Abstract
In this study, we have assessed the ability of two TAT-fused peptides PYC36-TAT and JNKI-1-TAT (JNKI-1 or XG-102), which respectively inhibit jun proto-oncogene (c-Jun) and c-Jun N-terminal kinase (JNK) activation, to reduce infarct volume and improve functional outcome (adhesive tape removal) after transient focal cerebral ischemia in Spontaneously Hypertensive (SH) rats. PYC36-TAT and JNKI-1-TAT peptide batches used for experiments were tested in vitro and protected cortical neurons against glutamate excitotoxicity. Rats were treated intravenously with three different doses of PYC36-TAT (7.7, 76, or 255 nmol/kg), JNKI-1-TAT (255 nmol/kg), -TAT peptide (255 nmol/kg), or saline (vehicle control), 10 minutes after reperfusion after 90 minutes of middle cerebral artery occlusion (MCAO). Contrary to other stroke models, no treatment significantly reduced infarct volume or improved functional score measurements compared with vehicle-treated animals when assessed 48 hours after MCAO. Additionally, assessment of the JNKI-1-TAT peptide, when administered 1 or 2 hours after reperfusion after 90 minutes of MCAO, also did not improve histological or functional outcomes at 48 hours after occlusion. This study is the first to evaluate the efficacy of PYC36-TAT and JNKI-1-TAT using the SH rat, which has recently been shown to be more sensitive to AMPA receptor activation rather than to NMDA receptor activation after cerebral ischemia, and which may have contributed to the negative findings.
Keywords: c-Jun, focal cerebral ischemia, JNK, JNKI-1-TAT, PYC36-TAT, Spontaneously Hypertensive rats
Introduction
Stroke is a leading cause of death and disability worldwide. Current treatments for ischemic stroke such as thrombolysis (tissue plasminogen activator), decompressive hemicraniectomy, and aspirin, are aimed at either restoring perfusion to the compromised tissue or preventing recurrence of the ischemic event (Donnan et al, 2008). Targeted neuroprotective therapies that are able to limit the damage to potentially salvageable brain tissue remain unresolved (Campbell et al, 2008). Therefore, agents that block specific cell death pathways, which are activated after cerebral ischemia, are of potential significance in terms of reducing ischemic infarction and improving motor and sensory function.
One well-characterized cell death pathway activated after cerebral ischemia is the mitogen-activated protein kinase pathway, with most attention being focused on the blockade of the c-Jun N-terminal kinase (JNK) protein of this pathway. Less focus has been on the activator protein-1 complex, namely the c-Jun protein, which is downstream of JNK. The activator protein-1 complex comprising homodimerized c-Jun protein (or heterodimerized with other activator protein-1 proteins; c-fos), which is activated after phosphorylation by JNK, promoting the expression of pro-cell death proteins, such as Fas, Fas-L, DP5, Bax, and c-Jun itself (Besirli et al, 2005; Gao et al, 2005). Meanwhile, JNK is also able to phosphorylate other proteins involved in cell death including p53 and Bcl-2 family members Bad, Bax, Bid, and Bim (Cao et al, 2002; Plesnila et al, 2001; Tsuruta et al, 2004; Wang et al, 2007). After cerebral ischemia, besides being phosphorylated by JNK, c-Jun can also be phosphorylated by other kinases such as VRK1, PLK3, p300, and p38δ, and thereby bypass JNK-mediated activation (Besirli et al, 2005; Bessero et al, 2010; Raivich, 2008; Sevilla et al, 2004). Therefore, repression of both JNK and c-Jun represents attractive targets to inhibit cell death after cerebral ischemia.
With respect to c-Jun, our laboratory has identified 19 peptides (hereafter referred to as c-Jun inhibitory peptides) that downregulate activator protein-1 transcription (Meade et al, 2010a). Furthermore, we have shown that several of these peptides when used synthesized to the cell penetrating peptide TAT, have in vitro neuroprotective activity in glutamate, kainic acid, and oxygen-glucose deprivation neuronal injury models (Craig et al, 2011; Meade et al, 2010a, 2010b). One of these c-Jun inhibitory peptides is PYC36-TAT, which, after glutamate exposure, generated an IC50 value of 1.3 μmol/L, which was 1.6-fold lower than the IC50 value we obtained for the JNKI-1-TAT (also known as JNKI-1 or XG-102) inhibitory peptide (Meade et al, 2010a). Since the TAT-fused JNK inhibitory peptide has been shown to be neuroprotective in animal cerebral ischemia and stroke models, we decided to evaluate our c-Jun inhibitory peptide PYC36-TAT in a rat transient middle cerebral artery occlusion (MCAO) stroke model. We administered PYC36-TAT intravenously at different doses while including the JNKI-1-TAT peptide as a positive control. In addition, a delayed treatment study involving JNKI-1-TAT was conducted to determine a therapeutic window in the stroke model.
Materials and methods
Rat Transient Focal Cerebral Ischemia Model
This study was approved by the Animal Ethics Committee of the University of Western Australia and conducted according to the guidelines established for the use of animals in experimental research as outlined by the Australian National Health and Medical Research Council. Male Spontaneously Hypertensive (SH) rats weighing 255 to 305 g were kept under controlled housing conditions with 12 hours light–dark cycle with free access to food and water. Experimental animals were fasted overnight and subjected to 90 minutes of MCAO as follows.
Anesthesia was induced with 4% isoflurane and a 2:1 mix of N2O and O2 via mask. Anesthesia was maintained at 1.8% to 2% isoflurane. Cerebral blood flow was monitored continuously using laser Doppler flowmetry (Blood FlowMeter, AD Instruments, Sydney, NSW, Australia). The probe was located 1 mm posterior to the bregma and 4 mm from the midline of the hemisphere. A cannula was inserted in the right femoral artery to continuously monitor blood pressure and to provide samples for blood glucose and blood gas readings. Blood glucose was measured using a glucometer (MediSense Products, Abbott Laboratories, Bedford, MA, USA) and blood gases were measured using a blood gas analyser (ABL5, Radiometer, Copenhagen, Denmark). Blood pressure was maintained at >90 mm Hg. During surgery, rectal temperature was maintained at 37.5±0.5°C and temporalis muscle temperature as measured with a needle muscle probe (Physitemp Instruments, Clifton, NJ, USA), was maintained at 37.0±0.5°C. A heating fan was used when necessary.
The external carotid artery was ligated and cauterized to create a stump, whereby a 4–0-nylon filament with a 0.39-mm diameter silicone tip (Doccol, Redlands, CA, USA) was inserted into the common carotid artery. The pterygopalatine artery was ligated before filament insertion. The filament was advanced into the left internal carotid artery until laser Doppler flowmetry recorded a drop in cerebral blood flow. The filament was withdrawn after 90 minutes. The procedure was considered successful with a >30% decrease from baseline of cerebral blood flow after insertion of filament and a blood flow increase of >30% of baseline after filament withdrawal. Animals were given postoperative analgesia consisting of pethidine (3 mg/kg intramuscular) and bupivacaine (1.5 mg/kg subcutaneously) at head and leg incision sites. Animals were allowed to recover in a quiet holding room controlled at 27°C. Neurological deficit was confirmed in each animal 1 hour after completion of surgery, by observing flexion of the contralateral forelimb (right side) when suspended by the tail. The rectal temperatures of all experimental animals were monitored for 3 hours postoperatively at 20 minutes intervals and were maintained at 37.5±0.5°C with a heating pad and, when necessary, a heating fan. Occasionally, a cooling water spray was also required if animals became hyperthermic (>38.1°C).
A total of 42 animals were used in the PYC36-TAT dose response experiment. Three animals were euthanased due to subarachnoid hemorrhage, one animal was excluded due to insufficient increase in laser Doppler flowmetry at reperfusion and one animal was excluded due to hyperthermia postsurgery. In the delayed JNKI-1-TAT treatment trials, a total of 35 animals were used. Three animals were euthanased due to subarachnoid hemorrhage and three animals were excluded due to insufficient increase and/or decrease in laser Doppler flowmetry at reperfusion or induction.
PYC36-TAT, JNKI-1-TAT, and -TAT Peptides
The peptides were synthesized fused to the TAT cell penetrating peptide (HIV-1 TAT(48–57)), in the protease-resistant -retro-inverso form and high-performance liquid chromatography purified by Mimotopes Pty Ltd (Melbourne, VIC, Australia). Peptide sequences were as follows: PYC36-TAT; H-PKISQYGQRRRGQLGGRRRQRRKKRG-NH2 and JNKI-1-TAT; H-TDQSRPVQPFLNLTTPRKPRPPRRRQRRKKRG-NH2 (Underlined single letter code indicates -isoform of the amino acid. TAT transduction domain is indicated by bold lettering). The -retro-inverso form of the TAT peptide (-TAT; H-GRRRQRRKKRG-NH2) was also synthesized and used as a control. All peptides were prepared in normal saline in 500 μL volumes for intravenous administration and stored at −80°C before use. PYC36-TAT and JNKI-1-TAT peptide batches used for experiments were previously confirmed to be active/neuroprotective using an in vitro glutamate excitotoxicity model (Meade et al, 2010a).
PYC36-TAT Dose Response Experiment
Treatment groups (N=5) consisted of vehicle (saline), PYC36-TAT at three different doses (7.6, 76, and 255 nmol/kg or 0.03, 0.3, and 1 mg/kg), JNKI-1-TAT (255 nmol/kg or 1 mg/kg), and -TAT (255 nmol/kg). Application of treatment/vehicle was performed 10 minutes after reperfusion in a single bolus injection (500 μL) via the right jugular vein. The jugular vein was surgically exposed and isolated to allow visual confirmation of intravenous injections. All treatments or vehicle were randomized and were administered in a blinded manner.
Delayed JNKI-1-TAT Treatment Experiment
Treatment groups (N=7 to 8) consisted of vehicle (saline) and JNKI-1-TAT (255 nmol/kg) administered at one of two different time points after reperfusion. The two treatment time points were 1 and 2 hours after reperfusion in a single bolus injection (500 μL) via the jugular vein. An indwelling cannula was inserted into the right jugular vein and secured onto an external tether/swivel system to allow for intravenous administration of vehicle/treatment at the appropriate time points after reperfusion.
Tissue Processing and Infarct Volume Measurement
Animals were killed 48 hours after reperfusion with intraperitoneal injections of sodium pentobarbitone (900 mg/kg). After euthanasia, the brain was removed and placed in a sterile container of 0.9% NaCl and then placed in an −80°C freezer for 7 minutes. The brain was then coronally sliced from the junction of the cerebellum and cerebrum to 12 mm rostral to this point in 2 mm thick slices.
Slices were immediately stained with 2% 2,3,5 triphenyltetrazolium chloride (Sigma, St Louis, MO, USA) at 37°C for 15 minutes, followed by fixation in 4% formalin at room temperature for at least 18 to 24 hours before infarct volume measurement. Slices were scanned and images were analyzed by an operator blind to treatment status using ImageJ 3rd edition (NIH, USA). The total infarct volume was determined by measuring the areas of infarcted tissue on both sides of the 2 mm sections. These measured areas were multiplied by half slice thickness (1 mm), and corrected for cerebral edema by multiplying the ratio of affected to normal hemisphere areas (Campbell et al, 2008).
Adhesive Removal Test
This test measured the detection of sensory parameter and reaction to motor component small pieces of adhesive tape placed on the forelimbs (Esneault et al, 2008). Animals had adhesive removal tests performed before surgery for MCAO and at 48 hours after reperfusion. Animals were placed in a transparent enclosure for 2 minutes before starting the tests in order to adapt to placement in a new enclosure (habituation). Adhesive tape (Diversified Biotech, Dedham, MA, USA) was placed on the palmar surface of the paw and the time from first contact (detection) of adhesive tape to time of removal of adhesive was measured and recorded for each forelimb (maximum of 180 seconds).
Statistical Analysis
For infarct volume measurements, each treatment group was compared with its respective vehicle control group by analysis of variance followed by post hoc Bonferroni/Dunn test. The data obtained are presented as mean±standard deviation. Analysis of variance was employed to compare physiological parameters between groups. For the adhesive tape test, data were analyzed using both univariate and multivariate linear regression with the statistical package R (version 2.11.1; http://www.R-project.org). A value of P<0.05 was considered significant for all data sets.
Results
Physiological Measurements
Preischemia, during ischemia, and after reperfusion, there were no significant differences between the treatment groups in arterial blood pressure, blood gases (PaCO2 and PaO2), and blood pH in the PYC36-TAT dose response experiment (Table 1) and the delayed JNKI-1-TAT treatment experiments (Table 2).
Table 1. Physiological data for PYC36-TAT dose response trial.
|
Experimental groups |
||||||
|---|---|---|---|---|---|---|
| Vehicle saline (N=5) | PYC36-TAT 7.6 nmol/kg (N=5) | PYC36-TAT 76 nmol/kg (N=5) | PYC36-TAT 255 nmol/kg (N=5) | JNKI-1-TAT 255 nmol/kg (N=5) | -TAT 255 nmol/kg (N=5) | |
| MABP (mm Hg) | ||||||
| Preischemia | 102.5±5.0 | 105±10.0 | 102.0±7.6 | 107.0±6.7 | 100.0±14.7 | 110.0±8.0 |
| During ischemia | 103.8±11.1 | 110±6.1 | 107.0±5.7 | 106.0±7.4 | 108.8±13.1 | 112.0±9.1 |
| Postischemia | 101.3±10.3 | 106±8.2 | 103.0±12.0 | 105.0±10.0 | 102.5±10.4 | 109.0±7.4 |
| PaCO2 (mm Hg) | ||||||
| Preischemia | 41.3±6.2 | 38.3±3.1 | 39.7±2.1 | 41.3±5.0 | 38.4±3.7 | 38.0±1.6 |
| During ischemia | 37.8±6.7 | 38.7±4.0 | 40.3±4.2 | 43.3±1.7 | 39.4±3.3 | 38.3±4.8 |
| Postischemia | 43.8±7.0 | 39.7±4.7 | 47.0±4.0 | 42.0±1.8 | 40.8±1.8 | 37.0±5.0 |
| PaO2 (mm Hg) | ||||||
| Preischemia | 102.8±15.9 | 120.8±7.0 | 112.0±15.0 | 116.4±7.1 | 121.8±9.5 | 114.0±20.4 |
| During ischemia | 95.4±17.0 | 119.0±14.1 | 112.0±16.4 | 106.6±13.8 | 116.2±2.4 | 119.4±20.6 |
| Postischemia | 92.8±11.4 | 116.3±8.1 | 99.0±13.1 | 105.2±14.4 | 110.8±13.8 | 117.0±26.7 |
| Blood glucose (mmol/L) | 6.56±1.0 | 6.28±0.5 | 6.48±1.1 | 5.98±0.9 | 6.16±0.3 | 6.06±0.4 |
MABP, mean arterial blood pressure.
There were no significant differences between groups for measurements preischemia, during ischemia, or postischemia; values are means±standard deviation.
Table 2. Physiological data for delayed JNKI-1-TAT treatment trials (1 and 2 hours).
|
Experimental groups |
||||
|---|---|---|---|---|
| Vehicle saline (1 hour: N=7) | JNKI-1-TAT, 255 nmol/kg (1 hour: N=7) | Vehicle saline (2 hours: N=8) | JNKI-1-TAT, 255 nmol/kg (2 hours: N=7) | |
| MABP (mm Hg) | ||||
| Preischemia | 112.8±10.3 | 114±18.5 | 118.6±22.7 | 111.4±16.5 |
| During ischemia | 107.1±6.4 | 107±7.0 | 109.3±11.0 | 104.3±1594 |
| Postischemia | 104.3±7.3 | 109±5.3 | 105.9±15.0 | 102.9±14.1 |
| PaCO2 (mm Hg) | ||||
| Preischemia | 44.0±6.0 | 39.1±5.0 | 37.3±5.5 | 38.7±3.4 |
| During ischemia | 43.0±4.8 | 39.0±3.4 | 35.0±1.7 | 37.7±5.3 |
| Postischemia | 41.0±3.1 | 37.3±3.3 | 35.1±2.2 | 35.4±3.9 |
| PaO2 (mm Hg) | ||||
| Preischemia | 111.4±15.1 | 113.0±19.6 | 127.0±16.3 | 125.0±11.5 |
| During ischemia | 113.1±9.8 | 116.1±17.8 | 122.4±15.7 | 124.3±11.7 |
| Postischemia | 114.8±9.4 | 119.6±18.4 | 123.4±13.0 | 127.40±9.6 |
| Blood glucose (mmol/L) | 6.5±0.5 | 6.1±0.8 | 7.0±0.7 | 6.7±2.0 |
MABP, mean arterial blood pressure.
PYC36-TAT Dose Response Experiment
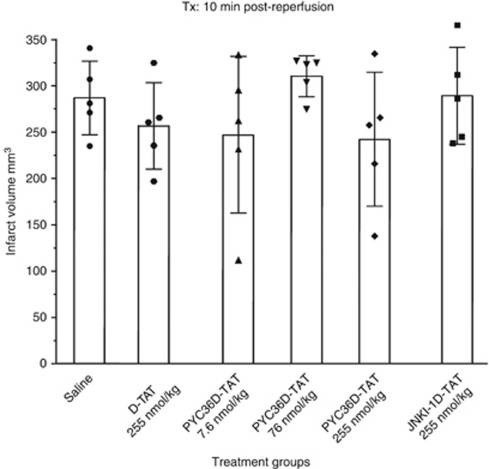
Intravenous administration of the PYC36-TAT peptide 10 minutes after reperfusion at three different doses did not significantly reduce infarct volume when measured 48 hours after 90 minutes of MCAO (Figure 1). Similarly, the JNKI-1-TAT and -TAT peptides also did not significantly reduce lesion volume when compared with the vehicle-treated controls (Figure 1).
Figure 1.
Effect of different doses of PYC36-TAT on infarct volume after 90 minutes of focal ischemia in Spontaneously Hypertensive (SH) rats assessed at 48 hours. Peptides administered 10 minutes after reperfusion. Values are the mean (±standard deviation) infarct volumes.
In the adhesive tape removal test, there were no significant differences between any of the treatment groups in the posttreatment reaction or removal times of both left and right sides (data not shown).
Delayed JNKI-1-TAT Experiment
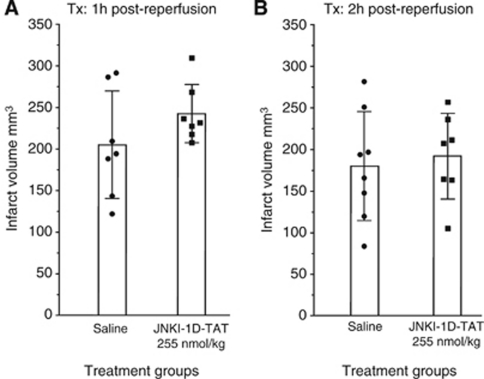
Delayed intravenous administration of the JNKI-1-TAT peptide at 1 or 2 hours after reperfusion at the 255-nmol/kg dose did not significantly reduce infarct volume when measured 48 hours after 90 minutes of MCAO (Figure 2). Although these two treatment groups show no significant difference, the mean infarct volume of JNKI-1-TAT-treated rats was higher than that of the mean infarct volume of vehicle-treated rats at both the 1- and 2-hour postreperfusion administration time points.
Figure 2.
Effect of JNKI-1-TAT on infarct volume after 90 minutes of focal ischemia in Spontaneously Hypertensive (SH) rats assessed at 48 hours. (A) Treatments administered 1 hour after reperfusion. (B) Treatments administered 2 hours after reperfusion. Values are the mean (±standard deviation) infarct volumes.
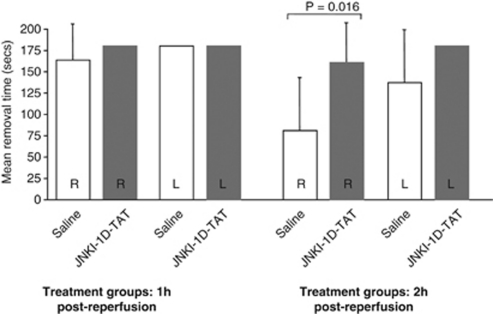
In the adhesive tape removal test, there was no statistically significant difference between saline and treatment groups in the 1-hour postreperfusion administration trial (data not shown). In the 2-hour postreperfusion administration trial, there was no difference in tape removal for the left side; however, vehicle-treated animals were significantly better at removing tape on their right side compared with JNKI-1-TAT-treated rats (P=0.028; Figure 3).
Figure 3.
Effect of JNKI-1-TAT treatment on adhesive tape removal time after 90 minutes of focal ischemia in Spontaneously Hypertensive (SH) rats assessed at 48 hours (1 and 2 hours after reperfusion treatment times). Values are the mean (±standard deviation) adhesive removal times. A maximum of 180 seconds was allowed for each trial.
Discussion
The initial focus of this study was to assess the neuroprotective potential of the c-Jun inhibitory peptide PYC36-TAT in an animal stroke model, after the demonstration of high neuroprotective efficacy of this peptide in an in vitro cortical neuronal glutamate excitotoxicity model (IC50: 1.3 versus 2.1 μmol/L for JNKI-1-TAT; Meade et al, 2010a). In addition, PYC36-TAT and JNKI-1-TAT showed similar efficacy to each other, albeit at a lower level than that in the glutamate model, in reducing cell death in kainic acid and oxygen-glucose deprivation neuronal culture injury models (Meade et al, 2010b; Craig et al, 2011). Despite the positive prior in vitro results, our experimental findings in the present study show that neither PYC36-TAT nor JNKI-1-TAT induced significant neuroprotection or improved motor and sensory function when administered at early (10 minutes) and/or delayed (1 or 2 hours) times after reperfusion in a rat transient MCAO stroke model. While PYC36-TAT was previously untested after cerebral ischemia, previous studies have shown that JNKI-1-TAT is neuroprotective in several stroke models (Esneault et al, 2008; Liu et al, 2010; Repici et al, 2007; Wiegler et al, 2008); and hence, the negative finding with this peptide was somewhat unexpected. However, several cerebral ischemia studies have reported negative outcomes when using JNKI-1-TAT (Ginet et al, 2009; Liu et al, 2010; Soriano et al, 2008).
Our experimental trial for the assessment of PCY36-TAT (and JNKI-1-TAT) was designed to increase the likelihood of generating a positive outcome. We chose to administer the peptide intravenously 10 minutes after reperfusion (100 minutes after ischemia) to allow access to the brain well before peak c-Jun and JNK activation, which start to increase 1 hour after reperfusion and peaking in the penumbra 6 to 8 hours after ischemia (Gao et al, 2005; Okuno et al, 2004; Repici et al, 2007). In addition, a dosing regimen over an ≈33-fold range (7.6, 76, and 255 nmol/kg) was used to minimize the risk of using a dose outside a potential effective range. The dosing range was selected based on previous effective doses reported for JNKI-1-TAT when administered intravenously or intraperitoneally before ischemia or after reperfusion in different rodent stroke models (Benakis et al, 2010; Esneault et al, 2008; Soriano et al, 2008; Wiegler et al, 2008). While the proposed positive control peptide JNKI-1-TAT was only used at one dose (255 nmol/kg), this dose is well within the effective does range reported for this peptide (Benakis et al, 2010; Esneault et al, 2008; Soriano et al, 2008; Wiegler et al, 2008). We also used the isoform for both peptides, which provides more stability and potency (Borsello et al, 2003; Meade et al, 2010a). Additionally, we confirmed that both batches of PCY36-TAT and JNKI-1-TAT peptide used in the animal experiments had potent neuroprotective activity in our in vitro glutamate excitotoxicity model (unpublished observation).
In an attempt to reveal any neuroprotective effects with these downstream mitogen-activated protein kinase pathway inhibitory peptides, we performed two additional trials with JNKI-1-TAT, using a delayed postreperfusion treatment regimen. The rationale for the trials was two-fold: First, by delaying treatment until 1 or 2 hours after reperfusion, delivery of JNKI-1-TAT into ischemic brain tissue would potentially be enhanced due to blood–brain barrier breakdown, which can occur as early as 30 minutes and as late as 2.5 hours after reperfusion (Neumann-Haefelin et al, 2000; Durukan et al, 2009). Second, it would allow for greater bioavailability of the peptide at a time closer to the 4- to 6-hour postischemia peak JNK activation time point, and thus maximize the ability of JNKI-1-TAT to counteract downstream signaling events. It should also be mentioned that previous studies using postreperfusion time points between 1.5 and 5.5 hours after transient MCAO have generated significant infarct reductions when JNKI-1-TAT was administered intravenously or intraperitoneally at doses ranging from 0.076 to 760 nmol/kg.
Based on our negative experimental outcomes with both PCY36-TAT and JNKI-1-TAT, and positive trials reported by other laboratories with the JNKI-1-TAT in stroke and cerebral ischemia models, several explanations for the conflicting results are possible. A unique aspect of our study was the use of the SH rat strain. The SH rat is commonly used in stroke studies because it provides consistent lesion size (Howells et al, 2010) and because hypertension is an important comorbidity in stroke. However, it has recently been shown that SH rats have an atypical patho-molecular response to glutamate receptor activation, a key feature of ischemic brain injury. Usually, overactivation of N-methyl--aspartic acid (NMDA) receptors leads to an increase in intracellular calcium levels and/or detrimental signaling pathways, which in turn can directly or indirectly cause much of the downstream damaging processes associated with ischemic stroke. In contrast, the SH rat has been shown to be much more sensitive than other strains to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation (which has different downstream effects to NMDA) and to be relatively resistant to NMDA activation (Lecrux et al, 2007). To this end, JNKI-1-TAT has shown high efficacy after NMDA excitotoxicity (Borsello et al, 2003) and we have also shown efficacy of PYC36-TAT (and JNKI-1-TAT) after NDMA exposure (unpublished observation). Consequently, there is a possibility that the negative results obtained in this study, at least in part, are due to the SH rat strain's-specific predominance in AMPA activated, rather than NMDA activated in ischemic brain injury. If so, comparison of this model with other models may prove useful in dissecting the mechanism of neuroprotection of different agents active in distinct pathways.
It is also possible that the JNKI-1-TAT and PYC36-TAT peptides have limited efficacy in vivo when the ischemic insult is severe and/or the peptides do not reach therapeutic levels (i.e., there was a technical rather than theoretical basis for the findings). For example, early studies used intraventricular delivery of JNKI-1-TAT and/or 14-day-old rats for MCAO stroke models (Borsello et al, 2003; Hirt et al, 2004; Gao et al, 2005; Repici et al, 2007; Soriano et al, 2008). Interestingly, in the Soriano et al (2008) study, 14-day-old rats administered JNKI-1-TAT (76 nmol/kg) intraperitoneally 6 hours after MACO were neuroprotective in a mild permanent focal ischemia model, but not when administered 30 minutes before or 6 hours after MCAO in a more severe ischemic form of the model. In addition, three different intraperitoneal doses of the JNKI-1-TAT peptide (125, 510, and 5100 nmol/kg) were not neuroprotective when administered 30 minutes before transient MCAO; however, the 510-nmol/kg dose was protective when administered 2.5 hours after reperfusion.
Despite the use of SH rats, which are considered to produce a consistent ischemic lesion (Howells et al, 2010), an unusual outcome of our study was the different mean infarct volumes obtained for control animals between trials. Mean infarct volume for saline-treated controls in the 10-minutes, 1-, and 2-hour treatment trials was 287, 206, and 180 mm3, respectively. The decreasing infarct volumes in each trial are suggestive that the ischemic severity was less and/or animals were more resistant to ischemia. Since the surgical protocol for each trial was the same and each trial was performed independently, any differences in ischemic severity/animal susceptibility would be expected to apply equally across all treatments within a trial, and are therefore not expected to confound treatment outcomes. One explanation for this may be seasonal fluctuations of brain neuropeptides or hormones. Although these animals are nonphotoperiodic rodents, maintained in rigorously controlled laboratory conditions for generations, studies have shown intrastrain variations of neuropeptides and hormones (Bissette et al, 1995; Vázquez et al, 2004a, 2004b; Wong et al, 1983), which may have a compounding factor on infarct volumes after MCAO. Moreover, the smaller infarct volumes obtained in the delayed JNKI-1-TAT 1 and 2 hours treatment trials would favor a treatment effect, but this was not the case.
Taken together, our findings reveal that two mitogen-activated protein kinase pathway inhibitory peptides PYC36-TAT and JNKI-1-TAT with potent in vitro neuroprotective activity in glutamate excitotoxic injury models, when administered as an intravenous bolus dose do not reduce infarct volume or improve functional outcome after transient focal ischemia in SH rats. These findings highlight the need for extensive preclinical animal stroke studies to better gauge the therapeutic potential of putative neuroprotective agents before consideration in clinical trials. To this end, additional experimental studies are warranted to better understand the mechanism of neuroprotection of these peptides and their relevance to clinical applications. Such studies include examining efficacy using a constant infusion over extended periods (12 to 24 hours) and using different doses, assessment in other experimental models (permanent MCAO, embolic occlusion models±tissue plasminogen activator) and in animals with comorbidities (aged animals and animals with diabetes). Finally, at least in the case of JNKI-1-TAT (XG-102), a further indication of this peptide's neuroprotective potential may be gleaned following the outcome of the phase I/II stroke trial assessing this agent (Wiegler et al, 2008; ARAMIS, 2011).
Acknowledgments
The authors would like to acknowledge Joanne Chieng for technical assistance.
PMW is an Executive Director for Phylogica Ltd Pty. NM is a Senior Scientist working for Phylogica. BPM is a Phylogica shareholder.
Footnotes
This study was supported by the Neuromuscular Foundation of Western Australia and by a grant from the National Heart Foundation (Australia).
References
- ARAMIS 2011Project number: 8909.1 . http://www.aramis.admin.ch
- Benakis C, Bonny C, Hirt L. JNK inhibition and inflammation after cerebral ischemia. Brain Behav Immun. 2010;24:800–811. doi: 10.1016/j.bbi.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Besirli CG, Wagner EF, Johnson EM., Jr The limited role of NH2-terminal c-Jun phosphorylation in neuronal apoptosis: identification of the nuclear pore complex as a potential target of the JNK pathway. J Cell Biol. 2005;170:401–411. doi: 10.1083/jcb.200501138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessero AC, Chiodini F, Rungger-Brändle E, Bonny C, Clarke PG. Role of the c-Jun N-terminal kinase pathway in retinal excitotoxicity, and neuroprotection by its inhibition. J Neurochem. 2010;113:1307–1318. doi: 10.1111/j.1471-4159.2010.06705.x. [DOI] [PubMed] [Google Scholar]
- Bissette G, Griff D, Carnes M, Goodman B, Lavine M, Levant B. Apparent seasonal rhythms in hypothalamic neuropeptides in rats without photoperiod changes. Endocrinology. 1995;136:622–628. doi: 10.1210/endo.136.2.7835296. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Campbell K, Meloni BP, Knuckey NW. Combined magnesium and mild hypothermia (35°C) treatment reduces infarct volumes after permanent middle cerebral artery occlusion in the rat at 2 and 4, but not 6 hours. Brain Res. 2008;1230:258–264. doi: 10.1016/j.brainres.2008.06.110. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AJ, Meloni BP, Watt PM, Knuckey NW. Attenuation of neuronal death by peptide inhibitors of AP-1 activation in acute and delayed in vitro ischaemia (oxygen/glucose deprivation) models. Int J Pept Res Ther. 2011;17:1–6. [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Durukan A, Marinkovic I, Strbian D, Pitkonen M, Pedrono E, Soinne L, Abo-Ramadan U, Tatlisumak T. Post-ischemic blood-brain barrier leakage in rats: one-week follow-up by MRI. Brain Res. 2009;1280:158–165. doi: 10.1016/j.brainres.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Esneault E, Castagne V, Moser P, Bonny C, Bernaudin M. D-JNKi, a peptide inhibitor of c-Jun N-terminal kinase, promotes functional recovery after transient focal cerebral ischemia in rats. Neuroscience. 2008;152:308–320. doi: 10.1016/j.neuroscience.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, Luo Y, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- Ginet V, Puyal J, Magnin G, Clarke PG, Truttmann AC. Limited role of the c-Jun N-terminal kinase pathway in a neonatal rat model of cerebral hypoxia-ischemia. J Neurochem. 2009;108:552–562. doi: 10.1111/j.1471-4159.2008.05797.x. [DOI] [PubMed] [Google Scholar]
- Hirt L, Badaut J, Thevenet J, Granziera C, Regli L, Maurer F, Bonny C, Bogousslavsky J. D-JNKI1, a cell-penetrating c-Jun-N-terminal kinase inhibitor, protects against cell death in severe cerebral ischemia. Stroke. 2004;35:1738–1743. doi: 10.1161/01.STR.0000131480.03994.b1. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Rewell SSJ, O'Collins V, Sena ES, van der Worp HB, Traystman RT, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, Nicole O, Chazalviel L, Catone C, Chuquet J, MacKenzie ET, Touzani O. Spontaneously Hypertensive Rats are highly vulnerable to AMPA-induced brain lesions. Stroke. 2007;38:3007–3015. doi: 10.1161/STROKEAHA.107.491126. [DOI] [PubMed] [Google Scholar]
- Liu J-R, Zhao Y, Patzer A, Staak N, Boehm G, Culman J, Bonny C, Herdegen T, Eschenfelder C. The c-Jun N-terminal kinase (JNK) inhibitor XG-102 enhances the neuroprotection of hyperbaric oxygen after cerebral ischaemia in adult rats. Neuropathol Appl Neurobiol. 2010;36:211–224. doi: 10.1111/j.1365-2990.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- Meade AJ, Meloni BP, Cross J, Bakker AJ, Fear MW, Mastaglia FL, Watt PM, Knuckey NW. AP-1 inhibitory peptides are neuroprotective following acute glutamate excitotoxicity in primary cortical neuronal cultures. J Neurochem. 2010a;112:258–270. doi: 10.1111/j.1471-4159.2009.06459.x. [DOI] [PubMed] [Google Scholar]
- Meade AJ, Meloni BP, Mastaglia FL, Watt PM, Knuckey NW. AP-1 inhibitory peptides attenuate in vitro cortical neuronal cell death induced by kainic acid. Brain Res. 2010b;1360:8–16. doi: 10.1016/j.brainres.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–1973. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, Chiarugi A, Thomas SS, Kohane DS, Korsmeyer SJ, Moskowitz MA. BID mediates neuronal cell death after oxygen/glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G. c-Jun expression, activation and function in neural cell death, inflammation and repair. J Neurochem. 2008;107:898–906. doi: 10.1111/j.1471-4159.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- Repici M, Centeno C, Tomasi S, Forloni G, Bonny C, Vercelli A, Borsello T. Time-course of c-Jun N-terminal kinase activation after cerebral ischemia and effect of D-JNKI1 on c-Jun and caspase-3 activation. Neuroscience. 2007;150:40–49. doi: 10.1016/j.neuroscience.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Sevilla A, Santos CR, Barcia R, Vega FM, Lazo PA. c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK) Oncogene. 2004;23:8950–8958. doi: 10.1038/sj.onc.1208015. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Martel MA, Papadia S, Vaslin A, Baxter P, Rickman C, Forder J, Tymianski M, Duncan R, Aarts M, Clarke P, Wyllie DJ, Hardingham GE. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. J Neurosci. 2008;28:10696–10710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez N, Debeljuk L, Díaz E, Fernández C, Díaz B. Seasonal changes of SP and NKA in frontalcortex, striatum and testes in the rat. Role of maternal pineal gland. Peptides. 2004a;25:997–1004. doi: 10.1016/j.peptides.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Vázquez N, Debelkuk L, Díaz E, Fernández C, Díaz B. Influence of maternal pineal gland on the developmental pattern of neurokinin A (NKA) and substance P (SP) in male-rat-offspring. Relationship to the season of the year. Neurosci Lett. 2004b;368:243–248. doi: 10.1016/j.neulet.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Wang XT, Pei DS, Xu J, Guan QH, Sun YF, Liu XM, Zhang GY. Opposing effects of Bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell Signal. 2007;9:1844–1856. doi: 10.1016/j.cellsig.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Wiegler K, Bonny C, Coquoz D, Hirt L. The JNK inhibitor XG-102 protects from ischemic damage with delayed intravenous administration also in the presence of recombinant tissue plasminogen activator. Cerebrovasc Dis. 2008;26:360–366. doi: 10.1159/000151639. [DOI] [PubMed] [Google Scholar]
- Wong CC, Döhler KD, Atkinson MJ, Geerlings H, Hesch RD, Von Zur Mühlen A. Circannual variations in serum concentrations of pituitary, thyroid, parathyroid, gonadal and adrenal hormones in male laboratory rats. J Endocrinol. 1983;97:179–185. doi: 10.1677/joe.0.0970179. [DOI] [PubMed] [Google Scholar]