Abstract
Cerebral cortical neurons have a heightened sensitivity to hypoxia and their survival depends on their ability to accommodate to changes in the concentration of oxygen in their environment. Tissue-type plasminogen activator (tPA) is a serine proteinase that activates the zymogen plasminogen into plasmin. Hypoxia induces the release of tPA from cerebral cortical neurons, and it has been proposed that tPA mediates hypoxic and ischemic neuronal death. Here, we show that tPA is devoid of neurotoxic effects and instead is an endogenous neuroprotectant that renders neurons resistant to the effects of lethal hypoxia and ischemia. We present in vitro and in vivo evidence indicating that endogenous tPA and recombinant tPA induce the expression of neuronal tumor necrosis factor-α. This effect, mediated by plasmin and the N-methyl--aspartate receptor, leads to increased expression of the cyclin-dependent kinase inhibitor p21 and p21-mediated development of early hypoxic and ischemic tolerance.
Keywords: cerebral ischemia, tissue-type plasminogen activator, tumor necrosis factor-α
Introduction
Tissue-type plasminogen activator (tPA) is a serine proteinase that activates the zymogen plasminogen into the broad-specificity protease plasmin. Tissue-type plasminogen activator is found in the intravascular space, where its main role is as a thrombolytic enzyme, and in the central nervous system, where its function is less clear. It has been demonstrated that tPA has an important role in synaptic activity (Qian et al, 1993). Indeed, tPA is stored in synaptic terminals, and neuronal tPA is connected to events associated with the development of synaptic plasticity, such as motor learning (Seeds et al, 1995) and long-term potentiation (Baranes et al, 1998).
Cerebral cortical neurons have a heightened vulnerability to hypoxia. Therefore, their survival depends on their ability to detect and accommodate to changes in the concentration of oxygen in their environment. A growing body of evidence indicates that hypoxia and ischemia have a direct effect on neuronal tPA. Indeed, exposure to hypoxic conditions induces the rapid release of tPA from cerebral cortical neurons (Echeverry et al, 2010), and the onset of cerebral ischemia is followed by a rapid increase in tPA activity in the ischemic tissue (Wang et al, 1998). Despite these observations, the effect of tPA on neuronal survival during hypoxic/ischemic conditions is poorly understood. Nevertheless, it has been postulated that tPA is neurotoxic, and that the presence of tPA in the ischemic tissue is associated with neuronal death and poor neurological outcome (Wang et al, 1998).
Ischemic preconditioning is a survival mechanism highly preserved among species whereby exposure to sublethal hypoxia/ischemia (preconditioning event) induces tolerance against a subsequent lethal hypoxic/ischemic injury (Kirino et al, 1996). The preconditioning event can protect the brain either very soon after its application (early preconditioning) or after a delay of 24 to 72 hours (delayed preconditioning). Unfortunately, despite the obvious clinical implications for the prevention and treatment of acute ischemic stroke patients, the mechanisms leading to the rapid induction of ischemic tolerance in the brain (early preconditioning) are poorly understood.
The onset of cerebral ischemia is followed by an inflammatory reaction in the ischemic brain. One of the best characterized mediators of this inflammatory response is the cytokine tumor necrosis factor-α (TNF-α), a member of the TNF superfamily of ligands synthesized as a monomeric type-2 transmembrane protein that is inserted into the membrane as a homotrimer and cleaved by the matrix metalloprotease TNF-converting enzyme (TACE) to a 51-kDa soluble circulating trimer (soluble TNF-α). Although it has been widely demonstrated that cerebral ischemia induces the expression of TNF-α in neurons, the effect of TNF-α on neuronal survival is unclear (Hallenbeck 2002). Accordingly, whereas some studies indicate that TNF-α has a deleterious effect in the acute phases of cerebral ischemia (Dawson et al, 1996; Barone et al, 1997), others have shown that induction of TNF-α is protective (Cheng et al, 1994; Nawashiro et al, 1997a; Ginis et al, 1999; Kirino 2002).
The cyclin-dependent kinase inhibitor p21cip/waf/sdi1 (hereafter p21) regulates cell cycle progression and inhibits apoptotic cell death (Gartel and Tyner, 2002). Experimental work indicates that p21 has a neuroprotective role in the ischemic brain (Rashidian et al, 2005). Hence, p21 has been associated with neuronal survival (van Lookeren and Gill, 1998) and regeneration (Qiu et al, 2004) in experimental models of hypoxia and cerebral ischemia.
The data presented here show that tPA induces the expression of neuronal TNF-α. This effect requires plasminogen and the N-methyl--aspartate receptor (NMDAR), and leads to p21-mediated development of early hypoxic and ischemic tolerance. Our findings indicate that tPA has a pivotal role in the development of tolerance against the damaging effects of lethal neuronal hypoxia and cerebral ischemia by inducing a sublethal inflammatory response mediated by TNF-α. These data have significant implications for the treatment of acute ischemic stroke patients.
Materials and methods
Animals, Reagents, and Doses
Murine strains were wild-type (Wt) C57BL/6J, tPA (tPA−/−), plasminogen (Plg−/−), and TNF-α (TNF-α−/−)-deficient mice, and their Wt littermate controls. Experiments were approved by the Institutional Animal Care and Use Committee of Emory University, Atlanta, GA, USA. Recombinant murine tPA (0 to 10 nmol/L), inactive tPA (itPA; 0 to 10 nmol/L). This form of tPA has an alanine for serine substitution at the active site Ser481; Lys plasmin (0 to 10 nmol/L), human α2-antiplasmin (140 nmol/L), and urokinase-type plasminogen activator (uPA) were purchased from Molecular Innovations (Novi, MI, USA). Other reagents were human recombinant tPA (Genentech, San Francisco, CA, USA), aprotinin (1.4 μmol/L; Calbiochem, Gibbstown, NJ, USA), kainic acid (KA; 250 μmol/L) and the NMDAR antagonist MK-801 (10 μmol/L; Tocris Bioscience, Ellisville, MO, USA), murine anti-TNF-α neutralizing antibodies (40 ng/mL) and recombinant TNF-α (1.2 nmol/L; R&D Systems; Minneapolis, MN, USA), 4′,6-diamidino-2-phenylindole and triphenyltetrazolium chloride (Sigma-Aldrich, St Louis, MO, USA), and p21 short hairpin RNA (shRNA) and shRNA lentiviral particles (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Neuronal Cultures and Determination of Neuronal Survival
Cerebral cortical neurons were cultured from E16-18 Wt, tPA−/−, and TNF-α−/− mice as described elsewhere (Echeverry et al, 2010). Briefly, the cerebral cortex was dissected, transferred into Hanks' balanced salt solution containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 mm HEPES, and incubated in trypsin containing 0.02% DNase at 37 °C for 15 minutes. Tissue was then triturated, and the supernatant was resuspended in B27-supplemented neurobasal medium containing 2 mmol/L -glutamine and plated onto 0.1 mg/mL poly--lysine-coated wells. To study the effect of tPA on neuronal survival, Wt cerebral cortical neurons were incubated with tPA 0 to 10 nmol/L and either kept under normoxic conditions or exposed in an anaerobic chamber (Hypoxygen, Frederick, MD, USA) to 55 minutes of oxygen–glucose deprivation (OGD) conditions (<0.1% oxygen, 94% N2, and 5% CO2 at 37 °C) in glucose-free media containing CaCl2 1.8 mmol/L, MgSO4 0.8 mmol/L, KCl 5.3 mmol/L, NaHCO3 44.05 mmol/L, and NaCl 110.34 mmol/L, followed 24 hours later by determination of cell survival/death with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (ATCC, Manassas, VA, USA) and the lactate dehydrogenase (LDH) release assays (Roche, Florence, SC, USA) following the manufacturer's instructions and as described elsewhere (Echeverry et al, 2010). Results are given as a percentage of cell survival or LDH release into the media compared with cultures maintained under normoxic conditions. Each experiment was performed in cultures from 3 different animals, and each observation was repeated 15 times.
In Vitro Model of Preconditioning
For tPA-induced preconditioning, Wt, tPA−/−, and TNF-α−/− cerebral cortical neurons were incubated for 5 minutes with 0 to 10 nmol/L of either active tPA or itPA or plasmin, or with a combination of tPA and either anti-TNF-α neutralizing antibodies (40 ng/mL) or an isotype immunoglobulin G control. A subgroup of neurons was incubated with 5 nmol/L or 50 nmol/L of uPA. The media was then changed to culture media without serum and the cells were placed back into the incubator for 0 to 60 minutes. After 0, 1, 5, 30, or 60 minutes, cells were placed in OGD media and exposed to hypoxic conditions for 55 minutes. At the end of the hypoxic injury, cells were placed in culture media and were placed back into the incubator for 24 hours. As controls, sister cultures were kept in culture media without serum for 0 to 60 minutes and then in culture media for 24 hours. Our preliminary studies indicate that exposure to serum-free culture media for 0 to 60 minutes does not have an effect on neuronal survival after 24 hours (data not shown).
To study the effect of tPA on KA-induced cell death, a subgroup of Wt neurons was incubated with tPA 5 nmol/L, followed 5 minutes later by treatment with KA 250 μmol/L. After 24 hours, neuronal survival and death were quantified with the MTT and LDH release assays, as described above. A subset of cultures was used for trypan blue viability assay and TdT-mediated dUTP nick-end labeling following the manufacturer's instructions (Calbiochem). Each experiment was repeated with cultures obtained from 3 different animals, and each observation was repeated 12 times. The number of either trypan blue- or terminal deoynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling-positive cells was expressed as a percentage over the total number of cells in each field at × 40 magnification in five different fields in five separate coverslips. For hypoxic preconditioning, neurons were exposed to OGD conditions for 5 minutes. A subset of cells was incubated with α2-antiplasmin 140 nmol/L, aprotinin 1.4 μmol/L, tPA 5 nmol/L, plasmin 10 nmol/L, MK-801 10 μmol/L, TNF-α 1.2 nmol/L, anti-TNF-α neutralizing antibodies (40 ng/mL), or an isotype immunoglobulin G control. Further, the media was changed to culture media without serum and the cells were returned to the incubator for 0 to 60 minutes. After 0, 5, 10, or 60 minutes, cells were placed in OGD media and exposed to hypoxic conditions for 55 minutes. At the end of the hypoxic injury, cells were placed in culture media and returned to the incubator for 24 hours. As controls, sister cultures were kept in OGD media without hypoxia for 5 minutes and then in serum-free culture media for 0 to 60 minutes. After 0, 5, 10, or 60 minutes, cells were placed in culture media again and neuronal survival was determined after 24 hours. Our preliminary studies indicate that exposure to OGD media for 5 minutes and then to serum-free culture media for 0 to 60 minutes, not followed by hypoxia, does not have an effect on neuronal survival after 24 hours (data not shown). A separate group of Wt neurons was infected with a lentiviral vector encoding a scrambled shRNA sequence or a silencing p21 shRNA, followed 96 hours later by either incubation with tPA 5 nmol/L or exposure to 5 minutes of OGD conditions (hypoxic preconditioning), 5 minutes before exposure to 55 minutes of OGD conditions. Knockdown of p21 gene was confirmed by reverse transcriptase PCR analysis. Neuronal survival/death was quantified 24 hours later with the MTT and LDH release assays. Each experiment was repeated from cultures obtained from 3 different animals, and each observation was repeated 10 times.
Middle Cerebral Artery Occlusion, In Vivo Model of Ischemic Preconditioning, and Determination of the Volume of the Ischemic Lesion
Transient occlusion of the middle cerebral artery (tMCAO) was induced with a 6 to 0 silk suture advanced from the external carotid artery into the internal carotid artery until the origin of the middle cerebral artery, as described elsewhere (Belayev et al, 1999). Briefly, a nylon monofilament (6 to 0, Ethicon, Issy Les Moulineaux, France), coated with silicone, was introduced through the external carotid artery and advanced up to the origin of the middle cerebral artery. The suture was withdrawn after 60 minutes of cerebral ischemia. Cerebral perfusion in the distribution of the middle cerebral artery was monitored throughout the surgical procedure and after reperfusion with a laser Doppler (Perimed, North Royalton, OH, USA), and only animals with a >70% decrease in cerebral perfusion after occlusion and complete recovery after suture withdrawn were included in this study. The rectal and masseter muscle temperatures were controlled at 37 °C with a homeothermic blanket. Heart rate, systolic, diastolic, and mean arterial blood pressures were controlled throughout the surgical procedure with an IITC 229 System (IITC-Lice Science, Woodland Hills, CA, USA). To induce ischemic tolerance (ischemic preconditioning), 1 hour before tMCAO a subgroup of Wt and TNFα−/− mice was exposed in an anaerobic chamber to 1 minute of either 1% O2, 5% CO2, and 94% N2, or 10% O2, 1% CO2, and 89% N2. A subset of Wt mice was injected with MK-801 1 μg/kg intraperitoneally. To test the effect of recombinant tPA (rtPA) on the induction of ischemic tolerance, a subset of Wt mice was injected into the tail vein with human rtPA 4.5 mg/kg intravenously, followed 1 hour later by tMCAO. To avoid the potential vasoactive effect of arginine present in the commercially available preparations, we used a SnakeSkin Dialysis Tubing (Thermo Scientific, Lafayette, CO, USA) to dialyze arginine from rtPA before its administration. To measure the volume of the ischemic lesion, 24 hours after tMCAO, animals were deeply anesthetized, the brains were harvested, cut into 2 μm sections, and stained with triphenyltetrazolium chloride. Each section was photographed, and the volume of the ischemic lesion was measured by a masked investigator with the National Institutes of Health Image Analyzer System as described elsewhere (Yepes et al, 2005). Each observation was repeated 10 times for the experiments with ischemic preconditioning and 12 times for the experiments with rtPA.
Determination of Tissue-Type Plasminogen activator and Tumor Necrosis Factor-α Concentrations
To measure the concentration of tPA in the ischemic brain, Wt mice (n=8) underwent tMCAO, followed 1 to 3 hours later by transcardial perfusion. A subgroup of animals was treated with rtPA 0.9 mg/kg intravenously. The concentration of active tPA was measured in the ischemic hemisphere with an ELISA kit, following the manufacturer's instructions (Molecular Innovations). Briefly, 100 μl of brain extracts was added to wells coated with plasminogen activator inhibitor-1 for 30 minutes, followed by the addition of an anti-tPA antibody and a horseradish peroxidase-conjugated anti-rabbit secondary antibody. Absorbance at 450 nm was measured after the addition of a substrate solution. Each experiment was performed with cultures from three different animals, and each observation was repeated eight times. To measure the release of TNF-α from cerebral cortical neurons, Wt neurons were incubated with tPA 5 nmol/L or plasmin 10 nmol/L, either alone or in combination with MK-801 10 μmol/L. A group of Wt and tPA−/− neurons was exposed to 5 minutes of OGD conditions, alone or in the presence of aprotinin 1.4 μmol/L, α2-antiplasmin 140 nmol/L, tPA 5 nmol/L or plasmin 10 nmol/L either alone or in combination with MK-801 10 μmol/L. After 2 hours, the concentration of TNF-α in the media was measured with an ELISA kit, following the manufacturer's instructions (Insight Genomics, Falls Church, VA, USA). Each experiment was repeated with neurons cultured from 3 different animals, and each observation was repeated 12 times.
Quantitative Reverse Transcriptase PCR for Tumor Necrosis Factor-α and p21 mRNA Expression
To study TNF-α mRNA expression, Wt neurons were incubated with 0–10 nm of active tPA or itPA. To study p21 expression, Wt and TNF-α−/− neurons were incubated with 5 nmol/L of active tPA or itPA, or 10 nmol/L of plasmin or 1.2 nmol/L of TNF-α. A group of Wt and tPA−/− neurons was exposed to 5 minutes of OGD conditions alone or in the presence of anti-TNF-α neutralizing antibodies (40 ng/mL). After 1 hour, total RNA was prepared using the RNAeasy kit (Qiagen, Valencia, CA, USA). Complementary DNA was generated using High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Reverse transcriptase PCR was performed wit TaqMan Gene Expression Assays (Applied Biosystems) using forward and reverse primers and an internal probe supplied by the manufacturer (Mm 00443258_m1 for TNF-α and Mm00432448_m1 for p21). Glyceraldehyde 3-phosphate dehydrogenase (Mm99999915_g1) was used as an internal control to standardize the samples. PCR was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems) under the following conditions: 50 °C for 2 minutes, 95 °C for 10 minutes, 40 cycles at 95 °C for 15 seconds, and 60 °C for 1 minute. The relative quantitation method (ΔΔCt) was used, with the ratio of the mRNA level for the gene of interest normalized to the level of internal control. Each observation was repeated 10 times.
Statistical Analyses
Data were analyzed either by a Wilcoxon–Mann rank-sum test or, in cases where more than one group was compared, by analysis of variance. P values <0.05 were considered significant.
Results
Tissue-Type Plasminogen Activator is not Neurotoxic to Cerebral Cortical Neurons
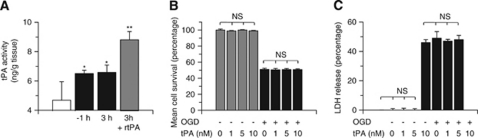
First, we used an enzyme-linked immunosorbent assay (ELISA) to quantify the concentration of active tPA in the brain under ischemic and non-ischemic conditions. Wild-type mice underwent tMCAO, followed 1 to 3 hours later by transcardial perfusion to wash tPA from the intravascular space. A subgroup of mice was intravenously injected 3 hours after tMCAO with rtPA at the same dose used for the treatment of acute ischemic stroke patients (0.9 mg/kg intravenously). We found that the concentration of active tPA increases from 4.68±1.2 ng/g tissue in the non-ischemic brain to 6.5±0.2 and 6.58±0.5 ng/g tissue 1 to 3 hours after tMCAO. Treatment with rtPA further increased the concentration of tPA to 8.7±0.5 ng/g (n=8 per experimental group; *P<0.05 versus non-ischemic brains and **P<0.01 versus ischemic brains not treated with rtPA; Figure 1A). Further, we studied the effect of tPA on neuronal survival. Wt cerebral cortical neurons were incubated with 0 to 10 nmol/L tPA and either maintained under normoxia or simultaneously exposed to OGD conditions for 55 minutes, followed 24 hours later by determination of cell survival and death with the MTT and LDH release assays, respectively. We found that incubation with tPA neither decreases cerebral cortical neuronal survival under normoxia nor increases neuronal death when cells are simultaneously exposed to lethal hypoxia (Figures 1B and C). Our results indicate that tPA is not directly neurotoxic to cerebral cortical neurons.
Figure 1.
Tissue-type plasminogen activator (tPA) is not neurotoxic to cerebral cortical neurons. (A) Mean concentration of active tPA in the brain of wild-type (Wt) mice either under non-ischemic conditions (white bar) or 1 or 3 hours after transient occlusion of the middle cerebral artery (tMCAO; black bars), or 3 hours after tMCAO and the intravenous administration of recombinant tPA (rtPA; gray bar); n=8 for each group. Data represent mean±s.d. *P<0.05 versus non-ischemic brains. **P<0.01 versus untreated ischemic brains 1 and 3 hours after tMCAO. Mean neuronal survival determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (B) and quantification of lactate dehydrogenase (LDH) release into the media (C) in Wt cerebral cortical neurons incubated with tPA 0 to 10 nmol/L and either maintained under normoxia (gray bars) or simultaneously exposed to oxygen–glucose deprivation (OGD) conditions for 55 minutes (black bars); n=15 for each group. Data represent mean±s.d. NS, nonsignificant.
Tissue-Type Plasminogen Activator Protects Neurons from Hypoxia- and Ischemia-Induced Cell Death
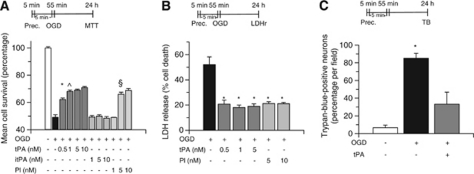
Because exposure to a brief period of hypoxia (hypoxic preconditioning) not only renders neurons resistant against a subsequent lethal hypoxic injury (Grabb and Choi, 1999) but also induces the rapid release of tPA from cerebral cortical neurons (Echeverry et al, 2010), we investigated whether tPA has an effect on the development of early tolerance to lethal hypoxia. To test this hypothesis, Wt neurons were incubated for 5 minutes with tPA 0 to 10 nmol/L, followed 1 to 60 minutes later by exposure to lethal hypoxia (55 minutes of OGD conditions), and cell survival was determined with the MTT, LDH release, and trypan blue exclusion assays. We found that treatment with tPA 5, but not 1, 30, or 60 minutes before exposure to lethal OGD conditions, induces a dose-dependent increase in cell survival from 49±1.87% in neurons exposed to OGD conditions without previous incubation with tPA to 62.22±1.1%, 68.1±2%, 69±0.82%, and 70.2±0.94% in cells incubated 5 minutes before exposure to OGD conditions with tPA 0.5, 1, 5, and 10 nmol/L, respectively (n=12 per experimental group; P<0.01 versus neurons exposed to OGD conditions without treatment with tPA; Figure 2A). Additionally, incubation with tPA 5 minutes before exposure to OGD conditions decreased OGD-induced LDH release into the media from 51.3±8% in neurons exposed to OGD conditions without previous treatment with tPA to 18±3% (n=10; P<0.01; Figure 2B), and the percentage of Trypan blue-positive neurons/field from 82±2% in neurons exposed to OGD conditions without previous treatment with tPA to 23±8% (n=10; P<0.01; Figure 2C). The protective effect of tPA was reproduced by treatment with plasmin 10 nmol/L (Figure 2B) and was not observed when neurons were incubated with proteolytically inactive tPA (Figure 2A). Because uPA also catalyzes the conversion of plasminogen into plasmin, we determined cell survival in Wt neurons left untreated or incubated with uPA 5 or 50 nmol/L 5 minutes before exposure to 55 minutes of OGD conditions. Our results indicate that cell survival in Wt neurons increases from 49.22±2.08% in neurons exposed to OGD conditions without preconditioning to 49.44±4.48% and 62.38±1.57% in cells preconditioned with 5 and 50 nmol/L of uPA, respectively (n=10; P<0.01 when cells treated with uPA 50 nmol/L are compared with non-preconditioned neurons). Our results indicate that uPA also induces preconditioning.
Figure 2.
Proteolytically active tissue-type plasminogen activator (tPA) induces hypoxic tolerance. Mean neuronal survival determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (A) and quantification of lactate dehydrogenase (LDH) release into the media (B) in wild-type (Wt) cerebral cortical neurons incubated during 5 minutes with 0 to 10 nmol/L of either active tPA or proteolytically inactive tPA (itPA), or plasmin (Pl), followed 5 minutes later by exposure to 55 minutes of oxygen–glucose deprivation (OGD) conditions; n=12 for each group. Data represent mean±s.d. (A) *P<0.01 versus cells maintained under OGD conditions without preconditioning with tPA, ^P<0.05 versus cells treated with tPA 0.5 nmol/L before exposure to lethal OGD conditions, and §P<0.01 versus untreated neurons exposed to OGD conditions. (B) *P<0.01 versus cells maintained under OGD conditions without previous incubation with tPA or Pl. Prec. denotes preconditioning and indicates the moment when neurons were treated. (C) Percentage of trypan blue-positive cells/field in Wt neuronal cultures maintained under normoxia (white bars) or exposed to lethal OGD conditions for 55 minutes (black bars) or incubated with tPA 5 nmol/L 5 minutes before exposure to 55 minutes of OGD conditions (gray bars); n=10 for each group. Data represent mean±s.d. *P<0.01 versus cells maintained under OGD conditions without treatment. Prec. denotes preconditioning and indicates the time of treatment with tPA. LDHr, LDH release assay; TB, trypan blue exclusion assay.
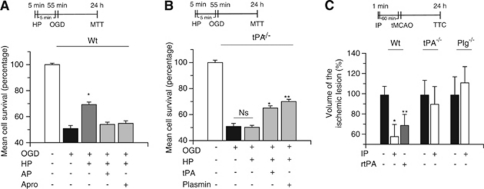
To investigate whether endogenous tPA also protects neurons from the deleterious effects of lethal hypoxia, we used an in vitro model of hypoxic preconditioning in which exposure to a brief period of sublethal hypoxia, also known to cause the release of tPA from cerebral cortical neurons (Echeverry et al, 2010), induces tolerance against a subsequent lethal hypoxic injury. We found that exposure of Wt neurons to 5 minutes of OGD conditions (hypoxic preconditioning), followed 5 minutes later by a lethal hypoxic injury (55 minutes of OGD), increases neuronal survival from 49.3±2.8% in non-preconditioned neurons to 69.38±7.56% (n=12; P<0.01), and that this effect is abrogated by treatment with either α2-antiplasmin or aprotinin, or by genetic deficiency of tPA (Figures 3A and B). Importantly, treatment with tPA or plasmin during the preconditioning phase (exposure to sublethal hypoxia) rescued the protective effect of hypoxic preconditioning in tPA−/− neurons.
Figure 3.
Endogenous tissue-type plasminogen activator (tPA) induces hypoxic tolerance. Mean cell survival determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in wild-type (Wt; A) and tPA-deficient (tPA−/−; B) neurons exposed to 5 minutes of oxygen–glucose deprivation (OGD) conditions (hypoxic preconditioning, HP), followed 5 minutes later by 55 minutes of OGD. A subgroup of neurons was incubated during the preconditioning phase with either α2-antiplasmin 100 nmol/L (AP) or aprotinin 1.4 μmol/L (Apro), or tPA 5 nmol/L or plasmin 10 nmol/L. HP denotes the moment when cells were exposed to sublethal hypoxia; n=12 for each observation. Data represent mean cell survival±s.d. (A) *P<0.01 versus cells exposed to OGD without hypoxic preconditioning. (B) *P<0.01 versus tPA−/− neurons preconditioned in the absence of tPA and **P<0.01 versus tPA−/− neurons preconditioned in the absence of plasmin. (C) Mean percentage decrease in the volume of the ischemic lesion compared with non-preconditioned mice (black bars) in Wt, tPA−/−, and plasminogen (Plg−/−) mice preconditioned by exposure to 1 minute of low oxygen concentration (ischemic preconditioning, IP), followed 60 minutes later by transient middle cerebral artery occlusion (tMCAO). Black bars depict mice undergoing tMCAO without preconditioning. A subgroup of Wt mice was preconditioned with recombinant tPA (rtPA) intravenously administered 60 minutes before tMCAO (gray bar); n=12 for each observation. Data represent mean percentage compared with stroke volume in non-preconditioned brains±s.d. *P<0.01 compared with non-preconditioned Wt mice. **P<0.05 versus non-preconditioned Wt mice. Ns, non significant; TTC, triphenyltetrazolium chloride staining.
To study the effect of endogenous tPA on the development of ischemic tolerance in vivo, we used the model of ischemic preconditioning described in the Materials and methods section in which mice are exposed for 1 minute to low oxygen concentrations (ischemic preconditioning), followed 60 minutes later by tMCAO. We found that, compared with non-preconditioned animals, ischemic preconditioning causes a 41.11±18% decrease in the volume of the ischemic lesion in Wt mice (n=12; P<0.01), and that this effect is abrogated by genetic deficiency of either tPA or plasminogen (Figure 3C). After its intravenous administration, rtPA permeates the brain parenchyma (Benchenane et al, 2005). Thus, to investigate whether treatment with rtPA also has an effect on the development of ischemic tolerance, we measured the volume of the ischemic lesion in Wt mice treated intravenously with rtPA 4.5 mg/kg 60 minutes before tMCAO. We found that, compared with non-treated animals, the intravenous administration of rtPA induces a 30.12±18% decrease in the volume of the ischemic lesion (n=10; P<0.05; Figure 3C). Our results indicate that either endogenous tPA or treatment with rtPA induces early tolerance to lethal hypoxia and ischemia via a plasminogen-dependent mechanism.
N-Methyl--Aspartate Receptor Mediates the Protective Effect of Tissue-Type Plasminogen Activator
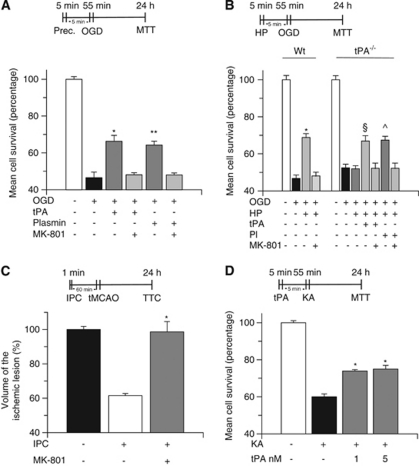
Because tPA and plasmin interact with the NMDAR (Nicole et al, 2001; Samson et al, 2008), we investigated whether the NMDAR mediates the neuroprotective effect of tPA. To study this possibility, we quantified cell survival in Wt neurons incubated with tPA or plasmin, alone or in combination with the NMDAR antagonist MK-801 10 μ, 5 minutes before exposure to lethal hypoxia. Our data show that MK-801 abrogates the protective effect of tPA and plasmin (Figure 4A). To determine whether the NMDAR also mediates the protective effect of endogenous tPA, we quantified cell survival in Wt neurons preconditioned with sublethal hypoxia (hypoxic preconditioning), either alone or with MK-801, followed 5 minutes later by exposure to lethal hypoxic conditions. In a parallel group of experiments, tPA−/− neurons underwent hypoxic preconditioning either alone or in the presence of tPA 5 nmol/L plasmin 10 nmol/L, or MK-801 in combination with either tPA or plasmin, followed 5 minutes later by exposure to lethal hypoxia as described above. We found that the protective effect of hypoxic preconditioning in Wt neurons is abrogated by treatment with MK-801. Similarly, MK-801 abolished the rescue effect of tPA and plasmin in tPA−/− neurons (Figure 4B). To study the effect of NMDAR antagonism in vivo, Wt mice were either left untreated or injected with MK-801 1 μg/kg intraperitoneally and exposed to ischemic preconditioning, as described above, followed 60 minutes later by tMCAO. Our results indicate that the protective effect of ischemic preconditioning (38.5±12% decrease in the volume of the ischemic lesion) is abrogated by NMDAR antagonism (Figure 4C; n=10; P<0.01). Because these data suggest that tPA protects neurons from excitotoxin-induced cell death, we quantified cell survival in Wt neurons treated with tPA 0 to 5 nmol/L, followed 1 to 30 minutes later by incubation with KA 250 μmol/L. We found that preconditioning with tPA 5 minutes before treatment with KA increases neuronal survival from 60±1.57% in cells incubated with KA without previous treatment with tPA to 73.97±0.72% and 75±1.72% in neurons preconditioned with tPA 1 and 5 nmol/L, respectively, indicating that tPA also induces tolerance against excitotoxin-induced neuronal death (n=10; P<0.01; Figure 4D).
Figure 4.
The N-methyl--aspartate receptor (NMDAR) is required for the neuroprotective effect of tissue-type plasminogen activator (tPA). (A) Mean cell survival in wild-type (Wt) neurons treated with tPA 5 nmol/L or plasmin 10 nmol/L, either alone or in combination with the NMDAR antagonist MK-801 10 μ, followed 5 minutes later by exposure to 55 minutes of oxygen–glucose deprivation (OGD) conditions; n=12 for each observation. *P<0.01 versus cells cotreated with tPA and MK-801. **P<0.01 versus cells cotreated with plasmin and MK-801. (B) Mean cell survival in Wt and tPA−/− neurons exposed to 5 minutes of OGD (hypoxic preconditioning, HP), followed 5 minutes later by incubation under OGD conditions for 55 minutes. A subgroup of tPA−/− cells was treated with tPA 5 nmol/L or plasmin 10 nmol/L either alone or in combination with MK-801 10 μmol/L; n=10 for each observation. Data represent mean±s.d. *P<0.01 versus cells cotreated with MK-801. §P<0.01 versus tPA−/− neurons preconditioned either in absence of tPA or with a combination of tPA and MK-801. ^P<0.01 versus preconditioned and non-preconditioned tPA−/− neurons and versus tPA−/− neurons cotreated with plasmin and MK-801 during the preconditioning phase. HP denotes the moment when cells were treated with tPA or plasmin alone or in combination with MK-801. Indicates the moment when cells were exposed to sublethal hypoxia. (C) Mean percentage decrease in the volume of the ischemic lesion in Wt mice either left untreated or injected with MK-801 1 μg/kg intraperitoneally, followed by ischemic preconditioning and transient occlusion of the middle cerebral artery (tMCAO) 1 hour later; n=10; *P<0.01 versus mice preconditioned without MK-801. (D) Mean cell survival in Wt neurons incubated with tPA 1 or 5 nmol/L, followed 5 minutes later by exposure to kainic acid (KA) 250 μmol/L; n=10; P<0.01 versus neurons exposed to KA without preconditioning with tPA. IPC, ischemic preconditioning.
Tumor Necrosis Factor-α Mediates Tissue-Type Plasminogen Activator-Induced Neuroprotection
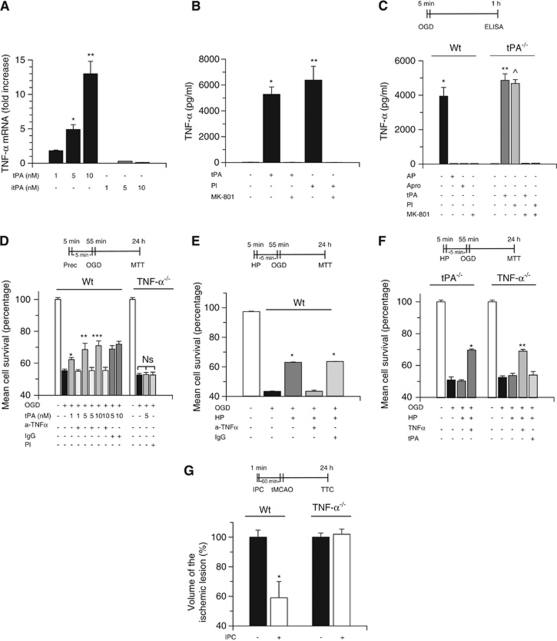
Because TNF-α mediates the development of some forms of hypoxic and ischemic tolerance (Nawashiro et al, 1997b; Liu et al, 2000), we studied whether TNF-α is downstream of tPA. First, Wt neurons were incubated with 0 to 10 nmol/L of either active tPA or itPA, followed 1 hour later by quantification of TNF-α mRNA expression in cell lysates by reverse transcriptase PCR analysis as described in the Materials and methods section. We found that treatment with 5 and 10 nmol/L of proteolytically active tPA induces a 4.9±0.7- and 13±1.8-fold increase in TNF-α mRNA expression, respectively, and that this effect is not observed with itPA (n=12; P<0.01; Figure 5A). Further, we used an ELISA to quantify the concentration of TNF-α in the culture media of Wt neurons after 1 hour of incubation with tPA 5 nmol/L or plasmin 10 nmol/L, alone or in combination with MK-801 10 μ. We found that incubation with either tPA or plasmin increases the concentration of TNF-α in the culture media to 5268±578 pg/ml and 6374±877 pg/ml, respectively, and that this effect is abrogated by NMDAR antagonism with MK-801 (n=12; P<0.01; Figure 5B).
Figure 5.
Tumor necrosis factor (TNF)-α mediates the neuroprotective effect of tissue-type plasminogen activator (tPA). (A) TNF-α mRNA expression in wild-type (Wt) neurons incubated with active tPA or inactive tPA (itPA) 1 to 10 nmol/L; n=12 for each observation. Data represent mean fold increase in TNF-α mRNA compared with control cells±s.d. *P<0.01 versus neurons treated with tPA 1 nmol/L. **P<0.01 versus neurons treated with tPA 5 nmol/L. (B) TNF-α concentration (pg/ml) in the culture media of Wt neurons incubated with tPA 5 nmol/L or plasmin (Pl) 10 nmol/L either alone or in combination with MK-801 10 μmol/L; n=12 for each observation. *P<0.01 versus neurons either left untreated or cotreated with tPA and MK-801. **P<0.01 versus neurons either left untreated or cotreated with Pl and MK-801. (C) Mean TNF-α concentration (pg/ml) in the media of Wt and tPA−/− neurons exposed to 5 minutes of oxygen–glucose deprivation (OGD) conditions (hypoxic preconditioning) alone or in the presence of either α2-antiplasmin 100 nmol/L (AP) or aprotinin 1.4 μmol/L (Apro) or tPA 5 nmol/L or Pl 10 nmol/L or MK-801 10 μmol/L; n=12 for each observation. Lines denote s.d. *P<0.01 versus neurons treated with either AP or Apro or MK-801. ** and ^P<0.01 versus untreated tPA−/− neurons. (D) Mean cell survival in Wt and TNF-α−/− neurons incubated with tPA 1 to 10 nmol/L, or with a combination of tPA 1 to 10 nmol/L and either anti-TNF-α-blocking antibodies 0.4 μg/ml (a-TNF-α) or an isotype immunoglobulin G (IgG) control (dark gray bars), or with Pl alone 5 nmol/L, followed 5 minutes later by exposure to lethal OGD conditions; n=12 for each observation. Lines denote s.d. *P<0.01 versus neurons cotreated with tPA 1 nmol/L and a-TNF-α antibodies. **P<0.01 versus neurons cotreated with tPA 5 nmol/L and a-TNF-α antibodies. ***P<0.01 versus neurons cotreated with tPA 10 nmol/L and a-TNF-α antibodies. Prec. indicates preconditioning and denotes the moment when cells where treated. (E) Mean cell survival in Wt neurons exposed to 5 minutes of OGD (hypoxic preconditioning, HP) alone or in the presence of either anti-TNF-α-neutralizing antibodies or an IgG isotype control, followed 5 minutes later by exposure to lethal OGD conditions; n=12 for each observation. *P<0.01 versus neurons treated with a-TNF-α antibodies during the preconditioning phase. Lines denote mean±s.d. (F) Mean cell survival in tPA−/− and TNF-α−/− neurons exposed to 5 minutes of OGD conditions (HP) either alone or in combination with tPA 5 nmol/L or TNF-α 20 ng/ml, followed 5 minutes later by exposure to 55 minutes OGD; n=10 for each observation. *P<0.01 versus preconditioned and non-preconditioned tPA−/− neurons. **P<0.01 versus non-preconditioned and preconditioned TNF-α−/− neurons and versus TNF-α−/− neurons treated with tPA during the preconditioning phase. Lines denote mean±s.d. (G) Mean percentage decrease in the volume of the ischemic lesion in Wt and TNF-α−/− mice exposed to ischemic preconditioning and transient occlusion of the middle cerebral artery (tMCAO) 1 hour later; n=12; P<0.01 versus non-preconditioned Wt mice and preconditioned and non-preconditioned TNF-α−/− mice. Ns, nonsignificant
To investigate the role of endogenous tPA on TNF-α expression, Wt and tPA−/− neurons were exposed to 5 minutes of OGD conditions, known to cause the release of endogenous tPA (Echeverry et al, 2010) and induce hypoxic tolerance (Figure 3A), followed 1 hour later by quantification of TNF-α concentration in the culture media with an ELISA as described in the Materials and methods section. We found that 5 minutes of exposure to OGD increases TNF-α concentration in the culture media of Wt neurons from 16.5±5 to 3939.5±512 pg/ml (n=12; P<0.01), and that this effect was abrogated by either NMDAR antagonism with MK-801 or genetic deficiency of tPA (n=12; P<0.01; Figure 5C). However, when tPA−/− neurons were incubated with tPA or plasmin during the exposure to OGD conditions, the concentration of TNF-α in the culture media increased to 4863±378 and 4684±226.6 pg/ml, respectively, and in both cases this rescue effect was abrogated by cotreatment with MK-801. These results indicate that tPA induces the expression of TNF-α in cerebral cortical neurons, and that this effect is mediated by plasmin and requires the engagement of the NMDAR.
To determine whether TNF-α mediates tPA-induced tolerance to lethal hypoxia, we studied cell survival in TNF-α−/− neurons treated with tPA 5 nmol/L or plasmin 10 nmol/L, and in Wt neurons treated with tPA 0 to 10 nmol/L alone or in combination with either anti-TNF-α antibodies 0.4 μg/ml or an immunoglobulin G isotype control, followed 5 minutes later by exposure to 55 minutes of OGD conditions. We found that tPA- and plasmin-induced tolerance to lethal hypoxia is abrogated either by cotreatment with anti-TNF-α-blocking antibodies or genetic deficiency of TNF-α (n=12; P<0.01; Figure 5D).
To investigate whether the protective effect of endogenous tPA is also mediated by TNF-α, we studied neuronal survival in Wt, tPA−/−, and TNF-α−/− neurons preconditioned with 5 minutes of OGD, alone or in the presence of anti-TNF-α-neutralizing antibodies (in Wt neurons), tPA (in TNF-α−/− neurons), or TNF-α 1.2 nmol/L (in tPA−/− neurons), followed 5 minutes later by exposure to lethal hypoxia. We found that the protective effect of hypoxic preconditioning is abrogated by treatment with anti-TNF-α antibodies (Figure 5E) or by genetic deficiency of either tPA or TNF-α (Figure 5F). Importantly, although treatment with TNF-α during the preconditioning phase (5 minutes of OGD) restored the protective effect of hypoxic preconditioning in tPA−/− and TNF-α−/− neurons, incubation with tPA had a rescue effect in tPA−/− but not in TNF-α−/− neurons. Our results indicate that the neuroprotective effect of tPA is mediated by TNF-α. To investigate whether TNF-α mediates the protective effect of ischemic preconditioning in vivo, we quantified the volume of the ischemic lesion in Wt and TNF-α−/− mice preconditioned in the anaerobic chamber as described above, followed 60 minutes later by tMCAO. We found that although ischemic preconditioning is associated with a 41±11% decrease in the volume of the ischemic lesion in Wt mice, this effect was absent in TNF-α−/− mice (Figure 5G; n=12; P<0.01).
Tissue-Type Plasminogen Activator Induces p21 Expression Via Neuronal Tumor Necrosis Factor-α
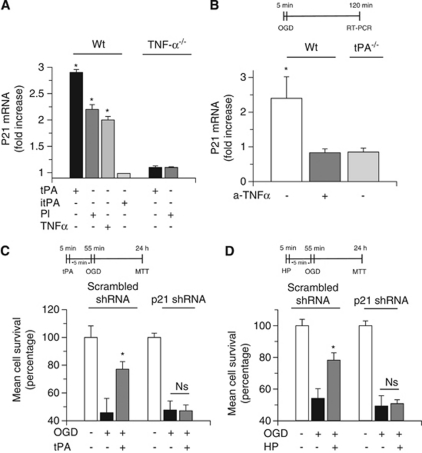
Because the cell cycle inhibitor p21 is associated with the development of tolerance to lethal hypoxia and ischemia (Tomasevic et al, 1999; Qiu et al, 2004), and experimental work in human colon cancer cells indicates a relation between TNF-α and p21 expression (Kobayashi et al, 2000), we studied p21 mRNA expression in Wt and TNF-α−/− neurons incubated with 5 nmol/L of active tPA or itPA, 10 nmol/L of plasmin, or 1.2 nmol/L of TNF-α. We found that proteolytically active tPA, plasmin, and TNF-α induce a 2.7±1.1-, 2.4±1.8-, and 1.98±1.1-fold increase, respectively, in p21 mRNA expression in Wt neurons. This effect was absent when TNF-α−/− cerebral cortical neurons were incubated with tPA or plasmin. Together, our results indicate that the effect of tPA on p21 expression is mediated by plasmin and TNF-α (n=10; P<0.05; Figure 6A). To investigate the effect of endogenous tPA on neuronal p21, we studied p21 mRNA expression in Wt and tPA−/− neurons exposed to 5 minutes of OGD conditions, known to induce hypoxic tolerance (Figures 3A and B). A subgroup of Wt neurons was incubated with anti-TNF-α-neutralizing antibodies. We found that hypoxic preconditioning induces a 2.4±0.6-fold increase in p21 mRNA expression (n=8; P<0.05), and that this effect is abrogated either by treatment with anti-TNF-α-neutralizing antibodies or by genetic deficiency of tPA (Figure 6B).
Figure 6.
p21 mediates the neuroprotective effect of tissue-type plasminogen activator (tPA). (A) Mean fold increase in p21 mRNA expression in wild-type (Wt) and tumor necrosis factor-α deficient (TNF-α−/−) neurons incubated with either active tPA 5 nmol/L or inactive tPA (itPA) 5 nmol/L or plasmin 10 nmol/L or TNF-α 20 ng/ml. Values represent mean±s.d; n=8 per group. *P<0.01 compared with p21 mRNA expression in neurons treated with itPA. (B) Mean fold increase in p21 mRNA expression in wild-type (Wt) and tPA-deficient (tPA−/−) neurons exposed to 5 minutes of oxygen–glucose deprivation (OGD) conditions. A subgroup of Wt neurons was incubated with anti-TNF-α antibodies 40 ng/ml. *P<0.01 versus Wt neurons incubated with anti-TNF-α antibodies and tPA−/− neurons; n=8 per group. Values represent mean±s.d. (C) Mean cell survival in Wt neurons infected with a lentiviral vector encoding a scrambled short hairpin RNA (shRNA) sequence that does not lead to the specific degradation of any known cellular mRNA or with a silencing p21 shRNA and treated with tPA 5 nmol/L followed 5 minutes later by exposure to 55 minutes of OGD conditions (lethal hypoxia). *P<0.01 versus non-preconditioned neurons; n=10 observations. Values represent mean±s.d. (D) Mean cell survival in Wt neurons infected with a lentiviral vector encoding a scrambled shRNA sequence or a silencing p21 shRNA and exposed to OGD conditions for 5 minutes (hypoxic preconditioning, HP), followed 5 minutes later by exposure to 55 minutes of OGD conditions (lethal hypoxia). *P<0.01 versus non-preconditioned neurons; n=12 observations. Values represent mean±s.d. Ns, nonsignificant
p21 Mediates the Neuroprotective Effect of Tissue-Type Plasminogen Activator
Next, we investigated whether p21 mediates tPA-induced hypoxic tolerance. To test this hypothesis, we quantified neuronal survival in Wt neurons infected with a lentiviral vector encoding either a scrambled shRNA sequence or a silencing p21 shRNA and incubated with tPA 5 nmol/L 5 minutes before exposure to 55 minutes of OGD conditions. We found that incubation with tPA increases the survival of scrambled shRNA-infected neurons from 45.76±10.3% in neurons exposed to OGD conditions without preconditioning with tPA to 77.1±5.5%. In contrast, tPA failed to induced hypoxic tolerance in p21 shRNA-infected cells (n=10; P<0.01; Figure 6C).
To investigate whether p21 also mediates the effect of endogenous tPA on the induction of early hypoxic tolerance, we quantified neuronal survival in scrambled shRNA- and p21 shRNA-infected Wt neurons exposed to 5 minutes of OGD (hypoxic preconditioning) 5 minutes before exposure to lethal hypoxia (55 minutes of OGD conditions). Our results indicate that hypoxic preconditioning induces tolerance to lethal hypoxia in scrambled shRNA- but not in p21 shRNA-infected neurons (Figure 6D). These data show that p21 mediates the neuroprotective effect of both exogenous and endogenous tPA. Because it has been demonstrated that p21 expression is associated with neuronal survival (van Lookeren and Gill, 1998), we investigated the effect of preconditioning with tPA on hypoxia-induced apoptotic cell death. Wt neurons were left untreated or incubated with tPA 5 nmol/L, followed 5 minutes later by exposure to 55 minutes of OGD conditions and study of apoptotic neuronal death with the terminal deoynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling assay as described in the Materials and methods section. Our results indicate that treatment with tPA 5 minutes before exposure to lethal OGD decreases the percentage of terminal deoynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling-positive neurons/field from 42±16% in neurons exposed to OGD conditions without previous treatment with tPA to 3.0±1.5% (n=16; P<0.01).
Discussion
Ischemic stroke is the second cause of mortality and a leading cause of disability in the world (Roger et al, 2011). Although the interruption of the blood supply to the brain has a deleterious impact on the survival of glial and endothelial cells, neurons are especially vulnerable to the devastating effects of the ischemic injury. Indeed, it is estimated that 1 minute of cerebral ischemia destroys ∼1.9 millions of neurons and 14 millions of synapses (Saver, 2006). It has long been recognized that a brief episode of sublethal hypoxia activates an endogenous neuroprotective response that renders neurons tolerant against a subsequent lethal hypoxic injury (Kirino et al, 1996). Thus, understanding the mechanisms leading to the development of this protective response may lead to the discovery of an effective neuroprotective strategy for the treatment of acute ischemic stroke patients.
In previous studies, we showed that exposure to sublethal hypoxia induces the rapid release of tPA from cerebral cortical neurons (Echeverry et al, 2010). Here, we report that this release of tPA is the first step of an endogenous neuroprotective response that renders neurons resistant against a subsequent lethal hypoxic/ischemic injury. Importantly, although our data do not indicate that tPA has a direct cytoprotective effect, they show that it promotes neuronal survival in the context of hypoxic and ischemic preconditioning by inducing a sublethal inflammatory response mediated by TNF-α. At this moment, we do not know why preconditioning with tPA induces tolerance when applied 5 but not 30 or 60 minutes before exposure to lethal hypoxia. However, our observations agree with the existence of a narrow ‘therapeutic window' for the protective effect of preconditioning (Kirino, 2002). Our findings are in discrepancy with reports by others, indicating that tPA mediates hypoxia- and ischemia-induced neuronal death (Wang et al, 1998; Nagai et al, 1999). Indeed, our data show that tPA does not induce neuronal death during neither normoxia nor hypoxia. We believe that this apparent disagreement may be explained by differences between experimental systems and the use of a wide range of doses and concentrations of tPA, some of them unlikely to be found in the brain even after cerebral ischemia and treatment with rtPA.
tPA has a beneficial thrombolytic effect in the intravascular space. Hence, treatment with rtPA within 3 to 4.5 hours of the onset of cerebral ischemia is associated with recovery in neurological function in a significant percentage of acute ischemic stroke patients (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995; Hacke et al, 2008). Accordingly, it is estimated that for every 100 patients with acute ischemic stroke treated with rtPA, 32 will experience some degree of neurological improvement (Saver, 2004). Unfortunately, only a small fraction of acute ischemic stroke patients currently benefit from treatment with rtPA (Reeves et al, 2005). Our data indicate that either endogenous tPA or treatment with rtPA, at doses not associated with the development of hemorrhagic complications, not only is not neurotoxic but instead induces ischemic tolerance. Because after its intravenous administration rtPA reaches the brain parenchyma (Benchenane et al, 2005), we propose that besides its beneficial thrombolytic properties, treatment with rtPA may also have a neuroprotective effect in the ischemic brain. Our findings are in apparent conflict with those by others indicating that genetic deficiency of tPA is associated with a better neurological outcome after experimental cerebral ischemia (Wang et al, 1998; Nagai et al, 1999). We propose that this discrepancy may be explained by a pleiotropic effect of tPA in the ischemic brain. Indeed, studies in animal models of cerebral ischemia (Yepes et al, 2003) and acute ischemic stroke patients (Kidwell et al, 2008) indicate that tPA also increases the permeability of the blood–brain barrier. Thus, we postulate that the neurotoxic effect of tPA observed in some experimental in vivo systems may be consequence of tPA-induced cerebral edema instead of a direct deleterious effect of tPA on neuronal survival.
We show that the neuroprotective effect of tPA is mediated by plasmin and requires engagement of the NMDAR, which agrees with reports by others indicating that plasmin mediates the interaction between tPA and the NMDAR (Samson et al, 2008), and that NMDAR antagonism abrogates the development of tolerance against lethal hypoxic and ischemic injuries (Grabb and Choi, 1999). However, our findings are in conflict with those of others indicating that the direct interaction between tPA and the NMDAR has a neurotoxic effect in the ischemic brain (Baron et al, 2010). We believe that this apparent disagreement may be explained by the doses of tPA used in some of these studies, as well as differences in the experimental design. Nevertheless, it is also possible that the interaction between tPA and the NMDAR has a pleiotropic effect in the ischemic brain according to the stage of the hypoxic/ischemic injury.
The onset of cerebral ischemia induces an inflammatory response in the brain, which has a critical effect on the final outcome of ischemic stroke. Our early work indicates that tPA has a proinflammatory effect in the ischemic brain (Polavarapu et al, 2005). Here, we report that tPA induces the expression of TNF-α in neurons, and that this effect, mediated by the interaction between plasmin and the NMDAR, leads to the development of hypoxic tolerance. Our data agree with studies in neuronal cultures (Ginis et al, 1999), ischemic stroke patients (Castillo et al, 2003), and experimental models of MCAO (Nawashiro et al, 1997b) indicating that the induction of a sublethal inflammatory reaction mediated by TNF-α has a protective effect in the ischemic brain.
A growing body of experimental work indicates that p21 expression is associated with neuronal survival and inhibition of apoptotic cell death (van Lookeren and Gill, 1998; Gartel and Tyner, 2002; Qiu et al, 2004). Our data indicate that tPA and plasmin induce the expression of p21 in neurons, and that this effect is mediated by TNF-α. More importantly, we found that p21 mediates the development of tPA-induced hypoxic tolerance. Furthermore, our results show that preconditioning with tPA attenuates the effect of hypoxia on apoptotic neuronal death.
In summary, here we show that tPA is not neurotoxic but instead is an endogenous neuroprotectant that renders neurons resistant against lethal hypoxia/ischemia. Our findings have significant implications not only for the understanding of the role of tPA in the ischemic brain, but also for the treatment of acute ischemic stroke patients.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by National Institutes of Health Grants NS-062073 and HL-095063, and VA MERIT award BX000474 (M Yepes), and National Natural Science Foundation of China 30900458 (J An).
References
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Baron A, Montagne A, Casse F, Launay S, Maubert E, Ali C, Vivien D. NR2D-containing NMDA receptors mediate tissue plasminogen activator-promoted neuronal excitotoxicity. Cell Death Differ. 2010;17:860–871. doi: 10.1038/cdd.2009.172. [DOI] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- Castillo J, Moro MA, Blanco M, Leira R, Serena J, Lizasoain I, Davalos A. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol. 2003;54:811–819. doi: 10.1002/ana.10765. [DOI] [PubMed] [Google Scholar]
- Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J Clin Invest. 2010;120:2194–2205. doi: 10.1172/JCI41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- Ginis I, Schweizer U, Brenner M, Liu J, Azzam N, Spatz M, Hallenbeck JM. TNF-alpha pretreatment prevents subsequent activation of cultured brain cells with TNF-alpha and hypoxia via ceramide. Am J Physiol. 1999;276:C1171–C1183. doi: 10.1152/ajpcell.1999.276.5.C1171. [DOI] [PubMed] [Google Scholar]
- Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J Neurosci. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von KR, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, Jahan R, Vinuela F, Kang DW, Warach S. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25:338–343. doi: 10.1159/000118379. [DOI] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kirino T, Nakagomi T, Kanemitsu H, Tamura A. Ischemic tolerance. Adv Neurol. 1996;71:505–511. [PubMed] [Google Scholar]
- Kobayashi N, Takada Y, Hachiya M, Ando K, Nakajima N, Akashi M. TNF-alpha induced p21(WAF1) but not Bax in colon cancer cells WiDr with mutated p53: important role of protein stabilization. Cytokine. 2000;12:1745–1754. doi: 10.1006/cyto.2000.0782. [DOI] [PubMed] [Google Scholar]
- Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000;278:C144–C153. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Martin D, Hallenbeck JM. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res. 1997a;778:265–271. doi: 10.1016/s0006-8993(97)00981-5. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997b;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Winkles JA, Yepes M. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappaB pathway activation. J Neurosci. 2005;25:10094–10100. doi: 10.1523/JNEUROSCI.3382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced asan immediate-early gene during seizure, kindling and long-termpotentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Qiu J, Takagi Y, Harada J, Rodrigues N, Moskowitz MA, Scadden DT, Cheng T. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199:937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian J, Iyirhiaro G, Aleyasin H, Rios M, Vincent I, Callaghan S, Bland RJ, Slack RS, During MJ, Park DS. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:14080–14085. doi: 10.1073/pnas.0500099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, Karp H, LaBresh KA, Malarcher A, Mensah G, Moomaw CJ, Schwamm L, Weiss P. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, De SG, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Michael HP, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, Roger VL, Turner MB. Executive summary: heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:459–463. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- Samson AL, Nevin ST, Croucher D, Niego B, Daniel PB, Weiss TW, Moreno E, Monard D, Lawrence DA, Medcalf RL. Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J Neurochem. 2008;107:1091–1101. doi: 10.1111/j.1471-4159.2008.05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL. Number needed to treat estimates incorporating effects overthe entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol. 2004;61:1066–1070. doi: 10.1001/archneur.61.7.1066. [DOI] [PubMed] [Google Scholar]
- Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Tomasevic G, Shamloo M, Israeli D, Wieloch T. Activation of p53 and its target genes p21(WAF1/Cip1) and PAG608/Wig-1 in ischemic preconditioning. Brain Res Mol Brain Res. 1999;70:304–313. doi: 10.1016/s0169-328x(99)00146-1. [DOI] [PubMed] [Google Scholar]
- van Lookeren CM, Gill R. Increased expression of cyclin G1 and p21WAF1/CIP1 in neurons followingtransient forebrain ischemia: comparison with early DNA damage. J Neurosci Res. 1998;53:279–296. doi: 10.1002/(SICI)1097-4547(19980801)53:3<279::AID-JNR2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Yepes M, Brown SA, Moore EG, Smith EP, Lawrence DA, Winkles JA. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am J Pathol. 2005;166:511–520. doi: 10.1016/S0002-9440(10)62273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]