Abstract
Vascular endothelial growth factor (VEGF)-induced neovasculature is immature and leaky. We tested if coexpression of angiopoietin-1 (ANG1) with VEGF improves blood–brain barrier (BBB) integrity and VEGF neuroprotective and neurorestorative effects using a permanent distal middle cerebral artery occlusion (pMCAO) model. Adult CD-1 mice were injected with 2 × 109 virus genomes of adeno-associated viral vectors expressing VEGF (AAV-VEGF) or ANG1 (AAV-ANG1) individually or together in a 1:1 ratio into the ischemic penumbra 1 hour after pMCAO. AAV-LacZ was used as vector control. Samples were collected 3 weeks later. Compared with AAV-LacZ, coinjection of AAV-VEGF and AAV-ANG1 reduced atrophy volume (46%, P=0.004); injection of AAV-VEGF or AAV-ANG1 individually reduced atrophy volume slightly (36%, P=0.08 and 33%, P=0.09, respectively). Overexpression of VEGF reduced tight junction protein expression and increased Evans blue extravasation. Compared with VEGF expression alone, coexpression of ANG1 with VEGF resulted in upregulation of tight junction protein expression and reduction of Evans blue leakage (AAV-ANG1/AAV-VEGF: 1.4±0.3 versus AAV-VEGF: 2.8±0.7, P=0.001). Coinjection of AAV-VEGF and AAV-ANG1 induced a similar degree of angiogenesis as injection of AAV-VEGF alone (P=0.85). Thus, coexpression of ANG1 with VEGF improved BBB integrity and resulted in better neuroprotection compared with VEGF expression alone.
Keywords: adeno-associated viral vector, angiopoietin-1, middle cerebral artery occlusion, tight junction protein, vascular integrity, VEGF
Introduction
Studies have shown that angiogenic factors, such as vascular endothelial growth factor (VEGF), increase during ischemic injury (Hayashi et al, 2003). Unfortunately, the innate increase of angiogenic factors is not sufficient to compensate for most clinically significant instances of cerebral ischemic injury (Zhang et al, 2000). Enhancing the innate response by exogenous delivery of angiogenic factors has been shown to attenuate ischemic injury and promote functional recovery (Greenberg and Jin, 2005; Simons and Ware, 2003; Wei et al, 2005).
Exogenous delivery of VEGF reduces ischemic brain injury by inducing neuroprotection, neurogenesis, and angiogenesis (Hayashi et al, 1998; Shen et al, 2006a; Sun et al, 2003; Zhang et al, 2000). The VEGF-induced neovasculature is usually immature and leaky, which may cause cerebral edema, augment brain injury, diminish the neuroprotective effect of VEGF at the early stage, and hamper the neurorestorative effect of VEGF at the latter stage of ischemic insult. Other factors may coordinate with VEGF to induce functional vessels.
The angiopoietins (ANG1, 2, 3, and 4) constitute a family of endothelial growth factors. In a mouse model of stroke, Ang1 inhibits leakage from cerebral vessels and decreases lesion size (Zhang et al, 2002a). Angiopoietin-1 also inhibits Vegf-induced permeability and inflammation-induced leakage from mature blood vessels in animal models (Thurston et al, 1999, 2005; Zacharek et al, 2007). Using a myocardial infarction mouse model, we have shown in a previous study that ANG1 reduces vascular leakage caused by VEGF overexpression (Su et al, 2009).
In this study, we hypothesized that coexpression of ANG1 with VEGF enhances blood–brain barrier (BBB) integrity and VEGF neuroprotective and restorative effects. To test these hypotheses, we used a permanent distal middle cerebral artery occlusion (pMCAO) mouse model. Adeno-associated viral vector (AAV) was used to deliver the payload.
Materials and methods
Experimental Groups
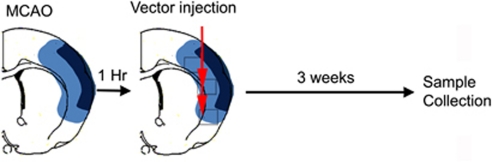
All experimental procedures involving animals were approved by the University of California, San Francisco Committee of Animal Research, and conformed to the NIH Guidelines for the use of animals in research. Male adult CD-1 mice (Charles River, Hollister, CA, USA) were divided into four groups: (1) AAV-LacZ: pMCAO plus injection of AAV-LacZ; (2) AAV-VEGF: pMCAO plus injection of AAV-VEGF; (3) AAV-ANG1: pMCAO plus injection of AAV-ANG1; (4) AAV-VEGF/AAV-ANG1: pMCAO plus injection of AAV-VEGF and AAV-ANG1 in 1:1 ratio. AAV vectors were injected into the ischemic border zone 1 hour after pMCAO, and brain samples were collected for analysis 3 weeks after vector injection (Figure 1).
Figure 1.
Schematic drawing showing the experimental design. Adeno-associated viral vectors (AAVs) were injected into two sites (red arrows) at the ischemic penumbra 1 hour after middle cerebral artery occlusion (MCAO). Dark blue highlights the infarct area and light blue highlights the penumbra. Brain samples were collected 3 weeks after vector injection. The squares show the regions used for histological analysis. The color reproduction of this figure is available at online version.
Mouse Permanent Distal Middle Cerebral Artery Occlusion Model
After anesthesia, the left middle cerebral artery was occluded using electrical coagulation just proximal to the pyriform branch. Body temperature was maintained at 37±0.5°C by using a thermal blanket throughout the surgical procedure. Surface cerebral blood flow was monitored during the procedure using a laser Doppler flowmeter (Vasamedics Inc., Saint Paul, MN, USA). Mice were excluded from the experiment when the surface cerebral blood flow in the ischemic core region was >15% of the baseline after MCA occlusion.
Adeno-Associated Viral Vector Construction and Production
AAV-VEGF, AAV-ANG1, and AAV-LacZ plasmids were constructed as previously described (Su et al, 2000, 2009). Human VEGF165 and human ANG1 cDNA were cloned in these vectors. CMV promoter was used to control the gene expression in all of these vectors. AAV vectors were produced using a three plasmid cotransfection system (Matsushita et al, 1998). Two helper plasmids, one with adenoviral VA, E2A, and E4 regions and the other with the AAV rep and AAV serotype one cap genes (provided by Xiao Xiao, University of North Carolina), were cotransfected with AAV plasmid into HEK293 cells to package the AAV vector. AAV vectors were purified using CsCl2 centrifugation. Viral titers were determined by dot blot analysis of DNA content and expressed as viral genome (vg).
Adeno-Associated Viral Vector Transduction
One hour after pMCAO, following anesthesia, the animals were placed in a stereotactic frame with a holder (David Kopf Instruments, Tujunga, CA, USA), and a burr hole was drilled in the pericranium, 2.5 mm lateral to the sagittal suture and 1 mm caudal to the coronal suture. A 10-μl Hamilton syringe was stereotactically inserted into the lateral caudate nucleus (3.0 mm deep from the dura) at the border of the infarct area (Figure 1). Four microliters AAV viral suspension containing 2 × 109 vg of virus was injected into the left caudate putamen at a rate of 0.2 μl/min. For the AAV-VEGF/AAV-ANG1 group, 2 × 109 vg of each vector was used. Brain specimens were harvested 3 weeks later.
Evaluation of Atrophy Volume
Obvious atrophy in the ischemic hemisphere can be seen at 7 days after pMCAO (Nonaka et al, 2009). Therefore, we and other investigators have used atrophic volume to evaluate brain injury at the subacute stage of ischemic stroke (Fan et al, 2010; Nonaka et al, 2009).
A series of 20-μm thick coronal sections start 2 mm caudal to the frontal pole was obtained. One of every ten sections was stained with cresyl violet. Sections were digitized, and the atrophic area was outlined using Image J software. The atrophic area was calculated by subtraction of the area of ipsilateral hemisphere from the area of contralateral hemisphere. The atrophy volume was reconstructed using serial sections (Pang et al, 2001).
Analysis of Blood–Brain Barrier Permeability
We used a method modified from a previously described Evans blue assay (Chan et al, 1991). Before the animals were killed, 4 mL/kg of 2% Evans blue (Sigma, St Louis, MO, USA) in normal saline was injected into the left jugular vein of anesthetized animals. Vessels were perfused with phosphate-buffered saline 60 minutes later. The brain was removed and divided into right and left hemispheres. After photographs were taken, brain tissues were washed in phosphate-buffered saline twice, blotted dry, weighed, homogenized in 1500 μl formamide, and incubated overnight in formamide at 55°C. The samples were centrifuged at 12,000 r.p.m for 45 minutes. A supernatant was used to measure the absorbance of Evans blue at 620 nm with a spectrophotometer (Genesis 10uv; Thermo Electron Corp., Madison, WI, USA). Evans blue content was expressed as micrograms per gram of brain tissue, which was calculated against a standard. To normalize the perfusion efficiency, the absorbance of the contralateral hemisphere was subtracted from the hemisphere ipsilateral to the pMCAO. As a secondary analysis, we normalized the means of Evans blue extravasation by the means of atrophic volume.
Vascular Density Assessments
We have established reliable methods to quantify vascular density in the brain using CD31-stained sections (Shen et al, 2006b). Coronal sections (20 μm thick) were stained with anti-CD31 antibody (1:50, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Two coronal sections, 0.5 mm rostral and 0.5 mm caudal to the needle track, were chosen for vascular quantification. Photographs were taken from three areas of each section around the injection site at the border of the infarct region (Figure 1) under a 20 × objective. The vascular density was quantified using NIH Image J software and calculated as the mean of vascular numbers obtained from the six areas.
Histological Analysis
Immunohistochemical staining was performed using a series of 20-μm thick coronal sections. Briefly, sections were incubated overnight with the following primary antibodies at 4°C: rabbit anti-ZO-1 (zonula occludens, 1:100, Invitrogen, Carlsbad, CA, USA), occludin (1:100, Invitrogen), claudin-5 (1:100, Invitrogen), rat anti-CD31 (1:50, Santa Cruz Biotechnology), rabbit anti-human ANG1 (1:100, Santa Cruz Biotechnology), rabbit anti-human VEGF (1:200, Santa Cruz Biotechnology), and rabbit anti-Ki67 (1:800, Sigma, St Louis, MO, USA). After washing, the sections were incubated sequentially with biotinylated secondary antibody (1:3,000, Vector Laboratories, Burlingame, CA, USA), horseradish peroxidase-labeled streptavidin complex (Vector Laboratories) and diaminobenzidine (Vector Laboratories). For dual immunofluorescent staining, after incubating with primary antibodies, sections were incubated with Alexa Fluor 594-conjugated or Alexa Fluor 488-conjugated IgG (1:500, Invitrogen). Negative controls were performed by omitting the primary antibodies.
LacZ protein was detected by X-gal (5-bromo-4-chloro-3-indolyl-β--galactoside) staining. Sections were fixed in 0.5% glutaraldehyde for 10 minutes, incubated for 2 hours in X-gal staining solution (5 mmol/L K3Fe (CN)6, 5 mmol/L K4Fe (CN)6, 2 mmol/L, MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-β--galactoside; Allstar Scientific, Sunnyvale, CA, USA) in phosphate-buffered saline, and photographed.
Western Blot
Proteins were isolated from the ipsilateral hemisphere of the pMCAO, separated in NuPAGE® Novex 4–12% Bis-Tris gel (Invitrogen), and electrotransferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA). After blocking in 5% milk, the membranes were incubated overnight with antibodies specific to human VEGF (1:200, Santa Cruz Biotechnology), human ANG1 (1:200, Santa Cruz Biotechnology), mouse ZO-1, occludin, and claudin-5, (1:200, Invitrogen) at 4°C. After incubating with horseradish peroxidase-conjugated secondary antibody (Amersham, Piscataway, NJ, USA), the membranes were treated with SuperSignal West Femto Maximum Sensitivity Substrate detection reagent (Pierce Biotechnology, Rockford, IL, USA). The membrane was then exposed to Hyperfilm ECL (Amersham). β-Actin (1:10,000, Sigma) was used as a loading control.
Statistical Analyses
Data are presented as mean±standard deviation (s.d.). Mean values were compared using one-way analysis of variance followed by Fisher's LSD post hoc correction. A P value ⩽0.05 was considered statistically significant. Sample sizes were n=3 for analysis of transgene expression and quantification of tight junction protein expression, n=6 for quantification of atrophic volume and Evans blue extravasation, and n=7 for quantification of vessel density analysis.
Results
Vascular Endothelial Growth Factor and Angiopoietin-1 Expression Detected at the Injection Sites
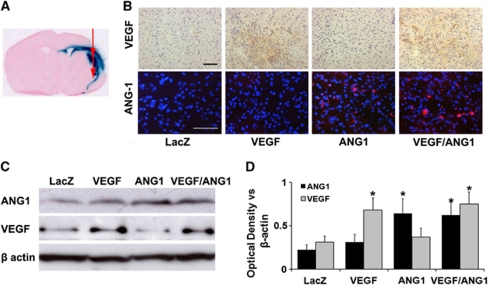
The VEGF, ANG1, and LacZ expression was detected around the injection sites at the border of the infarct area (Figures 2A and 2B). AAV-mediated human VEGF expression increased in the brain injected with AAV-VEGF, and human ANG1 expression increased in the brain injected with AAV-ANG1. Both VEGF and ANG1 expressions increased in the brain coinjected with AAV-VEGF and AAV-ANG1 (Figure 2B). Semiquantification using Western blot analysis showed that the levels of VEGF and ANG1 expression were comparable in the brain coinjected with AAV-VEGF and AAV-ANG1 (Figures 2C and 2D).
Figure 2.
Adeno-associated viral vector (AAV) mediated transgene expression. (A) A representative image of a coronal section shows LacZ expression around the injection sites at the border of the infarct area. (B) Representative images of vascular endothelial growth factor (VEGF) and angiopoietin-1 (ANG1) antibody-stained sections. VEGF expression was detected in the VEGF and VEGF/ANG1 groups (brown). ANG1 expression was detected in the ANG1 and VEGF/ANG1 groups (red). Scale bars are 100 μm. (C) Representative Western blots are shown. β-Actin was used as loading control. (D) Bar graph shows the quantification of the protein expression analyzed from Western blots. The expression of VEGF and ANG1 increased only in corresponding vector-injected brain tissues. *P<0.05 versus AAV-LacZ group, N=3 per group. LacZ: AAV-LacZ-injected group; ANG1: AAV-ANG1-injected group; VEGF: AAV-VEGF-injected group; and VEGF/ANG1: AAV-VEGF and AAV-ANG1-coinjected groups. The color reproduction of this figure is available at online version.
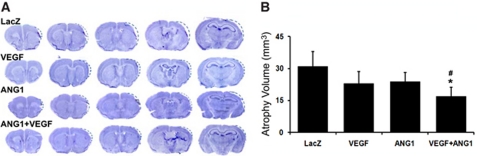
Coexpression of Angiopoietin-1 and Vascular Endothelial Growth Factor Reduced Atrophy Volume
Compared with injection of AAV-LacZ (control vector), coinjection of AAV-VEGF and AAV-ANG1 reduced the atrophy volume (18±5 mm3 versus AAV-LacZ: 31±7 mm3, P=0.004). Individual injection of AAV-VEGF (23±6 mm3, P=0.08) or AAV-ANG1 (24±4 mm3, P=0.09) resulted in a trend toward reduction of atrophy volume only. The coinjection group also had a significantly smaller atrophy volume than the AAV-ANG1 group (P=0.03, Figure 3). These indicate that coexpression of ANG1 and VEGF results in a greater reduction of atrophy volume than expression of either one alone.
Figure 3.
Coinjection of AAV-ANG1 and AAV-VEGF reduced atrophy volume. (A) Cresyl violet-stained sections are shown. Dotted lines outline the original brain size before atrophy of the infarct regions. (B) Bar graph shows the quantification of the atrophy volume; N=6. Compared with AAV-LacZ, injection of AAV-VEGF or AAV-ANG1 resulted in a trend toward reduction of infarct (P=0.08; P=0.09); coinjection of AAV-VEGF with AAV-ANG1 reduced the atrophy volume significantly compared with the AAV-LacZ and AAV-ANG1 groups (*P=0.004 versus AAV-LacZ group; #P=0.03 versus AAV-ANG1 group). LacZ: AAV-LacZ-injected group; ANG1: AAV-ANG1-injected group; VEGF: AAV-VEGF-injected group; and VEGF/ANG1: AAV-VEGF and AAV-ANG1-coinjected groups.
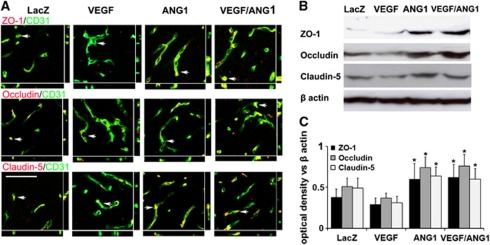
Coexpression of Angiopoietin-1 with Vascular Endothelial Growth Factor Improved Blood–Brain Barrier Integrity
We found that overexpression of VEGF reduced the expression of tight junction proteins, ZO-1, occludin, and claudin-5 (Figure 4; Supplementary Figure 1). Coexpression of ANG1 with VEGF restored the expression of tight junction proteins (Figure 4).
Figure 4.
Expression of tight junction proteins decreased in the AAV-VEGF-injected brain and was restored in the AAV-VEGF/AAV-ANG1-coinjected brain. (A) Representative images show tight junction protein expression. Vessels were visualized by immunolabeling endothelial cells with anti-CD31 antibody (green). ZO-1, occludin, and claudin-5 were detected by antibodies specific to the proteins (red). Arrows indicated the positions that were selected to show colocalization of endothelial cells and tight junction proteins. Scale bar=100 μm. (B) Representative images of Western blots are shown. β-Actin was used as loading control. (C) Bar graph shows quantification of the Western blot analysis. *P<0.05 versus AAV-VEGF-injected group. LacZ: AAV-LacZ-injected group; ANG1: AAV-ANG1-injected group; VEGF: AAV-VEGF-injected group; and VEGF/ANG1: AAV-VEGF and AAV-ANG1-coinjected groups. The color reproduction of this figure is available at online version.
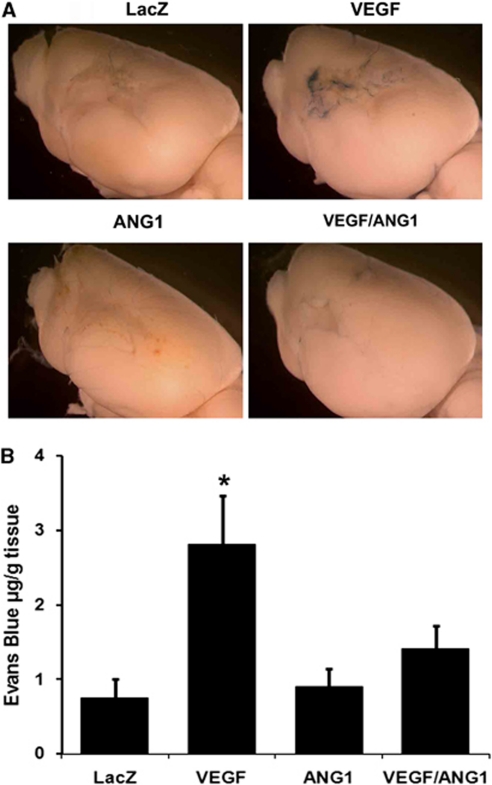
Injection of AAV-VEGF increased Evans blue extravasation compared with injection of AAV-LacZ (AAV-VEGF: 2.8±0.7 μg/g tissue versus AAV-LacZ: 0.7±0.3 μg/g tissue, P=0.001). The AAV-ANG1 (0.9±0.3 μg/g tissue) and AAV-LacZ groups had a similar degree of Evans blue extravasation (P=0.53). Coinjection of AAV-VEGF and AAV-ANG1 (1.4±0.3 μg/g tissue) reduced Evans blue extravasation compared with injection of AAV-VEGF alone (P=0.001) (Figure 5).
Figure 5.
Coexpression of angiopoietin-1 (ANG1) with vascular endothelial growth factor (VEGF) reduced blood–brain barrier (BBB) leakage caused by VEGF overexpression. (A) Images of the brain perfused with Evans blue are shown. More blue color was observed in the infarct region of the AAV-VEGF-injected group than other groups. (B) Bar graph shows the quantification of Evans blue extravasation; N=6. *P<0.01, versus AAV-LacZ- and AAV-ANG1-treated mice. Coinjection of AAV-VEGF and AAV-ANG1 reduced Evans blue extravasation compared with injection of AAV-VEGF alone (P=0.001). LacZ: AAV-LacZ-injected group; ANG1: AAV-ANG1-injected group; VEGF: AAV-VEGF-injected group; and VEGF/ANG1: AAV-VEGF and AAV-ANG1-coinjected groups. The color reproduction of this figure is available at online version.
After normalizing the means of Evans blue extravasation by the means of atrophic volume, we found that compared with injection of AAV-LacZ, the AAV-VEGF group had a higher Evans blue extravasation (AAV-VEGF: 0.12 μg/g tissue/mm3 atrophy versus AAV-LacZ: 0.02 μg/g tissue/mm3 atrophy). The normalized Evans blue extravasation for the AAV-ANG1 group was 0.04 μg/g tissue/mm3 atrophy and for the AAV-VEGF/AAV-ANG1-coinjected group was 0.08 μg/g tissue/mm3 atrophy (Supplementary Figure 3), both were less than that of the AAV-VEGF group. Hence, the data are consistent with the results before normalization.
Coexpression of Vascular Endothelial Growth Factor and Angiopoietin-1 Induced a Similar Degree of Angiogenesis as Vascular Endothelial Growth Factor Expression Alone
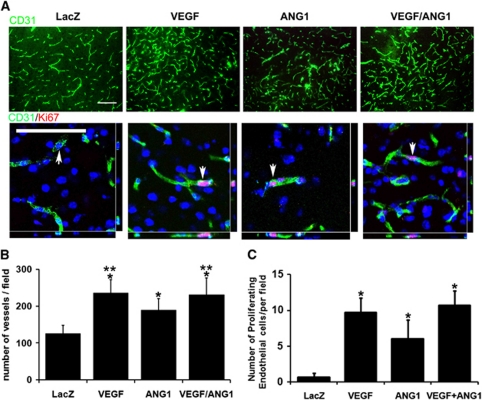
The vascular density was higher in the AAV-VEGF (235±34 per 20 × objective field) and AAV-ANG1 (189±29 per field) groups than in the AAV-LacZ group (126±20 per field, P<0.001; Figure 6). The AAV-VEGF group also had a higher vascular density than the AAV-ANG1 group (P=0.02). The AAV-VEGF/AAV-ANG1 group (231±42 per field) had similar vessel density as the AAV-VEGF group (P=0.85) and a higher vessel density than the AAV-ANG1 group (P=0.05; Figure 6). Compared with the AAV-LacZ group (0.7±0.6 per field), there were more Ki67-positive endothelial cells in the AAV-VEGF (9.7±2.0 per field, P=0.002), AAV-ANG1 (6±2.6 per field, P=0.027), and AAV-VEGF/AAV-ANG1 (10.7±2.1 per field, P=0.003; Figure 6; Supplementary Figure 2) groups. Thus, overexpression of VEGF and ANG1 individually or together increased endothelial cell proliferation. Coexpression of ANG1 with VEGF did not affect the angiogenic effect of VEGF, but improved the integrity of new blood vessels.
Figure 6.
Injection of AAV-VEGF, AAV-ANG1, or AAV-VEGF/AAV-ANG1 induced angiogenesis. (A) Representative images of CD31 and Ki67 antibody-stained sections are shown. There were more vessels and CD31/Ki67 double positive cells in the AAV-VEGF, AAV-ANG1, or AAV-VEGF/AAV-ANG1-injected brain than in the AAV-LacZ-injected brain. Arrows indicate cells that were selected to show colocalization of endothelial cells and Ki67-positive nuclei. Scale bars=100 μm. (B) Bar graph shows the quantification of vascular density. *P<0.05 versus LacZ group. **P⩽0.05 versus AAV-ANG1 group. (C) Bar graph shows the quantification of Ki67-positive endothelial cells. *P<0.05, versus LacZ group. N=7 for each group. LacZ: AAV-LacZ-injected group; ANG1: AAV-ANG1-injected group; VEGF: AAV-VEGF-injected group; and VEGF/ANG1: AAV-VEGF and AAV-ANG1-coinjected groups. The color reproduction of this figure is available at online version.
Discussion
Using a distal permanent MCAO mouse model, we have shown that AAV-mediated coexpression of ANG1 and VEGF resulted in reduced atrophy volume compared with expression of these factors individually. We also showed that overexpression of VEGF reduced the expression of the structural tight junction proteins and increased BBB leakage, and that coexpression of ANG1 with VEGF increased tight junction protein expression and reduced BBB leakage.
Endogenous VEGF expression rapidly increases within hours and remains elevated for as long as 1 month after stroke. Vascular endothelial growth factor and ANGs are expressed in a temporally and spatially orchestrated manner, which makes neurologic recovery possible (Hermann and Zechariah, 2009). Zhang et al (2002b) showed that Vegf mRNA increased 2 to 4 hours after onset of ischemia, accompanied by reduced Ang1 mRNA and increased BBB leakage. Increased expression of Vegf/Vegf receptors and Ang/Tie2 2 to 28 days after stroke correlate with the formation of enlarged and thin-walled vessels, suggesting that acute alteration of VEGF and ANG1 in the ischemic core may mediate BBB leakage, whereas upregulation of Vegf/Vegf receptors and Ang1/Tie2 at the boundary zone may regulate neovascularization in the ischemic brain.
Vascular endothelial growth factor also promotes neurogenesis after cerebral ischemia (Sun et al, 2003). However, the acute upregulation of VEGF after stroke is associated with an increase in BBB permeability (Zhang et al, 2000). We demonstrated in this study that overexpression of VEGF resulted in a trend toward reduction of atrophy volume. We also showed that overexpression of VEGF reduced tight junction protein expression and increased Evans blue extravasation. Thus, overexpression of VEGF alone is not adequate to protect against ischemic injuries and to induce functional cerebral angiogenesis that promotes neurorestoration in the ischemic brain.
The improved outcome we observed in this study was due to multiple factors. Gene expression mediated by AAV serotype 1 vector presented at day 1 after the vector injection and lasted beyond 3 weeks (Su et al, 2006), indicating that VEGF and ANG1 affect both acute and chronic stages.
At the acute stage, coexpression of ANG1 with VEGF reduces ischemic injury through (1) their additive effect on pro-survival and antiapoptotic functions leading to a reduction of neuronal cell death and vessel damage (Bartholdi and Schwab, 1995; Beierle et al, 2002; Han et al, 2010; Tao et al, 2011) and (2) reduction of vessel leakage and edema caused by VEGF leading to better tissue perfusion. Both VEGF and ANG1 have been shown to inhibit intrinsic apoptotic signaling (Tao et al, 2011; Beierle et al, 2002). Angiopoietin-1, through the Tie2 receptor, promotes endothelial survival (Han et al, 2010; Schubert et al, 2011). We recently showed in a porcine myocardial infarction model that AAV-mediated coexpression of VEGF and ANG1 stimulated cardiomyocyte proliferation and reduced apoptosis through activation of the Akt/Bcl-2 pathway (Tao et al, 2011). Angiopoietin-1 reduces the permeability of vessels induced by several stimuli such as Vegf, histamines, and inflammatory cytokines (Thurston et al, 1999, 2005). Thus, coexpression of ANG1 with VEGF reduces vessel leakage and edema caused by VEGF at the acute stage, which can also lead to reduction of infarct/atrophy volume.
At the chronic stage, coexpression of ANG1 with VEGF improves outcomes through induction of new nonleaky vasculature supporting the growth and differentiation of neuronal stem/progenitor cells (Zhao et al, 2011). Brain neurons and astrocytes critically depend on the integrity of a functional capillary system that supplies oxygen and energy-rich substrates to the tissue (Han and Suk, 2005). Thus, new healthy vessels can support the growth and differentiation of neuronal stem/progenitor cells that have been activated by endogenous Vegf upregulated at the early stage of ischemic insult, and exogenous VEGF expression by AAV-mediated VEGF gene transfer.
Other studies have revealed the benefits of coexpression of ANG1 with VEGF in reducing neuron injuries. Intravenous injection of human bone marrow stromal cells transduced with VEGF and ANG1 genes into MCAO rats results in smaller atrophy volume and better functional recovery than an injection of untransduced bone marrow stromal cells or bone marrow stromal cells transduced with either gene alone (Toyama et al, 2009).
Angiopoietin-1 alone has been shown to inhibit leakage of cerebral vessels and decrease lesion size after focal cerebral embolic ischemia in mice analyzed 24 hours after MCAO (Zhang et al, 2002a). In our study, we did not detect a difference of Evans blue extravasation between the AAV-LacZ and AAV-ANG1 groups 3 weeks after pMCAO. We surmise that the discrepancy could be due to the differences in models and vectors used in these studies, the time and the route of vector delivery, and the time of the sample analysis. Dr Zhang's group studied the effects of ANG1 at the acute stage, while we analyzed the effects at a relatively later stage.
Vascular endothelial growth factor-induced increases of BBB leakage have also been associated with VEGF-induced upregulation of matrix metalloproteinase-9 activity. Angiopoietin-1 counteracts VEGF-induced permeability and is associated with a reduction of matrix metalloproteinase-9 activity (Valable et al, 2005). In vitro studies show that Vegf mediates disruption of claudin 5 and promotes BBB breakdown (Argaw et al, 2009), and the ANG1 antipermeability effect is associated with a regulation of the expression of ZO-1 and occludin (Valable et al, 2005). Here, we show that coexpression of ANG1 and VEGF increases the expression of tight junction proteins in a mouse pMCAO model, suggesting that overexpression of ANG1 improves the BBB structural integrity of newly formed vessels induced by VEGF. However, matrix metalloproteinase-9 has biphasic roles in stroke pathophysiology, mediates injury during the acute phase, and promotes neurovascular remodeling during the recovery phase (Lo, 2008; Zhao et al, 2006). The mechanisms by which ANG1 reduces BBB alteration in the ischemic brain and exerts its protective effect are, therefore, not yet fully understood.
Angiopoietin-1 has been implicated in the remodeling of vessels, resulting in their enlargement (Baffert et al, 2006; Thurston et al, 2005) and, in some cases, stimulating an angiogenic response (Asahara et al, 1998; Cho et al, 2004). Han et al (2010) showed that neuroprotection provided by ANG1 protein is mainly through improvement of endothelial survival and reduction of inflammation after spinal cord injury, but it did not show an angiogenic effect for ANG1. Our findings in this study indicate that overexpression of ANG1 alone induces significant angiogenesis, and that ANG1 has a pro-angiogenic effect in the setting of murine MCAO. However, coexpression of ANG1 with VEGF did not induce more angiogenesis than expression of VEGF alone, but it improved vascular integrity.
In conclusion, our study has shown that AAV-mediated coexpression of ANG1 with VEGF in the MCAO brain improved BBB integrity and resulted in a better reduction of atrophy volume than expression of VEGF alone. Because this study has limitations, namely: (1) the outcomes were only analyzed at 3 weeks after pMCAO and (2) the functional recovery was not analyzed, in our future experiments we will endeavor to assess functional outcomes in connection with analyses of neurogenesis and neurorestoration.
Acknowledgments
The authors thank Tony Pourmohamad, MS, for assistance with statistical analysis, Voltaire Gungab for assistance with manuscript preparation, and the other members of the UCSF BAVM Study Project (http://avm.ucsf.edu.) for their support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by grants R21 NS070153 (HS), R01 NS27713 (WLY), P01 NS44155 (WLY and HS), and T32 GM008440-14 (EW) from the National Institutes of Health and AHA 10GRNT3130004 (HS) from the American Heart Association.
Supplementary Material
References
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006;290:H107–H118. doi: 10.1152/ajpheart.00542.2005. [DOI] [PubMed] [Google Scholar]
- Beierle EA, Strande LF, Chen MK. VEGF upregulates Bcl-2 expression and is associated with decreased apoptosis in neuroblastoma cells. J Pediatr Surg. 2002;37:467–471. doi: 10.1053/jpsu.2002.30868. [DOI] [PubMed] [Google Scholar]
- Chan PH, Yang GY, Chen SF, Carlson E, Epstein CJ. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing CuZn-superoxide dismutase. Ann Neurol. 1991;29:482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, Chen Y, Su H, Young WL, Yang GY. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res. 2005;2:409–423. doi: 10.2174/156720205774962647. [DOI] [PubMed] [Google Scholar]
- Han S, Arnold SA, Sithu SD, Mahoney ET, Geralds JT, Tran P, Benton RL, Maddie MA, D′Souza SE, Whittemore SR, Hagg T. Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain. 2010;133:1026–1042. doi: 10.1093/brain/awq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29:1620–1643. doi: 10.1038/jcbfm.2009.100. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- Nonaka Y, Koumura A, Hyakkoku K, Shimazawa M, Yoshimura S, Iwama T, Hara H. Combination treatment with normobaric hyperoxia and cilostazol protects mice against focal cerebral ischemia-induced neuronal damage better than each treatment alone. J Pharmacol Exp Ther. 2009;330:13–22. doi: 10.1124/jpet.109.151548. [DOI] [PubMed] [Google Scholar]
- Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- Schubert SY, Benarroch A, Monter-Solans J, Edelman ER. Primary monocytes regulate endothelial cell survival through secretion of angiopoietin-1 and activation of endothelial tie2. Arterioscler Thromb Vasc Biol. 2011;31:870–875. doi: 10.1161/ATVBAHA.110.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, Young WL, Yang GY. Adeno-associated viral vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006a;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006b;11:3190–3198. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- Su H, Huang Y, Takagawa J, Barcena A, Arakawa-Hoyt J, Ye J, Grossman W, Kan YW. AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene Ther. 2006;13:1495–1502. doi: 10.1038/sj.gt.3302787. [DOI] [PubMed] [Google Scholar]
- Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci USA. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Takagawa J, Huang Y, Arakawa-Hoyt J, Pons J, Grossman W, Kan YW. Additive effect of AAV-mediated angiopoietin-1 and VEGF expression on the therapy of infarcted heart. Int J Cardiol. 2009;133:191–197. doi: 10.1016/j.ijcard.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Chen B, Tan X, Zhao Y, Wang L, Zhu T, Cao K, Yang Z, Kan YW, Su H. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci USA. 2011;108:2064–2069. doi: 10.1073/pnas.1018925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Thurston G, Wang Q, Baffert F, Rudge J, Papadopoulos N, Jean-Guillaume D, Wiegand S, Yancopoulos GD, McDonald DM. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–3326. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol. 2009;216:47–55. doi: 10.1016/j.expneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, Adams LD, Gottlieb DI, Johnson EM, Jr, Yu SP, Choi DW. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–193. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002a;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002b;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao H, Bao XJ, Wang RZ, Li GL, Gao J, Ma SH, Wei JJ, Feng M, Zhao YJ, Ma WB, Yang Y, Li YN, Kong YG. Postacute ischemia vascular endothelial growth factor transfer by transferrin-targeted liposomes attenuates ischemic brain injury after experimental stroke in rats. Hum Gene Ther. 2011;22:207–215. doi: 10.1089/hum.2010.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.