Abstract
This study investigated the effects of acetylsalicylic acid (ASA) and clopidogrel, standardly used in the secondary prevention of vascular occlusions, on cerebral arteriogenesis in vivo and in vitro. Cerebral hypoperfusion was induced by three-vessel occlusion (3-VO) in rats, which subsequently received vehicle, ASA (6.34 mg/kg), or clopidogrel (10 mg/kg). Granulocyte colony-stimulating factor (G-CSF), which enhanced monocyte migration in an additional cell culture model, augmented cerebrovascular arteriogenesis in subgroups (40 μg/kg). Cerebrovascular reactivity and vessel diameters were assessed at 7 and 21 days. Cerebrovascular reserve capacity was completely abolished after 3-VO and remained severely compromised after 7 (−14±14%) and 21 (−5±11%) days in the ASA groups in comparison with controls (4±5% and 10±10%) and clopidogrel (4±13% and 10±8%). It was still significantly decreased when ASA was combined with G-CSF (1±4%) compared with G-CSF alone (20±8%). Posterior cerebral artery diameters confirmed these data. Monocyte migration into the vessel wall, improved by G-CSF, was significantly reduced by ASA. Acetylsalicylic acid, but not clopidogrel, inhibits therapeutically augmented cerebral arteriogenesis.
Keywords: arteriogenesis, brain ischemia, cerebral blood flow, cerebrovascular disease, platelet inhibitors, macrophages
Introduction
For the secondary prevention of vascular occlusive disease, antiplatelet therapy with either acetylsalicylic acid (ASA) or clopidogrel alone or a combination of both is recommended (Diener et al, 2007; Furie et al, 2011; Hirsch et al, 2006; Smith et al, 2006).
Clopidogrel, a thienopyridine derivative, inhibits platelet function by selective and irreversible binding to their ADP-dependent receptor P2Y12. Acetylsalicylic acid, in contrast, acts through an irreversible acetylation of cyclooxygenases, thereby inhibiting cyclooxygenase-1-mediated synthesis of thrombocytic thromboxane A2, which mediates platelet aggregation. Acetylsalicylic acid is used clinically for the treatment of acute ischemic events, such as myocardial infarction and thromboembolic stroke, for long-term antithrombotic treatment, and for its analgesic, antipyretic, and antiinflammatory properties (Chiang et al, 2004). Because of its antiinflammatory effects, ASA inhibits leukocyte–endothelial interactions, which, in turn, are among the initiating events of arteriogenesis, the growth of collateral vessels (Bergmann et al, 2006; Buschmann et al, 2001; Heil et al, 2002; Hoefer et al, 2005b).
After stenosis or occlusion of a conductance artery, hemodynamic factors, i.e., a flow-mediated increase in fluid shear stress acting on vascular endothelial cells, regulate the outgrowth of preexistent collateral arteries (Buschmann et al, 2010; Carmeliet 2000). During the subsequent endothelial cell activation and monocyte adhesion, attracted monocytes invade the vessel wall, cause a balanced proteolytic remodeling (Hillmeister et al, 2008), and aid SMC dedifferentiation to a proliferative phenotype. In contrast to other mechanisms of adaptive vascular growth, arteriogenesis might lead to a functionally significant restoration of blood flow to the ischemic area (Eitenmuller et al, 2006; Kolibash et al, 1982).
When adaptive arteriogenesis is not sufficient to prevent ischemic cellular damage, therapeutic stimulation through the exogenous application of promonocytic growth factors has yielded promising results in different vascular territories (Hoefer et al, 2005b).
The colony-stimulating factors (CSFs) granulocyte-CSF (G-CSF; Deindl et al, 2006) and granulocyte–macrophage-CSF (GM-CSF; Buschmann et al, 2003; Schneeloch et al, 2004) are among the most potent cytokines to augment the arteriogenic response, presumably through a stimulation of monocyte function and an increased cellular lifespan (Buschmann et al, 2001). Current clinical studies aim at avoiding potential pro-atherogenic side-effects (Schaper and Buschmann 1999). Granulocyte–macrophage colony-stimulating factor and G-CSF are among currently evaluated substances (Schirmer et al, 2009).
Acetylsalicylic acid severely interferes with the adaptive arteriogenic response in a rabbit hindlimb model (Hoefer et al, 2005a). This study was designed to evaluate the effects of ASA, as compared with clopidogrel, on (a) monocyte migration, (b) cerebral adaptive arteriogenesis, and (c) G-CSF-mediated, therapeutically augmented arteriogenesis.
Materials and methods
Ethics Statement
Animal study was in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines, and study permission was given by the Landesamt für Gesundheit und Soziales, Berlin, Germany (No. G 0316/05). The current study was performed in 252 male Sprague–Dawley rats (300 to 350 g, Harlan–Winkelmann, Borchen, Germany) in conformity with the German Law for the Protection of Animals and the National Institute of Health Guidelines for Care and Use of Laboratory Animals.
Hypoperfused Rat Brain Model
To induce cerebral hypoperfusion, rats underwent three-vessel (bilateral vertebral plus unilateral common carotid artery) occlusion (3-VO) as described in detail previously (Busch et al, 2003). Anesthesia was induced with 50 mg/kg ketamine (Ketavet, Pfizer, Berlin, Germany) and 4 mg/kg xylazine (Rompun 2%, Bayer HealthCare, Leverkusen, Germany) and maintained with 2% isoflurane in 70%/30% nitrous oxide/oxygen. After occlusion of both vertebral arteries by electrocoagulation, the left common carotid artery was ligated under constant laser Doppler flowmetry (LDF; Busch et al, 2003). To exclude confounding effects of the tested substances (ASA and clopidogrel) on the vasodilatory capacity of the cerebral vasculature (cerebral blood flow (CBF) measurements), animals of each group underwent unilateral occlusion of the left common carotid artery (n=6 each).
Groups and Treatment Protocol
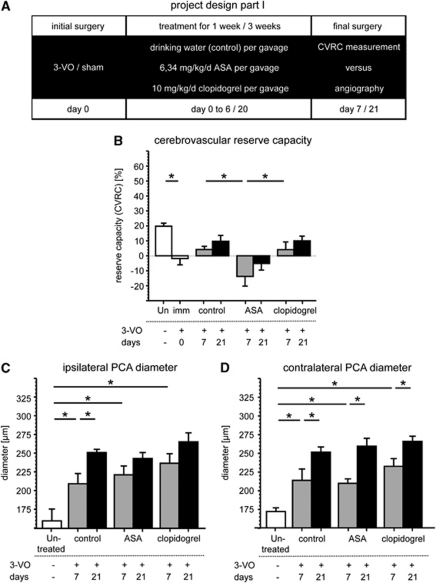
Animals were randomly assigned to the following groups: cerebrovascular hemodynamic reserve capacity (CVRC) measurement or postmortem angiography (n=6 each) was performed as baseline in ‘untreated' nonoperated rats. At day 0, either 3-VO or sham surgery was performed. Then, rats received either daily dosages of ASA, clopidogrel, or drinking water as control for 1 or 3 weeks. In all three cases in the 3-VO and sham groups, CVRC measurement or postmortem angiography was performed (n=6 each; Figure 2A).
In one control group, only to show the impaired reserve capacity immediately after 3-VO, CVRC measurement was performed ‘1 day after 3-VO' (n=6).
Dosage of ASA (6.34 mg/kg/day via gavage) was adjusted from a recommended human dosage to metabolic body weight, equaling 100 mg/day ASA for long-term antithrombotic prophylaxis in secondary stroke prevention (Chiang et al, 2004). In accordance with Hoefer et al (2005a), clopidogrel was used at a higher dose (10 mg/kg/day via gavage; Bristol–Myers Squibb) to exclude dose-dependent effects.
Project part II was completed with groups for the augmentation of cerebral arteriogenesis: the initial surgery (3-VO or sham) was followed by additional treatment with G-CSF and finalized with CVRC measurement or postmortem angiography after 1 week (n=6 each; Figure 3A).
For pharmacological augmentation of arteriogenesis, rats received G-CSF (Filgrastim; Sygnis, Heidelberg, Germany) at 40 μg/kg diebus alternis subcutaneously.
Cerebrovascular Hemodynamic Reserve Capacity
Cerebrovascular hemodynamic reserve capacity was calculated from CBF, measured by LDF, in six animals per group. In general anesthesia, left femoral vessels were catheterized for pre- and postprocedural arterial blood gas analysis and intravenous application of 30 mg/kg acetazolamide (Diamox, Sanofi Aventis, Frankfurt/Main, Germany), respectively. To monitor LDF, the probe was placed directly on the left temporal hemicranium. Recording of blood flow was started, acetazolamide was then injected, and LDF was observed for 6 minutes, when a plateau was reached. The relative blood flow changes were analyzed, and the mean of 4 minutes was calculated. When relative flow before stimulation is assumed as 100% normal blood flow, relative changes after acetazolamide injection were identified as CVRC.
Blood samples were taken before and after CVRC test for blood gas analysis.
Visualization of the Circle of Willis
To visualize cerebral angioarchitecture, the method of Maeda et al (1998) was adapted as described in detail elsewhere (Busch et al, 2003). In brief, in six animals of each group at 7 and 21 days after 3-VO, the ligated common carotid artery was cannulated in general anesthesia. Injection of a lethal dose of papaverine hydrochloride (50 mg/kg, Paveron, Linden, Heuchelheim, Germany) induced maximal vasodilation and was followed by pressure-controlled (150 mm Hg) perfusion with 5.5 to 6 mL/kg warm (37°C) latex milk (Chicago Latex Products, Crystal Lake, IL, USA) colorized with Indian ink. Animals were placed on ice for 15 minutes, brains were then removed, and fixed by immersion in RNAlater (Ambion, Austin, TX, USA). External posterior cerebral artery (PCA) diameters were measured at four different fixed points under a stereo-zoom microscope equipped with a calibrated eyepiece micrometer by volunteers in a masked manner. The mean of these four values per vessel was compared between all groups.
Cell Culture
Human monocytic THP-1 cells were purchased from DSMZ (German Resource Centre for Biological Material, Braunschweig, Germany) and cultivated in RPMI 1640 with 10% fetal calf serum, 2 mmol/L -glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (95% relative humidity and 5% CO2 at 37°C) as described (Kappert et al, 2008). Cells were rendered quiescent by serum starvation over night, followed by preincubation with 10 mg/mL ASA (30 min) or 5 μmol/L 2-MeSAMP (2-methylthio-AMP triethylammonium salt hydrate, antagonist for P2Y(AC) receptor), and were used in the following for migration assays. Experiments were performed in triplicates and were repeated at least three times.
Chemotaxis Assay
Chemotaxis experiments (toward either 100 ng/mL monocyte chemotactic protein-1 as positive control; 50 ng/mL GM-CSF; and 50 ng/mL G-CSF or 50 ng/mL M-CSF) were performed using transwell cell culture chambers with a gelatin-coated (0.2%) polycarbonate membrane (8 μm pores) as reported recently (Kappert et al, 2008). The number of monocytic THP-1 cells per high-power field (magnification × 320) that migrated after 4 hours to the lower surface of the filters was determined microscopically. Four randomly chosen high-power fields were counted per filter. Experiments were performed in triplicates and were repeated at least three times.
Histochemical Analysis
At 7 days after 3-VO, brains were removed for macrophage staining. Samples were fixed in 4% paraformaldehyde, embedded in paraffin, and cut in 5 μm sections. After heat-induced epitope retrieval (citrate buffer 10 mmol/L), the following antibodies were used for immunohistochemistry: mouse anti-rat CD 68 (AbD Serotec, Duesseldorf, Germany, MCA341), goat anti-mouse-Cy3 (Jackson Laboratories, West Grove, PA, USA, 115-165-166), and mouse anti-SMC–FITC (Sigma-Aldrich, St Louis, MO, USA, 1A4). The cell nucleus was identified by a Hoechst staining (Molecular Probes, Carlsbad, CA, USA, 33342). Three sections separated by 50 μm from three animals each were analyzed in a masked approach by three independent investigators. Within the area of interest, vessels were viewed, monocyte numbers were quantified, and representative sections obtained using an Olympus IX81 Microscope (Olympus Europa GmbH, Hamburg, Germany).
Data Analysis
All data are given as mean±s.d. Graphics are shown as mean±s.e.m.
Results obtained by measuring hemodynamic reserve capacity, vessel diameters, and monocyte migration were analyzed for statistical significance by using the SPSS 18 software package (PASW, IBM SPSS Statistics, NY, USA; analysis of variance with Bonferroni test as post hoc test). P values were adjusted using a false discovery rate procedure to achieve experiment-wide significance of P<0.05.
Results
Induction of Cerebral Arteriogenesis
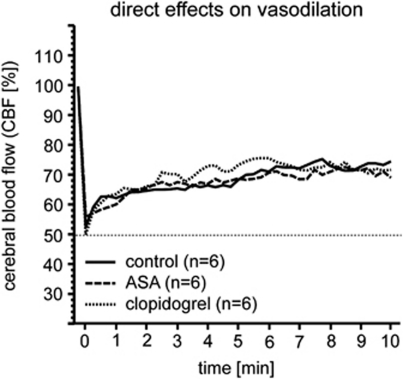
Induction of chronic nonlethal cerebral hypoperfusion by 3-VO (Busch et al, 2003) is mirrored by a persistent decrease in CBF to ∼50% (3-VO/control; n=12; data not shown). In contrast, occlusion of one common carotid artery (CCAO) is followed by a rapid recovery of CBF from 50% immediately after surgery to 70% to 75% within 10 minutes in all CCAO groups (CCAO/control, CCAO/ASA, CCAO/clopidogrel; n=6 each; Figure 1).
Figure 1.
Direct effects on vasodilation. Direct influence on vessel's ability to vasodilate was investigated after common carotid artery occlusion and no differences were found.
Immediately after 3-VO, no changes in external vessel diameter are observed (data not shown), as measurement at total vasorelaxation reflects anatomical vessel sizes and is not affected by vascular tone as described previously (Busch et al, 2003). At 7 and 21 days after surgery, increase in the vessel diameter of the PCA is notable in all the 3-VO groups (Figures 2C and 2D) in comparison with sham-operated controls (Table 1). Postmortem angiograms are demonstrated in Figure 3.
Figure 2.
Effects on adaptive cerebral arteriogenesis. Effects on adaptive cerebral arteriogenesis were observed 1 or 3 weeks after initial surgery (A). Reserve capacity is completely abolished 7 and 21 days after three-vessel occlusion by acetylsalicylic acid (B;*nominal P values <0.019). No differences in posterior cerebral artery diameter on ipsilateral (C; *nominal P values <0.017) or contralateral side (D; *nominal P values <0.025) can be detected between groups.
Table 1. Data sheet—effects on adaptive and therapeutically induced cerebral arteriogenesis.
|
Postmortem latex angiography—diameter |
Reserve capacity cerebral blood flow (%) | |||||
|---|---|---|---|---|---|---|
| PCA ipsi (μm) | PCA contra (μm) | PCA ipsi (μm) | PCA contra (μm) | |||
| Untreated |
160±39 |
172±12 |
160±39 |
172±12 |
120±5 |
|
| 3-VO | Sham | 3-VO | Sham | |||
| Immediately | — | — | — | — | 98±8* | — |
| 7-Day control | 210±34*∥# | 214±38*∥# | 146±12 | 142±9 | 104±5§# | 112±16 |
| 7-Day ASA | 222±29*∥# | 210±15*∥# | 150±25 | 178±23‡**†† | 86±14‡∥# | 105±8 |
| 7-Day clopidogrel | 238±29*∥ | 233±23*∥ | 138±13 | 174±15 | 104±13§# | 99±6*# |
| 21-Day control | 252±11†∥ | 252±17†∥ | 132±17 | 146±28 | 110±10 | 124±16 |
| 21-Day ASA | 244±20∥ | 260±26†∥ | 139±14 | 156±17 | 95±11∥ | 111±8 |
| 21-Day clopidogrel | 266±27∥ | 266±16†∥ | 170±30 | 152±34 | 110±8 | 117±12 |
| 7-Day G-CSF | 264±54‡§∥ | 248±27‡§∥** | 160±24 | 144±11§ | 120±8‡§** | 117±20 |
| 7-Day ASA and G-CSF | 230±13∥ | 209±14∥# | 156±35 | 130±18§ | 101±4# | 106±5 |
| 7-Day clopidogrel and G-CSF | 245±38∥ | 226±38∥ | 146±33 | 154±19 | 117±20** | 105±5 |
ASA, acetylsalicylic acid; contra, contralateral; G-CSF, granulocyte colony-stimulating factor; ipsi, ipsilateral; PCA, posterior cerebral artery; 3-VO, three-vessel occlusion.
Values are means±s.d., n=6; *P⩽0.05=immediately or 7-day control/ASA/clopidogrel compared with untreated; †P⩽0.05=21-day control/ASA/clopidogrel compared with 7-day control/ASA/clopidogrel; ‡P⩽0.05=7 or 21 days compared with control; §P⩽0.05=7 or 21 days compared with ASA; ∥P⩽0.05=3-VO compared with sham; #P⩽0.05=7 days compared with G-CSF; and **P⩽0.05=7-day ASA/G-CSF/clopidogrel and G-CSF compared with ASA and G-CSF.
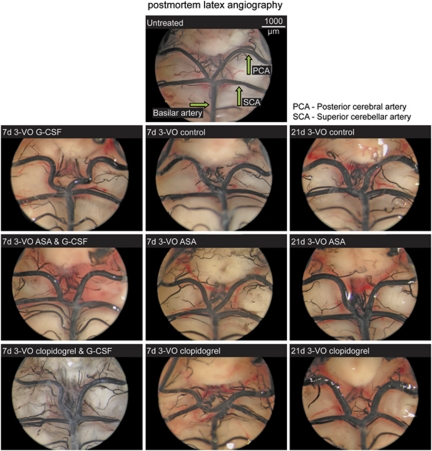
Figure 3.
Postmortem latex angiography. Pictures show growth of the posterior cerebral artery diameter by granulocyte colony-stimulating factor and their inhibition by acetylsalicylic acid.
Acetylsalicylic Acid Treatment Suppresses Recovery of Hemodynamic Reserve Capacity
Acute 3-VO completely abolishes CVRC (−2±8%) as measured by LDF after acetazolamide-induced arteriolar dilation (Figure 2B and Table 1). Hemodynamic reserve capacity slowly recovers over 3 weeks after surgery, to 4±5% (day 7) and 10±10% (day 21). Clopidogrel treatment does not influence CBF recovery (4±13% (day 7) and 10±8% (day 21)). Acetylsalicylic acid, in contrast, significantly interferes with CBF recovery, which reaches only −14±14% at day 7 and −5±11% at day 21, respectively.
Acetylsalicylic Acid Inhibits Cerebrovascular Blood Flow Recovery and Collateral Growth During Granulocyte Colony-Stimulating Factor-Stimulated Cerebral Arteriogenesis
Next, effects of ASA and clopidogrel on therapeutic cerebral arteriogenesis (stimulated by G-CSF treatment) were compared with control groups 7 days after 3-VO. Administration of G-CSF for 7 days after 3-VO surgery enhances cerebral arteriogenesis. Morphologically, PCA diameters increase to 264±54 μm/248±27 μm on ipsilateral/contralateral side in comparison with sham/G-CSF and to 210±34 μm/214±38 μm in 3-VO/controls (Figures 4C and 4D and Table 1). Functionally, CVRC is restored to near-normal values (20±8% in 3-VO/G-CSF versus 4±5% in 3-VO/control; Figure 4B and Table 1).
Figure 4.
Effects on therapeutically augmented cerebral arteriogenesis. Effects on therapeutically augmented cerebral arteriogenesis were analyzed 1 week after three-vessel occlusion or sham (A). For induction of arteriogenesis, additional granulocyte colony-stimulating factor (G-CSF) was given every other day. Reserve capacity is improved by combined treatment with acetylsalicylic acid (ASA) and G-CSF compared with ASA alone, but still significantly reduced compared with G-CSF alone (B; *nominal P values <0.030). Posterior cerebral artery diameters on ipsilateral side (C; *nominal P values <0.018) are smaller after treatment with ASA alone. Diameters on contralateral side (D; *nominal P values <0.021) are smaller after treatment combined with ASA and G-CSF compared with treatment with G-CSF alone.
Acetylsalicylic acid, in contrast to clopidogrel, interferes significantly with the G-CSF-augmented restoration of CVRC (20±8% in 3-VO/G-CSF versus 1±4% in 3-VO/ASA/G-CSF).
Accordingly, the increase in contralateral PCA diameter in G-CSF-treated 3-VO animals is significantly attenuated by additional ASA administration (248±27 μm in 3-VO/G-CSF versus 209±14 μm in 3-VO/ASA/G-CSF). For clopidogrel, no significant attenuation is detectable.
Acetylsalicylic Acid Disturbs the Growth-Factor-Mediated Stimulation of Monocyte Migration
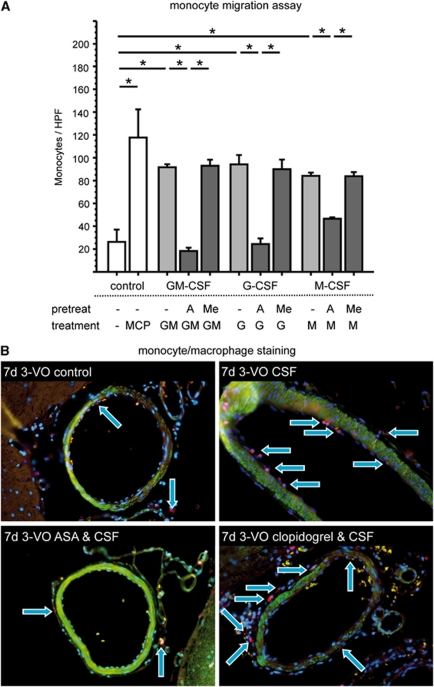
To examine whether ASA-mediated inhibition of therapeutically induced arteriogenesis is caused by inhibition of monocytic cell function, the impact of ASA on monocyte migration into the perivascular tissue of growing collaterals in the rat brain was tested. Therefore, 3-VO surgery and staining for CD68-positive cells was performed on sections taken from the brain collateral region up to 7 days postoperatively (Figure 5B). Herein, we identified that rats treated over time with G-CSF alone or clopidogrel combined with G-CSF show a noticeable invasion of macrophages into the perivascular tissue around collateral arteries. However, treatment with ASA alone or ASA combined with G-CSF led to considerably lower numbers of monocytes in the perivascular region. Furthermore, we performed a monocyte chemotaxis assay, in which the effect of ASA and the PY2 antagonist 2-MeSAMP on monocyte migration was examined in vitro. GM-CSF, G-CSF, and M-CSF (all 50 ng/mL) significantly induce migration of human monocytic THP-1 cells through gelatine-coated filters (all P<0.05 versus control), comparable to monocyte chemotactic protein-1 (100 ng/mL), used as standard (Kappert et al, 2008). Pretreatment of cells with ASA (10 mg/mL; 30 min.) strongly inhibits GM-CSF-, G-CSF-, and M-CSF-induced migration, whereas 2-MeSAMP (5 μmol/L) has no effect (Figure 5A and Table 2).
Figure 5.
Monocyte migration. Migration of human monocytic THP-1 cells is significantly improved by granulocyte colony-stimulating factor (G-CSF; G), granulocyte–macrophage-CSF (GM), and macrophage-CSF (M), but inhibited after pretreatment with acetylsalicylic acid (ASA; A). 2-methylthio-AMP triethylammonium salt hydrate (Me) has neutral effects (*nominal P values <0.035; A). After CD68 staining, more monocytes/macrophages can be detected after treatment with G-CSF compared with controls. Combined treatment with ASA and G-CSF reveals a lack in monocyte/macrophage findings, whereas after treatment with clopidogrel and G-CSF, count of cells are equal compared with G-CSF alone (B; blue: nuclei, green: smooth muscle cells, and red: monocytes/macrophages).
Table 2. Effects on monocyte migration.
| Control | GM-CSF | G-CSF | M-CSF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | — | — | — | A | Me | — | A | Me | — | A | Me |
| Treatment | — | MCP | GM | GM | GM | G | G | G | M | M | M |
| Monocytes per HPF | 26±18 | 118±43* | 92±5*† | 18±5 | 93±9† | 94±14*‡ | 24±8 | 90±15‡ | 84±5*§ | 47±0 | 84±6§ |
A, acetylsalicylic acid; GM-CSF, granulocyte–macrophage colony-stimulating factor; HPF, high-power field; MCP, monocyte chemoattractant protein; Me, 2-MeSAMP.
Values are means±s.d., n=3.
*P⩽0.05=compared with control.
†P⩽0.05=compared with ASA+GM-CSF.
‡P⩽0.05=compared with ASA+G-CSF.
§P⩽0.05=compared with ASA+M-CSF.
Discussion
This study investigated the differential effects of ASA and clopidogrel on adaptive and therapeutically augmented cerebral arteriogenesis.
Surgical 3-VO induces a nonlethal cerebral hypoperfusion in our rat model of cerebral collateral growth, causing a subsequent enhancement in diameter of the PCA and a restoration of hemodynamic reserve capacity over a period of ∼3 weeks as described in detail in previous studies from our group (Busch et al, 2003; Buschmann et al, 2003; Hillmeister et al, 2008; Schneeloch et al, 2004).
Treatment with ASA during the recovery period significantly interferes with the restoration of hemodynamic reserve capacity, which is usually brought about by adaptive collateral growth in the 3-VO model (Busch et al, 2003). For clopidogrel, a comparable effect is not demonstrable. These results are in line with those of Hoefer et al (2005a), who, for the rabbit hindlimb, demonstrated that ASA inhibits the postocclusive restoration of collateral conductance in a rabbit model of peripheral artery occlusive disease (PAOD), whereas vessel diameters remained unchanged. These data might be because of the fact that according to the law of Hagen and Poiseuille, even insignificant changes in vessel diameter, which determine the flow through a given vessel to the fourth power, might bring about significant changes in perfusion and thus in hemodynamic reserve capacity (Buschmann and Schaper 2000).
To exclude a mere effect on ASA on the vasodilatory capacity of the cerebral vasculature during CBF measurements, we assessed ASA and clopidogrel effects in a CCAO model, wherein no differences in vessel responsiveness were detectable.
Granulocyte colony-stimulating factor is a potent cytokine that stimulates the production and release of bone marrow-derived granulocytes and stem cells. It has been shown to promote collateral artery growth, presumably through the intercellular adhesion molecule-1-mediated attraction of cytokine-supplying leukocytes, a mechanism accounting for its beneficial long-term effects in patients with ischemic cardiomyopathy (Deindl et al, 2006). In this study, G-CSF was shown to significantly enhance the migratory function of monocytes, a key event in the induction and maintenance of collateral artery growth (Bergmann et al, 2006; Heil et al, 2002). Therefore, we used G-CSF in the 3-VO model to therapeutically enhance arteriogenesis. Granulocyte colony-stimulating factor treatment significantly augments cerebral collateral growth, as demonstrated by an enhanced outgrowth of the PCA, which leads to an augmented restoration of cerebrovascular reserve capacity in animals that were treated with G-CSF after 3-VO surgery.
In this setting, the effect of ASA was even more pronounced: not only does it impede the recovery of cerebrovascular reserve capacity, but also, in addition, significantly interferes with the morphologically detectable growth of the PCA, which, in the 3-VO model, is attributable to blood flow redistribution in the circle of Willis (Busch et al, 2003).
Supporting evidence was generated in vivo and in vitro, where ASA inhibits the migratory capacity of monocytic cells, cellular key mediators of arteriogenesis, in the presence as well as absence of G-CSF. These data might, in part, explain the results of Hoefer et al (2005a), who showed an ASA-mediated decrease of monocyte accumulation and proliferation in the perivascular tissues of recruited collateral arteries outside the ischemic area at risk.
First, an ASA-mediated inhibition of monocyte chemotaxis and adherence to the endothelium (Hascelik et al, 1994; Krumholz et al, 2000) have been attributed to its antiinflammatory effects. Acetylation of cyclooxygenase-2 by ASA generates aspirin-triggered epi-lipoxins (Chiang et al, 2004), which, in turn, might interfere with leukocyte-endothelial interactions via an nitrous-oxide-mediated pathway (Paul-Clark et al, 2004).
Second, a nitrous-oxide-independent ASA-mediated inhibition of leukocyte rolling and adhesion have been described (Fricchione et al, 1998). In line with these results, the role of nitrous oxide for arteriogenic vascular growth has yet to be elucidated and might be context specific, as conflicting results have been generated so far (Dai and Faber 2010; Mees et al, 2007; Troidl et al, 2010).
Third, Eisele et al (2004) and Mehta et al (2004) demonstrated an ASA-mediated downregulation of the endothelial adhesion molecules intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and the cytokine monocyte chemoattractant protein-1, whose crucial role for arteriogenesis has repeatedly been demonstrated (Hoefer et al, 2004; Voskuil et al, 2004).
Last, ASA seems to interfere with the Jak/STAT pathway activation (Kim et al, 2009), which is caused by the pro-arteriogenic cytokines G-CSF and GM-CSF (Marino and Roguin 2008; Valdembri et al, 2002), respectively, so as to counteract their neuroprotective effects.
In conclusion, we have demonstrated that ASA, in contrast to clopidogrel, interferes with adaptive as well as therapeutically induced arteriogenesis in the brain. These data might be of great clinical relevance, given the high numbers of patients taking ASA on a regular basis for the secondary prevention of atherosclerotic events. Current guidelines unequivocally recommend ASA use, warning only of gastrointestinal bleeding as a potential side-effect. A special up-to-the-minute relevance exists for diabetic patients, for whom current guidelines from the American Heart Association recommend ASA for primary prevention (Pignone et al, 2010), whereas diabetes itself severely compromises the arteriogenic capacity. Recent clinical alerts and the differences in the cost of care between ASA and clopidogrel have been generating further uncertainty among clinicians. Therefore, additional experimental studies to rapidly verify our data and extend their impact from bench to bedside are critical and might lead to a reconsideration of the current recommendations for the use of the antithrombotic agents ASA and clopidogrel, respectively.
Acknowledgments
We thank Mrs V Furundzija for expert technical assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by Art.Net., the Deutsche Forschungsgemeinschaft, Germany (Grant DFG BU1141/4-2) and the Volkswagen Foundation, Germany.
References
- Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab. 2003;23:621–628. doi: 10.1097/01.WCB.0000057741.00152.E4. [DOI] [PubMed] [Google Scholar]
- Buschmann I, Pries A, Styp-Rekowska B, Hillmeister P, Loufrani L, Henrion D, Shi Y, Duelsner A, Hoefer I, Gatzke N, Wang H, Lehmann K, Ulm L, Ritter Z, Hauff P, Hlushchuk R, Djonov V, van Veen T, le Noble F. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137:2187–2196. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis) J Pathol. 2000;190:338–342. doi: 10.1002/(SICI)1096-9896(200002)190:3<338::AID-PATH594>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- Buschmann IR, Hoefer IE, van Royen N, Katzer E, Braun-Dulleaus R, Heil M, Kostin S, Bode C, Schaper W. GM-CSF: a strong arteriogenic factor acting by amplification of monocyte function. Atherosclerosis. 2001;159:343–356. doi: 10.1016/s0021-9150(01)00637-2. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. 2010;106:1870–1881. doi: 10.1161/CIRCRESAHA.109.212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller-Hoecker J, Franz WM. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. Faseb J. 2006;20:956–958. doi: 10.1096/fj.05-4763fje. [DOI] [PubMed] [Google Scholar]
- Diener HC, Allenberg JR, Bode C, Busse O, Forsting M, Grau AJ, Hennerici M, Grond M, Haberl RL, Hamann GF, Ringelstein EB, Ringleb PA. Recommendations of the German Neurological Society and the German Stroke Society for Primary and Secondary Stroke Prevention: update 2007. Akt Neurol, Georg Thieme Verlag KG Stuttgart New York. 2007;34:8–12. [Google Scholar]
- Eisele G, Schwedhelm E, Schieffer B, Tsikas D, Boger RH. Acetylsalicylic acid inhibits monocyte adhesion to endothelial cells by an antioxidative mechanism. J Cardiovasc Pharmacol. 2004;43:514–521. doi: 10.1097/00005344-200404000-00006. [DOI] [PubMed] [Google Scholar]
- Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- Fricchione GL, Bilfinger TV, Stefano GB. Aspirin inhibits granulocyte and monocyte adherence to saphenous vein endothelia in a process not mediated by nitric oxide. Int J Cardiol. 1998;64 (Suppl 1:S29–233. doi: 10.1016/s0167-5273(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. A Guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- Hascelik G, Sener B, Hascelik Z. Effect of some anti-inflammatory drugs on human neutrophil chemotaxis. J Int Med Res. 1994;22:100–106. doi: 10.1177/030006059402200206. [DOI] [PubMed] [Google Scholar]
- Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- Hillmeister P, Lehmann KE, Bondke A, Witt H, Duelsner A, Gruber C, Busch HJ, Jankowski J, Ruiz-Noppinger P, Hossmann KA, Buschmann IR. Induction of cerebral arteriogenesis leads to early-phase expression of protease inhibitors in growing collaterals of the brain. J Cereb Blood Flow Metab. 2008;28:1811–1823. doi: 10.1038/jcbfm.2008.69. [DOI] [PubMed] [Google Scholar]
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- Hoefer IE, Grundmann S, Schirmer S, van Royen N, Meder B, Bode C, Piek JJ, Buschmann IR. Aspirin, but not clopidogrel, reduces collateral conductance in a rabbit model of femoral artery occlusion. J Am Coll Cardiol. 2005a;46:994–1001. doi: 10.1016/j.jacc.2005.02.094. [DOI] [PubMed] [Google Scholar]
- Hoefer IE, Grundmann S, van Royen N, Voskuil M, Schirmer SH, Ulusans S, Bode C, Buschmann IR, Piek JJ. Leukocyte subpopulations and arteriogenesis: specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis. 2005b;181:285–293. doi: 10.1016/j.atherosclerosis.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res. 2004;94:1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- Kappert K, Meyborg H, Clemenz M, Graf K, Fleck E, Kintscher U, Stawowy P. Insulin facilitates monocyte migration: a possible link to tissue inflammation in insulin-resistance. Biochem Biophys Res Commun. 2008;365:503–508. doi: 10.1016/j.bbrc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kim SR, Bae MK, Kim JY, Wee HJ, Yoo MA, Bae SK. Aspirin induces apoptosis through the blockade of IL-6-STAT3 signaling pathway in human glioblastoma A172 cells. Biochem Biophys Res Commun. 2009;387:342–347. doi: 10.1016/j.bbrc.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Kolibash AJ, Bush CA, Wepsic RA, Schroeder DP, Tetalman MR, Lewis RP. Coronary collateral vessels: spectrum of physiologic capabilities with respect to providing rest and stress myocardial perfusion, maintenance of left ventricular function and protection against infarction. Am J Cardiol. 1982;50:230–238. doi: 10.1016/0002-9149(82)90171-0. [DOI] [PubMed] [Google Scholar]
- Krumholz W, Szalay G, Ogal H, Menges T. [Effect of migraine medications on monocyte chemotaxis] Anaesthesiol Reanim. 2000;25:102–104. [PubMed] [Google Scholar]
- Maeda K, Hata R, Hossmann KA. Differences in the cerebrovascular anatomy of C57black/6 and SV129 mice. Neuroreport. 1998;9:1317–1319. doi: 10.1097/00001756-199805110-00012. [DOI] [PubMed] [Google Scholar]
- Marino VJ, Roguin LP. The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J Cell Biochem. 2008;103:1512–1523. doi: 10.1002/jcb.21542. [DOI] [PubMed] [Google Scholar]
- Mees B, Wagner S, Ninci E, Tribulova S, Martin S, van Haperen R, Kostin S, Heil M, de Crom R, Schaper W. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1926–1933. doi: 10.1161/ATVBAHA.107.145375. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Chen J, Yu F, Li DY. Aspirin inhibits ox-LDL-mediated LOX-1 expression and metalloproteinase-1 in human coronary endothelial cells. Cardiovasc Res. 2004;64:243–249. doi: 10.1016/j.cardiores.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, Rosenson RS, Williams CD, Wilson PW, Kirkman MS. Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol. 2010;55:2878–2886. doi: 10.1016/j.jacc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Schaper W, Buschmann I. Arteriogenesis, the good and bad of it. Eur Heart J. 1999;20:1297–1299. doi: 10.1053/euhj.1999.1686. [DOI] [PubMed] [Google Scholar]
- Schirmer SH, van Nooijen FC, Piek JJ, van Royen N. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart. 2009;95:191–197. doi: 10.1136/hrt.2007.136119. [DOI] [PubMed] [Google Scholar]
- Schneeloch E, Mies G, Busch HJ, Buschmann IR, Hossmann KA. Granulocyte-macrophage colony-stimulating factor-induced arteriogenesis reduces energy failure in hemodynamic stroke. Proc Natl Acad Sci USA. 2004;101:12730–12735. doi: 10.1073/pnas.0404880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- Troidl K, Tribulova S, Cai WJ, Ruding I, Apfelbeck H, Schierling W, Troidl C, Schmitz-Rixen T, Schaper W. Effects of endogenous nitric oxide and of DETA NONOate in arteriogenesis. J Cardiovasc Pharmacol. 2010;55:153–160. doi: 10.1097/FJC.0b013e3181c9556f. [DOI] [PubMed] [Google Scholar]
- Valdembri D, Serini G, Vacca A, Ribatti D, Bussolino F. In vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSF. Faseb J. 2002;16:225–227. doi: 10.1096/fj.01-0633fje. [DOI] [PubMed] [Google Scholar]
- Voskuil M, Hoefer IE, van Royen N, Hua J, de Graaf S, Bode C, Buschmann IR, Piek JJ. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9:287–292. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]