Abstract
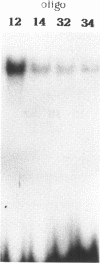
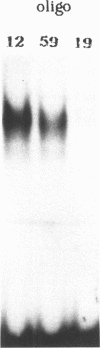
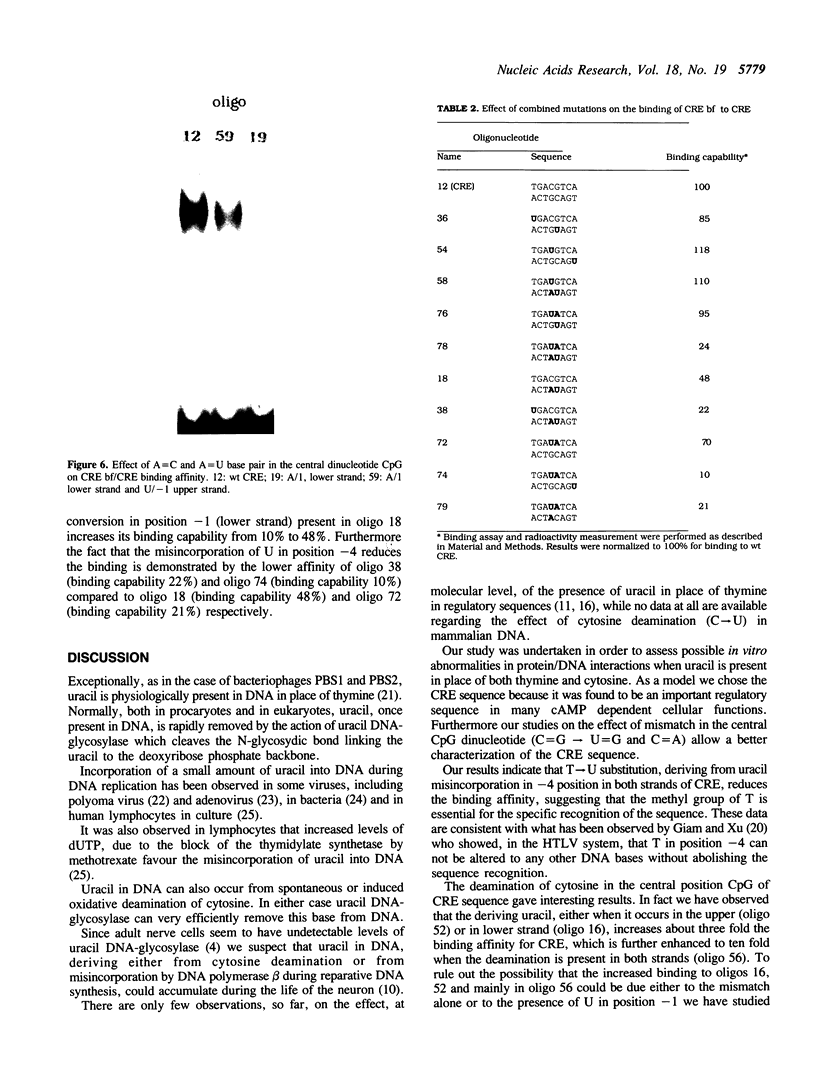
Point mutations in the cAMP-responsive element (CRE) of the rat somatostatin gene promoter/enhancer sequence (TGACGTCA) were used as a model for assessing the effect of uracil, deriving either from misincorporation during DNA synthesis (T----U) or cytosine deamination (C----U), on the binding of sequence/specific regulatory proteins. The results show that the T----U conversion in both strands of the CRE palindromic sequence reduces its affinity for the CRE binding factor(s), suggesting the crucial role of the methyl group contributed by T for the correct recognition of the sequence. On the other hand, deamination of C in the CpG central dinucleotide (CpG----UpG) causes an increase of binding affinity which is further enhanced by the contemporary deamination in both strands. Then, both uracil misincorporation and cytosine deamination alter the binding to CRE sequence in vitro, suggesting that uracil, if not removed by uracil DNA-glycosylase, could be dangerous for cellular functions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Shimojo H. Incorporation of uracil into the growing strand of adenovirus 12 DNA. Biochem Biophys Res Commun. 1979 Mar 30;87(2):588–597. doi: 10.1016/0006-291x(79)91835-7. [DOI] [PubMed] [Google Scholar]
- Brynolf K., Eliasson R., Reichard P. Formation of Okazaki fragments in polyoma DNA synthesis caused by misincorporation of uracil. Cell. 1978 Mar;13(3):573–580. doi: 10.1016/0092-8674(78)90330-6. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focher F., Mazzarello P., Verri A., Hübscher U., Spadari S. Activity profiles of enzymes that control the uracil incorporation into DNA during neuronal development. Mutat Res. 1990 Mar;237(2):65–73. doi: 10.1016/0921-8734(90)90012-g. [DOI] [PubMed] [Google Scholar]
- Giam C. Z., Xu Y. L. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989 Sep 15;264(26):15236–15241. [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1956–1960. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Stimulation of the nuclear uracil DNA glycosylase in proliferating human fibroblasts. Cancer Res. 1981 Aug;41(8):3133–3136. [PubMed] [Google Scholar]
- Hurst H. C., Jones N. C. Identification of factors that interact with the E1A-inducible adenovirus E3 promoter. Genes Dev. 1987 Dec;1(10):1132–1146. doi: 10.1101/gad.1.10.1132. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Limacher W., Scherrer P., Spadari S. Functions of DNA polymerases alpha, beta, and gamma in neurons during development. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):625–629. doi: 10.1101/sqb.1979.043.01.069. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Functional roles of DNA polymerases beta and gamma. Proc Natl Acad Sci U S A. 1979 May;76(5):2316–2320. doi: 10.1073/pnas.76.5.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Variation of DNA polymerases-alpha, -beta. and -gamma during perinatal tissue growth and differentiation. Nucleic Acids Res. 1977 Aug;4(8):2917–2929. doi: 10.1093/nar/4.8.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Schaffner W. CpG methylation of the cAMP-responsive enhancer/promoter sequence TGACGTCA abolishes specific factor binding as well as transcriptional activation. Genes Dev. 1989 May;3(5):612–619. doi: 10.1101/gad.3.5.612. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Kanei-Ishii C., Sudo T., Yoshimura T., Fujisawa J., Yoshida M., Ishii S. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 1989 Jul;8(7):2023–2028. doi: 10.1002/j.1460-2075.1989.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarello P., Focher F., Verri A., Spadari S. Misincorporation of uracil into DNA as possible contributor to neuronal aging and abiotrophy. Int J Neurosci. 1990 Feb;50(3-4):169–174. doi: 10.3109/00207459008987169. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risse G., Jooss K., Neuberg M., Brüller H. J., Müller R. Asymmetrical recognition of the palindromic AP1 binding site (TRE) by Fos protein complexes. EMBO J. 1989 Dec 1;8(12):3825–3832. doi: 10.1002/j.1460-2075.1989.tb08560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sirover M. A. Induction of the DNA repair enzyme uracil-DNA glycosylase in stimulated human lymphocytes. Cancer Res. 1979 Jun;39(6 Pt 1):2090–2095. [PubMed] [Google Scholar]
- Spadari S., Focher F., Hübscher U. Developmental activity profile of DNA polymerases delta and alpha in rat neurons suggests a coordinated in vivo function. In Vivo. 1988 Sep-Oct;2(5):317–320. [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- Waser J., Hübscher U., Kuenzle C. C., Spadari S. DNA polymerase beta from brain neurons is a repair enzyme. Eur J Biochem. 1979 Jul;97(2):361–368. doi: 10.1111/j.1432-1033.1979.tb13122.x. [DOI] [PubMed] [Google Scholar]
- Weiss B., el-Hajj H. H. The repair of uracil-containing DNA. Basic Life Sci. 1986;38:349–356. doi: 10.1007/978-1-4615-9462-8_38. [DOI] [PubMed] [Google Scholar]