Abstract
In mouse hippocampal CA1 pyramidal neurons, the activity of synaptic small-conductance Ca2+-activated K+ channels type 2 (SK2 channels) provides a negative feedback on N-methyl--aspartate receptors (NMDARs), reestablishing Mg2+ block that reduces Ca2+ influx. The well-established role of NMDARs in ischemia-induced excitotoxicity led us to test the neuroprotective effect of modulating SK2 channel activity following cerebral ischemia induced by cardiac arrest and cardiopulmonary resuscitation (CA/CPR). Administration of the SK channel positive modulator, 1-ethyl-benzimidazolinone (1-EBIO), significantly reduced CA1 neuron cell death and improved CA/CPR-induced cognitive outcome. Electrophysiological recordings showed that CA/CPR-induced ischemia caused delayed and sustained reduction of synaptic SK channel activity, and immunoelectron microscopy showed that this is associated with internalization of synaptic SK2 channels, which was prevented by 1-EBIO treatment. These results suggest that increasing SK2 channel activity, or preventing ischemia-induced loss of synaptic SK2 channels, are promising and novel approaches to neuroprotection following cerebral ischemia.
Keywords: cardiac arrest, electrophysiology, excitotoxicity, global ischemia, hippocampus, potassium channels
Introduction
Sudden cardiac arrest (CA) is a leading cause of mortality and morbidity. Each year ∼200,000 people in the United States suffer severe cardiac arrest, requiring cardiopulmonary resuscitation (CPR) (Lloyd-Jones et al, 2009). Transient global ischemia following CA/CPR elicits selective, delayed neuronal cell death of specific subpopulations of vulnerable neurons, particularly hippocampal CA1 neurons (for review see Crepel et al, 2003). While the precise mechanisms are not fully understood, several Ca2+-permeable membrane proteins have been implicated, including N-methyl--aspartate receptors (NMDARs), Ca2+-permeable AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors, acid sensing ion channels (ASIC) and transient receptor potential M7 channels (TRPM7) (for reviews see Szydlowska and Tymianski, 2010; Moskowitz et al, 2010). The most well-characterized pathway mediating ischemia-induced damage involves the rapid rise in extracellular glutamate that follows reperfusion in the ischemic brain (Benveniste et al, 1984). This causes excessive excitatory glutamatergic neurotransmission that results in excitotoxic influx of Ca2+ through NMDARs, triggering the production of free radicals and activation of other enzymatic processes that leads to cell death (Lo et al, 2003). Consistent with this model, a large number of animal studies have shown that glutamate receptor antagonists effectively prevent excitotoxic neuronal cell death (Choi et al, 1988; Gill et al, 1988; Zipfel et al, 1999; Planells-Cases et al, 2002), and decrease injury following focal (Gill et al, 1988; Boast et al, 1988) and global (Hicks et al, 1999; Janac et al, 2008) cerebral ischemia. Indeed, overstimulation of NMDARs represents an important common pathway in several acute and chronic neurologic disorders, such as stroke, brain trauma, epilepsy, Alzheimer's disease, and Parkinson's disease (Waxman and Lynch, 2005). Unfortunately, human clinical trials with compounds that inhibit glutamate receptors have proven unsuccessful, largely due to unacceptable side effects (Ginsberg, 2008). Therefore, alternative mechanisms that limit NMDAR-induced excitotoxicity may provide targets for novel intervention strategies to minimize the consequences of cerebral ischemia.
SK channels are K+ channels that are activated solely by intracellular Ca2+ ions. They are selectively blocked by the peptide toxin, apamin (Deschaux and Bizot, 1997), and potentiated by compounds such as 1-ethyl-benzimidazolinone (1-EBIO) (Pedarzani et al, 2001). In hippocampal CA1 pyramidal neurons, SK2 channels are colocalized with NMDARs in the synaptic membrane of dendritic spines, in the postsynaptic density (PSD) SK channels are K+ channels that are activated solely by intracellular Ca2+ ions. Synaptically evoked Ca2+ influx activates synaptic SK2 channels; their repolarizing activity diminishes AMPA receptor-mediated depolarization and promotes Mg2+ block of NMDARs thereby limiting Ca2+ influx. Thus, in CA1 pyramidal neurons, synaptic SK2 channels are endogenous use-dependent inhibitors of NMDAR activity and Ca2+ influx (Ngo-Anh et al, 2005). This leads to the hypothesis that increased SK2 channel activity may be neuroprotective against ischemia-induced cell death.
To test the effects of altering SK channel activity on neuronal survival and cognitive outcome following CA/CPR, SK channel activity was pharmacologically enhanced before or after CA/CPR-induced cerebral ischemia. The results show that enhancement of SK channel activity significantly reduces CA/CPR-induced damage to CA1 pyramidal neurons and improves cognitive outcome. Electrophysiological recordings show that CA/CPR-induced ischemia causes a reduction of functional synaptic SK channels within hours of resuscitation, and treating the mice with neuroprotective doses of 1-EBIO prevents this reduction. These functional data are supported by immunogold-electron microscopy (iEM) showing that SK2 immunoparticles are absent from the PSD following CA/CPR and that 1-EBIO treatment prevents their removal.
Materials and methods
Experimental Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University in accordance with the National Institutes of Health guidelines for the care and use of animals in research. All experiments were performed in a blinded randomized manner using male C57Bl/6(J) wild-type (WT), and SK2-OE mice ∼12 weeks old (20 to 25 g). SK2 transgenic mice have been backcrossed >20 generations into C57Bl/6J background, therefore this strain used as WT. The 1-EBIO experiments were performed in C57Bl/6 mice.
Cardiac Arrest and Treatment Groups
Male mice were subjected to CA/CPR as previously described (Kofler et al, 2004; Kelley et al, 2008). Briefly, anesthesia was induced with 3% isoflurane and maintained with 1% to 1.5% isoflurane in O2 enriched air via face mask. Temperature probes were inserted into the left temporalis muscle and rectum to monitor head and body temperature simultaneously. For drug administration, a PE-10 catheter was inserted into the right internal jugular vein and flushed with heparinized 0.9% saline. A second PE-10 catheter was introduced into the right fermoral artery and connected to a pressure transducer to continuously monitor mean arterial blood pressure (Gould Instruments, Valley View, OH, USA). Animals were then endotracheally intubated, connected to a mouse ventilator (Minivent, Hugo Sachs Elektronik, March-Hugstetten, Germany) and set to a respiratory rate of 160/min. Cardiac arrest was induced by injection of 70 μL cold (4°C) 0.5 mol/L KCl via the jugular catheter, and confirmed by appearance of asystole on the EKG monitor and no spontaneous breathing. During CA, body temperature was cooled to 28°C and the head temperature increased to 38.8°C. CPR was begun 8 minutes after induction of CA by injection of 0.5 to 1 mL prewarmed epinephrine solution (16 μg epinephrine/mL 0.9% saline), chest compressions at a rate of 300/min, and ventilation with 100% oxygen at a respiratory rate of 190/min and a 25% increased tidal volume. Cardiac massage was stopped as soon as spontaneous circulation was restored. Return of spontaneous circulation was assessed by reappearance of electrical activity on the ECG monitor, rapid decreasing of head temperature, and observation of the chest for visible cardiac contractions to ascertain that electrical activity of the heart was accompanied by appropriate mechanical activity. CPR was abandoned if spontaneous circulation was not restored within 2.5 minutes. A separate group of mice received a femoral artery catheter for continuous monitoring of blood pressure and obtaining blood samples to measure arterial blood gases, pH, sodium, potassium, lactate, and glucose levels. Samples were taken 10 minutes before CA and 30 minutes after resuscitation.
Apamin (0.05 mg/kg) or 1-EBIO (16 mg/kg) was administered via two intraperitoneal injections (100 μL/10 g body weight) 30 minutes before or 30 minutes after CA, and a second injection 6 hours after resuscitation. Apamin is a specific SK channel blocker that crosses the blood–brain barrier (Cheng-Raude et al, 1976). 1-Ethyl-benzimidazolinone increases SK channel activity (Vick et al, 2010). It remains to be directly demonstrated that 1-EBIO crosses the blood–brain barrier; however, multiple studies show functional effects consistent with a rapid effect in the brain (Walter et al, 2006; Vick et al, 2010).
Histological Analysis
Three or 10 days after CA/CPR, mice were deeply anesthetized with 3% isoflurane and transcardially perfused and fixed with 10% formalin as previously described (Kofler et al, 2004). Brains were removed, embedded in paraffin, and 6 μm coronal sections were serially cut. The CA1 region of the hippocampus was analyzed, three levels (100 μm apart) from −1.5 mm bregma. Sections were stained with hematoxylin and eosin for analysis of damaged neurons, determined by the presence of pink eosinophilic cytoplasm and dark pyknotic nucleus. All viable and nonviable neurons were counted for each microscopic field, and the percentage of nonviable neurons was calculated for the entire CA1 region (average of three levels/region). The investigator was masked to treatment and genotype before analyzing neuronal damage.
Behavioral Evaluations
We selected novel object recognition because this test has consistently provided reliable evaluations of outcome across experimental ischemia studies. All mice undergoing behavioral testing were single housed in a 12/12-hour light/dark cycle, and all assessments were performed during the second half of the light cycle (noon to 1800 hours). The observer who performed and scored the mouse behavior tests was masked to the treatment group. All equipment was cleaned with 10% ethanol between trials.
Locomotor activity in the open field
To evaluate spontaneous locomotor activity, we used the open field protocol. Mice were placed individually into a 41-cm (W) × 41 cm (D) × 38 cm (H) plastic enclosure equipped with video cameras mounted above to record movement in four arenas simultaneously and video analyzed off-line using Noldus software (Ethovision 2.3, Noldus, Leesburg, VA, USA). Baseline performance was assessed at pre. locomotor activity (total distance moved and velocity) of each mouse was assessed on day 7.
Novel object recognition test
Mice were placed individually into a 41-cm (W) × 41 cm (D) × 38 cm (H) plastic enclosure and allowed to habituate to the arena before novel object recognition testing. During the sample session 6 days after CA/CPR, two identical objects were placed in opposite corners of the arena, ∼1 in. from the wall. Time spent exploring each object during the sample session was hand scored with stopwatches and each mouse was removed from the arena after accumulating 38 seconds of exploration time, maximum of 10 minutes. After a 24-hour delay, one object was replaced with a new object and a 5-minute test session was performed. Novel object preference ratio is the time spent exploring the new object divided by the total exploration time during the test session. All objects used in this study were characterized previously in our laboratory to ensure that mice prefer each object equally.
Additional behavior tests
A neurologic deficit score was recorded for consciousness, interaction, eye appearance, breathing, food/water intake, and overall activity in each mouse on days 1 and 3 after surgery (day 1 and day 3). The graded scoring systems ranged from either 0 to 3 or 0 to 4 depending on the behavior assessed with 0 always indicating no deficit and 3 or 4 indicating most impaired. In addition, latency-to-move was assessed at day 1 and day 3 by placing each mouse in the center of a 12-cm diameter circle on a flat surface and recording the time required for the mouse to move outside the circle.
Hippocampal slice preparation
Hippocampal slices were prepared from 3- to 8-week-old C57BL/6 mice. Animals were anesthetized with an intraperitoneal injection of ketamine–xylazine cocktail before being perfused with ice cold artificial cerebrospinal fluid (ACSF) (in mmol/L: 125 NaCl, 2.5. KCl, 25 NaHCO3, 1.25 NaH2PO4, 2.0 CaCl2, 1.0 MgCl2, 12 glucose) equilibrated with 95%O2/5%CO2. Hippocampi were removed and transferred into a slicing chamber containing sucrose-ACSF (in mmol/L: 75 sucrose, 87 NaCl, 2.5 KCl, 21.4 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 1.3 ascorbic acid, 20 glucose) equilibrated with 95%O2/5%CO2. Transverse hippocampal slices (300 μm) were cut with a Vibratome 1000+ (myNeuroLab, St Louis, MO, USA) and transferred into a holding chamber containing regular ACSF and equilibrated with 95%O2/5%CO2. Slices were incubated at 34°C for 35 minutes and were allowed to recover at room temperature for 1 hour before recordings were performed.
Electrophysiology
For synaptically evoked recordings, CA1 pyramidal cells were visualized with infrared differential interference contrast optics (Leica DMLFS, Buffalo Grove, IL, USA) and a charge-coupled device camera. Whole-cell patch-clamp recordings were obtained from CA1 pyramidal cells using a Multiclamp 700B amplifier (MDS Inc, Sunnyvale, CA, USA), digitized using a Digidata 1322A analog-to-digital converter (MDS Inc) and transferred to a computer using pCLAMP 9.2. software (MDS Inc). Patch pipettes (2–3 MΩ) were filled with a solution containing (mmol/L) 135 K-gluconate, 8 NaCl, 1 MgCl2, 10 HEPES, 4 MgATP, 0.3 Na2GTP, and 10 phosphocreatine, pH 7.26. Series resistance was not electronically compensated and recordings with series resistance that changed >20% during the experiment were discarded. Electrophysiological records were filtered at 5 kHz and sampled at 20 kHz. The input resistance was determined from an ∼30-pA (500 milliseconds) hyperpolarizing current injection pulse interspersed between events. All recordings were from cells with a resting membrane potential between −75 and −55 mV and a stable input resistance. A bias current was applied to maintain the membrane potential at 60 mV.
Synaptic stimulation
Excitatory post-synaptic potential (EPSPs) were recorded in whole-cell current-clamp mode. Presynaptic axons in stratum radiatum were stimulated by a capillary glass pipette filled with ACSF with a tip diameter of ∼5 μm connected to an Iso-Flex stimulus isolation unit (AMPI). Stimulation was ∼100 μm away from the soma of the recorded cell. SR95531 (2 μmol/L; Tocris, Ellisville, MO, USA) and CGP55845 (1 μmol/L; Tocris) were present to reduce the contributions of γ-aminobutyric acid receptors (GABAA and GABAB, respectively).
Electron microscopy
Antibodies used were anti-SK2 raised in guinea pig (1 to 2 μg/mL) and anti-PSD-95 raised in mouse (1 to 2 μg/mL; NeuroMab, Davis, CA, USA). Ultrastructural analyses were performed with a TEM-JEOL 1010 electron microscope (Alcobendas, Spain). Electron photomicrographs were captured with a charge-coupled device camera (Mega View III, Soft Imaging System, Munster, Germany). Digitized electron images were modified for color, brightness, and contrast with Adobe Photoshop version 7.0 (Adobe, San Jose, CA, USA). Reproducibility of immunolabeling was assessed with tissue from at least three mice of the same treatment group. Labeled structures were classified based on unambiguous morphological information in each section, as described elsewhere (Lin et al, 2008).
Postembedding immunohistochemistry
Sham-operated control mice and mice at varying times following CA/CPR were perfuse-fixed with 4% paraformaldehyde, 0.1% glutaraldehyde, and ∼15% picric acid made up in 0.1 mol/L phosphate buffer (pH 7.4) for 10 hours. CA1 slices were incubated in 1 mol/L sucrose/phosphate buffer solution overnight, slammed onto copper blocks cooled in liquid nitrogen and processed for osmium-free embedding Lowicryl resin (Electron Microscopic Sciences, Hatfield, PA, USA). Ultrathin sections (70–90 nm) from three Lowicryl-embedded blocks from each experimental group were cut on an Ultramicrotome and processed for immunocytochemical detection of SK2 and/or PSD-95, as described previously (Lin et al, 2008). To establish the relative abundance of synaptic and intracellular SK2 channels in the different experimental groups, quantitative analysis was performed in the stratum radiatum from 80 nm ultrathin sections. For each of the three animals, three samples of tissue were obtained (nine total blocks). Areas were randomly chosen and were digitally captured at a magnification of × 50,000. The radial distribution of SK2 channels in dendritic spines of pyramidal cells, that is the distance of SK2 channels in the spine relative to the PSD, was calculated.
Statistical analysis
Analysis of CA1 neuronal damage following ischemia and treatment was performed masked; the identity of treatment group was concealed and coded by a separate investigator. All data are expressed as mean±s.e.m. Statistical significance of all histological and electrophysiological data was determined using both the parametric Student's t-test and the nonparametric Wilcoxon–Mann Whitney two-sample rank test for two group comparisons. Statistical analysis of behavioral data was determined using a one-way analysis of variance and post hoc Newman–Keuls test for comparisons of multiple groups. Differences were considered statistically significant with P<0.05.
Results
Increased SK Channel Activity Protects CA1 Pyramidal Neurons Following Ischemia
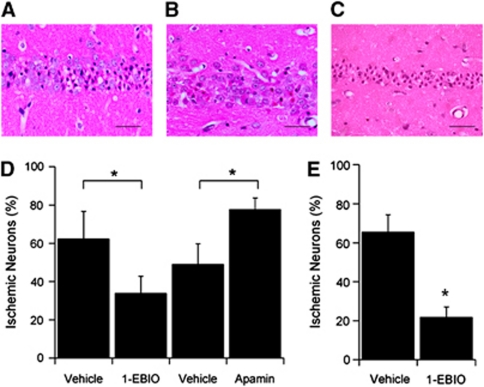
To assess whether pharmacological enhancement of SK channel activity is neuroprotective, mice were administered either vehicle or the SK channel potentiatior 1-EBIO, via intraperitoneal injection 30 minutes before CA/CPR and given a second injection 6 hours after resuscitation. Immediate asystolic arrest was observed in all mice after a bolus injection of KCl. Body weight, CPR duration, epinephrine dose, and survival were not different between groups (Table 1). Global cerebral ischemia in this model exhibited selective, delayed cell death of hippocampal CA1 pyramidal neurons, as described previously (Kofler et al, 2004) (Figures 1A–1C). Quantification of ischemic CA1 pyramidal neurons was assessed with hematoxylin and eosin staining 3 days after resuscitation and revealed that 1-EBIO treatment significantly reduced neuronal injury compared with vehicle (0.01% dimethyl sulfoxide in 0.9% saline)-treated mice, reducing damage from 62.4%±8.6% (n=9) for vehicle to 33.9%±8.9% (n=9, P<0.05) for 1-EBIO-treated mice (Figure 1D). Similarly, increased expression and activity of SK2 in transgenic mice (SK2-OE) exhibited significantly less neuronal injury compared with WT littermates (10.5%±5.2% (n=7) and 36.9%±6.1% (n=10, P<0.05), respectively (Supplementary Figure 1). The effect of decreasing SK channel activity was examined by administration of the selective SK channel blocker apamin to a separate cohort of mice. Mice were administered either vehicle (0.9% saline) or 0.05 mg/kg apamin via intraperitoneal injection 30 minutes before CA/CPR and given a second injection 6 hours after resuscitation. This dose of apamin did not result in overt behavioral or physiological abnormalities compared with larger doses used previously (Stackman et al, 2002; Vick et al, 2010). Apamin administration significantly increased neuronal injury compared with vehicle-treated mice; the percentage of damaged CA1 neurons rose from 49.1%±8.5% for vehicle (n=10) to 77.8%±5.9% (n=7, P<0.05) for apamin-treated mice (Figure 1D). We observed very few damaged neurons in sham-operated mice, regardless of treatment group (Supplementary Figure 2) and no significant differences in a range of physiological parameters (arterial blood gases, pH, glucose, lactate, sodium or potassium, blood pressure) were observed between treatment groups (vehicle, 1-EBIO, or apamin; Table 2).
Table 1. Body weight, cardiac arrest parameters, and survival rates.
| WT | SK2-OE | SK2 null | WT+vehicle | WT+apamin | WT+EBIO | |
|---|---|---|---|---|---|---|
| Body weight (g) | 22.7±0.7 | 22.0±0.9 | 21.4±0.6 | 24.5±0.5 | 24.2±0.5 | 24.7±0.6 |
| Total ischemia time (minutes) | 9.1±0.2 | 9.3±0.10 | 9.2±0.1 | 9.5±0.1 | 9.8±0.2 | 9.7±0.1 |
| Epinephrine (μg) | 11.2±0.4 | 12.2±0.6 | 12.3±0.4 | 10.5±0.3 | 9.9±0.4 | 11.3±0.4 |
| Survival | 10/14 (71%) | 7/10 (70%) | 8/12 (67%) | 9/15 (60%) | 7/14 (50%) | 9/10 (90%) |
EBIO, ethyl-benzimidazolinone; WT, wild type.
No differences are found among groups. Total ischemia time represents total time from induction of cardiac arrest to successful resuscitation (spontaneous circulation). Data are presented as mean±s.e.m.
Figure 1.
Pharmacological enhancement of SK2 channel activity increases survival of CA1 neurons following cardiac arrest (CA). (A) Representative photomicrograph of hippocampal CA1 neurons from a wild-type (WT) mouse injected with vehicle (intraperitoneal injection) 30 minutes before and 6 hours after CA and cardiopulmonary resuscitation (CA/CPR) and stained with hematoxylin and eosin (H&E) 3 days later. Damaged neurons identified by the presence of pink eosinophilic cytoplasm and a dark pyknotic nucleus. (B) Representative photomicrograph of hippocampal CA1 neurons from a WT mouse injected with the SK2, Ca2+-sensitivity-enhancing drug 1-ethyl-benzimidazolinone (1-EBIO) (16 mg/kg, intraperitoneally), 30 minutes before and 6 hours after CA/CPR and stained with H&E 3 days later. Damaged neurons identified by the presence of pink eosinophilic cytoplasm and a dark pyknotic nucleus. (C) Representative photomicrograph of hippocampal CA1 neurons from a WT mouse injected with the SK2 antagonist apamin (0.1 mg/kg, intraperitoneally), 30 minutes before and 6 hours after CA/CPR and stained with H&E 3 days later. (D) Quantification of ischemic neurons in the CA1 region of the hippocampus 3 days after CA/CPR. (E) Quantification of ischemic neurons in the CA1 region of the hippocampus 3 days after CA/CPR in mice treated with 16 mg/kg 1-EBIO 30 minutes after resuscitation, with a second injection given 6 hours after resuscitation. Data are presented as mean±s.e.m. *P<0.05 compared with vehicle.
Table 2. 1-EBIO has little effect on arterial blood pressure (BP) or blood gases.
|
Vehicle |
1-EBIO |
|
|---|---|---|
| Average s.e.m. | Average s.e.m. | |
| Baseline (10 minutes before induction of CA) | ||
| BP | 74.9±2.2 | 74.0±1.4 |
| pH | 7.303±0.014 | 7.279±0.028 |
| PaCO2 | 44.0±3.0 | 43.6±2.0 |
| PaO2 | 148.1±9.0 | 138.2±7.3 |
| HCO3– | 21.1±0.9 | 20.1±1.4 |
| BE | –5.1±0.6 | –6.4±1.6 |
| Na | 150.7±1.5 | 148.3±1.7 |
| K | 5.2±0.2 | 5.2±0.1 |
| Cl | 115.4±1.7 | 111.7±1.7 |
| Glu | 206.6±15.5 | 255.0±11.9 |
| Lac | 3.9±0.3 | 3.6±0.6 |
| Recovery (30 minutes after recovery of spontaneous circulation) | ||
| BP | 82.1±4.8 | 83.8±1.4 |
| pH | 7.001±0.042966 | 7.0153±0.025 |
| PaCO2 | 56.0±3.3 | 48.6±1.5 |
| PaO2 | 306.2±15.5 | 291.2±1.1 |
| HCO3− | 13.7±1.3 | 12.2±0.8 |
| BE | −17.9±1.9 | −18.8±1.1 |
| Na | 150.1±1.3 | 149.0±1.4 |
| K | 6.3±0.4 | 5.7±0.4 |
| Cl | 122.0±1.6 | 118.0±1.2 |
| Glu | 271.5±25.3 | 324.3±48.2 |
| Lac | 4.9±0.9 | 4.2±0.9 |
CA, cardiac arrest; 1-EBIO, 1-ethyl-benzimidazolinone.
To examine the therapeutic potential of using SK channel positive modulators on neuronal cell death following ischemia, 1-EBIO was administered 30 minutes after resuscitation while the control group received vehicle injection. The results showed that post-CPR treatment with 1-EBIO significantly reduced neuronal injury compared with mice treated with vehicle, reducing damage from 65.6%±8.8% (n=8) for vehicle to 21.8%±5.3% (n=8, P<0.05) for 1-EBIO-treated mice (Figure 1E). Thus, systemic administration of an SK channel positive modulator before or after CA/CPR resulted in similar and significant improvements in the survival of CA1 pyramidal neurons.
1-Ethyl-Benzimidazolinone Improves Cognitive Outcome
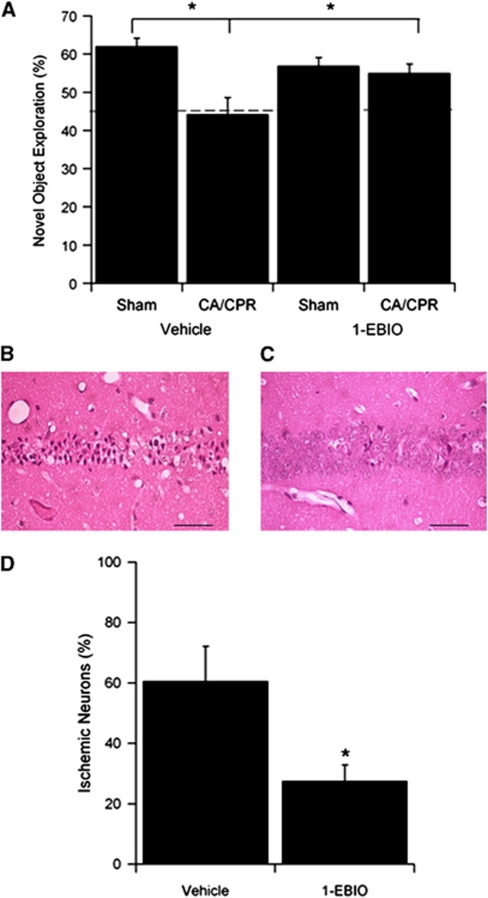
To determine whether 1-EBIO treatment improves cognitive outcome following CA/CPR, novel object recognition was tested following CA/CPR (and sham-operated) in mice treated with vehicle or 1-EBIO 30 minutes after resuscitation. No difference in survival rates was observed for each group and neurologic deficit scores for consciousness, interaction, eye appearance, breathing, food/water intake, and overall activity recorded in each mouse on days 1 and 3 post-CA/CPR were similar in vehicle and 1-EBIO-treated mice. The novel object recognition task was performed on days 6 and 7 after CA/CPR. Compared with vehicle-treated sham control mice, vehicle-treated CA/CPR mice showed a decline in novel object preference 24 hours after training, indicating impaired memory retention (Figure 2A). Vehicle-treated sham control mice explored the novel object for 62.0%±2.0% (n=14) of the time during the test session, while vehicle-treated CA/CPR mice explored only 44.0%±4.0% (n=14, P<0.05) of the time. Post-CA/CPR treatment with 1-EBIO resulted in a significant increase in novel object exploration following CA/CPR compared with vehicle-treated CA/CPR mice. Indeed, 1-EBIO prevented the CA/CPR-induced decrement in novel object recognition, exploring the novel object 56.9%±2.0% (n=16) of the time for 1-EBIO-treated sham-operated mice and 55.0%±2.1% (n=17) of the time in 1-EBIO-treated CA/CPR mice. The open field test was performed before CA/CPR and on day 5 after CA/CPR as a control for recovery of motor function, to avoid this possible confounder during cognitive testing. No significant change in total distance moved or velocity of movement was observed during a 30-minute open field test, regardless of treatment . Finally, CA1 neuronal damage was assessed at the conclusion of behavioral testing (10-day survival). Post-CPR treatment with EBIO significantly reduced neuronal injury 10 days after CA/CPR compared with mice treated with vehicle, reducing damage from 61.9%±11.5% (n=8) for vehicle to 27.4%±8.9% (n=8, P<0.05) for 1-EBIO-treated mice (Figure 2B).
Figure 2.
Pharmacological enhancement of SK channel activity improves long-term cognitive recovery. (A) Novel object recognition was assessed 7 days after cardiac arrest and cardiopulmonary resuscitation (CA/CPR) or sham-operated in vehicle or 1-ethyl-benzimidazolinone (1-EBIO) (16 mg/kg administered 30 minutes after resuscitation)-treated mice. The dashed line represents a preference ratio of 50%, indicating a lack of memory of the novel object. CA/CPR significantly reduced novel object preference in vehicle-treated mice, which was significantly higher in 1-EBIO-treated mice after CA/CPR. (B) Representative photomicrograph of hippocampal CA1 neurons from a wild-type (WT) mouse injected with vehicle 30 minutes after CA/CPR and stained with hematoxylin and eosin (H&E) 10 days later. Damaged neurons identified by the presence of pink eosinophilic cytoplasm and a dark pyknotic nucleus. (C) Representative photomicrograph of hippocampal CA1 neurons from a WT mouse injected with 1-EBIO (16 mg/kg, intraperitoneally), 30 minutes after after CA/CPR and stained with H&E 10 days later. Damaged neurons identified by the presence of pink eosinophilic cytoplasm and a dark pyknotic nucleus. (D) Quantification of ischemic neurons in the CA1 region of the hippocampus 10 days after CA/CPR in mice treated with 1-EBIO 30 minutes and again 6 hours after resuscitation. Data are presented as mean±s.e.m. *P<0.05 compared with vehicle.
Ischemia Causes Delayed Loss of Synaptic SK2 Channels
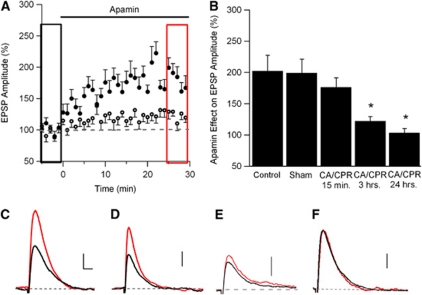
SK2-containing channels are expressed in the PSD of dendritic spines on CA1 pyramidal neurons where they modulate Ca2+ influx through NMDARs (Ngo-Anh et al, 2005; Lin et al, 2008). To determine whether synaptic SK channel activity is altered by ischemia, whole-cell current-clamp recordings of CA1 neurons were performed in acute hippocampal slices prepared at varying times following CA/CPR, or from sham-operated mice. A stimulating electrode placed in the stratum radiatum was used to evoke subthreshold EPSPs every 30 seconds. After establishing a stable baseline of EPSP amplitude (5 to 10 minutes), apamin (100 nmol/L), the selective SK channel blocker, was applied to the bath solution (Figure 3). In sham-operated control mice, apamin increased the amplitude of the EPSP by 99%±22% of control (n=12, P<0.05) (Figures 3A and 3B). The magnitude of the apamin effect on EPSP amplitude was not different than that observed in control mice that did not undergo a surgical procedure (102%±25%, n=6; Figure 3B). The effect of apamin on EPSP amplitude was not significantly altered in recordings from slices taken from mice 15 minutes after CA/CPR (Figure 3B) when apamin increased the amplitude of EPSPs by 76%±15% (n=10). In contrast, the effect of apamin was significantly reduced by 3 hours post-CA/CPR, increasing the EPSP by only 22%±7% (n=10, P<0.05 compared with Sham and 15 minutes post-CA/CPR). The effect of apamin remained diminished at 24 hours post-CA/CPR, increasing EPSPs by only 3%±7% of baseline (n=6, P<0.05 compared with Sham and 15 minutes post-CA/CPR) (Figures 3C–3E). These data demonstrate that CA/CPR results in reduced synaptic SK channel activity by 3 hours after resuscitation. Minimal differences in input resistance, resting membrane potential, or access resistance were observed across treatment groups (Supplementary Table 2).
Figure 3.
Ischemia is associated with the loss of synaptic SK2 channel activity in CA1 neurons by 3 hours, but not by 15 minutes. (A) Time course of EPSP amplitude (mean±s.e.m.) from sham-operated mice (solid circles) and mice 3 hours after cardiac arrest and cardiopulmonary resuscitation (CA/CPR) (empty circles) before (black rectangle) and after (red rectangle) a 30-minute application of apamin (100 nmol/L). Apamin was applied at time 0. Each point is the average of two adjacent, normalized EPSPs. (B) Quantification of blocking synaptic SK2 channels with apamin on EPSP amplitude. Average EPSP amplitude (mean±s.e.m.) 30 minutes after adding apamin (100 nmol/L, red square in (A) normalized to baseline (black square in (A). *P<0.05 compared with sham controls. (C) Example EPSPs from sham-operated mice before (black) and after (red) a 30-minute application of apamin (100 nmol/L). All example EPSPs are the average of 15 EPSPs. (D) Example EPSPs from mice 15 minutes after CA/CPR before (black) and after (red) a 30-minute application of apamin (100 nmol/L). (E) Example EPSPs from mice 3 hours after CA/CPR before (black) and after (red) a 30-minute application of apamin (100 nmol/L). (F) Example EPSPs from mice 24 hours after CA/CPR before (black) and after (red) a 30-minute application of apamin (100 nmol/L). Scale bars on traces are vertical: 1 mV; horizontal: 50 milliseconds (scale same in all traces). The color reproduction of this figure is available at the Journal of Cerebral Blood Flow and Metabolism journal online. EPSP, excitatory post-synaptic potential.
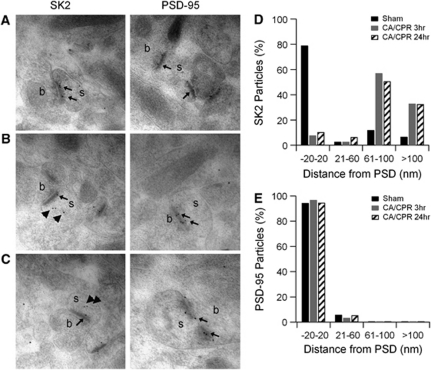
Synaptic SK2 channels in CA1 pyramidal neurons undergo activity- and NMDAR-dependent endocytosis (Lin et al, 2008, 2010). To determine the spine distribution of SK2 following CA/CPR, postembedding iEM was performed. In sham control mice, SK2 immunoparticles were observed predominantly in the PSD region of CA1 pyramidal neuron dendritic spines (Figures 4A and 4D). In contrast, at 3 or 24 hours, post-CA/CPR SK2 immunoparticles were observed predominantly in the intracellular space of the spines, distant from the PSD (Figures 4B–4D). Quantification of radial distance from the PSD for SK2 particles showed that CA/CPR causes a significant shift of SK2 immunoparticle distribution away from the PSD, with 79% of SK2 particles observed within 20 nm of the PSD under control conditions, compared with 8% and 10% at 3 and 24 hours post-CA/CPR, respectively (Figure 4D). Cardiac arrest and cardiopulmonary resuscitation had no effect on the location of the PSD protein PSD-95 (Figures 4A–4C and 4E). These results suggest that the reduction in functional synaptic SK channel activity following ischemia is caused by endocytosis and removal from synapses.
Figure 4.
SK2 channels are removed from the postsynaptic density following cardiac arrest and cardiopulmonary resuscitation (CA/CPR). (A) Single immunogold labeling for SK2 or postsynaptic density (PSD)-95 in representative asymmetric synapses of hippocampal CA1 neurons in sham control mice or 3 (B) or 24 hours (C) following CA/CPR. Small arrows indicate labeling near the PSD and bold arrows indicate SK2 immunogold particles that appear distant from the PSD in post-CA/CPR mice. (D) Quantification of immunogold particles for SK2 expressed as percentage of total particles in each of four distance bins from the PSD (in nm). Black bars are the sham control condition, gray bars 3 hours after CA/CPR, and stripped bars 24 hours after CA/CPR. (E) Quantification of immunogold particles for PSD-95 expressed as percentage of total particles in each of four distance bins from the PSD (in nm). Black bars are the control condition, gray bars 3 hours after CA/CPR, and stripped bars 24 hours after CA/CPR.
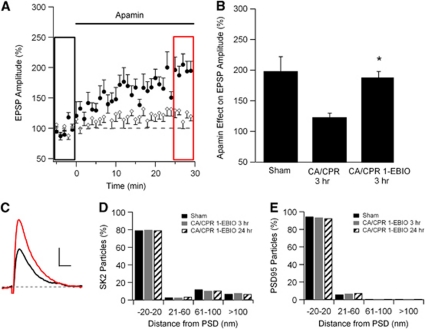
1-Ethyl-Benzimidazolinone Prevents Cardiac Arrest and Cardiopulmonary Resuscitation-Induced Endocytosis and Loss of Functional SK2 Channels
The results presented above show that the SK channel positive modulator 1-EBIO protects CA1 neurons from CA/CPR-induced damage. While the precise mechanism of 1-EBIO actions is not known, 1-EBIO is thought to interact with the intracellular C-terminal domain of SK channels to increase activity. To determine whether 1-EBIO treatment prevents the loss of synaptic SK2 channels following CA/CPR, iEM and current-clamp recordings were performed from mice following CA/CPR, with or without 1-EBIO treatment. Mice were administered 1-EBIO (16 mg/kg) 30 minutes before CA/CPR and whole-cell current-clamp recordings were made from 3 hours post-CA/CPR. The effect of apamin on EPSP amplitude was not significantly different than that measured in recordings from slices taken from sham-operated control mice (Figures 5A–5C). On average, apamin increased the amplitude of EPSPs by 87%±10% of control (n=9). These data show that neuroprotective doses of 1-EBIO prevent the CA/CPR-induced reduction of functional synaptic SK channels. Postembedding iEM results were consistent with this, showing that 1-EBIO treatment stabilizes synaptic SK2 channels. At 3 or 24 hours post-CA/CPR in mice treated with 1-EBIO, SK2 immunoparticles were observed predominantly in the PSD. Quantification of radial distance from the PSD for SK2 particles showed that 79% of SK2 particles were within 20 nm of the PSD under sham control conditions, compared with 80% and 79% at 3 and 24 hours post-CA/CPR treated with 1-EBIO, respectively (Figure 5D). Cardiac arrest and cardiopulmonary resuscitation had no effect of the PSD protein PSD-95 (Figure 5E).
Figure 5.
Injection with 1-ethyl-benzimidazolinone (1-EBIO) rescues the internalization of SK2 channels following cardiac arrest and cardiopulmonary resuscitation (CA/CPR). (A) Time course of EPSP amplitude (mean±s.e.m.) before (black rectangle) and after (red rectangle) a 30-minute application of apamin (100 nmol/L). Mice that received CA/CPR following an injection with 1-EBIO (solid circles) 30 minutes beforehand are compared with mice that received only CA/CPR (empty circles). Both groups are assessed 3 hours after CA/CPR. Apamin was applied at time 0 and each point is the average of two adjacent, normalized EPSPs. (B) Quantification of blocking synaptic SK2 channels with apamin on EPSP amplitude. Sham and CA/CPR 3 hours time point data from Figure 3 are replotted for better comparison with 1-EBIO preinjected 3 hours time point data. Average EPSP amplitude (mean±s.e.m.) 30 minutes after adding apamin (100 nmol/L, red square in Figures 3A or 5A) is normalized to baseline (black square in Figures 3A or 5A). *P<0.05 compared with CA/CPR 3 hours. (C) Example EPSPs from mice injected 30 minutes before CA/CPR with 1-EBIO and assessed 3 hours after resuscitation before (black) and after (red) a 30-minute application of apamin (100 nmol/L). All example EPSPs are the average of 15 EPSPs. (D) Quantification of immunogold particles for SK2 expressed as percentage of total particles in each of four distance bins from the postsynaptic density (PSD) (in nm). Black bars are the sham control condition, gray bars 3 hours after CA/CPR+1-EBIO, and stripped bars 24 hours after CA/CPR+1-EBIO. (E) Quantification of immunogold particles for PSD-95 expressed as percentage of total particles in each of four distance bins from the PSD (in nm). Black bars are the control condition, gray bars 3 hours after CA/CPR, and stripped bars 24 hours after CA/CPR. The color reproduction of this figure is available at the Journal of Cerebral Blood Flow and Metabolism journal online. EPSP, excitatory post-synaptic potential.
Discussion
The results presented here show that pharmacological maintenance of SK channel activity provides sustained neuroprotection to hippocampal CA1 pyramidal neurons, and improved cognitive outcome following global cerebral ischemia induced by CA/CPR. The data also show that cerebral ischemia causes a sustained reduction of functional synaptic SK2 channels that may contribute to NMDAR-dependent Ca2+-mediated excitotoxicity. Thus, increasing or maintaining SK2 channel activity provides a promising therapeutic strategy to minimize the neuronal death that accompanies CA/CPR-induced ischemia.
How does enhanced SK channel activity protect CA1 neurons from ischemic damage? The most parsimonious explanation is that maintaining synaptic SK2 channel activity limits Ca2+ influx through NMDARs, overcoming the increased glutamatergic signaling induced by ischemia and blunting the biochemical cascade that leads to excitotoxicity. SK2 channels mediate synaptic SK channel activity in spines on CA1 pyramidal neurons (Bond et al, 2004; Ngo-Anh et al, 2005) yet, 1-EBIO does not discriminate among the four members of the SK family (SK1–3 and IK1), and systemic administration might result in consequences mediated by other members of the SK channel family. While we cannot rule out contributions from other SK family members, for instance endothelial SK3 or IK1 channels, neuroprotection is also afforded by transgenic SK2 overexpression, in the absence of 1-EBIO (Supplementary Figure 1). This suggests that the effects of 1-EBIO are mediated at least in part by SK2 channels. Indeed, we have previously demonstrated that apamin has a larger effect on EPSPs recorded from SK2-OE mice compared with WT mice, indicating increased functional synaptic SK2 channels in SK2-OE mice (Hammond et al, 2006). Taken together, the data show that SK2 channels represent endogenous neuroprotectants that, when enhanced either pharmacologically or genetically, reduce damage following ischemia. Importantly, administration of 1-EBIO 30 minutes after resuscitation provided benefits comparable to prophylactic administration. This 30-minute therapeutic window used here is consistent with the timing of hypothermia, the only clinically approved intervention for CA (Bernard et al, 2002; Safar and Kochanek, 2002). To assess cognitive outcome, we used the novel object recognition task at 7 days after CA/CPR, a task that is sensitive to hippocampal injury and has been utilized previously to assess outcome following global cerebral ischemia (Gulinello et al, 2006; Plamondon et al, 2008). The significant decrease in novel object exploration following CA/CPR was prevented in mice treated with 1-EBIO 30 minutes after CA/CPR. These data are consistent with our histological analysis and indicate that therapeutic 1-EBIO treatment provides significant benefit to mice after global cerebral ischemia induced by CA/CPR.
Extrasynaptic NR2B-containing NMDARs have been implicated as the major contributors to ischemia-induced excitotoxic damage (Hardingham et al, 2002; Liu et al, 2007). However, recent studies indicate that both synaptic and extrasynaptic NMDARs are capable of mediating glutamate excitotoxicity (von Engelhardt et al, 2007; Martel et al, 2009). In this respect, SK2 channels are expressed throughout the dendrites and in the extrasynaptic spine membrane (Allen et al, 2011). Thus, it is possible that extrasynaptic SK2 channels contribute to the neuroprotective effects of 1-EBIO or SK2 overexpression. As the differential roles in ischemia-induced cell death as well as their and subspine localization are presently unresolved, it will be important to determine the relative contributions of synaptic and extrasynaptic SK2 channels following CA/CPR. Interestingly, increasing the activity of two other classes of nonsynaptic K+ channels, KATP channels or BK channels, has been shown to reduce ischemia-induced cell death in rodent models, although both targets failed in clinical trials (Gribkoff et al, 2001; Ballanyi, 2004). Different from these examples, synaptic SK2 channels are functionally coupled to NMDARs making them uniquely positioned for exploitation as therapeutic targets.
Long-term potentiation (LTP), the activity-dependent increase in synaptic transmission, is the leading model for the cellular basis of learning and memory (Kerchner and Nicoll, 2008). At many synapses, the induction of LTP requires Ca2+ influx through NMDARs that initiates a biochemical cascade to strengthen synaptic transmission in a synapse-specific manner. In CA1 pyramidal neurons, this is largely accomplished by the rapid insertion of AMPA receptors (Kerchner and Nicoll, 2008) and dynamin-dependent endocytosis of SK2 channels from the PSD (Lin et al, 2008, 2010). In vitro ischemia causes a similar synaptic potentiation, ischemic LTP (Di et al, 2008), which shares many mechanistic features of physiological LTP. It seems probable that ischemic LTP, albeit more global than physiological LTP, is also induced in this in vivo model of CA/CPR-induced ischemia, and our results suggest, then, that decreased synaptic SK2 channels contribute to ischemic LTP. Similar to physiological LTP, synaptic SK2 channels are largely absent from the PSD following global ischemia, within minutes (>15 minutes) following CA/CPR. Postembedding iEM results support the functional findings by demonstrating that following CA/CPR most SK2 immunoparticles are found within CA1 pyramidal neuron spines, away from the PSD. The reduction of synaptic SK2 channels is apparently complete 3 hours following CA/CPR and is sustained for at least 24 hours after CA/CPR. Interestingly, we observed that neuroprotective doses of 1-EBIO prevented CA/CPR-induced internalization of SK2 and reduction of functional synaptic channels. Whether this is a consequence of the neuroprotective effects of acutely increased SK channel activity and reduced Ca2+ influx through NMDARs, or if stabilization of SK2 channels contributes to neuroprotection, remains to be determined.
In summary, the native coupling between synaptic SK2 channels and NMDARs may be exploited by increasing and/or stabilizing SK2 channel activity following CA/CPR-induced ischemia to spare CA1 neurons from delayed cell death and improve long-term cognitive outcome.
Acknowledgments
The monoclonal antibody PSD-95 (Clone K28/43) was obtained from the UC Davis/NIH NeuroMab Facility supported by NIH Grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behaviour, College of Biological Sciences, University of California, Davis, CA 95616, USA.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by NIH Grant RO1NS065855 (JPA and PSH), and by the Spanish Ministry of Science and Technology (CONSOLIDER CSD2008-00005), and Junta de Comunidades de Castilla-La Mancha (PAI08-0174-6967) to RL.
Supplementary Material
References
- Allen D, Bond CT, Lujan R, Ballesteros-Merino C, Lin MT, Wang K, Klett N, Watanabe M, Shigemoto R, Stackman RW, Maylie J, Adelman JP. The SK2-long isoform directs synaptic localization and function of SK2-containing channels. Nature Neuroscience. 2011;14:744–749. doi: 10.1038/nn.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K. Protective role of neuronal KATP channels in brain hypoxia. J Exp Biol. 2004;207:3201–3212. doi: 10.1242/jeb.01106. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Boast CA, Gerhardt SC, Pastor G, Lehmann J, Etienne PE, Liebman JM. The N-methyl-D-aspartate antagonists CGS 19755 and CPP reduce ischemic brain damage in gerbils. Brain Res. 1988;442:345–348. doi: 10.1016/0006-8993(88)91522-3. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent after hyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Raude D, Treloar M, Habermann E. Preparation and pharmacokinetics of labeled derivatives of apamin. Toxicon. 1976;14:467–476. doi: 10.1016/0041-0101(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel V, Epsztein J, Ben-Ari Y. Ischemia induces short- and long-term remodeling of synaptic activity in the hippocampus. J Cell Mol Med. 2003;7:401–407. doi: 10.1111/j.1582-4934.2003.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaux O, Bizot JC. Effect of apamin, a selective blocker of Ca2+-activated K+-channel, on habituation and passive avoidance responses in rats. Neurosci Lett. 1997;227:57–60. doi: 10.1016/s0304-3940(97)00301-7. [DOI] [PubMed] [Google Scholar]
- Di FM, Tozzi A, Costa C, Belcastro V, Tantucci M, Picconi B, Calabresi P. Plasticity and repair in the post-ischemic brain. Neuropharmacology. 2008;55:353–362. doi: 10.1016/j.neuropharm.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Gill R, Foster AC, Woodruff GN. MK-801 is neuroprotective in gerbils when administered during the post-ischaemic period. Neuroscience. 1988;25:847–855. doi: 10.1016/0306-4522(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE, Jr, Dworetzky SI, Hewawasam P, Boissard CG, Cook DA, Frantz SW, Heman K, Hibbard JR, Huston K, Johnson G, Krishnan BS, Kinney GG, Lombardo LA, Meanwell NA, Molinoff PB, Myers RA, Moon SL, Ortiz A, Pajor L, Pieschl RL, Post-Munson DJ, Signor LJ, Srinivas N, Taber MT, Thalody G, Trojnacki JT, Wiener H, Yeleswaram K, Yeola SW. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat Med. 2001;7:471–477. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hicks CA, Ward MA, Ragumoorthy N, Ambler SJ, Dell CP, Dobson D, O'Neill MJ. Evaluation of glycine site antagonists of the NMDA receptor in global cerebral ischaemia. Brain Res. 1999;819:65–74. doi: 10.1016/s0006-8993(98)01329-8. [DOI] [PubMed] [Google Scholar]
- Janac B, Selakovic V, Radenovic L. Temporal patterns of motor behavioural improvements by MK-801 in Mongolian gerbils submitted to different duration of global cerebral ischemia. Behav Brain Res. 2008;194:72–78. doi: 10.1016/j.bbr.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Kelley MH, Taguchi N, Ardeshiri A, Kuroiwa M, Hurn PD, Traystman RJ, Herson PS. Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and allopregnanolone neuroprotection is associated with GABAA receptor stabilization. J Neurochem. 2008;107:668–678. doi: 10.1111/j.1471-4159.2008.05617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Frerking M, Maylie J, Adelman JP. Coupled activity-dependent trafficking of synaptic SK2 channels and AMPA receptors. J Neurosci. 2010;30:11726–11734. doi: 10.1523/JNEUROSCI.1411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update. A report from the American Heart Association. Circulation. 2009;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Martel MA, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158:334–343. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- Plamondon H, Davignon G, Khan S, Charron C. Cerebral ischemic preconditioning induces lasting effects on CA1 neuronal survival, prevents memory impairments but not ischemia-induced hyperactivity. Behav Brain Res. 2008;189:145–151. doi: 10.1016/j.bbr.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Montoliu C, Humet M, Fernandez AM, Garcia-Martinez C, Valera E, Merino JM, Perez-Paya E, Messeguer A, Felipo V, Ferrer-Montiel A. A novel N-methyl-D-aspartate receptor open channel blocker with in vivo neuroprotectant activity. J Pharmacol Exp Ther. 2002;302:163–173. doi: 10.1124/jpet.302.1.163. [DOI] [PubMed] [Google Scholar]
- Safar PJ, Kochanek PM. Therapeutic hypothermia after cardiac arrest. N Engl J Med. 2002;346:612–613. doi: 10.1056/NEJM200202213460811. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Vick KA, Guidi M, Stackman RW., Jr In vivo pharmacological manipulation of small conductance Ca(2+)-activated K(+) channels influences motor behavior, object memory and fear conditioning. Neuropharmacology. 2010;58:650–659. doi: 10.1016/j.neuropharm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Kohr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA-receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Zipfel GJ, Lee JM, Choi DW. Reducing calcium overload in the ischemic brain. N Engl J Med. 1999;341:1543–1544. doi: 10.1056/NEJM199911113412011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.