Abstract
Diffusion-weighted imaging (DWI) is commonly used to assess irreversibly infarcted tissue but its accuracy is challenged by reports of diffusion lesion reversal (DLR). We investigated the frequency and implications for mismatch classification of DLR using imaging from the EPITHET (Echoplanar Imaging Thrombolytic Evaluation Trial) and DEFUSE (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution) studies. In 119 patients (83 treated with IV tissue plasminogen activator), follow-up images were coregistered to acute diffusion images and the lesions manually outlined to their maximal visual extent in diffusion space. Diffusion lesion reversal was defined as voxels of acute diffusion lesion that corresponded to normal brain at follow-up (i.e., final infarct, leukoaraiosis, and cerebrospinal fluid (CSF) voxels were excluded from consideration). The appearance of DLR was visually checked for artifacts, the volume calculated, and the impact of adjusting baseline diffusion lesion volume for DLR volume on perfusion–diffusion mismatch analyzed. Median DLR volume reduced from 4.4 to 1.5 mL after excluding CSF/leukoaraiosis. Visual inspection verified 8/119 (6.7%) with true DLR, median volume 2.33 mL. Subtracting DLR from acute diffusion volume altered perfusion–diffusion mismatch (Tmax>6 seconds, ratio>1.2) in 3/119 (2.5%) patients. Diffusion lesion reversal between baseline and 3 to 6 hours DWI was also uncommon (7/65, 11%) and often transient. Clinically relevant DLR is uncommon and rarely alters perfusion–diffusion mismatch. The acute diffusion lesion is generally a reliable signature of the infarct core.
Keywords: brain ischemia, cerebrovascular disease, diffusion-weighted MRI, MRI, thrombolysis
Introduction
Definition of the irreversible infarct core is of critical importance in the assessment of potential risk and benefit associated with thrombolysis in acute ischemic stroke. Diffusion-weighted magnetic resonance imaging (MRI) is the most widely used measure of infarct core. Various MRI methods of selecting patients with salvageable ‘ischemic penumbra' for reperfusion therapies have been proposed. These include perfusion–diffusion mismatch (Donnan and Davis, 2008; Hjort et al, 2005; Warach, 2002), magnetic resonance angiography–diffusion mismatch (Lansberg et al, 2008), and clinical–diffusion mismatch (Davalos et al, 2004; Ebinger et al, 2009; Lansberg et al, 2007; Prosser et al, 2005; Tei et al, 2007). All depend on diffusion-weighted imaging (DWI) for an accurate assessment of infarct core volume. In addition, large infarct core volume has been associated with symptomatic intracerebral hemorrhage (Singer et al, 2008) and is a feature of the ‘malignant profile' that predicts poor clinical response to thrombolysis (Mlynash et al, 2011). However, a recent review emphasized the uncertainty in the literature about the relationship of diffusion restriction to irreversible injury (Kranz and Eastwood, 2009). An initial analysis of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) trial imaging data suggested that, on average, 43% of the acute diffusion lesion reversed (Olivot et al, 2009). In a previous analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) imaging data, we found that coregistration inaccuracy and infarct atrophy over time were major contributors to apparent diffusion lesion reversal (DLR) and that true DLR in this population of 60 patients 3 to 6 hours after the onset of ischemic stroke was rare (Chemmanam et al, 2010). Nonetheless, the question of reversibility of diffusion lesions remains controversial. Given the importance of this issue to clinical practice, we substantially enlarged the data set by pooling the EPITHET and DEFUSE trials and used an optimized methodology, including analysis of DLR in the early hours following thrombolysis.
Materials and methods
EPITHET was a prospective, double-blind, multicenter trial with acute ischemic stroke patients randomized to IV tissue plasminogen activator (tPA) or placebo 3 to 6 hours after symptom onset. DEFUSE was an open-labeled study of IV tPA for acute ischemic stroke 3 to 6 hours after the symptom onset. Methodological details have been reported previously (Albers et al, 2006; Davis et al, 2008). In brief, in both studies, a baseline noncontrast computed tomography scan was used to exclude patients with acute hemorrhage and major early ischemic change >1/3 MCA territory. Patients had acute MRI at 1.5 T with DWI, PWI, T2-weighted sequences and magnetic resonance angiography, and clinical assessments, with the National Institutes of Health Stroke Scale performed before the treatment. In EPITHET, these assessments were repeated between days 3 to 5 (subacute) and day 90 (follow-up). In DEFUSE, these assessments were repeated between 3 to 6 hours (subacute) and at day 30 (follow-up) after the treatment. Treatment with tPA (or randomization to tPA/placebo in EPITHET) was carried out without the knowledge of the MRI results, which were analyzed centrally before unblinding, to determine the presence of perfusion–diffusion mismatch. The studies were approved by the institutional review boards at all centers and informed consent was obtained from all the participants.
For this analysis, coregistration of the follow-up images to the acute DWI was performed using MINC tools (Montreal Neurological Institute, McGill University, Montreal, Canada) and judged by two expert imaging scientists. Images that failed to register in the first attempt using a rigid body 3D registration were subjected to a standardized sequence of alternate registration procedures, including manual initialization as well as scaling and shear transforms to correct for echoplanar imaging artifacts. Cases that failed all attempts were excluded from this study. Regions of interest were manually drawn using careful windowing to outline the maximal visual extent of the acute DWI (B1000 trace-weighted) lesion with reference to the apparent diffusion coefficient (ADC) image to avoid regions of T2 shine-through. The B1000 image was used as the primary template as quantitative ADC thresholds tend not to accurately outline the visually evident lesion and have been shown to vary with time after stroke onset and perfusion status (An et al, 2011). Manual regions of interest were also drawn to the maximal extent of the final infarct on the coregistered day 90 T2 (EPITHET) or day 30 fluid attenuated inversion recovery (FLAIR) (DEFUSE) images. This was performed in DWI space to avoid the step artifact sometimes introduced by transforming a binary region of interest.
Apparent DWI lesion reversal was defined as the region of acute DWI lesion that did not lie within the coregistered final infarct at follow-up. The purpose of this analysis was to determine the amount of diffusion-restricted tissue that returns to a normal radiological appearance. We, therefore, limited ‘apparent reversal' to normal-appearing brain tissue at follow-up by the use of a cerebrospinal fluid (CSF) and gliosis mask created by manual thresholding of the follow-up image. This reduced the impact of infarct atrophy where it led to ex vacuo dilatation of ventricles and sulci, and excluded preexisting gliosis or leukoaraiosis where reversal could not be assessed. Infarct core was defined as the region of acute DWI lesion that lay within the coregistered final infarct. Mismatch was defined as a ratio of perfusion lesion volume (threshold Tmax >6 seconds) to diffusion lesion volume >1.2 and absolute difference >10 mL as employed in the current EXTEND randomized trial of IV thrombolysis in patients 4.5 to 9 hours after the stroke onset (Ma et al, 2011). To analyze the effect of DLR on mismatch classification, mismatch was compared using two definitions of the diffusion lesion volume: (1) the raw diffusion lesion volume and (2) the raw diffusion lesion volume after subtraction of the apparent reversal volume. The subacute DWI images from the DEFUSE trial (obtained 3 to 6 hours after tPA) were also coregistered to the acute DWI and processed similarly to examine acute reversal of diffusion lesions.
Assessment of reperfusion was an important cofactor in the analysis. To standardize the assessment of perfusion imaging between trials, all data were reprocessed using a fully automated software package (RAPID, Stanford University), which produced Tmax maps deconvolved using an arterial input function (Straka et al, 2011). The perfusion lesion was outlined using a Tmax >6 seconds threshold with manual exclusion of artifacts by a single stroke neurologist. As the timing of reperfusion assessment varied between the two studies, the original trial definitions for reperfusion were used (a reduction in perfusion lesion volume between the acute and subacute MRI of >90% in EPITHET and >30% in DEFUSE, (Lansberg et al, 2011).
Finally, all available acute, subacute, and late imaging data were visually reviewed slice-by-slice using interactive image blending of the coregistered images by consensus of a neuroradiologist and stroke neurologist to distinguish cases with ‘true DWI lesion reversal,' defined as a distinct region of a DWI lesion not included in the follow-up infarct and not attributable to lesion shrinkage or coregistration inaccuracy. Cases with true reversal between acute and 3 to 6 hours posttreatment time points (DEFUSE subgroup only) were carefully examined visually to determine whether the reversal was sustained on follow-up FLAIR. Regions of true DWI lesion reversal were manually outlined and the volume was calculated.
Statistical analysis was performed using PASW (v.18, SPSS, Chicago, IL, USA). Nonparametric (Wilcoxon) testing was applied to the proportion of apparent DWI lesion reversal in baseline perfusion categories and after reperfusion.
Results
Of 175 patients in the pooled EPITHET and DEFUSE studies, there were 119 patients suitable for this analysis of whom 83 were treated with intravenous tPA (Table 1). The reasons for exclusion of patients were absent follow-up imaging (43), no lesion on baseline DWI (8), coregistration failure (4), and withdrawal of consent before baseline imaging (1).
Table 1. Clinical characteristics of patients (n=119).
| tPA | Placebo | P-value* | |
|---|---|---|---|
| Number (%) | 83 (70%) | 36 (30%) | |
| Age (mean (s.d.)) (years) | 69.0 (14.7) | 70.4 (13.5) | 0.59 |
| Gender (% men) | 42 (49%) | 19 (47%) | 0.85 |
| Hypertension (%) | 55 (66%) | 22 (61%) | 0.68 |
| Diabetes (%) | 20 (24%) | 6 (17%) | 0.47 |
| Smoking (%) | 33 (40%) | 14 (39%) | 0.99 |
| Dyslipidemia (%) | 29 (35%) | 12 (33%) | 0.99 |
| Atrial fibrillation (%) | 26 (31%) | 10 (28%) | 0.83 |
| Time to treatment (mean (s.d.)) (minutes) | 310 (37) | 295 (50) | 0.41 |
| Baseline NIHSS (median (IQR)) | 12 (8) | 10 (9) | 0.29 |
| Acute DWI volume (mean (s.d.)) (mL) | 27 (32) | 38 (45) | 0.13 |
DWI, diffusion-weighted imaging; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator.
*P-value Mann–Whitney or Fisher's exact for categorical variables.
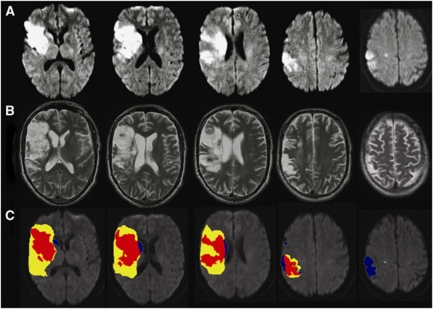
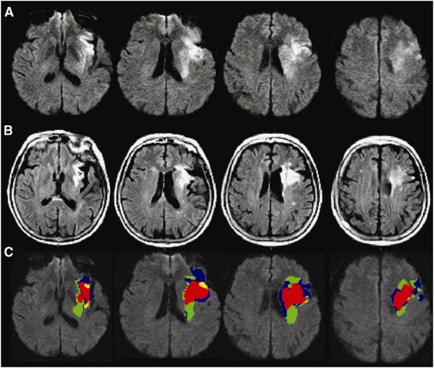
A CSF/gliosis exclusion mask was utilized to reduce the impact of infarct atrophy, as well as gliosis and leukoaraiosis on the follow-up scan, where reversal could not be reliably assessed (Figure 1). This reduced the median volume of apparent reversal from 4.4 mL (interquartile range 1.6 to 8.1 mL) to 1.5 mL (interquartile range 1.5 to 3.0 mL, paired Wilcoxon, P<0.0001). There was no difference in the proportion of the diffusion lesion with apparent reversal between EPITHET and DEFUSE patients (median 13.8% versus 14.3%, respectively, P=0.85). In the 95 patients where reperfusion could be assessed, apparent reversal was greater in cases with reperfusion (median 2.43 versus 0.75 mL, P=0.003). Subtracting the volume of apparent reversal from the acute diffusion lesion volume rarely altered perfusion–diffusion mismatch. Only 3/119 (2.5%) patients shifted to ‘mismatch' from ‘no mismatch.' There were 4/119 (3.4%) with apparent reversal >10 mL and >10% of baseline diffusion volume. These cases were visually reviewed, and in two cases, a component of the apparent reversal was judged to be beyond that attributable to infarct atrophy, that is there was true DWI reversal (Figure 2). Visual inspection of the entire data set with acute and follow-up imaging verified 8/119 (6.7%) with true DLR, median volume 2.3 mL, representing a median 20% of baseline DWI volume in these cases. The regions of true reversal were generally in the periventricular white matter (Figure 2) and the ADC map was used to confirm that this did not simply represent T2 shine-through. Reperfusion occurred in all eight of these cases (before baseline imaging in one case). Overall, true reversal of a component of the diffusion lesion occurred in 13.8% of cases with reperfusion, altering mismatch classification in only one case.
Figure 1.
A patient with apparent diffusion lesion reversal due to infarct atrophy (A) baseline diffusion-weighted imaging (DWI), (B) follow-up T2, (C) composite image with red indicating stable infarction, yellow indicating infarct growth, green indicating diffusion lesion reversal, and blue indicating regions of acute DWI lesion that, rather than ‘reversing,' correspond to cerebrospinal fluid (CSF) or gliosis at follow-up.
Figure 2.
A patient with true diffusion lesion reversal (A) baseline diffusion-weighted imaging (DWI), (B) follow-up fluid attenuated inversion recovery (FLAIR), (C) composite image with red indicating stable infarction, yellow indicating infarct growth, green indicating diffusion lesion reversal in the deep white matter, and blue indicating regions of acute DWI lesion that correspond to cerebrospinal fluid (CSF) or gliosis at follow-up.
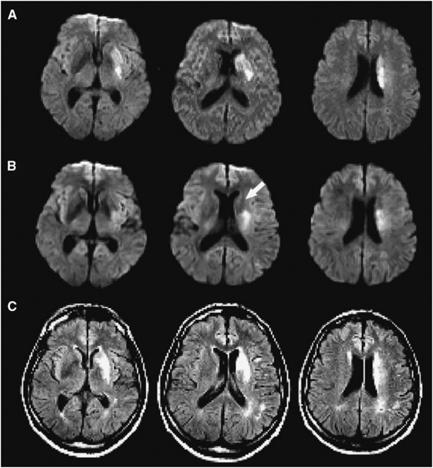
Diffusion lesion reversal between baseline and 3 to 6 hours DWI was also reviewed in 65 DEFUSE cases with available data. The median volume of apparent reversal was 1.1 mL (interquartile range 0.64 to 2.5). Adjusting baseline DWI volume for apparent reversal only altered perfusion–diffusion mismatch classification in 2/65 cases (3%). Visual checking verified the reversal in seven cases, all but one of whom had reperfused. The median volume affected in these cases of true ‘acute' reversal was 4.2 mL. Adjusting baseline DWI for this volume reclassified mismatch in the same single case as in the acute to follow-up analysis. The seven cases of true acute reversal included the only two cases of true permanent reversal identified in the acute to follow-up analysis that had 3 to 6 hours imaging. Importantly, in the other five cases of acute DLR, the region in question had abnormal FLAIR signal at follow-up (Figure 3). Thus, the majority of acute DLR did not translate to permanent tissue salvage.
Figure 3.
An example of temporary diffusion lesion reversal in a patient who reperfused with tissue plasminogen activator (tPA). (A) Baseline diffusion-weighted imaging (DWI), (B) DWI 12 hours later (after reperfusion), arrow indicating temporary reversal in the region of the caudate nucleus, (C) follow-up fluid attenuated inversion recovery (FLAIR).
Discussion
In this study, the largest addressing the issue of DLR after tPA, we found that DLR was uncommon using both a quantitative volumetric approach and a more subjective expert visual assessment. When it did occur, the volume of brain tissue affected was small and unlikely to be clinically relevant. In addition, temporary DLR was observed but, in the majority of cases, did not lead on to true tissue salvage. When permanent diffusion reversal did occur, it was universally associated with early reperfusion and generally affected small volumes of tissue in the deep white matter. These are important findings as the use of MRI-based selection for thrombolysis in extended time windows is increasing, although further validation is required (Mishra et al, 2010). The reliability of infarct core assessment in MRI-based selection depends on the irreversibility of the diffusion lesion. This is regardless of whether the strategy for patient selection is perfusion–diffusion mismatch, magnetic resonance angiography–diffusion mismatch, or clinical–diffusion mismatch. We have demonstrated that the impact of apparent DLR on perfusion–diffusion mismatch classification is minimal.
The determination of DLR is highly dependent on the definition of diffusion abnormality employed. Outlining the diffusion lesion can be performed in a number of ways. However, no automated thresholding approach currently available can reliably segment the entire visually apparent lesion. This relates to heterogeneity of ADC across the individual's brain and between individuals, especially with increasing age (Thomas et al, 2010). The optimal ADC threshold to define infarction also appears to vary with time from stroke onset (An et al, 2011). We have, therefore, used manual outlining, a technique with good reproducibility (Butcher et al, 2008), that leads to regions of interest considerably larger than automated segmentation (Lansberg et al, 2011). This means that DLR volumes in this study are, if anything, overestimated compared with automated segmentation techniques. Likewise, the effect of any coregistration inaccuracy would be to increase the volume of apparent DLR.
A limitation of this study is that interpretation is restricted to patients beyond 3 hours from stroke onset. It is possible that diffusion restriction after less prolonged ischemia may have a degree of reversibility in humans. However, in practical terms, MRI is rarely used to select patients in earlier time windows, given that intravenous tPA is a proven therapy based simply on a noncontrast computed tomography brain (Lees et al, 2010). It could be argued that the exclusion of gliosis and leukoaraiosis on follow-up images biases the results against finding diffusion reversal. However, whether these lesions date from the incident stroke or were preexisting cannot be reliably determined. At any rate, the gliotic tissue is not likely to be functioning normally. The follow-up imaging differed between EPITHET and DEFUSE in both time (90 versus 30 days) and technique (T2 versus FLAIR). However, there is evidence that most infarct atrophy has already occurred by day 30 with minimal further evolution (Gaudinski et al, 2008). Although lesion volumes using T2 and FLAIR may differ slightly, the proportions of apparent DLR after exclusion of CSF and gliosis were very similar between studies (median 14.3% in DEFUSE and 13.8% in EPITHET).
The most impressive reports of DLR have occurred after reperfusion induced by intraarterial therapy (Kidwell et al, 2000). However, in many cases, the initial reversal was transient and did not herald tissue salvage, as the diffusion restriction subsequently returned or FLAIR hyperintensity developed (Kidwell et al, 2002). This is consistent with our study findings that the majority of early DLR was not sustained at follow-up. Indeed, there are important methodological considerations affecting most previous reports of diffusion reversal in humans. In some cases, follow-up was early and fell during a period when temporary reversal is now recognized to be common (Chalela et al, 2004). Other studies used delayed follow-up without accounting for infarct atrophy (Ritzl et al, 2004; Rivers et al, 2006). The imaging modality used for follow-up is also critical as ADC normalizes after a few days and infarct volume using T2 images varies depending on the windowing employed.
The lack of correlation between early DLR and tissue salvage is also supported by animal data. In rats, diffusion restriction after 10 and 30 minutes of ischemia demonstrated initial reversal after reperfusion (Li et al, 2000). The diffusion restriction later returned in the 30-minute group but not the 10-minute group. However, there was histologically evident neuronal loss in both groups of rats. In our study and previous reports (Fiehler et al, 2004), white matter seemed to be more likely to exhibit DLR. This may reflect an increased tolerance to ischemia. It is possible that reversal of diffusion restriction represents a potentially salvageable state and that subsequent return of imaging abnormalities represents a secondary injury (Kidwell et al, 2002). Support for this comes from molecular pathology as cytotoxic edema (the putative substrate of diffusion restriction) is theoretically reversible with restoration of cellular energy status (Simard et al, 2007). Future developments in neuroprotection may allow further investigation of this possibility. However, for the moment, the presence of diffusion restriction can be taken as a clinically reliable indicator of irreversible ischemic damage.
Acknowledgments
tPA was supplied at no charge by Boehringer Ingelheim (Australia, New Zealand, and European sites) and Genentech (US and Canada sites). Neither Boehringer Ingelheim, Genentech nor the NIH played a role in the design and the conduct of the studies; collection, management, analysis, and interpretation of the data; or preparation or approval of the manuscript.
The authors declare no conflict of interest.
Footnotes
The EPITHET study was supported by the National Health and Medical Research Council of Australia, National Stroke Foundation and National Heart Foundation of Australia. The DEFUSE study was funded by National Institutes of Health (NIH) Grants RO1 NS39325, Principal Investigator, Gregory W Albers; K24 NS044848, Principal Investigator, Gregory W Albers; K23 NS051372, Principal Investigator Maarten G Lansberg and the RAPID software development was supported by R01 EB002711, Principal Investigator, Roland Bammer. Bruce Campbell is supported by the National Health and Medical Research Council of Australia Postgraduate Scholarship 567156, the Heart Foundation of Australia, a Cardiovascular Lipid (CVL) Australia grant and the Neuroscience Foundation of the Royal Melbourne Hospital.
References
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- An H, Ford AL, Vo K, Powers WJ, Lee JM, Lin W. Signal evolution and infarction risk for apparent diffusion coefficient lesions in acute ischemic stroke are both time- and perfusion-dependent. Stroke. 2011;42:1276–1281. doi: 10.1161/STROKEAHA.110.610501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher K, Parsons M, Allport L, Lee SB, Barber PA, Tress B, Donnan GA, Davis SM. Rapid assessment of perfusion-diffusion mismatch. Stroke. 2008;39:75–81. doi: 10.1161/STROKEAHA.107.490524. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Kang DW, Luby M, Ezzeddine M, Latour LL, Todd JW, Dunn B, Warach S. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55:105–112. doi: 10.1002/ana.10781. [DOI] [PubMed] [Google Scholar]
- Chemmanam T, Campbell BCV, Christensen S, Nagakane Y, Desmond PM, Bladin CF, Parsons MW, Levi CR, Barber PA, Donnan GA, Davis SM. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology. 2010;75:1040–1047. doi: 10.1212/WNL.0b013e3181f39ab6. [DOI] [PubMed] [Google Scholar]
- Davalos A, Blanco M, Pedraza S, Leira R, Castellanos M, Pumar JM, Silva Y, Serena J, Castillo J. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology. 2004;62:2187–2192. doi: 10.1212/01.wnl.0000130570.41127.ea. [DOI] [PubMed] [Google Scholar]
- Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Davis SM. Breaking the 3 h barrier for treatment of acute ischaemic stroke. Lancet Neurol. 2008;7:981–982. doi: 10.1016/S1474-4422(08)70230-8. [DOI] [PubMed] [Google Scholar]
- Ebinger M, Iwanaga T, Prosser JF, De Silva DA, Christensen S, Collins M, Parsons MW, Levi CR, Bladin CF, Barber PA, Donnan GA, Davis SM. Clinical-diffusion mismatch and benefit from thrombolysis 3 to 6 hours after acute stroke. Stroke. 2009;40:2572–2574. doi: 10.1161/STROKEAHA.109.548073. [DOI] [PubMed] [Google Scholar]
- Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, Eckert B, Wittkugel O, Weiller C, Zeumer H, Rother J. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35:514–519. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- Gaudinski MR, Henning EC, Miracle A, Luby M, Warach S, Latour LL. Establishing final infarct volume: stroke lesion evolution past 30 days is insignificant. Stroke. 2008;39:2765–2768. doi: 10.1161/STROKEAHA.107.512269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjort N, Butcher K, Davis SM, Kidwell CS, Koroshetz WJ, Rother J, Schellinger PD, Warach S, Ostergaard L. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36:388–397. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, Villablanca JP, Liebeskind DS, Gobin YP, Vinuela F, Alger JR. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52:698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]
- Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am J Neuroradiol. 2009;30:1206–1212. doi: 10.3174/ajnr.A1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, Bammer R, Olivot JM, Desmond P, Davis SM, Donnan GA, Albers GW. RAPID Automated Patient Selection for Reperfusion Therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke. 2011;42:1608–1614. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Thijs VN, Bammer R, Olivot JM, Marks MP, Wechsler LR, Kemp S, Albers GW. The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke. 2008;39:2491–2496. doi: 10.1161/STROKEAHA.107.508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Thijs VN, Hamilton S, Schlaug G, Bammer R, Kemp S, Albers GW. Evaluation of the clinical-diffusion and perfusion-diffusion mismatch models in DEFUSE. Stroke. 2007;38:1826–1830. doi: 10.1161/STROKEAHA.106.480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- Li F, Liu KF, Silva MD, Omae T, Sotak CH, Fenstermacher JD, Fisher M, Hsu CY, Lin W. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke. 2000;31:946–954. doi: 10.1161/01.str.31.4.946. [DOI] [PubMed] [Google Scholar]
- Ma H, Parsons M, Christensen S, Campbell B, Churilov L, Connelly A, Yan B, Bladin C, Phan T, Barber A, Read S, Hankey G, Markus R, Wijeratne T, Grimley R, Mahant N, Kleinig T, Sturm J, Lee A, Blacker D, Gerraty R, Krause M, Desmond PD, Carey L, Howell D, Davis SM, Donnan GA.on behalf of the EXTEND investigators (2011A multicentre, randomized, double blinded, placebo controlled phase 3 study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) Int J Stroke(in press) [DOI] [PubMed]
- Mishra NK, Albers GW, Davis SM, Donnan GA, Furlan AJ, Hacke W, Lees KR. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke. 2010;41:e25–e33. doi: 10.1161/STROKEAHA.109.566869. [DOI] [PubMed] [Google Scholar]
- Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, Campbell BCV, Bammer R, Olivot JM, Desmond PM, Donnan GA, Davis SM, Albers GW. Refining the definition of the malignant profile-insights from the defuse-EPITHET pooled data set. Stroke. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivot JM, Mlynash M, Thijs VN, Purushotham A, Kemp S, Lansberg MG, Wechsler L, Bammer R, Marks MP, Albers GW. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke. 2009;40:1692–1697. doi: 10.1161/STROKEAHA.108.538082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J, Butcher K, Allport L, Parsons M, MacGregor L, Desmond P, Tress B, Davis S. Clinical-diffusion mismatch predicts the putative penumbra with high specificity. Stroke. 2005;36:1700–1704. doi: 10.1161/01.STR.0000173407.40773.17. [DOI] [PubMed] [Google Scholar]
- Ritzl A, Meisel S, Wittsack HJ, Fink GR, Siebler M, Modder U, Seitz RJ. Development of brain infarct volume as assessed by magnetic resonance imaging (MRI): follow-up of diffusion-weighted MRI lesions. J Magn Reson Imaging. 2004;20:201–207. doi: 10.1002/jmri.20096. [DOI] [PubMed] [Google Scholar]
- Rivers CS, Wardlaw JM, Armitage PA, Bastin ME, Carpenter TK, Cvoro V, Hand PJ, Dennis MS. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke. Stroke. 2006;37:98–104. doi: 10.1161/01.STR.0000195197.66606.bb. [DOI] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, Rovira A, Liebeskind DS, Gass A, Rosso C, Derex L, Kim JS, Neumann-Haefelin T. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63:52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2011;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei H, Uchiyama S, Usui T. Clinical-diffusion mismatch defined by NIHSS and ASPECTS in non-lacunar anterior circulation infarction. J Neurol. 2007;254:340–346. doi: 10.1007/s00415-006-0368-8. [DOI] [PubMed] [Google Scholar]
- Thomas RGR, Lymer K, Armitage PA, Karaszewski B, Carpenter TK, Wardlaw JM. Apparent diffusion coefficient thresholds do not identify accurately diffusion lesion volume in acute stroke patients. Cerebrovasc Dis. 2010;29 (Suppl 2:498. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Warach S. Thrombolysis in stroke beyond three hours: targeting patients with diffusion and perfusion MRI. Ann Neurol. 2002;51:11–13. doi: 10.1002/ana.10109. [DOI] [PubMed] [Google Scholar]