Abstract
As the average age of the population grows, the incidence of osteoporosis and skeletal diseases continues to rise. Current treatment options for skeletal repair include immobilization, rigid fixation, alloplastic materials and bone grafts, all which have significant limitations, especially in the elderly. Adipose derived stromal cells (ASCs) represent a readily available abundant supply of mesenchymal stem cells which demonstrate the ability to undergo osteogenesis in vitro and in vivo, making ASCs a promising source of skeletal progenitor cells. Current protocols allow for the harvest of over 1 million cells from only 15cc of lipoaspirate. Despite the clinical use of ASCs to treat systemic inflammatory diseases, no large human clinical trials exist using ASCs for skeletal tissue engineering. The aim of this review is to define ASCs, to describe the isolation procedure of ASCs, to review the basic biology of their osteogenic differentiation, discuss cell types and scaffolds available for bone tissue engineering and lastly to explore imaging of ASCs and their potential future role in human skeletal tissue engineering efforts.
Keywords: Adipose derived stromal cells, Skeletal tissue engineering, Tissue regeneration, Multipotent stromal cells, adipogenic differentiation, Subcutaneous fat depots
Introduction
Over the last 50 years, the number of Americans over 65 has increased from 12 to 37 million and continues to grow at an average rate of 2.0% per year.1 With a more aged population comes a significant increase in people living with osteoporosis and suffering from fractures and other skeletal disabilities. Current methods for the treatment of fractures largely rely on immobilization, rigid fixation and bone grafts, all of which have associated drawbacks particularly in aged patients. Concurrent with the increase in age of the population, the weight of the average American continues to grow as does surgical procedures to address this excess adipose tissue with over 200,000 liposuction procedures performed annually in the United States and over a million procedures performed annually worldwide. 2
Fat has long been felt to be an inert tissue, and for years lipoaspirate has been discarded as surgical waste. When a surgeon harvests lipoaspirate, they are harvesting numerous cell types, including preadipocytes, adipocytes, fibroblasts, vascular smooth muscle cells or pericytes, endothelial cells and resident monocytes, macrophages and lymphocytes.3 Within the stromal vascular layer, scientists have begun to investigate a vast population of cells with the potential to differentiate into mesodermal tissues. Importantly, these multipotent adipose derived stromal cells (ASCs) have been shown to have similar morphology, differentiation capacity and phenotype to those mesenchymal stem cells isolated from bone marrow and umbilical cord blood. Clinical trials have already been established for intravenous administration of ASCs for autoimmune and inflammatory disorders such as multiple sclerosis and arthritis.4 Despite promising preliminary results using ASCs as a therapeutic modality for inflammatory disease applications, a large clinical trial has yet to be established to address fractures and skeletal diseases.
While considering therapies for skeletal deficits, scientists must understand the composition of bone and how to define the formation of bone de novo from ASCs. Bone is defined broadly as a specialized cell type, the osteoblast, and surrounding extracellular matrix that these cells secrete and remodel. Osteoblasts function to secrete calcified matrix (termed osteoid), proteins and growth factors necessary for osteogenesis to occur. The extracellular matrix consists of proteins such as collagen type I, ostocalcin, osteopontin and bone sialoprotein as well as mineralized matrix or hydroxyapatite. As mineralization occurs, osteblasts are surrounded by its own calcifying osteoid matrix, becoming an osteocyte. The molecular mechanisms that occur during osteoblast maturation have been thoroughly studied. Runx2/Cfba, a helix-loop-helix nuclear factor initiates a pluripotent mesenchymal stem cell to start differentiating down an osteoblastic lineage and Osterix (Osx) is also required for mesenchymal cell osteogenic differentation.5
ASCs have been found to undergo osteogenesis rapidly and with minimal stimulation by exogenous cytokines and thus represent a promising option for skeletal tissue engineering trials.6 The current, clinical gold standard for the treatment of non-healing skeletal defects is an autogenous bone graft. Autogenous sources from which to harvest bone grafts are limited and thus clinicians have turned to allogenic bone substitutes such as demineralized bone matrix which consists of extracellular matrix proteins and growth factors without any cells. In an attempt to augment acellular options, surgeons have begun to use Bone Morphogenic Protein-2 (BMP-2) absorbed on a collagen sponge for non-unions and spinal fusion. Despite promising results, collagen sponges are not rigid and BMP-2 release is limited in duration failing to provide long term growth. The shortcomings of current methods indicate the need to combine our understanding of what cell types can form bone and what scaffold best facilitates the differentiation of these cells.
One osteoprogenitor cell that is easily harvested and abundant in large quantities is the ASC. The ultimate translational goal is to harvest subcutaneous adipose tissue from the ideal anatomic location, enrich for ASCs with an improved osteogenic potential, treat the cells with ideal small molecules or cytokines and implant these cells on a scaffold into the skeletal defect in the same patient without leaving to the operating room. In this review we will discuss the definition of ASCs, review how they are harvested, examine the molecular underpinnings of their osteogenic differentiation, analyze the effect of harvest techniques on osteogenic differentiation in vitro and in vivo, and finally detail future clinical correlates.
Definitions
Caplan et al. originally described mesenchymal cells when observing bone formation after bone marrow cells were transplanted to a heterotopic location. These cells were named “mesenchymal” stem cells because of the belief that they could differentiate into skeletal muscle, smooth muscle fat, cartilage, connective tissues, tendon and bone.7 Within bone marrow, mesenchymal cells are located in the stromal compartment and are unique from the hematopoietic compartment. Mesenchymal stem cells (MSCs) harvested from the marrow compartment of bone, have been used for tissue engineering, including the study of bone formation for the spine. 8 MSC harvest, however, requires aspiration from the iliac crest which only yields 10–40 ml of marrow or from bone marrow biopsies, both of which can be painful and yield low numbers.9 As an alternative to marrow derived MSCs, laboratories have identified a similar multi-lineage mesenchymal progenitor cells from adipose tissue. A wide variety of terms have been used to describe the multipotent cells derived from white adipose tissues that adhere to plastic. Such descriptions include preadipocytes, adipose derived mesenchymal cells, adipose derived adult stem cells, processed lipoaspirate cells, human adipose-derived adherent stromal cells and adipose derived stromal cells (ASC).10 At the Second Annual International Fat Applied Technology Society meeting, scientists reached a consensus to refer to these cells as ASCs which is how they will be referred throughout this review.11 It is crucial to differentiate ASCs from the stromal vascular fraction which refers to the minimally processed cells that have not yet been exposed to plastic. These, ASCs are both mesenchymal stem cells (in that they are a postnatal progenitor of mesoderm derived cell types) as well as stromal cells (as they are fibroblastic cell type in vitro and likely derived from adipose connective tissue).12
Characterizing ASCs based on surface proteins
Previous studies have demonstrated similar phenotypes between human MSCs and ASCs as well as similar adhesion and receptor profiles.13 Numerous investigators have described ASC surface antigen expression profiles, but a definitive surface antigen profile that completely defines ASCs and allows prospective isolation has been lacking. Cultured ASCs are STRO-1 negative, however, cultured bone marrow derived MSCs (BMSCs) are reported as STRO-1 positive despite comprising less than 5% of the total BMSC population.13 Scientists have also noted CD106 by flow cytometry,9 while others did not detect this surface antigen. Such discrepancies underlie the need to indicate the cell passage, proliferative stage and patient profile. Despite inconsistencies, scientists generally define ASCs as those cells that express the surface receptor molecules CD44 (hyaluronate) and CD90, as well as integrin β1 (CD29), endoglin (CD105) and integrin α4 (CD49) and to not to express the hematopoietic markers CD45, CD34 and cKit (CD117).2 Despite certain cell surface panels being used to define ASCs, there has been observed a significant amount of drift when looking at ASC markers immediately after harvest compared to later passage cells making it difficult to define a “pure population.” 2
ASC Harvest
Human ASC harvest can be derived from lipoaspiration or surgically resected adipose tissue. Once harvested, the adipose tissue settles into two layers: supernatant or processed lipoaspirate layer, consists of the suctioned adipocytes as well as their surrounding endothelium and stroma. The bottom layer, or liposuction aspirate fluid, consists of injected saline, erythrocytes and denser pieces of the processed lipoaspirate layer. 2 ASCs can be harvested from both layers, however, the yield of adherent ASCs is significantly higher in the adipocyte layer than in the liposuction aspirate fluid cells.2
Following harvest, adipose specimens should first be washed, undergo enzymatic digestion followed by neutralization, straining and plating. The specimen that is plated is the stromal vascular fraction (SVF) and those adherent cells are the ASCs. On average, 15cc of starting lipoaspirate can be plated onto 1x 10cm cell culture plates which three days later, can be split to three 10cm plates. The total number of cells from adipose harvest has been described as 308,849 per ml of lipoaspirate or 1.5 × 1010 from 1000ml of lipoaspirate after two passages.11 At a plating density of 1.85 × 103 cells/cm2, ASCs reach confluence by day 13.14
When using human tissues in the laboratory, it is critical to use biohazard precautions and to sterilize or discard all equipment with bleach before and after usage as this tissue is considered Biosafety Level 2 (BSL-2). Collection containers and tubing should be disposed or sterilized with bleach after each patient use.
Differences in hASCs based on tissue location, and preparation
Fat distribution between men and women and within each gender demonstrate significant heterogeneity. Adipose tissue from subcutaneous locations, or depots, have different blood supplies, cytokine signaling and gene expression profiles leading to differences in osteogenic capacity. 15 Stimulated by the rising incidence of obesity and the need for a more thorough understanding of adipose biology, a growing number of studies have investigated the differences between subcutaneous and visceral fat depots with visceral fat depots having a greater osteogenic potential.15 The potential breakthroughs in the use of stromal cells have prompted some individuals to store their own tissues in a fee-for-service fashion and allowed the formation of biotechnology companies specializing in stromal cell storage. The most prevalent example is the cryostorage of umbilical cord derived blood. The process of cell freezing and the long-term storage under such conditions inevitably alters cellular processes and characteristics.16 In vitro cellular parameters, including proliferation, osteo- and adipogenic differentiation have also been assessed after the freeze-thaw process demonstrating a decreased capacity to proliferate and differentiate after cryostorage. 17, 18 Data from our laboratory confirm that the freezing process of hASCs, has deleterious effects on the ability of these cells to undergo osteogenic differentiation even if only in cryostorage for two weeks.19 Thus, though cryopreserved cells can undergo osteogenic differentiation, the fresh harvest of hASCs and immediate use may allow for a more robust osteogenic reconstruction.
Osteogenic Differentiation In Vitro
Osteogenesis is defined by a series of events which starts with a commitment to an osteogenic lineage by mesenchymal cells. Subsequently, these cells proliferate and demonstrate an up regulation of osteoblast-specific genes and mineralization. After attachment, ASCs can be treated with osteogenic differentiation medium (ODM) containing DMEM, 10% FBS, 100μg/mL ascorbic acid, 10mM β-glycerophosphate, and 100 U/mL penicillin/streptomycin. Retinoic acid can be supplemented to augment mASC differentiation but is not necessary for hASCs.
Multiple signaling pathways have been demonstrated to participate in the differentiation of an osteoblast progenitor to a committed osteoblast including TGFB/BMP, Wnt/B-Catenin, Notch, Fibroblast growth factor and Hedgehog. BMPs in particular have been demonstrated to play a significant role in osteoblast differentiation and osteogenesis, most notably BMP-2,4 and 7. BMP is a member of the TGF-B superfamily and initiates its signaling cascade through BMPR receptors types I and II. These activated receptor kinases subsequently phosphorylate transcription factors Smad 1,5 and 8.20 These phosphorylated Smads form a heterodimeric complex with Smad4 and stimulate target genes. Such downstream genes include Msx2, Dlx5 and Id proteins, (inhibitor of DNA binding/differentiation helix-loop-helix proteins) which during early osteogenesis, regulate proliferation of osteoprogenitors.21 Investigators have demonstrated that inhibition of these Id proteins promote osteogenesis in vivo.22
BMPs have also been shown to activate Cbfa1/AML3 or Runt-Related Protein 2 (Runx-2) and osteopontin as well as stimulate osteogenic differentiation. Runx-2 and Osterix (Osx) are considered the master regulation genes for bone formation.23 There are two major isoforms of Runx2 that vary in their tissue localization. Compared with isoform II of Runx2, isoform I is not as tissue specific as it is also detected in sperm, brain, testis, breast, prostate, and melanoma cancer cells.24 Runx-2 isoform II is considered one of the earliest markers of the osteoblast lineage and is considered a key transcriptional activator of osteogenic differentiation and is seen two to three days prior to osteogenesis.24 Binding sites for Runx-2 are present in genes whose gene products play a role in extracellular matrix mineralization such as collagen type I and osteopontin (OPN) as well as genes involved cell growth, and angiogenesis. 23
Cells that express Runx-2 display characteristics of a future osteoblast or chondrocyte, however, later in development, only those cells which differentiate into osteoblasts show evidence of Runx-2 expression.25 Transfection of Runx-2 in fibroblasts stimulates expression of osteoblast specific genes such as Osteocalcin whereas inhibition of Runx-2 during development leads to the absence of osteoblasts and has a severe phenotype of cleidocranial dysplasia that is associated with perinatal death.25
Specific to mesenchymal stem cells, studies have shown that Runx2 remains present during mitosis and may induce lineage differentiation of multipotent cells.23 It is felt that after mesenchymal cell differentiation down an osteoblastic pheonotype, Runx2 promotes departure from the cell cycle and decreases proliferation but increases osteogeneic differentiation.
Osx is a zinc-finger containing transcription factor that is expressed in osteoblasts during mouse bone development. Up regulation of Osx has been demonstrated to stimulate the osteogenic differentiation of ES cells in vitro and a retroviral transduction of Osx into marrow derived MSCs has been shown to increase their osteoblastic markers such as alkaline phosphatase, osteocalcin and osteopontin.26 Osx-null mice, similar to Runx-2 null mice have no cortical or trabelcular bone. 5 Osx, however, appears to play a role more downstream as Osx-null mice had normal cartilage and normal Runx-2 expression whereas Runx-2 null mice display delayed chondrocyte maturation and decreased Osx expression.5 More recent studies using Osx knockdown demonstrated significantly impaired osteogenesis during osteogenic differentiation of fetal bone cells.27
To assess the osteogenic differentiation of ASCs, RNA of these osteogenic genes can be analyzed at different time points. Specific gene markers of early ostoeogenesis include, RUNX-2 and Alkaline Phosphatase (ALP), intermediate markers include, OPN and late osteogenesis is best determined by Osteocalcin (OCN) expression. Alkaline phosphatase and Alizarin Red stains also allow assessment of early osteogenic differentiation and terminal bone mineralization respectively.
Adipocyte Differentiation of ASCs
There has been increasing interest in examining the interdependency between adipogenesis and osteogenesis as it is thought there was an inverse relationship between adipocytes and osteoblasts in bone marrow.28 Peroxisome proliferator activated receptor gamma (Ppar γ) has been shown to play a key role in adipogenic differentiation as scientists have demonstrated that Ppar γ-deficient ES cells failed to differentiate into adipocytes, but spontaneously differentiated into osteoblasts. Furthermore, these ES had restoration of their adipogenic potential with reintroduction of the Ppar γ gene.29 Subsequent findings indicate that a shared co-activator protein, transcriptional co-activator with PDZ binding motif (TAZ) functions as a link between Runx2 and PPAR γ and that TAZ activated Runx2 and osteogenesis while suppressing Ppar γ and adipogenesis.28 Specific mutations in Ppar γ allowed scientists to demonstrate that young heterozygous Ppar γ +/− mice had increased bone mass and elevated mRNA of Runx2 and Osx.30 These studies into adipogenesis and osteogenesis indicate that Ppar γ down-regulation may be a future target in order to enhance the osteogenic capability of ASCs.
In vivo models
To adequately translate in vitro findings to the clinical realm, robust in vivo data must be obtained to demonstrate the osteogenic capacity of ASCs. Long bone skeletal defect models offer the benefit of using bones that are under load bearing stress. Many groups use femoral defects as well as tibial defects as a load bearing bone.31 The ideal model should represent that clinical condition of the bone injury or defect. Thus, if interested in treating long bone non-unions, similar appendicular skeletal non-union models should be used in animals. Alternatively, if treating a calvarial defect where other tissues such as dura mater play a role, a similar calvarial model should be utilized.
When studying long bone non-unions, injuries should be created in a load bearing location, and stable fixation must be used. Benefits of the femoral bone are its larger shaft than the tibia thus tolerating a larger defect and allowing for the placement of an external fixator device. Several groups have used this defect model to seed ASCs on an osteoconductive scaffold. Peterson et al demonstrated the use of ASCs genetically modified to over-express BMP-2 to heal a femoral critical sized defect, though this same study showed that BMP-2 alone on a scaffold also allowed for significant healing.32 Other laboratories attempting to enhance osteogenesis using ASCs seeded on a BMP-2 releasing scaffold failed to demonstrate improved healing over using the BMP-2 laden scaffold alone, however, the authors failed to demonstrate viability of the ASCs in vivo.33 Thus, it is known that BMP-2 stimulates surrounding tissues, however, more robust data is needed to demonstrate that BMP-2 also augments the osteogenic potential of implanted ASCs. Human ASCs promoted fracture healing and improved biomechanical function in a rat femur non-union, however, ASCs failed to improve healing in the spinal fusion model.8
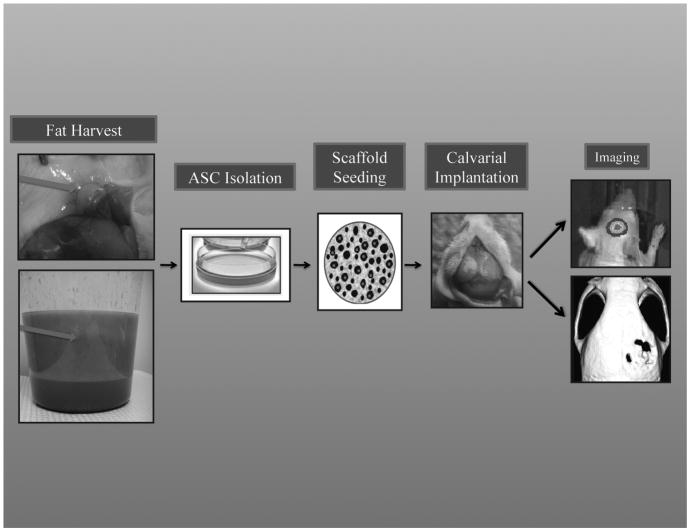
Several models exist to assess calvarial defects, and we believe a 4mm mouse parietal bone model offers a reliable, easily replicated and easily followed defect model (Figure 1). This model functions as a critical sized defect (4mm) in that it will undergo less than 5% healing, even if followed for 8 weeks. Thus, if any healing is seen, it is likely due to the treatment used. When a hydroxyappatite coated PLGA scaffold is seeded with 150,000 human or mouse ASCs, we have observed over 80% healing at 8 weeks.6 In comparison, those treated with a PLGA scaffold alone healed less than 20% proving the osseous healing is likely due to the implanted ASCs.
Figure 1.
In vivo model for skeletal tissue engineering.(Left, Top) Superior view of mouse left inguinal fat pad. Red arrow points to the fat pad which is dissected and subsequently digested for ASC harvest. (Left, Bottom) Human lipoaspirate in sterile collection canister settled into two layers. The upper yellow layer (Green Arrow) is the adipose tissue from which ASCs are harvested. Subsequently ASCs are isolated in vitro. 150,000 cells can then be seeded on an osteoconductive scaffold (Middle) and then the ASC laden scaffold can be placed inside a critical sized calvarial defect in syngeneic for mASCs or nude athymic mice for hASCs (Right). Mice can then be imaged using IVIS systems to detect cell viability and MicroCT to quantify osseous healing (Right).
When using human cells, nude or athymic animals should be used to decrease the inflammatory response which may confound results. The pro-inflammatory environment is a significant feature in bone repair and is initiated by the innate immune system. Similar to injuries to other tissues, an increase in intereukin-1 and 6 as well as tumor necrosis factor alpha is seen during bone remodeling.34 Injured bone repair requires the participation of both the immune and hematopoeitic niche of immune marrow cells as well as osteogenic precursor cells from the surrounding periosteum and surrounding osteoblasts and soft tissue. Nude or athymic animals may demonstrate a blunted inflammatory response, however, they only lack a T-cell response and can still mount an inflammatory response with regards to B-cells and NK-cells and still possess the surrounding osteogenic precursor cells from the periosteum.
Imaging
Although histology and histomorphometry remain the basis of in vivo analysis of pathways and defining tissues at a specific time point, such methods are less able to demonstrate physiologic whole body effects.35 Furthermore, such hostologic methods require chemical fixation and examination of tissues under nondynamic conditions.35 Imaging techniques, such as Live micro-computed tomography (microCT), however, allow scientists to serially follow osseous healing without sacrificing the animal and 3-D reconstructions allows for high resolution images. Unlike histology, imaging techniques allow the repetitive study of the same animal model allowing a dynamic analysis.35 Simultaneously, cells can be tagged with reporters allowing studies of the implanted cells to continue while osteogenesis occurs. Along with osseous healing, scientists and surgeons must demonstrate the viability of the ASCs in vivo to prove that the donor ASCs participate in healing of the recipient defect rather than just secreting cytokines to stimulate the surrounding host tissues. One way to demonstrate viability is to stably transduce ASCs with a lentivirus carrying triple fusion reporter genes, including firefly luciferase (Fluc), red fluorescence protein (RFP) and herpes simplex virus truncated thymidine kinase (HSV-ttk) genes.35 Stably expressed ASCs can then be purified by fluorescence activated cell sorting based on the RFP expression (Figure 1). Bioluminesence allows monitoring in vivo whereas the RFP or GFP allows for marking of these cells during histologic examination to determine if the host or implanted cells are located in the region of osteoid formation.
Bone Tissue Engineering: Scaffolds
Stem cells exist in tightly controlled environmental niches and alterations in this microenvironment can dramatically modify their behavior and differentiation capacities. Furthermore, stem cells are often utilized in the setting of disease or injury where inflammatory signals may be prevalent altering their function. Biomaterial scaffolds can potentially provide a controlled environment protecting implanted cells from harmful stimuli. Biomaterial matrices are also used to deliver genetic material and/or inductive biochemical cues which allow for some degree of developmental control over the delivered stem cells.
Numerous studies have demonstrated the benefits of utilizing stem cell-scaffold constructs in regenerative medicine. Scaffolds provide a highly modifiable vehicle for inductive factors as well. One cytokine of great interest and promise in skeletal tissue engineering is BMP-2. In a laboratory setting, several investigators have used BMP-2 delivery methods to enhance the osteogenic capability of ASCs. 32 BMP-2, however, is osteoinductive and likely enhances the osteogenesis of surrounding osteoblasts, periosteal cells and underlying dura in calvarial defects. Thus, further studies must determine if the enhanced osteogenesis seen with BMP-2 is due to its paracrine effect on implanted cells or host tissues.
In a clinical setting, InfuseR, a rBMP-2 with an absorbable collagen sponge carrier has been approved and is frequently used in spine surgeries. Original clinical studies in 2002 analyzed a prospective randomized analysis of 279 patients with degenerative lumbar disc disease who were treated with either InFUSE, or autogenous iliac bone grafts. The authors demonstrate that at 24 months, 94.5% fusion rate in the InFUSE treated group compared to 88.7% in the bone graft treated group. Furthermore, mean operative time and blood loss were shown to be lower in the InFUSE treated patients.36 A more recent randomized, controlled trial of lumbar spine fusion in patients over sixty, also demonstrated that the InFUSE group had lower complication rates likely related to the lack of a secondary defect created by harvesting iliac bone. Interestingly, this study also demonstrated a similar total cost in care between the two groups despite the expense of BMP-2.37
Other studies analyzing the interaction of scaffolds and cells include a study by Fang et al. where the authors utilized degradable matrices containing a BMP-4 plasmid DNA and demonstrated successful in vivo genetic manipulation of fibroblasts to induce bone formation in rats.38 Schek et al. demonstrated that bone formation was greater with hydrogel delivery of BMP-7-expressing adenovirus in mice compared to non-hydrogel controls.39 These are just a handful of examples of the benefits of using scaffold-based applications to enhance the regenerative potential of stem cells.
Bone Tissue Engineering: Cells
Investigations into cell-based skeletal tissue engineering must identify a cell type with the ability to generate bone. Shortcomings of cell types often involve their availability, viability, vascularization and elicitation of immune response upon implantation, and directed differentiation. Options for skeletal tissue engineering include osteoblasts, embryonic stem cells and other postnatal mesenchymal cells. Though osteoblasts have already undergone further differentiation than MSCs down an osteogenic pathway, osteoblasts are limited in availability and their harvest requires loss of bone from the donor site. Attempts to enhance the osteogenic capability of osteoblasts using mitogens and cytokines also failed to improve osteogenesis in aged osteoblasts.
The capability of ESCs to differentiate into almost any cell type found in the human body has led to many promising areas of investigation which may yield deeper understanding of cellular biology and potential cures for diseases. Previous studies have demonstrated the ability of ESCs to undergo osteogenic differentiation in vivo and in vitro, however, their pluripotency predisposes them to differentiating into any three of the embryonic germ layers (ectoderm, endoderm or mesoderm) thus forming a teratoma. 40 Use of ESCs in a clinical capacity, also potentially raises the challenging issue of graft-versus-host disease associated with allogeneic stem cell transplantation. While investigations continue to reduce donor-host rejection, concerns regarding xenogeneic contamination also exist. ESCs have been traditionally cultured in vitro in the presence of mouse embryonic fibroblast (MEF) feeder layers because in their absence, ESCs have been found to undergo rapid differentiation.40 Eliminating this MEF layer necessitated the creation of a stem-cell line under animal-free conditions. To accomplish this, scientists developed extracellular matrix coated plates from MEFs and sterilized prior to use.41 Other strategies proposed to avoid animal products and graft versus host reactions include the use of autologous donor adult stem cells or the more recently described induced pluripotent stem (iPS) cell.42
Periostium derived progenitor cells may also serve as a cell source for tissue engineering. Periosteum is a specialized cell type that covers bone surfaces and has the potential to differentiate into multiple mesenchymal tissues including bone. These cells have been of particular interest to dentists and oral surgeons given the increased ease of harvesting periosteal cells compared to bone marrow cells during periodontal surgery. Furthermore, studies have demonstrated an improved proliferation rate of periosteal cells compared to MSCs and demonstrate a robust capability to undergo osteogenesis.43 Despite this proliferative and osteogenic capability and promising use in alveolar bone treatment, periostium is limited in availability and this cell type may not be ideal if treating a large non-union or calvarial defect.
Bone Marrow Derived MSCs have also been used successfully for osteogenic differentiation in vitro and in vivo. MSCs implanted on Matrigel and placed into the lumbar fusion bed of rats have been shown to result in enhanced fusion compared to animals receiving mixed marrow stromal cells alone.44 A study following 51 long bones (tibia and femur) undergoing distraction osteogenesis of long bones, and injection of MSCs along with platelet-rich plasma into the callus during both lengthening and consolidation phases, resulted in accelerated mature bone formation and a reduction in the incidence of complications in the femur.45 Horwitz and colleagues have also investigated the use of MSCs in the treatment of osteogenesis imperfecta, a genetic disorder of type I collagen resulting in fragile bones and skeletal deformities.46 While there is no current cure for osteogenesis imperfecta, preliminary reports have shown MSCs to be potentially successful in enhancing bone formation in these patients.46 Thus, the success of marrow derived MSCs can hopefully be translated to the much more readily available and more easily accessible ASCs.
Scientists have demonstrated that ASCs can be easily isolated from human adipose tissue and can undergo differentiation into bone, cartilage, tendon, muscle and fat cells when grown in the appropriate conditions. 10 Though the number of ASCs harvested per gram is the same as marrow derived MSCs, adipose tissue is more readily available in larger quantities.14 With regards to skeletal tissue engineering, some groups have shown ASCs to have similar growth kinetics, cell senescence, and osteogenic differentiation capacity in vitro to bone marrow derived MSCs, though others have shown marrow derived MSCs to have superior growth kinetics and differentiation capacities.14 Similarly, some have demonstrated the increased osteogenic potential of marrow derived MSCs whereas others have shown ASCs have been shown to be more osteogenic in vivo.6, 14 In vitro studies of ASCs have demonstrated their robust capability of undergoing osteogenic differentiation as early as 3 days after induction and those ASCs from humans have proven more osteogenic than those harvested from mice.47 In vivo studies have demonstrated that ASCs loaded on a PLGA scaffold led to the development of osteoid material. Subsequent studies have demonstrated the ability of ASCs from mouse and human sources to heal critical sized defects with those treated with human derived ASCs showing significant bone formation as early as 2 weeks post implantation. 6
To achieve clinical translation of hASCs, ideally surgeons would harvest the cells, seed them on an osteoinductive scaffold and reconstruct the skeletal injury/defect in the same patient without leaving the operating room. Similar cell saving and re-use devices such as the Cell SaverR, are used in trauma and complicated surgeries in which blood loss gets cycled through this FDA approved machine and the erythrocytes can be transfused back into the patient. One example of a potential clinical strategy would be to harvest lipoaspirate, isolate ASCs intraoperatively, place them on a BMP-2 laden scaffold and place the BMP-2 and ASC loaded scaffold back into the patient’s skeletal defect in one step, without leaving the operating room. Interestingly, ASCs have been shown to up-regulate osteogenic genes in response to shear stress making them capable of responding to a mechanical load.48 Such ability to undergo osteogenesis without in vitro differentiation and responsiveness to mechanical stress makes ASCs an appealing cell source for skeletal tissue engineering.
Clinical correlates
Case report studies have examined the potential use of hASCs to heal skeletal defects in the human patient. Defects of facial bones and the cranium49 have been demonstrated to heal or stimulate healing with the use of ASCs. Though promising, current studies are limited as many countries have not yet approved the use of ASCs. Furthermore, the methods of ASC usage have varied dramatically and have included combination with bone grafts, the use of various osteoconductive scaffolds as well as recombinant proteins. Mesimaki et al. used a microvascular flap with hASCs, beta-tricalcium phosphate and BMP-2 to heal a large defect in the maxilla, reporting promising outcomes up to 8 months post-operatively. 50 Though encouraging, these early studies offer only level 4 and 5 data and lack significant power to help tailor clinical practice. Larger scale studies including prospective, randomized control trials, must verify these findings, and to determine the optimum cell delivery method and cytokine stimuli for hASC driven osteogenesis.
Conclusions
ASCs are a readily available, multipotent, abundant cell type with the capability to undergo robust osteogenesis. The fact that they can undergo osteogenic differentiation so rapidly in vitro makes them an exciting candidate for in vivo studies. Even more importantly, their ability to undergo osteogenic differentiation without any stimulation when placed on an osteoconductive scaffold in vivo make ASCs a promising candidate for skeletal tissue engineering. Further studies of the mechanism of osteogenic differentiation and ways to improve ASC osteodifferentiation by eliminating the heterogeneity and stimulating the BMP pathway offer potential for future studies.
Acknowledgments
We would like to thank the following people for their outstanding work in our laboratory: Emily R. Nelson, B.S., Victor Wong, M.D. and Aaron W. James, M.D.
Sources of Support:
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grant 1 R21 DE019274-01, 1 RC2 DE020771-01, the Oak Foundation and Hagey Laboratory for Pediatric Regenerative Medicine to M.T.L. B.L was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1F32AR057302-02. National Endowment of Plastic Surgery
Footnotes
Author Contributions:
Benjamin Levi: Conception and design, manuscript writing, final approval of manuscript, collection and/or assembly of data
Michael T. Longaker: Conception and design, manuscript writing, final approval of manuscript, collection and/or assembly of data
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.NCHS. Health, United States; Services. UDoHaH. 2007 with Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2007. [PubMed] [Google Scholar]
- 2.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 3.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 6.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 8.Hsu WK, Wang JC, Liu NQ, et al. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J Bone Joint Surg Am. 2008;90:1043–1052. doi: 10.2106/JBJS.G.00292. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 12.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gronthos S, Simmons PJ. The growth factor requirements of STRO-1-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood. 1995;85:929–940. [PubMed] [Google Scholar]
- 14.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 15.Peptan IA, Hong L, Mao JJ. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast Reconstr Surg. 2006;117:1462–1470. doi: 10.1097/01.prs.0000206319.80719.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996;36:303–308. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 17.Oishi K, Noguchi H, Yukawa H, et al. Cryopreservation of mouse adipose tissue-derived stem/progenitor cells. Cell transplantation. 2008;17:35–41. doi: 10.3727/000000008783906937. [DOI] [PubMed] [Google Scholar]
- 18.Thirumala S, Gimble JM, Devireddy RV. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. Journal of tissue engineering and regenerative medicine. 2009 doi: 10.1002/term.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James AW, Levi B, Nelson ER, et al. Deleterious Effects of Freezing on Osteogenic Differentiation of Human Adipose-Derived Stromal Cells In Vitro and In Vivo. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0082. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 21.Peng Y, Kang Q, Luo Q, et al. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 22.Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 23.Lian JB, Stein GS, Javed A, et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 24.Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 25.Karsenty G. Minireview: transcriptional control of osteoblast differentiation. Endocrinology. 2001;142:2731–2733. doi: 10.1210/endo.142.7.8306. [DOI] [PubMed] [Google Scholar]
- 26.Tai G, Polak JM, Bishop AE, et al. Differentiation of osteoblasts from murine embryonic stem cells by overexpression of the transcriptional factor osterix. Tissue Eng. 2004;10:1456–1466. doi: 10.1089/ten.2004.10.1456. [DOI] [PubMed] [Google Scholar]
- 27.Chan CK, Chen CC, Luppen CA, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gimble JM, Zvonic S, Floyd ZE, et al. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 29.Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawaguchi H, Akune T, Yamaguchi M, et al. Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J Bone Miner Metab. 2005;23:275–279. doi: 10.1007/s00774-005-0599-2. [DOI] [PubMed] [Google Scholar]
- 31.Niemeyer P, Fechner K, Milz S, et al. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31:3572–3579. doi: 10.1016/j.biomaterials.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 32.Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 33.Chou YF, Zuk PA, Chang TL, et al. Adipose-derived stem cells and BMP2: Part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect Tissue Res. doi: 10.3109/03008207.2010.484514. [DOI] [PubMed] [Google Scholar]
- 34.Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 35.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 36.Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Glassman SD, Carreon LY, Djurasovic M, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine (Phila Pa 1976) 2008;33:2843–2849. doi: 10.1097/BRS.0b013e318190705d. [DOI] [PubMed] [Google Scholar]
- 38.Fang J, Zhu YY, Smiley E, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schek RM, Hollister SJ, Krebsbach PH. Delivery and Protection of Adenoviruses Using Biocompatible Hydrogels for Localized Gene Therapy. Mol Ther. 2004;9:130–138. doi: 10.1016/j.ymthe.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 41.Klimanskaya I, Chung Y, Meisner L, et al. Human embryonic stem cells derived without feeder cells. Lancet. 2005;365:1636–1641. doi: 10.1016/S0140-6736(05)66473-2. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Agata H, Asahina I, Yamazaki Y, et al. Effective bone engineering with periosteum-derived cells. Journal of dental research. 2007;86:79–83. doi: 10.1177/154405910708600113. [DOI] [PubMed] [Google Scholar]
- 44.Cui Q, Ming Xiao Z, Balian G, et al. Comparison of lumbar spine fusion using mixed and cloned marrow cells. Spine (Phila Pa 1976) 2001;26:2305–2310. doi: 10.1097/00007632-200111010-00003. [DOI] [PubMed] [Google Scholar]
- 45.Kitoh H, Kitakoji T, Tsuchiya H, et al. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007;27:629–634. doi: 10.1097/BPO.0b013e318093f523. [DOI] [PubMed] [Google Scholar]
- 46.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 47.Wan DC, Shi YY, Nacamuli RP, et al. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006;103:12335–12340. doi: 10.1073/pnas.0604849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knippenberg M, Helder MN, Doulabi BZ, et al. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780–1788. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 49.Lendeckel S, Jodicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. International journal of oral and maxillofacial surgery. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
Additional Recommended reading
- Mizuno H. The Potential for Treatment of Skeletal Muscle Disorders with Adipose-Derived Stem Cells. Current stem cell research & therapy. 2009 doi: 10.2174/157488810791268573. [DOI] [PubMed] [Google Scholar]
- Menton DN, Simmons DJ, Chang SL, et al. From bone lining cell to osteocyte--an SEM study. Anat Rec. 1984;209:29–39. doi: 10.1002/ar.1092090105. [DOI] [PubMed] [Google Scholar]
- Levi B, James AW, Nelson ER, et al. Human Adipose-Derived Stromal Cells Stimulate Autogenous Skeletal Repair via Paracrine Hedgehog Signaling with Calvarial Osteoblasts. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Shao-qing G, Geng-ting D. In vivo evaluation of bone marrow stromal-derived osteoblasts-porous calcium phosphate ceramic composites as bone graft substitute for lumbar intervertebral spinal fusion. Spine (Phila Pa 1976) 2003;28:1653–1658. doi: 10.1097/01.BRS.0000083168.37329.B4. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hyakusoku H. Mesengenic potential and future clinical perspective of human processed lipoaspirate cells. J Nippon Med Sch. 2003;70:300–306. doi: 10.1272/jnms.70.300. [DOI] [PubMed] [Google Scholar]
- Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Palou M, Priego T, Sanchez J, et al. Gene expression patterns in visceral and subcutaneous adipose depots in rats are linked to their morphologic features. Cell Physiol Biochem. 2009;24:547–556. doi: 10.1159/000257511. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, et al. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Jager M, Zilkens C, Bittersohl B, et al. Cord blood--an alternative source for bone regeneration. Stem cell reviews. 2009;5:266–277. doi: 10.1007/s12015-009-9083-z. [DOI] [PubMed] [Google Scholar]
- Quarto N, Wan DC, Longaker MT. Molecular mechanisms of FGF-2 inhibitory activity in the osteogenic context of mouse adipose-derived stem cells (mASCs) Bone. 2008;42:1040–1052. doi: 10.1016/j.bone.2008.01.026. [DOI] [PubMed] [Google Scholar]
- atija NK, Gurudutta GU, Sharma S, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16:7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- Alliston T, Choy L, Ducy P, et al. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kim HJ, Li QL, et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippenberg M, Helder MN, Zandieh Doulabi B, et al. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902–908. doi: 10.1016/j.bbrc.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Minireview: transcriptional control of osteoblast differentiation. Endocrinology. 2001;142:2731–2733. doi: 10.1210/endo.142.7.8306. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Young DW, Choi JY, et al. Intranuclear trafficking: organization and assembly of regulatory machinery for combinatorial biological control. J Biol Chem. 2004;279:43363–43366. doi: 10.1074/jbc.R400020200. [DOI] [PubMed] [Google Scholar]
- Beresford JN, Bennett JH, Devlin C, et al. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102 ( Pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- Dorheim MA, Sullivan M, Dandapani V, et al. Osteoblastic gene expression during adipogenesis in hematopoietic supporting murine bone marrow stromal cells. J Cell Physiol. 1993;154:317–328. doi: 10.1002/jcp.1041540215. [DOI] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- Einhorn TA, Majeska RJ, Rush EB, et al. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–1281. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- Liew Cg, Moore H, Ruban L, et al. Human embryonic stem cells: Possibilities for human cell transplantation. Annals of Medicine. 2009;37:521–532. doi: 10.1080/07853890500379463. [DOI] [PubMed] [Google Scholar]
- Bielby RC, Boccaccini AR, Polak JM, et al. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- Karner E, Unger C, Sloan AJ, et al. Bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Stem Cells Dev. 2007;16:39–52. doi: 10.1089/scd.2006.0010. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Nguyen T, Gillen K, et al. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auquier P, Macquart-Moulin G, Moatti JP, et al. Comparison of anxiety, pain and discomfort in two procedures of hematopoietic stem cell collection: leukacytapheresis and bone marrow harvest. Bone Marrow Transplant. 1995;16:541–547. [PubMed] [Google Scholar]
- Erickson GR, Gimble JM, Franklin DM, et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Ten Dijke P, Heldin CH, et al. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Tsuji K, Nifuji A, et al. Inhibitory helix-loop-helix transcription factors Id1/Id3 promote bone formation in vivo. J Cell Biochem. 2004;93:337–344. doi: 10.1002/jcb.20154. [DOI] [PubMed] [Google Scholar]
- Gori F, Thomas T, Hicok KC, et al. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res. 1999;14:1522–1535. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Boquest AC, Shahdadfar A, Fronsdal K, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Role of Cbfa1 in osteoblast differentiation and function. Semin Cell Dev Biol. 2000;11:343–346. doi: 10.1006/scdb.2000.0188. [DOI] [PubMed] [Google Scholar]
- White AP, Vaccaro AR, Hall JA, et al. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Ming Xiao Z, Balian G, et al. Comparison of lumbar spine fusion using mixed and cloned marrow cells. Spine (Phila Pa 1976) 2001;26:2305–2310. doi: 10.1097/00007632-200111010-00003. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wan DC, Siedhoff MT, Kwan MD, et al. Refining retinoic acid stimulation for osteogenic differentiation of murine adipose-derived adult stromal cells. Tissue Eng. 2007;13:1623–1631. doi: 10.1089/ten.2006.0283. [DOI] [PubMed] [Google Scholar]
- Cho HH, Kim YJ, Kim SJ, et al. Endogenous Wnt signaling promotes proliferation and suppresses osteogenic differentiation in human adipose derived stromal cells. Tissue Eng. 2006;12:111–121. doi: 10.1089/ten.2006.12.111. [DOI] [PubMed] [Google Scholar]
- Levi B, James AW, Nelson ER, et al. Human Adipose-Derived Stromal Cells Stimulate Autogenous Skeletal Repair via Paracrine Hedgehog Signaling with Calvarial Osteoblasts. Stem Cells Dev. doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Jin JS, Kim HN, et al. Expression of Runx2 transcription factor in non-skeletal tissues, sperm and brain. J Cell Physiol. 2008;217:511–517. doi: 10.1002/jcp.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn TA, Majeska RJ, Rush EB, et al. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–1281. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- Levi B, James AW, Nelson ER, et al. Human Adipose-Derived Stromal Cells Stimulate Autogenous Skeletal Repair via Paracrine Hedgehog Signaling with Calvarial Osteoblasts. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Satija NK, Gurudutta GU, Sharma S, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16:7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Kogler C, Diel I, et al. Age-associated changes in the stimulatory effect of transforming growth factor beta on human osteogenic colony formation. Mech Ageing Dev. 1999;110:73–85. doi: 10.1016/s0047-6374(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Lees JG, Lim SA, Croll T, et al. Transplantation of 3D scaffolds seeded with human embryonic stem cells: biological features of surrogate tissue and teratoma-forming potential. Regen Med. 2007;2:289–300. doi: 10.2217/17460751.2.3.289. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]