Abstract
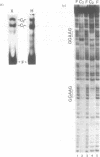
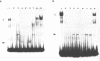
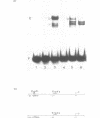
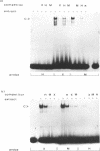
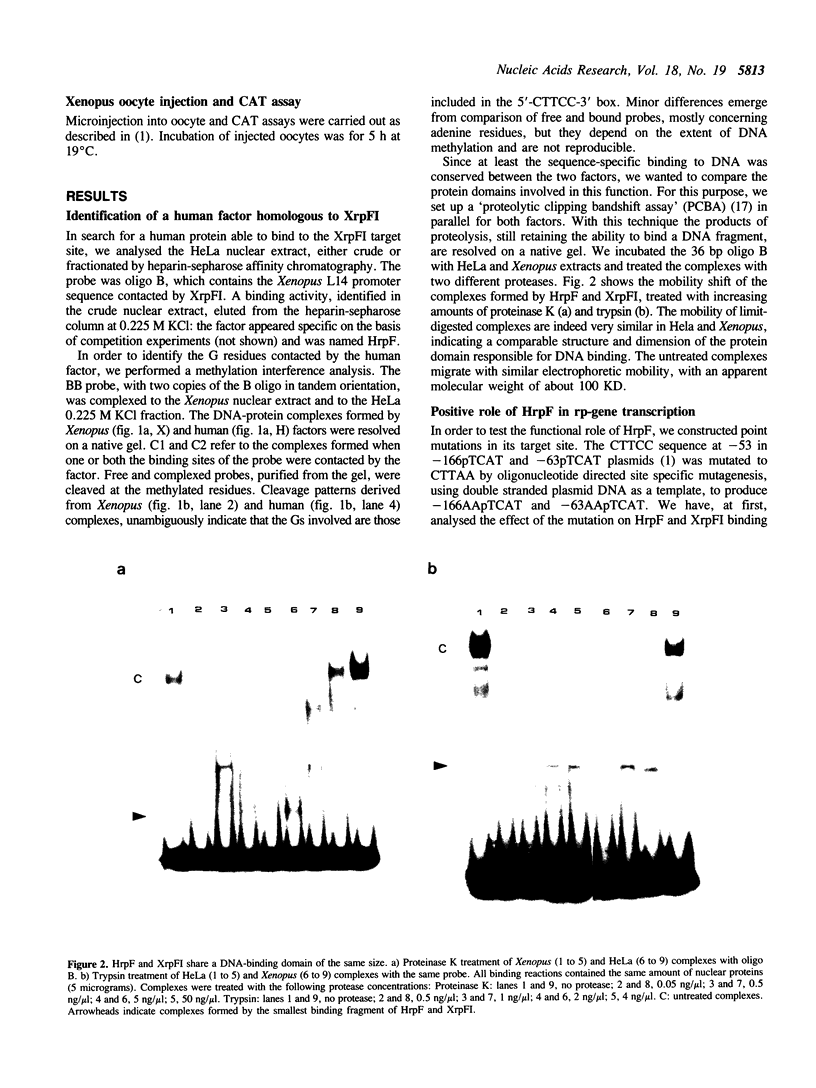
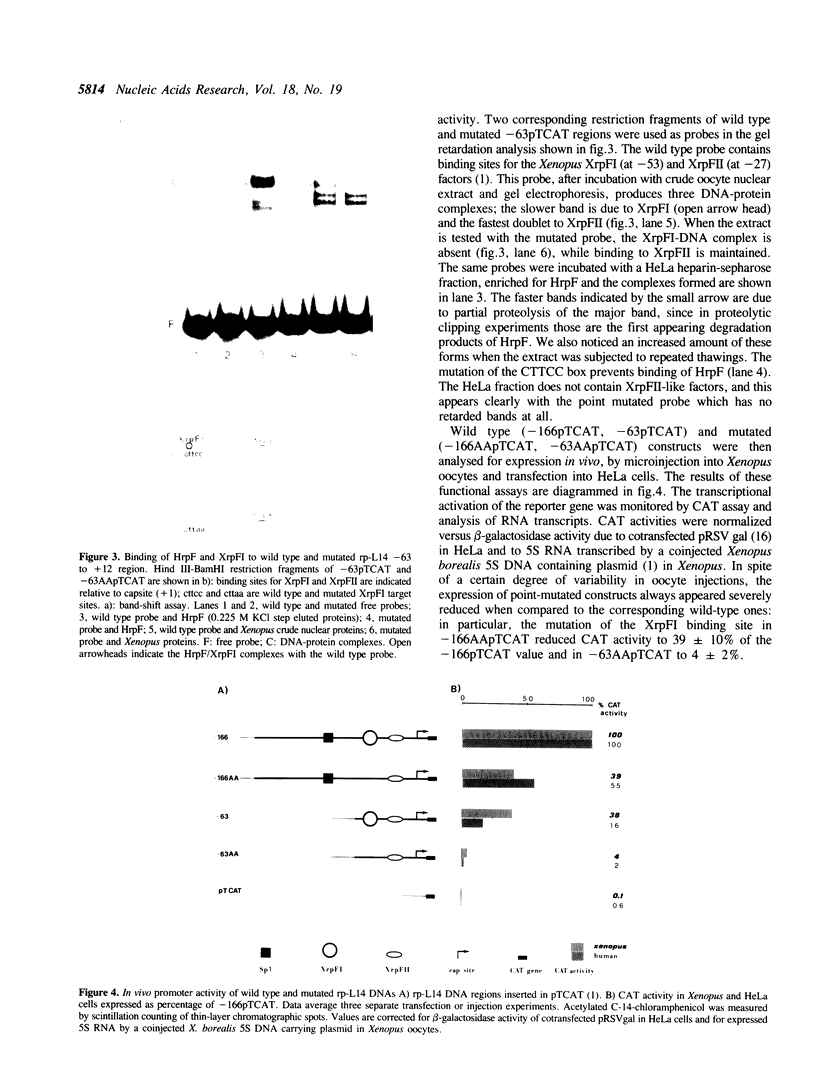
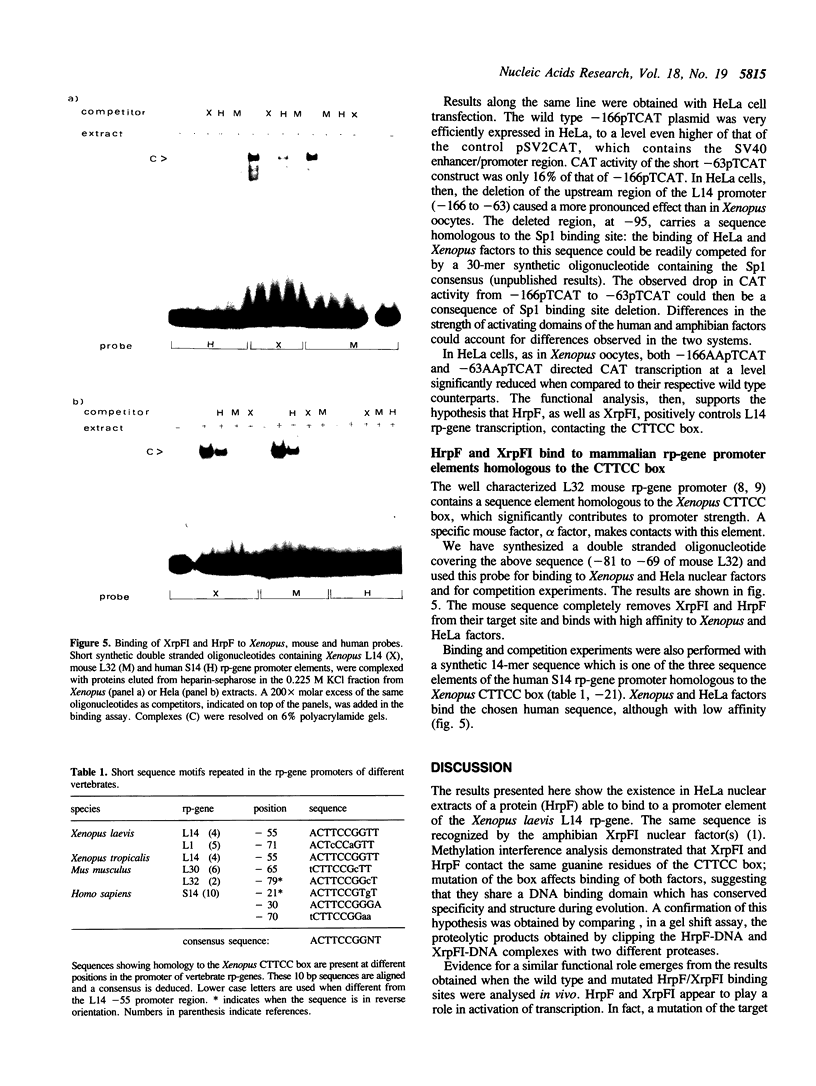
The identification in HeLa nuclei of a novel DNA-binding protein, designated HrpF, is presented. This factor recognizes and binds a sequence of the Xenopus laevis L14 ribosomal protein (r-p) gene promoter bound by the Xenopus r-p transcription factor I (XrpFI). We show here that XrpFI and HrpF share a conserved DNA-binding domain. We also present evidences suggesting that the two factors perform similar functions in the cell. We discuss the hypothesis that closely related factors might be involved in the control of rp-gene transcription in vertebrates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison M. L., Meyuhas O., Perry R. P. Localization of transcriptional regulatory elements and nuclear factor binding sites in mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2067–2074. doi: 10.1128/mcb.9.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari E., Mazzetti P., Mileo A., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Sequences coding for the ribosomal protein L14 in Xenopus laevis and Xenopus tropicalis; homologies in the 5' untranslated region are shared with other r-protein mRNAs. Nucleic Acids Res. 1986 Oct 10;14(19):7633–7646. doi: 10.1093/nar/14.19.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali F., La Porta C., Ilardi V., Beccari E. Nuclear factors specifically bind to upstream sequences of a Xenopus laevis ribosomal protein gene promoter. Nucleic Acids Res. 1989 Oct 25;17(20):8171–8184. doi: 10.1093/nar/17.20.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Perry R. P. Importance of introns for expression of mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2075–2082. doi: 10.1128/mcb.9.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Benoist C., Mathis D. New B-lymphocyte-specific enhancer-binding protein. Mol Cell Biol. 1989 Jan;9(1):312–320. doi: 10.1128/mcb.9.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreni F., Ruberti I., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Nucleotide sequence of the L1 ribosomal protein gene of Xenopus laevis: remarkable sequence homology among introns. EMBO J. 1985 Dec 16;4(13A):3483–3488. doi: 10.1002/j.1460-2075.1985.tb04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager W. H. Control of ribosomal protein gene expression. Biochim Biophys Acta. 1988 Jan 25;949(1):1–15. doi: 10.1016/0167-4781(88)90048-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto K. W., Kaulen H., Roeder R. G. Positive and negative regulation of the gene for transcription factor IIIA in Xenopus laevis oocytes. Genes Dev. 1989 May;3(5):651–662. doi: 10.1101/gad.3.5.651. [DOI] [PubMed] [Google Scholar]
- Wagner M., Perry R. P. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol. 1985 Dec;5(12):3560–3576. doi: 10.1128/mcb.5.12.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]