Abstract
Deimination refers to conversion of protein-bound arginine into citrulline. An mRNA carrier, RNA binding export factor (REF), present on mitochondria undergoes loss of deimination with impaired ATP5b mRNA transport in ND4 mice (model of multiple sclerosis) compared with the controls. We present evidence of (1) reduced ATP5b mRNA binding strength of non-deiminated REF compared with deiminated REF, (2) impaired ATP5b mRNA transport in ND4 mice and (3) reduced mitochondrial ATP synthase activity on inhibition of deimination in PC12 cells. Impaired deimination of REF and defect in mitochondrial mRNA transport are critical factors in mitochondrial dysfunction in ND4 mice.
Keywords: deimination, mitochondria, ATP synthase, multiple sclerosis, RNA binding export factor
Introduction
Mitochondria (mt) are main energy providers for the cells and are involved in a variety of basic cellular processes in higher organisms. The crosstalk between nuclear and mt has important role for integrity of mt genome and mt biogenesis. Nuclear-coded mt mRNAs are translated in the cytoplasm and usually transported in the mt in the form of nascent polypeptide-associated complex [1]. In yeast, select mt proteins via mt surface-bound ribosomes have been shown to be co-translationally imported into mt [2, 3]. Several studies have shown importance of the sequences within the mRNA transcript in their specific subcellular localization. The 3′ untranslated region of the mRNA (conserved from yeast to humans) have a key role for targeting of the mRNA to the vicinity of mt [4]. The transport of mRNA to their surface and co-translational protein import into the mt are important for mitochondrial structural integrity and function.
Multiple sclerosis (MS) is a late-onset disease associated with demyelination. Mitochondrial defects have been detected in MS [5] that remain to be further investigated. ND4 is a transgenic mouse model of MS overexpressing the DM 20 variant of myelin proteolipid protein, which has been recently clinically characterized using non-invasive methods [6]. Deimination refers to the conversion of protein-bound arginine into citrulline and is a long-term posttranslational modification due to lack of any known reversal enzyme [7]. Aberrant deimination has been reported in human MS [8] and in the ND4 mice. ND4 mice show elevated deimination in the brain with presence of aberrant simultaneous hypo- and hyper-deimination in the retina. Cell-type-specific simultaneous elevation (astroglial cells) and decreased deimination (neuronal cells) have been proposed to explain occurrence of simultaneous hypo- and hyper-deimination [8].
Previously, we have identified RNA binding export factor (REF, in mice, refbp1 is the long form and refbp2 is the short form analogue of human REF) as one of the several candidate proteins that undergo the loss of deimination in MS cadaver eyes. REF belongs to a family of ribonucleoprotein-type RNA binding proteins. It is known to have a role in mRNA processing and export [9]. A conserved protein complex termed THO/TREX complex in eukaryotes is involved in mRNA metabolism and export. REF/Aly is a component of the THO/TREX complex [9]. In contrast to the studies that have been reported in the nucleous, the role of REF in the cytoplasm is not clear. Whether deimination of the REF has a role in mitochondrial surface mRNA transport and thereby might contribute to mitochondrial respiratory defects remains to be investigated. Current research is aimed to test whether loss of deimination in REF leads to mitochondrial dysfunction in ND4 model and to determine deimination in REF-mediated mitochondrial mRNA binding and transport as an important mechanism in transport of ATP5b mRNA on mitochondrial surface.
Results And Discussion
Evidence of REF association in ATP5b mRNA transport
Cytosolic and mitochondrial protein extracts were prepared from ND4 and wild-type control mice retinas. We performed immunoprecipitation (IP) to determine the presence of REF in mitochondrial and cytosolic fraction and it was found to be present in both fractions (Fig 1A; supplementary Fig S1A online) and associated with different mRNAs. Negative control IP with antialdehyde dehydrogenase (ALDH1) showed lack of REF pull down (supplementary Fig S1B online). We identified several nuclear-coded mitochondrial mRNAs, specially a series of ATP synthase complex mRNAs, which showed a significantly increased binding with deiminated REF compared with control REF (supplementary Table S1 online, GEO accession number GSE11843). Mitochondrial mRNAs have been shown to be transported to the mitochondrial surface and locally translated [10]. ATP2 and ATM1 are two mRNAs that have been shown [11] to be transported onto the mitochondrial surface in yeast. We sought to determine whether both ATP5b (homolog of ATP2 in mouse) and ATM1 bind to REF protein in cells. Existence of ATP5b and ATM1 mRNAs at mitochondrial surface were confirmed using reverse transcription–polymerase chain reaction (PCR; Fig 1Ba). Usually long mRNA prefer to localize to the vicinity of mt, ATP5b has a short 3′ untranslated region (269 bases) compared with ATM1 (3479 bases). ATP5b falls among 10% lowest membrane versus free index (a synthetic potential value for localization of mRNA onto mt surface) for mRNA that localize to mt [12]. IP of REF-associated protein–mRNA complexes were carried out with two different mitochondrial fractions: synaptic and non-synaptic as well as with cytosolic fraction. ATP5b and ATM1 mRNAs from the eluents were detected by PCR (Fig 1Bb). ATP5b has been shown in both cytosolic and mitochondrial fractions (Fig 1Bb). In contrast, actin mRNA (control) and ATM1 was detected only in the cytosolic but not in mitochondrial fractions (Fig 1Bb; supplementary Fig S1C online). Detection of actin and ATM1 in cytosolic fraction supports the mRNA export function of REF in the cytosol, where REF is expected to associate with most mRNAs. However, our PCR analyses showed stronger association of REF for ATP5b compared with that for ATM1 mRNA at mitochondrial surface, suggesting that ATM1 and ATP5b are possibly transported by two different pathways. These results indicate that REF is involved in the transport of ATP5b.
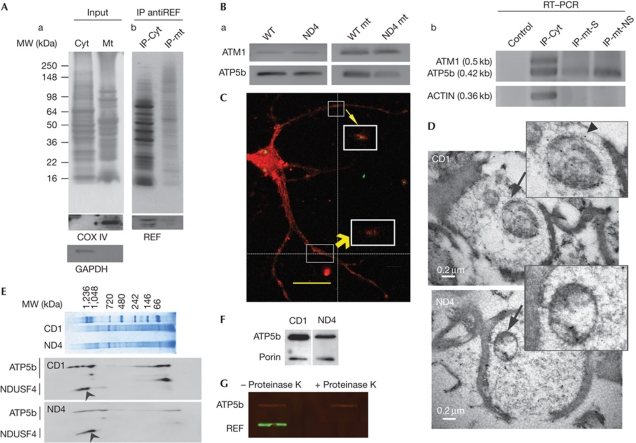
Figure 1.
REF and ATP5b status on mitochondrial fraction. (A) Demonstration of RNA binding export factor (REF) in mitochondrial fraction. AntiREF immunoprecipitation (IP) from cytosolic or mitochondrial fraction. (a) The input retinal cytosolic and brain mitochondrial protein extracts were separated on a 10% SDS–PAGE and detected with antiCOX IV and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as indicated. (b) IP products from inputs as shown in panel a were gel fractionated and visualized using silver staining. Bottom panel shows recovery of REF using western analysis. (B) Detection of ATP5b and ATM1 mRNA by reverse transcription–polymerase chain reaction (RT–PCR). (a) ATM1 and ATP5b with wild-type (WT) and ND4 mitochondrial (mt) fraction. (b) Detection of ATP5b, ATM and actin in normal mouse complementary DNA derived from three IP products: Retinal cytosolic (cyt), non-synaptic (mt-NS) and synaptic (mt-S) mitochondria as indicated were carried out using appropriate primer pairs. Control represents RT–PCR from an IP performed with an antibody unrelated to any known mammalian protein. Bottom panel shows amplification of actin used as a control. The product size has been provided in the parentheses. (C) Localization of REF on the mitochondrial surface. Primary hippocampal neuron culture was probed with Porin (red) and REF (green) antibodies. Merged figure has been shown. White boxes indicated co-localization of REF and Porin. Z axis and magnified view indicated by yellow arrows. Scale bar, 20 μm. (D) Electron microscopy in detection of REF at mitochondrial surface. Upper and lower panel indicate CD1 and ND4 mice sections, respectively. Scale bar, 0.2 μm. Arrow and arrowhead indicate the region shown in the inset and a mitochondrial gold particle, respectively. (E) Blue native gel electrophoresis of mitochondrial protein extract from CD1 and ND4 mice as indicated (upper panel). Bottom panel shows western blot analyses from a posttransfer two-dimensional SDS–PAGE. Arrowhead indicate NADH dehydrogenase ubiquinone iron-sulphur protein 4 (NDUSF4); proteins were probed with antibodies as indicated. (F) Western blot analyses of mt proteins from CD1 and ND4 mice with ATP5b and Porin as indicated. (G) Control and mt subjected to digestion with Proteinase K as indicated probed for REF and ATP5b, with secondary antibodies coupled with IR-700, IR-800 and scanned on an Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE). MW, molecular weight.
Localization of REF at mitochondrial surface
Localization of REF on mt was detected by immunohistochemistry, confocal (Fig 1C) and electron microscopy (EM; Fig 1D). Mitochondrial surface markers Porin/VDAC (Fig 1C) and Tomm20 (supplementary Fig S1D online) were used to determine their co-localization with REF at the mt surface in primary neurons isolated from rat hippocampus. REF was detected with different antibodies (Fig 1C, monoclonal antiREF (Abcam); supplementary Fig S1 online, rabbit polyclonal antiREF). REF was found to be present at the mitochondrial surface in addition to other locations also in elongating neurites (Fig 1C). In EM detection, the REF was shown to present at mitochondrial surface (Fig 1D), consistent with findings using confocal microscopy (Fig 1C; supplementary Fig S1 online). No primary control EM sections showed a lack of gold particles (supplementary Fig S1F,G online). ATP5b was found to co-localize with mitochondrial marker Tomm20 (supplementary Fig S1E online). Proteins from isolated mitochondrial preparations were characterized with respect to ATPase assembly (Fig 1E,F). Isolated mitochondrial preparations were subjected to proteinase K digestion (Fig 1G) to demonstrate presence of REF on the mitochondrial surface. Whereas REF was completely digested by proteinase K treatment after 10 min, ATP5b was largely left intact (Fig 1G) suggesting REF to be present on mitochondrial surface. The lower levels of ATP5b in the mitochondrial surface of ND4 compared with control CD1 (Fig 1F) mice could be as a consequence of mutation in ATP5b structural sequence [13]. Our restriction fragment length polymorphism or sequencing has not shown any difference in the ATP5b gene or mRNA between ND4 and control CD1 mice.
Reduced level of deimination of REF in ND4 mice
Western analyses are consistent with loss of REF deimination in the ND4 mouse model compared with the normal controls (Fig 2A); however, the expression of REF did not show significant difference (Fig 2A). Endogenous REF from wild-type and ND4 mice neurons was purified by fast protein liquid chromatography and subjected to mass spectrometry of intact REF in linear mode (solid-state ionization) for comparison. REF derived from wild-type mice showed two main peaks with m/z ratio 23,730.25 and 23,737.41, in contrast, ND4 REF showed only one main peak with m/z ratio 23,730.28 (Fig 2B). Unmodified REF is expected to have m/z ratio 23,730, which is consistent with m/z ratio at 23,730.25 and 23,730.28. The peak with m/z ratio 23,737.41 from wild type represents the deiminated REF (Fig 2B), and seven arginines were deiminated based on calculation, which was consistent with our amino acid analysis. The REF might possess more modifications (methylation, myristolation) that either do not appear in the fast protein liquid chromatography purified REF fractions or is possibly too low in concentration compared with main peaks (non-modified and deiminated peaks).
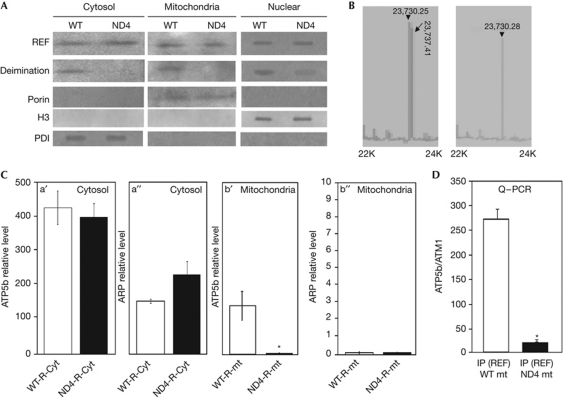
Figure 2.
Comparison of ATP5b expression and ATP synthase activity between wild-type and ND4 mice. (A) Loss of RNA binding export factor (REF) deimination in ND4 mice. Western analysis of deimination and REF in the cytosol, nuclear fraction and mitochondria of wild-type (WT) and ND4 mice as indicated. The deimination status of REF was detected using anticitrulline antibody (bottom panel), blots were probed with Porin, histone H3 and protein disulphide isomerase (PDI) as indicated. (B) Analysis of intact purified REF using mass spectrometry on a matrix-assisted laser desorption/ionization–time of flight device in linear mode. Arrowheads indicated non-deiminated form, arrows indicated deiminated form with m/z ratios. Cyt, retinal cytosolic soluble proteins; mt, mitochondria; IP, immunoprecipitation. (C) ATP5b transcript levels were quantified and normalized by ATM1 mRNA levels by real-time reverse transcription–polymerase chain reaction (PCR) and compared between control (WT) and ND4 mice as indicated. (a′, a″) ATP5b and actin-related protein (ARP) mRNA levels in retinal cytosolic fraction and (b′, b″) mitochondrial fraction as indicated. ARP mitochondrial level is ∼2,000-fold less compared with ATP5b or cytosolic ARP levels. (D) Comparison of REF and ATP5b association between WT (CD1) and ND4 mice. ATP5b expression from complementary DNA recovered by reverse transcription from IP products: wild-type mitochondria (WT mt) and ND4 mitochondria (ND4 mt) as indicated was quantified by real-time PCR and normalized by ATM1 levels. NS, non-synaptic mitochondria; S, synaptic mitochondria. All comparisons were made from at least three independent measurements with analysis of variance expressed as mean±s.d. All data were subjected to two-tailed paired t-test against controls (and were considered significant *P⩽0.05).
Deficient ATP5b mRNA transport in ND4
We next determined whether there is a difference in ATP5b mRNA transport between ND4 and wild-type mice by real-time PCR (Fig 2C). Although the total ATP5b mRNAs in the cytosol (Fig 2Ca′) did not show a significant difference, ATP5b mRNA located on mitochondrial surface was decreased in ND4 compared with the controls (Fig 2Cb′), indicating that the synthesis of ATP5b mRNA was normal but the ATP5b transport pathway was impaired. The relative level of another mRNA, an actin-related protein (whether cytosolic or mitochondrial) showed lack of any significant change either (Fig 2Ca″,b″) between CD1 and ND4 mice. To determine whether there is a loss of ATP5b mRNA on mitochondrial surface in ND4 mice due to the loss of deimination of REF, we carried out the IP experiment (using antiREF to determine REF-bound mRNA fraction) comparing the ATP5b mRNA between ND4 and control mt. The mitochondrial fractions were prepared using a similar protocol used for RT–PCR analyses (Fig 2D). The antiREF IP from ND4 retinal mitochondrial fraction contained less ATP5b compared with controls, suggesting the decreased association between mRNA and REF protein in ND4 (Fig 2D). This is most likely due to the loss of deimination of REF. To confirm these findings, we also determined steady-state levels of ATP5b. The levels of mitochondrial ATP5b were significantly lower in ND4 as compared with CD1 mice (Fig 2Cb′), suggesting impaired ATP5b transport in ND4 mice.
Decreased ATP synthesis efficiency in ND4 mice
Mitochondrial respiratory defects have been proposed to occur in MS [5]. We investigated the function of mitochondrial respiratory chain in ND4 and control mice by measuring the rate of oxidation of different substrates in mt isolated from mouse brain and spine. Our attempts showed that mitochondrial respiratory defects are associated with ND4 mice but not to CD1 controls (supplementary Fig S2 online). The adenosine diphosphate-oxygen (ADP-O) ratio was also lower in ND4 mt compared with control and corroborated severity of defect in synaptic mt consistent with observation of respiratory defects (Fig 3A and Table 1). The measures of mitochondrial respiratory chain complexes I, III and IV efficacy are state 3 respiration rates determined in the presence of pyruvate+malate. Our results thus indicate marginal decrease in efficacy of these three complexes together (Fig 3A, Table 1) and suggest that efficacy of complexes I, III and IV is not affected by ATP5b (a part of complex V). We confirmed these observations by measuring individual complex activity (supplementary Fig S3 online). The activity measurements of complexes did not show a significant difference between CD1 and ND4; however, the enzymatic analysis of ATP synthase activity indicated a defect in ND4 mice (Fig 3B). Overall, our results indicate decreased mitochondrial respiratory chain efficiency in ND4 mice. It is possible that observed lower ATP synthase activity in ND4 mice might be due to improper assembly of this respiratory chain complex. We used blue native gel to address whether the respiratory complexes are decreased in their relative abundance or fail to assemble (Fig 1E). The ratio of ATP5b levels in assembled versus non-assembled fraction was found to be same in mt isolated from CD1 or ND4 mice in comparison with NADH dehydrogenase ubiquinone iron-sulphur protein 4 (Fig 1E). These results indicate that observed lower complex V activity is due to lower levels of ATP5b (Fig 1F) and not due to impaired complex V assembly.
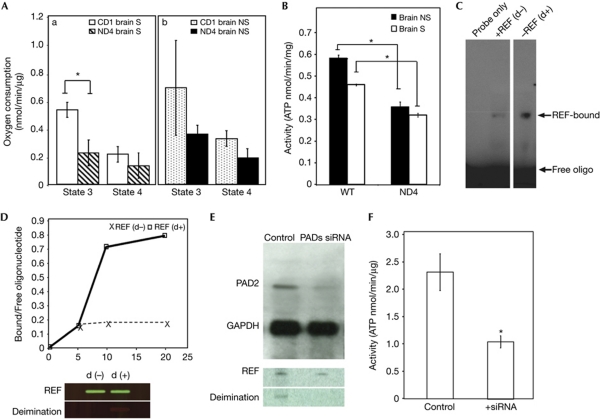
Figure 3.
Deiminated REF in regulation of ATP synthase expression. (A) Comparison of mean mitochondrial states 3 and 4 respiration rates between wild-type (WT; CD1) and ND4 mice. (a) Respiration rates (states 3 and 4 as indicated) in synaptic (S) mitochondria isolated from ND4 (n=4) and CD1 (n=6) brains. (b) Respiration rates (states 3 and 4 as indicated) in non-synaptic (NS) mitochondria isolated from ND4 (n=4) and CD1 (n=6) brains. Rate of oxygen consumption was measured in the presence and in the absence of ADP. (B) Comparison of mean mitochondrial ATP synthase activity between WT (CD1) and ND4 mice. Isolated mitochondria from brain non-synaptic (NS, solid column) and synaptosomes (S, hollow column) were applied to ATP synthase measurement as indicated. (C) Electrophoretic mobility shift assay (EMSA) analysis of deiminated REF using an oligonucleotide probe 5′-TGGGCAGAATCATGAATGTC-3′. EMSA of non-deiminated (d−) and deiminated (d+) REF as indicated in lane 2 and 3; the control lane was loaded only with probe. (D) Time course was generated by the ratio of bound and free probe (bound/free oligonucleotide) and incubation time and compared between non-deiminated REF (d−; cross and dashed line) and deiminated REF (d+; square; solid line). Protein loading from an EMSA gel was probed for REF and its deimination status (lower panel) using antiREF and anticitrulline with secondary antimouse and antirabbit coupled with IR-700 and IR-800 scanned on an Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE). (E) The mRNA expression after siRNA inhibition. Representative PAD2 and GAPDH protein as indicated were compared between the control and transfected cells. The expression level was quantified by ImageJ program (supplementary Fig S4D online). Control and PAD siRNA inhibited product were probed with antiREF and anticitrulline antibody, respectively (bottom panel). (F) The ATP synthase activity of PC12 cell line after PAD2 and PAD4 siRNA treatment. Control indicates the cells transfected with nonspecific negative siRNA, + siRNAs indicates the cells transfected with PAD2 and PAD4 siRNAs. All comparisons were made from at least three independent measurements with analysis of variance expressed as mean±s.d. All data were subjected to two-tailed paired or unpaired t-test with respect to controls (*), *P⩽0.05. ATP, adenosine triphosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; REF, RNA binding export factor; siRNA, short interfering RNA.
Table 1. ADP-O ratios.
| Samples | ADP/O±s.d. |
|---|---|
| CD1 brain-NS | 3.1±0.3 |
| CD1 brain-S | 2.8±1.3 |
| CD1 spine-NS | 3.1±0.2 |
| CD1 spine-S | 3.4±0.6 |
| ND4 brain-NS | 2±0.9 |
| ND4 brain-S | 2.1±1.5 |
| ND4 spine-NS | 1.7±0.9 |
| ND4 spine-S | 1.6±0.9 |
| NS, non-synaptic; S, synaptic; n=9 in each group. | |
Stronger ATP5b–REF association with deiminated REF
We also attempted to determine the RNA binding ability of deiminated (d+) and non-deiminated REF using microarray analyses (GEO accession number GSE11843). Compared with non-deiminated REF, deiminated REF showed stronger binding and enrichment for several mRNAs. The binding strength of REF was determined by electrophoretic mobility shift assay (EMSA; Fig 3C). Purified recombinant REF was prepared and used as non-deiminated form. Deiminated REF was prepared in vitro by incubating the recombinant REF protein with purified PAD2 in the presence of calcium ions. Equal amounts of non-deiminated and deiminated REF was incubated with a 0.5 nmol ATP5b probe separately. Both reactions reached their equilibrium states in the first 10 min (Fig 3D). The time course comparison between deiminated REF and non-deiminated REF revealed that deiminated REF had a higher affinity to the ATP5b probe (Fig 3D). Calculations based on EMSA showed a greater dissociation constant for ATP5b mRNA and non-deiminated REF compared with deiminated REF, that is, 0.28 versus 0.15 nM under our test conditions, suggesting stronger binding between ATP5b mRNA and the deiminated protein.
Decreased deimination and reduced ATP synthase activity
To test whether deiminations have a role in another kind of neuronal cells, we used PC12 cells. We used short interfering RNAs (siRNAs) against PAD2 and PAD4, the main deiminases in neuronal cells, and determined the effect of consequent reduced deimination on cellular function (Fig 3E). The transfected cells had less neurite outgrowth (supplementary Fig S4A–C online), reduced PAD2 level (Fig 3E; supplementary Fig S4D online) and reduced deimination of REF (Fig 3E, bottom panel) compared with the controls. ATP synthase activity in the transfected cells has been found to be significantly reduced compared with the controls (Fig 3F), which is consistent with our observation in ND4 mice (Fig 3B).
The role of deimination in ATP5b mRNA transport
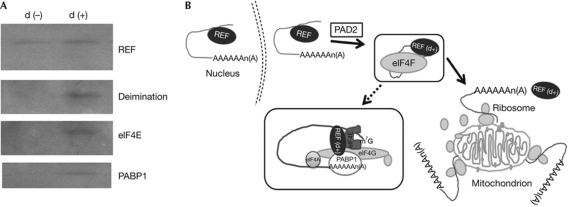
We also attempted to determine the association of components of ribosomal translation machinery with REF in deiminated and non-deiminated state using in vitro purified REF or deiminated REF as bait (Fig 4A). We found stronger association of translational machinery components with deiminated REF than controls (Fig 4A). On the basis of these experiments we propose a model in which deiminated REF contributes to the transport of select mRNA, such as ATP5b on mitochondrial surface (Fig 4B), thereby augmenting the translation of the latter. The siRNA treatment against REF in primary hippocampal neurons show reduced mitochondrial ATP5b levels (supplementary Fig S5A online). In REF siRNA–treated cells, the cytosolic ATP5b is also slightly reduced but not that of cytosolic or mitochondrial ATM1 levels (supplementary Fig S5A online). We found a reduction in ATPase activity in the mt (supplementary Fig S5b online) in cells treated with REF siRNA compared with controls. A coupled transcription translation system with added mt showed increased ATP5b accumulation in the presence of exogenously added in vitro deiminated REF, but not control recombinant non-deiminated REF (supplementary Fig S5C online), suggesting a role for deiminated REF for ATP5b translation. Our current work demonstrates that deiminated REF has a role in transport of ATP5b mRNA on mitochondrial surface in generic neurons. We propose a potential mechanism that the mitochondrial health and biogenesis can be affected by change at mRNA transport due to loss of protein deimination. Future experiments will show whether this is likely to extend to other select nuclear-coded mitochondrial mRNAs.
Figure 4.
Association of deiminated REF with protein translational machinery components. (A) Western analyses of antiREF (Refbp2) immunoprecipitation products with deiminated REF (d+) and control non-deiminated REF (d−) as bait. (B) Schematic diagram of REF mediate mRNA export at mitochondrial surface. The REF (indicated) exports polyadenylated cargo from the nucleus. REF becomes additionally deiminated (indicated as d+) by PAD2 and recruits members of protein translation initiation complex elf4F, facilitate the mRNA (ATP5b) transport to mitochondrial surface; details of REF interacting protein complex have been shown in the enlarged box. REF, RNA binding export factor.
Methods
Cell culture and siRNA treatment. Primary neurons and PC12 cells were grown following established protocols. The siRNA experiments were performed using siRNA against PAD2/4 sequences (Stealth Select RNAi (Invitrogen Inc.)), a siRNA unrelated to any known mammalian sequence served as control. Blue native gel was performed following published protocols [14]. All procedures used in this study are described in detail in supplementary information online. All experiments were repeated and results presented are at least from three independent measurements subjected to statistical analyses.
Mouse, mitochondrial and other studies. All animal experiments were approved by Institutional Animal Care and Use Committee. Brain mt were isolated following a previously described procedure [15]. All mitochondrial respiration experiments were performed in synaptosomes permeabilized with 0.007% digitonin [16]. Oxidation rates and phosphorylating capacities were determined by polarograph following published procedures [17]. Following antibodies were used in this study: REF/Thoc4 (Aviva Systems Biology), Tomm20: Porin: REF and ALDH1 (Abcam), COXIV (Sigma), GAPDH (Cell Signaling), Citrulline (Millipore), DAPI (Vector Laboratories).
Electrophoretic gel mobility shift, filter binding assay. The non-radioactive light shift chemiluminescent EMSA kit (Pierce Biotechnology, Rockland, IL, USA) and 5′-biotin end-labelled oligonucleotides were used according to the manufacturer's recommended protocol to detect RNA and protein interactions.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Antonio Barrientos, Ralf Landgraf for their suggestions, Margaret Bates and Gabriel Gaidosh for their technical assistance. This work was supported by a career award and unrestricted funds from Research to Prevent Blindness and NIH grant EY14801.
Author contributions: D.D., M.E.-A., K.R.D. and S.K.B. designed and executed experiments, interpreted results, K.R.D. and M.P.-P. contributed reagents and assisted in interpreting results and writing the manuscript. S.K.B. conceived the overall design and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Franke J, Reimann B, Hartmann E, Kohlerl M, Wiedmann B (2001) Evidence for a nuclear passage of nascent polypeptide-associated complex subunits in yeast. J Cell Sci 114: 2641–2648 [DOI] [PubMed] [Google Scholar]
- Ding D, Dave KR, Bhattacharya SK (2009) On message ribonucleic acids targeting to mitochondria. Biochem Insights 2: 1–11 [Google Scholar]
- Fujiki M, Verner K (1993) Coupling of cytosolic protein synthesis and mitochondrial protein import in yeast. Evidence for cotranslational import in vivo. J Biol Chem 268: 1914–1920 [PubMed] [Google Scholar]
- Sylvestre J, Margeot A, Jacq C, Dujardin G, Corral-Debrinski M (2003a) The role of the 3′ untranslated region in mRNA sorting to the vicinity of mitochondria is conserved from yeast to human cells. Mol Biol Cell 14: 3848–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R et al. (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59: 478–489 [DOI] [PubMed] [Google Scholar]
- Enriquez-Algeciras M, Ding D, Chou TH, Wang J, Padgett KR, Porciatti V, Bhattacharya SK (2011) Evaluation of a transgenic mouse model of multiple sclerosis with noninvasive methods. Invest Ophthalmol Vis Sci 52: 2405–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ (2003) PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 25: 1106–1118 [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK (2009) Retinal deimination in aging and disease. IUBMB Life 61: 504–509 [DOI] [PubMed] [Google Scholar]
- Levesque L, Guzik B, Guan T, Coyle J, Black BE, Rekosh D, Hammarskjold ML, Paschal BM (2001) RNA export mediated by tap involves NXT1-dependent interactions with the nuclear pore complex. J Biol Chem 276: 44953–44962 [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M (2007) mRNA specific subcellular localization represents a crucial step for fine-tuning of gene expression in mammalian cells. Biochim Biophys Acta 1773: 473–475 [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M, Blugeon C, Jacq C (2000) In yeast, the 3′ untranslated region or the presequence of ATM1 is required for the exclusive localization of its mRNA to the vicinity of mitochondria. Mol Cell Biol 20: 7881–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre J, Vialette S, Corral Debrinski M, Jacq C (2003b) Long mRNAs coding for yeast mitochondrial proteins of prokaryotic origin preferentially localize to the vicinity of mitochondria. Genome Biol 4: R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Delaveau T, Goussard S, Jacq C (2010) Mitochondrial presequence and open reading frame mediate asymmetric localization of messenger RNA. EMBO Rep 11: 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvaruso MA, Smeitink J, Nijtmans L (2008) Electrophoresis techniques to investigate defects in oxidative phosphorylation. Methods 46: 281–287 [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Heath JW, Harrison SM, Jarvie PE, Glenfield PJ, Rostas JA (1988) A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Res 441: 59–71 [DOI] [PubMed] [Google Scholar]
- Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, Perez-Pinzon MA (2008) Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci 28: 4172–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, Perez-Pinzon MA (2001) Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cereb Blood Flow Metab 21: 1401–1410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.