal segment that is not found in other ERM proteins (Fig 1a).
Figure 1. Merlin and ERM protein domain organization and phosphoregulation.
(A) Merlin and other canonical ERM proteins have similar domain organizations consisting of an amino-terminal (N-term) FERM domain that is divided into three subdomains, an α-helical coiled-coil domain and a carboxy-terminal (C-term) hydrophilic tail. Canonical ERM proteins contain an actin-binding C-terminal ERM-associated domain (C-ERMAD; pink), whereas Merlin does not. It also has an extended N-terminal motif (green) unique among ERM proteins, illustrating its divergent structure with respect to other ERM proteins. Merlin phosphorylation sites are indicated. (B) Canonical ERM proteins are maintained in an inactive state by intramolecular interaction between the C-terminal tail and FERM domain. Phosphorylation of a C-terminal threonine by Rho kinase—which might be aided by ERM protein recruitment to membrane regions rich in phosphatidylinositol 4,5-bisphosphate—activates the protein by disrupting the head-to-tail interaction. (C) Conversely, Merlin's dephosphorylated and closed form is active and functions in tumour suppression and contact inhibition. Phosphorylation by PAK and PKA at Ser 518 renders the protein inactive in its putatively open form. ERM, Ezrin/Radixin/Moesin; FERM, 4.1 protein/Ezrin/Radixin/Moesin; MYPT1, myosin phosphatase targeting subunit 1; PAK, p21-activated kinase; PKA, protein kinase A; PIP2, phosphatidylinositol 4,5-bisphosphate; RhoK, Ras homologue gene family, member K.
Canonical ERM proteins are maintained in a dormant state by an intramolecular association between the FERM domain and the C-terminal tail [38,39,40]. In response to upstream activation of Rho, Rho kinase phosphorylates a threonine residue in the C-terminal domain of ERM proteins, disrupting the head-to-tail association that maintains the closed conformation (Fig 1b). Once ERM proteins adopt the open conformation, their FERM domain can associate with the cytoplasmic segment of cell-adhesion receptors—such as CD44 and ICAM—and their C-terminal domain can interact with actin filaments, regulating the organization of the cortical cytoskeleton [38,41,42].
Biochemical and mutational studies suggest that Merlin undergoes a similar conformational transition in response to PAK-mediated phosphorylation of Ser 518 (Fig 1c; [43]). However, recent evidence suggests that additional post-translational modifications might be required to fully disengage the α-helical segment of Merlin from the FERM domain [44]. The putatively open form of Merlin might associate with the cytoskeleton by forming head-to-tail heterodimers with canonical ERM proteins [45,46,47], as well as through additional potential mechanisms [48], whereas the closed form of Merlin seems to mediate growth inhibition in vitro and is thereby considered active [25,43,49,50,51]. Several lines of evidence support the latter point. Mutation of Ser 518 to alanine enhances the growth inhibitory activity of Merlin, whereas its change to aspartic acid eliminates this activity [52]. Overexpression of a Blue box mutant form of Merlin—presumed to be constitutively open [37,39,40]—induces overproliferation in the wing of Drosophila, and the murine analogue promotes cellular transformation in vitro, both presumably through a dominant-negative mechanism [4,53]. Finally, numerous missense mutations detected in patients affected by NF2 map to the F2 segment of the FERM domain and the most parsimonious truncation mutants lack only the C-terminal segment, which is predicted to interact with the F2 segment (Fig 2). Furthermore, structural considerations based on the analysis of the crystal structure of the closed form of Moesin—which is the only closed structure available—suggest that virtually all pathogenic missense mutations found in NF2, including the few affecting the α-helical portion of Merlin, disrupt the extended surface that mediates the interaction of the FERM domain with the C-terminal segment of the protein [39,40]. Interestingly, a recent study found that Merlin isoform 2—which lacks the five C-terminal residues found in the canonical Merlin isoform 1 necessary for interdomain binding in vitro [50]—suppresses Nf2−/− Schwann cell proliferation to the same extent as Merlin isoform 1 [54]. As these results contradict previous findings [50,55], further investigation is required to understand how Merlin's conformation affects its activity and how Merlin isoform 2 functions (Sidebar C).
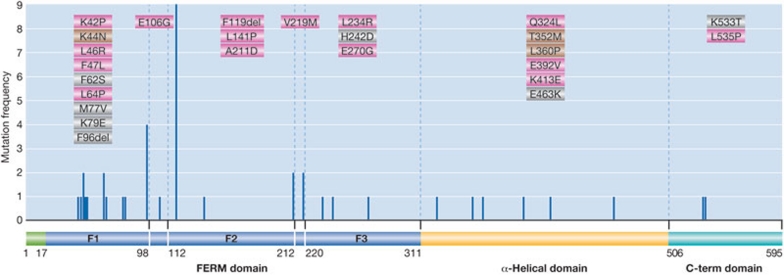
Figure 2. Merlin missense mutations and single residue deletions in NF2.
The position, frequency and type of mutation are plotted on a schematic diagram of Merlin. The pathogenicity of mutations supported by biological evidence is shown in orange, by genetic evidence in blue and by both biological and genetic evidence in green. These mutations either correlate with disease in multiple members of a family, are found in two or more unrelated patients and/or there is biological evidence of their pathogenicity. Data were obtained from Ahronowitz et al, 2007 [18] and Li et al, 2010 [35]. FERM, 4.1 protein/Ezrin/Radixin/Moesin.
Sidebar C | In need of answers.
What is the atomic structure of unphosphorylated, closed Merlin?
What is the impact of tumour-derived missense mutations?
Which conformational changes underlie the transition from the closed to the putatively open form of Merlin?
Which post-translational events drive these conformational changes?
What are the mechanisms that govern the association of Merlin with the cortical cytoskeleton at various subcellular locations?
Which mechanisms govern Merlin nuclear entry and exit?
Does Merlin mediate contact inhibition and tumour suppression through distinct or overlapping mechanisms?
How does Merlin contribute to each of these two functions?
Why does Merlin deficiency drive tumour development in only a subset of tissue types and anatomical locations?
Which oncogenic mutations cooperate with inactivation of Merlin to drive tumorigenesis in sensitive tissues?
The levels of closed, active Merlin increase substantially in cells undergoing growth arrest due to contact inhibition, loss of matrix adhesion, deprivation of growth factors or exposure to hyaluronic acid [6,56], suggesting that Merlin integrates various anti-mitogenic signals (Fig 3a). Promitogenic signals—which are initiated by integrins and receptor tyrosine kinases and transduced by Cdc42 and Rac—activate PAK, which directly phosphorylates Merlin at Ser 518. This phosphorylation disrupts the binding between the N-terminal FERM domain and the C-terminal tail, thereby inactivating Merlin [7,25]. Conversely, engagement of cadherins or loss of mitogenic signalling inactivates PAK, leading to an accumulation of the closed form of Merlin [56]. Although most studies have placed PAK upstream from Merlin, there is also evidence suggesting that Merlin can suppress PAK activation [24,57]. Recent studies suggest that this potential feed-forward mechanism is restricted to epithelial cells that express Erbin. Binding to Erbin allows Merlin to inactivate PAK2, disabling one branch of non-canonical TGF-β signalling [58]. Ser 518 can also be phosphorylated by PKA, suggesting that Merlin can also be inactivated by the cyclic AMP–PKA pathway, a signalling axis that regulates gene expression, cell growth and cell cycle progression in Schwann cells [59]. Increases in dephosphorylated Merlin might be a result not only of PAK inhibition but also of the activation of a Merlin phosphatase—such as MYPT1-PP1δ—which dephosphorylates Ser 518, thus activating Merlin [26]. Overexpression of CPI-17, an MYPT1 cellular inhibitor, induces neoplastic transformation in vitro, underscoring the importance of Ser 518 dephosphorylation [29]. However, whether MYPT1-mediated dephosphorylation of Merlin is necessary and sufficient for activation of Merlin in vivo remains to be determined.
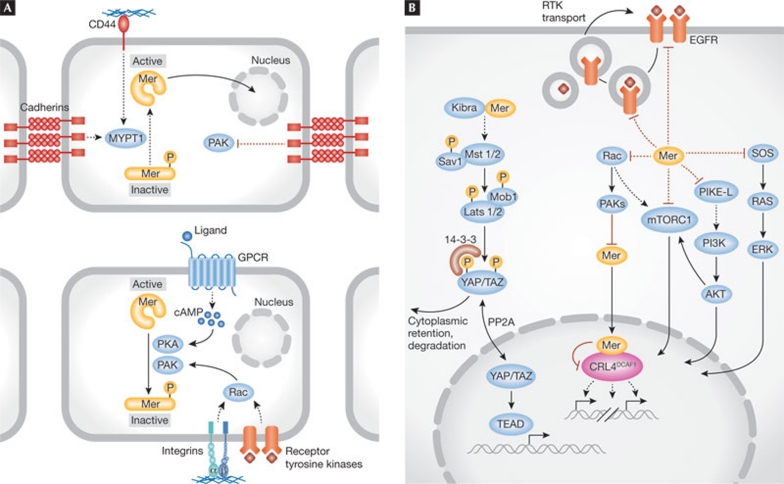
Figure 3. Merlin activation and downstream signalling.
The convergence of several upstream adhesion receptors regulates Merlin (Mer) activation and subsequently controls downstream mitogenic pathways. (A) The assembly of cell-to-cell adhesions and CD44 activation by hyaluronic-acid-rich matrix activates MYPT1, which dephosphorylates Merlin Ser 518 and maintains it in a closed and active conformation. Conversely, in sparse cells exposed to growth factors, integrins and receptor tyrosine kinases activate PAK, phosphorylating Ser 518. PKA—activated by increased cAMP—also phosphorylates Ser 518. (B) Merlin can affect a variety of mitogenic signalling pathways, including Rac–PAK signalling, mTORC1, EGFR–Ras–ERK and the PI3K–Akt pathway. In addition, Merlin contributes to the activation of the Hippo tumour-suppressor pathway. The active form of Merlin can enter the nucleus, bind to and inactivate the E3 ubiquitin ligase CRL4DCAF1. Akt, protein kinase B; cAMP, cyclic AMP; CRL4, cullin-ring E3 ligase 4; DCAF1, DDB1- and CUL4-associated factor 1; EGFR, epidermal growth factor receptor; ERK, extracellular-signal-regulated kinase; mTORC1, mammalian target of Rapamycin complex 1; MYPT1, myosin phosphatase targeting subunit 1; PAK, p21-activated kinase; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; Rac, Ras-related C3 botulinum toxin substrate; Ras, rat sarcoma.
Phosphorylation at Ser 518 seems to be the primary post-translational modification that drives the activation–inactivation cycle. Nevertheless, additional phosphorylation events or other post-translational modifications might be required to disengage the extended contacts that the α-helical and C-terminal segments of Merlin form with the FERM domain in the closed conformation (Sidebar C; [39]). In agreement with this hypothesis, band-shift experiments suggest that Merlin is post-translationally modified at multiple sites in response to mitogenic stimuli [56]. Overexpression studies suggest that AKT can phosphorylate Merlin at Thr 230 and Ser 315, stabilizing the open conformation and disrupting binding to PIKE-L—a GTPase that binds to PI3K and enhances its activity [60]. These observations suggest that constitutive activation of PI3K–Akt signalling, as occurs in cells lacking PTEN or carrying activating mutations in PI3K, could lead to inactivation of Merlin and potentially eliminate a negative-feedback loop that restrains the activation of PI3K. In addition, Akt-mediated phosphorylation has been proposed to promote Merlin ubiquitination and proteasome-mediated degradation [60]. However, the significance of these events remains unclear because cycloheximide chase experiments suggest that endogenous Merlin is not significantly degraded by the ubiquitin–proteasome system (UPS; [35]). Finally, PKA phosphorylation of Ser 10 was recently shown to alter the organization of the actin cytoskeleton without affecting Ser 518 phosphorylation [61].
Merlin localization
Due to its homology with classical ERM proteins and seemingly prevalent co-localization with cortical actin below the plasma membrane, Merlin has been proposed to regulate mitogenic signalling by organizing membrane domains [48]. The accumulation of Merlin at cell-to-cell junctions in confluent epithelial and endothelial cells [5,7], within lamellipodia in various types of cell [62,63,64], and on the surface of endocytic vesicles in Drosophila epithelial tissues [53,65] is consistent with this general hypothesis (Sidebar C).
Recent studies have shown that the distribution of Merlin can change dynamically in response to various signals and is more diverse than previously anticipated. For example, analysis of the behaviour of the Blue box and the S518A mutant forms of Merlin suggests that the open conformer of Merlin generated by integrin-mediated activation of PAK accumulates underneath matrix adhesions [7]. Skin-epithelium-specific deletion of Merlin in mouse shows that the protein transiently associates with primordial intercellular adhesions and regulates their maturation into adherens junctions, as well as the formation of tight junctions [16]. Although this study failed to detect any association of Merlin with mature adherens or tight junctions, another recent study indicated that Merlin is enriched at tight junctions in confluent MDCK cells and co-localizes with E-cadherin at the paranodes and Schmidt–Lanterman incisures of myelinating Schwann cells [34]. Finally, a significant fraction of Merlin is known to be associated with the soluble, cytosolic fraction in many cell types [64,66], and a smaller amount can move to a detergent-insoluble fraction that has been interpreted as lipid rafts in response to contact inhibition [67].
The above results support the hypothesis that Merlin functions at or near the plasma membrane. Nevertheless, the closed form of Merlin has recently been shown to suppress tumorigenesis by translocating to the nucleus, where it inhibits the E3 ubiquitin ligase CRL4DCAF1 [35]. These findings are fully consistent with the widely held view that only the closed form of Merlin is able to suppress tumorigenesis [43,50,52] and with reports suggesting that Merlin shuttles in and out of the nucleus [68,69]. However, most previous studies had failed to detect Merlin in the nucleus, possibly because the antibodies used are directed against an epitope of nuclear Merlin that is masked under standard fixation and permeabilization conditions. In fact, Merlin was observed in the nucleus in multiple cell types by using an antibody that recognizes its C-terminus and an enhanced permeabilization technique [35]. Similar problems initially hampered the detection of β-catenin in the nucleus [70,71]. In addition, consistent with the nucleo-cytoplasmic shuttling model, Merlin was recently found to move along microtubules towards the nucleus in Drosophila, in a kinesin-1- and dynein-dependent manner [72]. Thus, Merlin could potentially have a similar mechanism of intracellular transport in mammals (Sidebar C). Although Merlin does not have a canonical nuclear localization sequence, a deletion mutant lacking four amino acids near the N-terminus does not localize to the nucleus, suggesting that these residues are essential for Merlin nuclear translocation (W.L. and F.G.G., unpublished observation). In addition, Merlin contains a motif in exon 15 that promotes export via the CRM1–exportin pathway [68], and truncation mutants lacking this sequence have a prominent nuclear localization [35,54]. In the closed form, intramolecular interactions could block the recognition of this export sequence, contributing to Merlin nuclear translocation.
Recent mutational analyses have attempted to distinguish between plasma membrane and nuclear models of Merlin function. Merlin has been reported to associate with the plasma membrane by binding to phosphoinositides, particularly PIP2, through six charged residues within the FERM domain that are conserved in other ERM proteins. Although the interaction of Merlin with phosphoinositides is dispensable for Ser 518 phosphoregulation, it seems necessary for Merlin's localization to the plasma membrane and for some aspects of growth suppression [73]. In addition, deletion of the 17 N-terminal residues that are not found in other ERM proteins has been shown to prevent the association of Merlin with the cortical cytoskeleton in confluent hepatocytes, preventing downregulation of EGFR [21]. However, an elegant analysis of a series of Merlin–Ezrin chimeric constructs revealed that the suppression of proliferation exerted by Merlin does not require this N-terminal sequence or association with the actin cytoskeleton in Schwann cells, which are a primary target of NF2-dependent tumorigenesis [54]. In fact, this study has confirmed that the sequences of Merlin that are involved in the intramolecular interaction—the F2 subdomain of the FERM domain and the C-terminal segment—are essential for growth inhibition in Schwann cells.
Inhibition of receptor tyrosine kinase activation
Merlin has been proposed to suppress proliferation by reducing the amount of transmembrane growth factor receptors at the plasma membrane [22,74,75]. Genetic analysis in Drosophila showed that Merlin, cooperating with the FERM domain protein Expanded, promotes endocytosis of several transmembrane receptors, including some involved in mitogenic signalling [75]. Imaginal epithelial cells lacking functional Merlin and Expanded have elevated levels of Notch, EGFR, Patched, Smoothened and DE-cadherin at their surface. Pulse–chase labelling of Notch in living tissue indicated that Notch protein clearance from the membrane and degradation is deficient in these double mutant cells. This suggested that Merlin, cooperating with Expanded, controls the clearance of transmembrane receptors, regardless of their activation status. A similar phenomenon was observed in mouse Schwann cells, which were found to accumulate elevated levels of ErbB2, ErbB3, IGF1R and PDGFR at their surface when Nf2 is deleted [74]. However, at least in Schwann cells, loss of Merlin was proposed to not decrease internalization but rather accelerate export of membrane receptors to the cell surface. Thus, although the results of both studies attribute to Merlin a role in regulating subcellular trafficking of multiple transmembrane receptors, the underlying mechanisms appear to be divergent. Genetic studies in Drosophila indicate that such trafficking regulation functions through the Hippo signalling pathway [76,77], as receptor accumulation on the surface of imaginal epithelial cells can similarly be seen when other components of the Hippo pathway are depleted. Importantly, such accumulation depends on Yorkie [77]. Finally, studies on contact inhibition suggest that Merlin controls EGFR signalling by regulating the availability of activatable EGFR at the cell surface [21,22]. Activated EGFR was found to partition in a higher density membrane fraction in MEFs, osteoblasts and liver-derived cells that have become fully confluent. Loss of Merlin attenuates this effect, leading to the hypothesis that Merlin sequesters EGFR at a specific membrane compartment where the activated receptor cannot access its downstream targets. Notably, membrane extracts, rather than total cell lysates, were used to examine the activation state of downstream signalling components—such as ERK and Akt—leaving open the question of whether effectors in the cytosolic compartment are also influenced. Thus, both genetic studies in Drosophila and cell biological studies in mammalian cells support the hypothesis that Merlin controls the number of activatable growth factor receptors at the cell surface (Fig 3b), but the biochemical mechanisms underlying this phenomenon are unclear and its significance in tumour suppression remains to be tested (Sidebar C).
Inhibition of Rac signalling
It has long been known that cultured schwannoma and meningioma cells have abundant lamellipodia and membrane ruffles, which are reminiscent of fibroblasts expressing activated forms of Rac [78,80]. Loss of Merlin was later shown to activate Rac signalling, which has been attributed to the release of negative regulation that Merlin exerts on PAK, consistent with the hypothesis that Merlin functions both upstream and downstream from PAK in a positive feedback loop (Fig 3b; [7,23,24,25,79]). Furthermore, biochemical and imaging studies indicated that the closed form of Merlin inhibits the recruitment of Rac to the plasma membrane in confluent cells [7]. Notably, inactivation of Merlin enables normal confluent cells to exit contact inhibition and re-enter the cell cycle, which could occur at least partly through the activation of Rac signalling [7]. In agreement with this model, the expression of a membrane-targeted, but not constitutively active, form of Rac enables exit from contact inhibition, albeit not as efficiently as inactivation of Merlin [7]. Another mechanism for the regulation of Rac signalling by Merlin was recently reported [34]. Merlin can form a tight-junction-associated protein complex with Angiomotin, Patj and Pals1; Merlin binds tightly to Angiomotin and displaces Rich1, a small GTPase-activating protein (GAP) for Rac1, thereby inhibiting Rac1 and PAK. As anticipated from previous studies, inhibition of PAK attenuates Raf to ERK signalling [7]. These results suggest that loss of Merlin activates Rac signalling by multiple, potentially synergistic mechanisms.
Inhibition of mTORC1 signalling
Merlin is a negative regulator of the mTORC1 kinase complex, which regulates cell growth, proliferation, motility and survival by phosphorylating p70–S6K1 and 4E-BP1 [81]. Integrin-mediated adhesion was found to promote activation of mTORC1 signalling through PAK-mediated inactivation of Merlin, and Merlin-deficient mesothelioma cell lines were shown to have activated mTORC1 signalling, whereas other cell lines do not [27]. Similarly, constitutive activation of mTORC1 signalling was found in meningioma and vestibular schwannoma cells from NF2 patients [26,27]. Surprisingly, loss of Merlin was shown to activate mTORC1 independently of AKT or ERK, which activate mTORC1 signalling in response to various mitogenic stimuli. Furthermore, re-expression of Merlin attenuates mTORC1 activity in Merlin-deficient cells, which requires the TSC1–TSC2 complex. The mechanism by which loss of Merlin induces hyperactivation of mTORC1 remains unknown. However, recent studies have shown that Rac can recruit mTORC1 to the plasma membrane [82], raising the possibility that Merlin antagonizes mTORC1 signalling by limiting Rac recruitment to the plasma membrane (Fig 3b; [7]). The significance of mTORC1 as an important downstream effector of Merlin is emphasized by the observation that the mTORC1 inhibitor rapamycin inhibits the proliferation of meningioma cells from NF2 patients and mesothelioma cells lacking Merlin [26,27].
Activation of the Hippo pathway
Increasing evidence implicates the Hippo signalling pathway as a major mediator of contact inhibition of growth. In agreement with this model, genetic analysis in Drosophila and mice showed that this pathway restrains cell proliferation and promotes apoptosis to limit organ size and suppress tumorigenesis [83,84,85]. The core kinase cascade of this pathway—Hippo (MST1/2)–Salvador (WW45)–Warts (Lats1/2)—has been well characterized in Drosophila and is conserved in mammals, whereas its upstream regulation, which is rather complex, seems to have diverged after the separation of arthropods and chordates. In Drosophila, the atypical cadherin Fat and the apical polarity protein Crumbs activate the core kinase cascade through the FERM domain protein Expanded [84,86]. Interestingly, genetic epistasis experiments showed that Merlin cooperates with Expanded to activate the Hippo pathway in the fly [32]. Mammalian cells lack a clear functional homologue of Fat [87,88]. A recent study suggested that the FERM6/Willin protein—which is a putative homologue of human Expanded—can activate the Hippo pathway in HEK-293 cells [89], but this property of FERM6 cannot be recapitulated in the human breast cancer cell line MDA-MB-231, in which FERM6 expression inhibits proliferation [90]. Therefore, whether FERM6/Willin is a functional homologue of Expanded is still uncertain. Similarly, engagement of E-cadherin has been reported to be sufficient to activate the Hippo pathway in human MCF10A mammary epithelial cells and MDA-MB-231 cells [91]. However, studies in the HaCaT human keratinocyte cell line did not support this role of E-cadherin in activation of the Hippo pathway [92], so the issue remains unresolved. Nevertheless, loss of Crumbs [93] or the tight junction component Angiomotin [94,95,96] inactivates the Hippo pathway in mammalian cells, suggesting that signals originating from both adherens and tight junctions can contribute to its activation.
Although several studies support the hypothesis that Merlin can activate the Hippo pathway, the molecular mechanisms remain largely undefined. Overexpression of Merlin promotes phosphorylation and nuclear extrusion of YAP, a downstream target of the Hippo kinase cascade [33,94], which functions as a co-activator of TEAD transcription factors (Fig 3b; [97,98]). By contrast, silencing of Merlin induces TEAD-dependent transcription [92]. Two studies have recently shown that liver-specific deletion of Nf2 using albumin-Cre results in massive liver overgrowth, followed by the development of multiple malignant tumours that seem to arise from hyperplastic lesions of a relatively differentiated ductal population [99], and finally overt hepatocellular carcinoma [17,18]. Interestingly, simultaneous deletion of Yap suppresses liver overgrowth and the ensuing tumorigenesis in Nf2 mutant mice [18]. This effect is remarkable, as loss of a single Yap allele is sufficient to suppress liver overgrowth and tumorigenesis triggered by Nf2 loss, suggesting that YAP is necessary for this Merlin-deficient phenotype. This robust genetic evidence, and the fact that Nf2 deficiency in the liver reduces YAP phosphorylation, suggests that Merlin could regulate YAP signalling [18]. However, as YAP is necessary for the expansion of liver epithelial progenitors [18], its deficiency might suppress Merlin-dependent tumorigenesis by reducing the size of the stem cell or transit-amplifying compartment that sustains the enlargement of the liver, as well as its subsequent neoplastic transformation. Furthermore, treatment with the EGFR kinase inhibitor Erlotinib inhibits AKT activation and reduces hepatic tumour cell proliferation in mice with a liver-specific deletion of Nf2, suggesting that upregulation of EGFR signalling might also contribute to tumour development [17]. Although most of the genetic evidence suggests that YAP is necessary for liver overgrowth and tumorigenesis driven by loss of Merlin [18], further studies will be needed to compare the effect of conditional ablation of YAP or the EGFR on hepatomegaly and liver tumorigenesis. It will also be important to assess whether YAP is necessary for tumorigenesis in other mouse models of Merlin-deficient tumorigenesis.
Several mechanisms have been invoked to explain the effect that Merlin exerts on YAP-dependent transcription. Two additional upstream components of the Hippo pathway in Drosophila have been recently identified: Kibra [100,102,102] and Angiomotin [94,95,96]. Genetic analysis in Drosophila suggests that Kibra functions upstream from the Hippo kinase cascade. Although Merlin is required for some functions of Kibra, including control of organ size [100], evidence supports that both Kibra and Merlin cooperate with Expanded. Indeed, Merlin, Expanded and Kibra interact to form a protein complex [100,101,102] that subsequently binds to the Hippo–Salvador complex [102]. The biochemical mechanism underlying this interaction and how it can activate the kinase cascade are unknown, but overexpression studies suggest that Merlin can bind to Kibra and activate the canonical Hippo kinase cascade also in mammalian cells (Fig 3b; [18]).
Angiomotin has been shown to have a distinct role in Hippo signalling in mammalian cells [94,95,96,103]. It localizes to primordial tight junctions in response to the assembly of the apical polarity complex [104] and retains YAP and the related co-activator TAZ at the cell cortex, preventing them from acting in the nucleus [96,103]. Furthermore, it has been suggested that Angiomotin can also bind to Mst2 and Lats2 and function as a scaffold to enhance signal propagation through the canonical kinase cascade to YAP [94].
Nuclear inhibition of CRL4DCAF1
Tandem affinity purification followed by mass spectrometry showed that wild-type Merlin—but not mutants obtained from tumours, such as L64P—interacts with the E3 ubiquitin ligase CRL4DCAF1 with high affinity [35]. Merlin binds directly to DCAF1, the substrate receptor subunit of CRL4DCAF1, and inhibits CRL4DCAF1-mediated ubiquitination of target proteins. The closed form of Merlin, which is able to mediate growth inhibition, translocates to the nucleus and binds to CRL4DCAF1, whereas the putatively open form, which is inactive, does so to a much more limited extent. Genetic epistasis analysis in human Schwann cells, endothelial cells, mesothelial cells and mouse schwannoma cells indicates that Merlin inhibits growth and suppresses tumorigenesis by inhibiting CRL4DCAF1. For example, depletion of DCAF1 blocked the hyperproliferation caused by loss of Merlin in human Schwann cells and endothelial cells. Conversely, enforced expression of a Merlin-insensitive mutant of DCAF1 counteracted the anti-mitogenic effect of Merlin in human mesothelioma cells. In addition, re-expression of Merlin and silencing of DCAF1 induced an overlapping tumour-suppressive gene expression programme in Merlin-deficient mouse schwannoma cells, suggesting that Merlin inactivation induces oncogenic gene expression by deregulating CRL4DCAF1 activity. Notably, a detailed biochemical and functional analysis of several tumour-derived mutants of Merlin revealed that pathogenic mutations fall into three main classes: some of the missense mutants that map to the FERM domain are defective in nuclear translocation; others fail to bind to DCAF1; and the C-terminal truncation mutants accumulate in the nucleus and bind to DCAF1 but fail to suppress E3 ligase activity. Finally, depletion of DCAF1 suppresses the ability of Merlin-deficient schwannoma cells to hyperproliferate in vitro, to grow in soft agar or to form tumours after subcutaneous injection in nude mice. Together, these findings suggest that Merlin needs to enter the nucleus, bind to DCAF1 and suppress CRL4DCAF1 in order to inhibit tumorigenesis, although additional mechanisms are also possible.
CRL4DCAF1 belongs to a large subfamily of cullin-ring E3 ligases. These ligases consist of a catalytic subunit (Roc1/Rbx1), a scaffold (cullin 4), an adaptor protein (DDB1) and one of multiple WD40-domain-containing substrate receptors [105,106]. DCAF1 is the substrate receptor of CRL4DCAF1. Members of the CRL4 E3 ligase family regulate chromatin remodelling, DNA replication and the response to DNA damage. Although the physiological substrates of CRL4DCAF1 have not yet been identified, gene expression analysis suggests that CRL4DCAF1 regulates a broad gene expression programme, consisting of more than 1,000 genes [35]. CRL4DCAF1 could exert this effect by promoting the poly- or mono-ubiquitination of histones, chromatin-remodelling factors or transcription factors, as it has been established for other members of the CRL4 subfamily [107,108,109,110]. Indirect targets of CRL4DCAF1 include important growth regulators, such as receptor tyrosine kinases (RTKs), their downstream target-effectors, and various cell cycle regulators and anti-apoptotic proteins. For example, AXL, a RTK recently found to regulate mesothelioma proliferation and invasiveness [111], seems to be inhibited at the transcriptional level by Merlin expression as well as DCAF1 knockdown [35]. Irrespective of the specific mechanism by which CRL4DCAF1 regulates gene expression, the breadth of the oncogenic gene expression programme it induces and the identity of some of the genes regulated suggest that Merlin could suppress several mitogenic signalling pathways by inhibiting CRL4DCAF1. Notably, Merlin expression or silencing of DCAF1 coordinately regulates a subset of Hippo pathway target genes, suggesting a connection between CRL4DCAF1 and YAP-dependent transcription [35].
Cortical and nuclear models
In addition to rescuing normal cells from contact inhibition, loss of Merlin accelerates their transit through the G1 phase of the cell cycle [26,27,112], suggesting that Merlin functions as a brake on cell cycle progression in both sparse and confluent cells. In addition, Merlin promotes the maturation of adherens junctions and the assembly of tight junctions in skin epithelium [5,16], suggesting that loss of Merlin can contribute to disruption of cell adhesion and polarity during tumorigenesis. These observations imply that Merlin restrains tumorigenesis by promoting epithelial adhesion and polarity and by restraining proliferation. As discussed above, Merlin exerts these effects through multiple, non-mutually exclusive mechanisms (Fig 4).
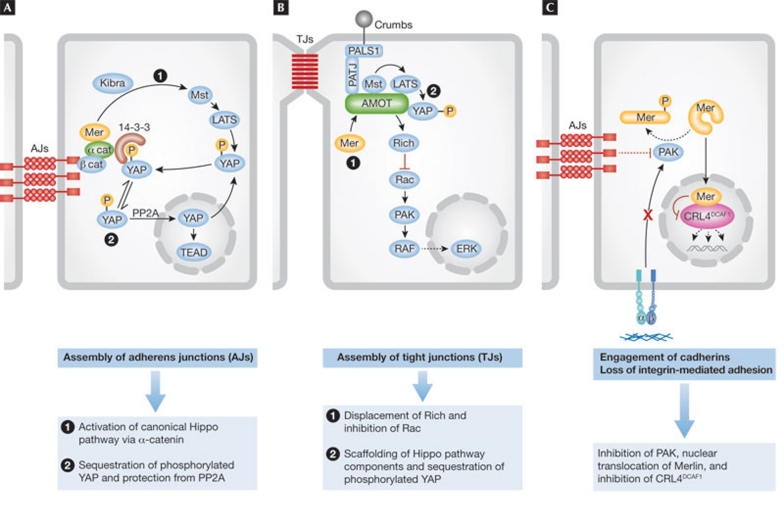
Figure 4. Emerging mechanisms of Merlin-mediated epithelial adhesion, polarity and inhibition of proliferation.
(A) Merlin (Mer) is recruited to nascent adherens junctions by α-catenin and contributes to the activation of Hippo signalling by cooperating with Kibra to activate the classical core kinase cascade, or by enabling α-catenin to sequester phosphorylated YAP in the cytoplasm through 14-3-3 proteins. (B) Upon assembly of tight junctions, Merlin binds to Angiomotin and displaces the Rac GAP Rich, thereby inhibiting Rac. In addition, Merlin might assist Angiomotin in coordinating the activation of the Hippo core kinase cascade. (C) Engagement of E-cadherin or loss of integrin-mediated adhesion leads to inactivation of PAK, promoting an accumulation of the de-phosphorylated (active) form of Merlin. Active Merlin enters the nucleus and inhibits the E3 ubiquitin ligase CRL4DCAF1, thereby suppressing the expression of multiple pro-oncogenic genes. CRL4, cullin-ring E3 ligase 4; DCAF1, DDB1- and CUL4-associated factor 1; ERK, extracellular-signal-regulated kinase; LATS1/2, large tumour suppressor 1/2; Mst1/2, macrophage stimulating 1/2; PAK, p21-activated kinase; PALS1, protein associated with Lin-7 1; PATJ, Pals1-associated tight junction protein; Rac, Ras-related C3 botulinum toxin substrate; TEAD, TEA domain family member; YAP, Yes-associated protein.
The first model of Merlin function, which emphasizes the role of contact inhibition in tumour suppression, places it downstream from the E-cadherin–catenin complex and upstream from Hippo signalling (Fig 4a). In one variant of this model, Merlin is recruited to nascent adherens junctions by binding to α-catenin [16] and activates the canonical Hippo kinase cascade through Kibra [18,91]. In the other variant, Merlin fosters maturation of adherens junctions [16], enabling α-catenin to bind to 14-3-3 and thereby to the phosphorylated inactive form of YAP [92]. Disassembly of the junctions or loss of α-catenin—which is a tumour suppressor on its own [113]—releases phosphorylated YAP from adherens junctions. Finally, dephosphorylation by PP2A allows activation and nuclear accumulation of YAP [92]. These mechanisms probably mediate contact inhibition, but their contribution to Merlin-mediated tumour suppression remains to be examined.
The second model proposes that Merlin enforces contact inhibition and suppresses tumorigenesis by binding to Angiomotin (Fig 4b). In one variant of this model, the binding of Merlin to Angiomotin displaces the Rac GAP Rich, suppressing Rac–PAK signalling [34]. In another variant, binding of Merlin is necessary for Angiomotin to function as a scaffold in the activation of the Hippo pathway. This latter possibility is consistent with the ability of Angiomotin to function as an upstream component of the Hippo pathway [94,95,95,96,103] but inconsistent with the fact that Angiomotin is required for tumour development in a xenograft model of NF2 [34]. Although Merlin binding to Angiomotin might contribute to contact inhibition, as suggested by studies implicating inhibition of Rac in this process [7], its role in tumour suppression remains to be fully investigated. In fact, Merlin binds to Angiomotin through the α-helical and C-terminal segments, independently of whether it is in its open or closed conformation [34].
The third model is based on the fact that the closed form of Merlin translocates into the nucleus to bind to DCAF1—thereby suppressing CRL4DCAF1-mediated E3 ligase activity—whereas the putatively open form does not (Fig 4c; [35]). The contribution of this pathway to contact inhibition remains to be tested; however, its involvement in tumour suppression is supported by strong genetic evidence, including the analysis of a large number of tumour-derived missense mutants of Merlin. Furthermore, this model is compatible with a role for YAP in NF2-dependent tumorigenesis. In fact, the observation that some of the genes regulated by YAP are concordantly regulated by CRL4DCAF1 suggests that CRL4DCAF1 could regulate YAP-dependent transcription [35]. As Merlin can promote phosphorylation of YAP independently of MST1/2 [91], CRL4DCAF1 could inhibit the Hippo pathway by acting on a target downstream from MST. In addition, CRL4DCAF1 probably ubiquitinates other targets. Future challenges include the identification of the physiological targets of CRL4DCAF1 and understanding how it regulates oncogenic gene expression.
Conclusions and perspectives
Recent studies strengthen the idea that Merlin can function in the nucleus as well as at the cell cortex. At the cell cortex, Merlin promotes the assembly of cell junctions by recruiting Par3–aPKC [16] as well as by locally inhibiting Rac signalling [7,34]. Once formed, both adherens and tight junctions can function as signalling hubs for the initiation of antimitogenic signals. Merlin can contribute to the initiation of some of these signals through interactions with Kibra, α-catenin or Angiomotin at the cell cortex, although additional signals are probably required to enforce contact inhibition. In particular, inactivation of PAK allows the accumulation of the closed form of Merlin, which migrates into the nucleus to inhibit CRL4DCAF1 and induce a growth-suppressive programme of gene expression [35]. Conversely, mitogenic stimulation induces activation of PAK and thereby accumulation of the putatively open form of Merlin, which remains in the cytoplasm, removing a block to cell cycle progression [7,25]. Taken together, these findings suggest that Merlin-mediated tumour suppression functions in an opposite manner to the Wnt–β-catenin signalling pathway [114,115]. Confirmation of this general model will require a careful assessment of the contribution of each of the signalling mechanisms outlined above to both contact inhibition and tumour suppression (Sidebar C). Considering the recent progress made in this field, it is reasonable to anticipate a quick answer to the most important outstanding questions. With a more definitive view of Merlin-mediated tumour suppression, it will be possible to design therapies that inhibit the oncogenic signalling pathways activated by the loss of Merlin [116].

Jonathan Cooper, Filippo G. Giancotti & Wei Li

Matthias A. Karajannis
Acknowledgments
We apologize to colleagues whose work could not be cited here due to space limitations. We thank members of our laboratory for discussions. This work was supported by National Institutes of Health Grant R01 CA152975 (to F.G.G.) and Cancer Center Support Grant P30 CA08748. W.L. is the recipient of a Young Investigator Award from the Children's Tumor Foundation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hanahan D, Weinberg R (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Rouleau GA et al. (1993) Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type2. Nature 363: 515–521 [DOI] [PubMed] [Google Scholar]
- Trofatter J, MacCollin M, Rutter J, Murrell J (1993) A novel moesin-, exrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor-suppressor. Cell 72: 791–800 [DOI] [PubMed] [Google Scholar]
- Johnson KC, Kissil JL, Fry JL, Jacks T (2002) Cellular transformation by a FERM domain mutant of the Nf2 tumor suppressor gene. Oncogene 21: 5990–5997 [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI (2003) NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev 17: 1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H, Herrlich P (2001) The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev 15: 968–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, Giancotti FG (2005) Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol 171: 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP (2007) Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat 28: 1–12 [DOI] [PubMed] [Google Scholar]
- Bianchi AB et al. (1994) Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet 6: 185–192 [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Lee WC, Klein MA, Cheng GZ, Jhanwar SC, Testa JR (1999) Frequent mutations of NF2 and allelic loss from chromosome band 22q12 in malignant mesothelioma: evidence for a two-hit mechanism of NF2 inactivation. Genes Chromosomes Cancer 24: 238–242 [PubMed] [Google Scholar]
- Dalgliesh GL et al. (2010) Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463: 360–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LB, MacCollin M, Barone R, Ramesh V, Gusella JF (1996) Frequency and distribution of NF2 mutations in schwannomas. Genes Chromosomes Cancer 17: 45–55 [DOI] [PubMed] [Google Scholar]
- Lau Y-KI, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q (2008) Merlin is a potent inhibitor of glioma growth. Cancer Res 68: 5733–5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustgi AK, Xu L, Pinney D, Sterner C, Beauchamp R, Schmidt S, Gusella JF, Ramesh V (1995) Neurofibromatosis 2 gene in human colorectal cancer. Cancer Genet Cytogenet 84: 24–26 [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T (1997) The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev 11: 1253–1265 [DOI] [PubMed] [Google Scholar]
- Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI (2010) The NF2 tumor suppressor, merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell 19: 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, McClatchey AI (2010) Nf2/merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev 24: 1718–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D (2010) The merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 19: 27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Ohishi M, Garrison B, Aspling M, Janzen V, Adams GB, Curto M, McClatchey AI, Schipani E, Scadden DT (2008) Nf2/merlin regulates hematopoietic stem cell behavior by altering microenvironmental architecture. Cell Stem Cell 3: 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599 [DOI] [PubMed] [Google Scholar]
- Cole BK, Curto M, Chan AW, McClatchey AI (2008) Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol 28: 1274–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI (2007) Contact-dependent inhibition of EGFR signaling by Nf2/merlin. J Cell Biol 177: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO (2003) Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet 12: 1211–1221 [DOI] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T (2003) Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell 12: 841–849 [DOI] [PubMed] [Google Scholar]
- Shaw RJ et al. (2001) The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 1: 63–72 [DOI] [PubMed] [Google Scholar]
- James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, Gusella JF, Ramesh V (2009) NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol 29: 4250–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG (2009) Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol 29: 4235–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO (2008) Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res 68: 5236–5245 [DOI] [PubMed] [Google Scholar]
- Jin H, Sperka T, Herrlich P, Morrison H (2006) Tumorigenic transformation by CPI17 through inhibition of a merlin phosphatase. Nature 442: 576–579 [DOI] [PubMed] [Google Scholar]
- Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR (2006) Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene 25: 5960–5968 [DOI] [PubMed] [Google Scholar]
- Rong R, Tang X, Gutmann DH, Ye K (2004) Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3kinase through binding to PIKE-L. Proc Natl Acad Sci USA 101: 18200–18205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G (2006) The tumour-suppressor genes NF2/merlin and expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8: 27–36 [DOI] [PubMed] [Google Scholar]
- Zhao B et al. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C et al. (2011) A tight junction-associated merlinangiomotin complex mediates merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 19: 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W et al. (2010) Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 140: 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11: 276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Seto A, Maita N, Hamada K, Tsukita S, Tsukita S, Hakoshima T (2002) Structural basis for neurofibromatosis type 2. Crystal structure of the merlin FERM domain. J Biol Chem 277: 10332–10336 [DOI] [PubMed] [Google Scholar]
- Gary R, Bretscher A (1995) Ezrin self-association involves binding of an Nterminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell 6: 1061–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nance MR, Kulikauskas R, Nyberg K, Fehon R, Karplus PA, Bretscher A, Tesmer JJG (2007) Self-masking in an intact ERM-merlin protein: an active role for the central [alpha]-helical domain. J Mol Biol 365: 1446–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MA, Reczek D, Bretscher A, Karplus PA (2000) Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101: 259–270 [DOI] [PubMed] [Google Scholar]
- Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O (1998) Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). J Biol Chem 273: 21893–21900 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A (1994) ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K (2004) Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene 23: 8447–8454 [DOI] [PubMed] [Google Scholar]
- Hennigan RF, Foster LA, Chaiken MF, Mani T, Gomes MM, Herr AB, Ip W (2010) Fluorescence resonance energy transfer analysis of merlin conformational changes. Mol Cell Biol 30: 54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronholm M, Sainio M, Zhao F, Heiska L, Vaheri A, Carpen O (1999) Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin. J Cell Sci 112: 895–904 [DOI] [PubMed] [Google Scholar]
- Meng J-J, Lowrie DJ, Sun H, Dorsey E, Pelton PD, Bashour A-M, Groden J, Ratner N, Ip W (2000) Interaction between two isoforms of the NF2 tumor suppressor protein, merlin, and between merlin and ezrin, suggests modulation of ERM proteins by merlin. J Neurosci Res 62: 491–502 [DOI] [PubMed] [Google Scholar]
- Nguyen R, Reczek D, Bretscher A (2001) Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J Biol Chem 276: 7621–7629 [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Giovannini M (2005) Membrane organization and tumorigenesis—the NF2 tumor suppressor, merlin. Genes Dev 19: 2265–2277 [DOI] [PubMed] [Google Scholar]
- Kissil JL, Johnson KC, Eckman MS, Jacks T (2002) Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem 277: 10394–10399 [DOI] [PubMed] [Google Scholar]
- Sherman L, Xu HM, Geist RT, Saporito-Irwin S, Howells N, Ponta H, Herrlich P, Gutmann DH (1997) Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene 15: 2505–2509 [DOI] [PubMed] [Google Scholar]
- Xiao GH, Beeser A, Chernoff J, Testa JR (2002) p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem 277: 883–886 [DOI] [PubMed] [Google Scholar]
- Surace EI, Haipek CA, Gutmann DH (2004) Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene 23: 580–587 [DOI] [PubMed] [Google Scholar]
- LaJeunesse DR, McCartney BM, Fehon RG (1998) Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. J Cell Biol 141: 1589–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand D, Saint-Amaux AL, Giovannini M (2009) Tumor-suppression functions of merlin are independent of its role as an organizer of the actin cytoskeleton in Schwann cells. J Cell Sci 122: 4141–4149 [DOI] [PubMed] [Google Scholar]
- Bashour AM, Meng JJ, Ip W, MacCollin M, Ratner N (2002) The neurofibromatosis type 2 gene product, merlin, reverses the F-actin cytoskeletal defects in primary human Schwannoma cells. Mol Cell Biol 22: 1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, McClatchey AI, Jacks T (1998b) Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J Biol Chem 273: 7757–7764 [DOI] [PubMed] [Google Scholar]
- Hirokawa Y, Tikoo A, Huynh J, Utermark T, Hanemann CO, Giovannini M, Xiao GH, Testa JR, Wood J, Maruta H (2004) A clue to the therapy of neurofibromatosis type 2: NF2/merlin is a PAK1 inhibitor. Cancer J 10: 20–26 [DOI] [PubMed] [Google Scholar]
- Wilkes MC, Repellin CE, Hong M, Bracamonte M, Penheiter SG, Borg JP, Leof EB (2009) Erbin and the NF2 tumor suppressor merlin cooperatively regulate cell-type-specific activation of PAK2 by TGF-beta. Dev Cell 16: 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O (2004) Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin–ezrin heterodimerization. J Biol Chem 279: 18559–18566 [DOI] [PubMed] [Google Scholar]
- Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, Brat D, Gutmann DH, Ye K (2007) Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol 9: 1199–1207 [DOI] [PubMed] [Google Scholar]
- Laulajainen M, Muranen T, Carpen O, Gronholm M (2008) Protein kinase A-mediated phosphorylation of the NF2 tumor suppressor protein merlin at serine 10 affects the actin cytoskeleton. Oncogene 27: 3233–3243 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Agosti C, Xu L, Pinney D, Beauchamp R, Hobbs W, Gusella J, Ramesh V (1996) The merlin tumor suppressor localizes preferentially in membrane ruffles. Oncogene 13: 1239–1247 [PubMed] [Google Scholar]
- Scherer SS, Gutmann DH (1996) Expression of the neurofibromatosis 2 tumor suppressor gene product, merlin, in Schwann cells. J Neurosci Res 46: 595–605 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, McClatchey AI, Jacks T (1998a) Localization and functional domains of the neurofibromatosis type II tumor suppressor, merlin. Cell Growth Differ 9: 287–296 [PubMed] [Google Scholar]
- McCartney B, Fehon R (1996) Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol 133: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gonzalez-Agosti C, Beauchamp R, Pinney D, Sterner C, Ramesh V (1998) Analysis of molecular domains of epitope-tagged merlin isoforms in Cos-7 cells and primary rat Schwann cells. Exp Cell Res 238: 231–240 [DOI] [PubMed] [Google Scholar]
- Stickney JT, Bacon WC, Rojas M, Ratner N, Ip W (2004) Activation of the tumor suppressor merlin modulates its interaction with lipid rafts. Cancer Res 64: 2717–2724 [DOI] [PubMed] [Google Scholar]
- Kressel M, Schmucker B (2002) Nucleocytoplasmic transfer of the NF2 tumor suppressor protein merlin is regulated by exon 2 and a CRM1-dependent nuclear export signal in exon 15. Hum Mol Genet 11: 2269–2278 [DOI] [PubMed] [Google Scholar]
- Muranen T, Gronholm M, Renkema GH, Carpen O (2005) Cell cycle-dependent nucleocytoplasmic shuttling of the neurofibromatosis 2 tumour suppressor merlin. Oncogene 24: 1150–1158 [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM (1995) Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol 128: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P (1996) Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57: 191–198 [DOI] [PubMed] [Google Scholar]
- Bensenor LB, Barlan K, Rice SE, Fehon RG, Gelfand VI (2010) Microtubule-mediated transport of the tumor-suppressor protein merlin and its mutants. Proc Natl Acad Sci USA 107: 7311–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani T, Hennigan RF, Foster LA, Conrady DG, Herr AB, Ip W (2011) FERM domain phosphoinositide binding targets merlin to the membrane and is essential for its growth-suppressive function. Mol Cell Biol 31: 1983–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, Lampin A, Niwa-Kawakita M, Kalamarides M, Giovannini M (2009) Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene 28: 854–865 [DOI] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG (2006) The tumor suppressors merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol 16: 702–709 [DOI] [PubMed] [Google Scholar]
- Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, Pichaud F, Tapon N (2009) The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci 122: 2360–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G (2009) The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J Cell Sci 122: 2351–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MF, Lelke JM, Maccollin M, Plotkin SR, Stemmer-Rachamimov AO, Ramesh V, Gusella JF (2008) Modeling NF2 with human arachnoidal and meningioma cell culture systems: NF2 silencing reflects the benign character of tumor growth. Neurobiol Dis 29: 278–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton PD, Sherman LS, Rizvi TA, Marchionni MA, Wood P, Friedman RA, Ratner N (1998) Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human schwannoma cells. Oncogene 17: 2195–2209 [DOI] [PubMed] [Google Scholar]
- Rosenbaum C, Kluwe L, Mautner VF, Friedrich RE, Muller HW, Hanemann CO (1998) Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis 5: 55–64 [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saci A, Cantley LC, Carpenter CL (2011) Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell 42: 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K, Tapon N (2007) The Salvador–Warts–Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191 [DOI] [PubMed] [Google Scholar]
- Pan D (2010) The Hippo signaling pathway in development and cancer. Dev Cell 19: 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan K-L (2010) The Hippo–YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 24: 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD (2010) Yorkie: the final destination of Hippo signaling. Trends Cell Biol 20: 410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, Allen S, Basson MA, Francis-West P, Irvine KD (2011) Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1–Fat4 signaling during mammalian development. Development 138: 947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H (2008) Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015 [DOI] [PubMed] [Google Scholar]
- Angus L et al. (2012) Willin/FRMD6 expression activates the Hippo signaling pathway kinases in mammals and antagonizes oncogenic YAP. Oncogene 31: 238–250 [DOI] [PubMed] [Google Scholar]
- Visser-Grieve S, Hao Y, Yang X (2011) Human homolog of Drosophila expanded, hEx, functions as a putative tumor suppressor in human cancer cell lines independently of the Hippo pathway. Oncogene [Epub ahead of print] doi:; DOI: 10.1038/onc.2011.318 [DOI] [PubMed] [Google Scholar]
- Kim N-G, Koh E, Chen X, Gumbiner BM (2011) E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA 108: 11930–11935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K et al. (2011) Yap1 acts downstream of [alpha]-catenin to control epidermal proliferation. Cell 144: 782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL (2010) The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-[beta]–SMAD pathway. Dev Cell 19: 831–844 [DOI] [PubMed] [Google Scholar]
- Paramasivam M, Sarkeshik A, Yates JR 3rd, Fernandes MJ, McCollum D (2011) Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell 22: 3725–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang J, Chen J (2011) Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem 286: 4364–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu C-Y, Lei Q, Guan K-L (2011) Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev 25: 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML (2001) TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev 15: 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B et al. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuhahn K, Schirmacher P (2010) A cellular view of Nf2 in liver homeostasis and tumorigenesis. Dev Cell 19: 363–364 [DOI] [PubMed] [Google Scholar]
- Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H (2010) The WW domain protein Kibra acts upstream of hippo in Drosophila. Dev Cell 18: 309–316 [DOI] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N (2010) Kibra is a regulator of the salvador/warts/hippo signaling network. Dev Cell 18: 300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D (2010) Kibra functions as a tumor suppressor protein that regulates hippo signaling in conjunction with merlin and expanded. Dev Cell 18: 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W (2011) Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem 286: 7018–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CD et al. (2006) A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125: 535–548 [DOI] [PubMed] [Google Scholar]
- Lee J, Zhou P (2007) DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell 26: 775–780 [DOI] [PubMed] [Google Scholar]
- O'Connell BC, Harper JW (2007) Ubiquitin proteasome system (UPS): what can chromatin do for you? Curr Opin Cell Biol 19: 206–214 [DOI] [PubMed] [Google Scholar]
- Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H (2006) CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol 8: 1277–1283 [DOI] [PubMed] [Google Scholar]
- Jin J, Arias EE, Chen J, Harper JW, Walter JC (2006) A family of diverse Cul4–Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell 23: 709–721 [DOI] [PubMed] [Google Scholar]
- Sugasawa K et al. (2005) UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell 121: 387–400 [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhang Z, Dornan D, Arnott D, Deshaies RJ, Dixit VM (2004) Human de-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science 303: 1371–1374 [DOI] [PubMed] [Google Scholar]
- Ou WB, Corson JM, Flynn DL, Lu WP, Wise SC, Bueno R, Sugarbaker DJ, Fletcher JA (2011) AXL regulates mesothelioma proliferation and invasiveness. Oncogene 30: 1643–1652 [DOI] [PubMed] [Google Scholar]
- Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, Jhanwar S, Testa JR (2005) The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol 25: 2384–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien W-H, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V (2011) {alpha}-Catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal 4: ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S, Hanemann CO (2011) Emerging therapeutic targets in schwannomas and other merlin-deficient tumors. Nat Rev Neurol 7: 392–399 [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, Jacks T (1998) Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev 12: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G (2000) Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev 14: 1617–1630 [PMC free article] [PubMed] [Google Scholar]
- Kalamarides M, Niwa-Kawakita M, Leblois H, Abramowski V, Perricaudet M, Janin A, Thomas G, Gutmann DH, Giovannini M (2002) Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev 16: 1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury-Feith J, Lecomte C, Renier A, Matrat M, Kheuang L, Abramowski V, Levy F, Janin A, Giovannini M, Jaurand MC (2003) Hemizygosity of Nf2 is associated with increased susceptibility to asbestos-induced peritoneal tumours. Oncogene 22: 3799–3805 [DOI] [PubMed] [Google Scholar]
- Jongsma J, van Montfort E, Vooijs M, Zevenhoven J, Krimpenfort P, van der Valk M, van de Vijver M, Berns A (2008) A conditional mouse model for malignant mesothelioma. Cancer Cell 13: 261–271 [DOI] [PubMed] [Google Scholar]
- Selvanathan SK, Shenton A, Ferner R, Wallace AJ, Huson SM, Ramsden RT, Evans DG (2009) Further genotype–phenotype correlations in neurofibromatosis 2. Clin Genet [Epub ahead of print] doi:; DOI: 10.1111/j.1399-0004.2009.01327.x [DOI] [PubMed] [Google Scholar]
- Roche PH, Bouvier C, Chinot O, Figarella-Branger D (2008) Genesis and biology of vestibular schwannomas. Prog Neurol Surg 21: 24–31 [DOI] [PubMed] [Google Scholar]
- De Vitis LR, Tedde A, Vitelli F, Ammannati F, Mennonna P, Bigozzi U, Montali E, Papi L (1996) Screening for mutations in the neurofibromatosis type 2 (NF2) gene in sporadic meningiomas. Hum Genet 97: 632–637 [DOI] [PubMed] [Google Scholar]
- Lekanne Deprez RH et al. (1995) Cytogenetic, molecular genetic and pathological analyses in 126 meningiomas. J Neuropathol Exp Neurol 54: 224–235 [DOI] [PubMed] [Google Scholar]
- Papi L, De Vitis LR, Vitelli F, Ammannati F, Mennonna P, Montali E, Bigozzi U (1995) Somatic mutations in the neurofibromatosis type 2 gene in sporadic meningiomas. Hum Genet 95: 347–351 [DOI] [PubMed] [Google Scholar]
- Tos M, Stangerup SE, Caye-Thomasen P, Tos T, Thomsen J (2004) What is the real incidence of vestibular schwannoma? Arch Otolaryngol Head Neck Surg 130: 216–220 [DOI] [PubMed] [Google Scholar]
- Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57: 1088–1095 [DOI] [PubMed] [Google Scholar]