Abstract
[11C]PHNO is a D2/D3 agonist positron emission tomography radiotracer, with higher in vivo affinity for D3 than for D2 receptors. As [11C]-(+)-PHNO is an agonist, its in vivo binding is expected to be more affected by acute fluctuations in synaptic dopamine than that of antagonist radiotracers such as [11C]raclopride. In this study, the authors compared the effects of an oral dose of the dopamine releaser amphetamine (0.3 mg/kg) on in vivo binding of [11C]-(+)-PHNO and [11C]raclopride in healthy subjects, using a within-subjects, counterbalanced, open-label design. In the dorsal striatum, where the density of D3 receptors is negligible and both tracers predominantly bind to D2 receptors, the reduction of [11C]-(+)-PHNO binding potential (BPND) was 1.5 times larger than that of [11C]raclopride. The gain in sensitivity associated with the agonist [11C]-(+)-PHNO implies that ∼65% of D2 receptors are in the high-affinity state in vivo. In extrastriatal regions, where [11C]-(+)-PHNO predominantly binds to D3 receptors, the amphetamine effect on [11C]-(+)-PHNO BPND was even larger, consistent with the higher affinity of dopamine for D3. This study indicates that [11C]-(+)-PHNO is superior to [11C]raclopride for studying acute fluctuations in synaptic dopamine in the human striatum. [11C]-(+)-PHNO also enables measurement of synaptic dopamine in D3 regions.
Keywords: brain imaging, dopamine, neurochemistry, positron emission tomography, receptor imaging
Introduction
Amphetamine-induced reduction in the specific binding of positron emission tomography (PET) and single photon emission computed tomography antagonist ligands such as [11C]raclopride or [123I]IBZM has been well validated as a measure of change in endogenous dopamine in both nonhuman primates and humans (Laruelle, 2000). A dose-dependent relationship between amphetamine dose and changes in ligand binding (Breier et al, 1997; Hartvig et al, 1997; Laruelle et al, 1997), as well as a consistent relationship between the concentration of extracellular dopamine change and the change in radioligand binding (Breier et al, 1997; Laruelle et al, 1997) have been demonstrated. Peak extracellular dopamine concentration increases of ∼40% were required for each percentage decrease in [123I]IBZM binding potential (BP) using amphetamine doses between 0.3 and 1.0 mg/kg (Laruelle et al, 1997). For [11C]raclopride, the corresponding figures were ∼50% increase in extracellular dopamine concentration for each percentage decrease in BP, using amphetamine doses of 0.2 to 0.4 mg/kg. These results show the limited sensitivity of this technique; large increases in extracellular dopamine are associated with relatively small effects on [11C]raclopride or [123I]IBZM binding.
This low sensitivity may be attributed to the fact that the D2 receptor is a member of the G-protein-coupled receptor family, and as such shows a low affinity for agonists, such as dopamine, when not coupled to G proteins (i.e., in a D2low state) (Creese et al, 1978, 1979; Sibley et al, 1982; Zahniser and Molinoff, 1978). Antagonists such as raclopride and IBZM are not expected to show a decreased affinity for the D2low state of the receptor compared with the G protein-coupled (or the D2high) state. Dopamine would have limited facility to compete effectively with antagonist ligands [11C]raclopride or [123I]IBZM at D2low sites. This hypothesis is consistent with the finding of a ∼1.4-fold greater change in the binding of the agonist radioligand [11C]-N-propyl-norapomorphine ([11C]NPA) than that of [11C]raclopride, after an amphetamine challenge in the anaesthetised baboon (Narendran et al, 2004). A similar result was obtained in awake humans, in whom the ratio of reduction in BP (ΔBPNDNPA/ΔBPNDRAC) after amphetamine challenge was between 1.5 and 1.9 (Narendran et al, 2010). However, not all studies support the notion that agonist ligands have differential binding to antagonist ligands. [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol([11C]-(+)-PHNO) and [3H]raclopride-specific binding ratios were inhibited to the same degree in response to amphetamine challenge in an ex vivo study in conscious rats (McCormick et al, 2008). In addition, in anaesthetised cynomologous monkeys, the D2/3 agonist apomorphine inhibited specific binding of the agonist ligand [11C]MNPA to a similar extent to [11C]raclopride (Finnema et al, 2009).
[11C]-(+)-PHNO was originally developed as an agonist ligand for imaging the D2high receptor (Wilson et al, 2005). Subsequent examination showed that [11C]-(+)-PHNO has preferential affinity for the D3 over the D2 receptor (Narendran et al, 2006; Rabiner et al, 2009; Searle et al, 2010). The endogenous agonist dopamine also has a greater affinity for the D3 receptor than for the D2 in vitro (Schotte et al, 1992, 1996), with selectivity estimated as ∼20-fold for the D3 receptor (Sokoloff et al, 1992). Thus, (+)-PHNO may have a higher sensitivity to the effects of synaptic dopamine fluctuations because of its selectivity for the D3 receptor, as well as its agonist profile. Comparisons of [11C]-(+)-PHNO with [11C]raclopride in anaesthetised animals found that after an amphetamine challenge, ΔBPNDPHNO/ΔBPNDRAC is ∼1.5 (Ginovart et al, 2006; Narendran et al, 2006). A human study examining the effects of amphetamine on [11C]-(+)-PHNO BP reported significant reductions in striatal regions of interest (ROIs) (ventral striatum (VST) 24.9%, caudate 13.2%, putamen 20.8%) (Willeit et al, 2008). These figures are larger than those reported in [11C]raclopride studies using similar doses of amphetamine, which generally lie between 5% and 15% (Drevets et al, 2001; Leyton et al, 2002; Munro et al, 2006; Oswald et al, 2005).
An additional factor to explain the increased sensitivity of [11C]-(+)-PHNO and [11C]NPA to the effects of an amphetamine challenge in animal studies may be the effects of anaesthesia. The volatile anaesthetic isoflurane was shown to significantly increase the effect of amphetamine on the specific binding of the agonist [11C]-(+)-PHNO, but not of the antagonist [3H]-raclopride in rats (McCormick et al, 2008). In a follow-up study by the same group, the effect of amphetamine on the binding of the agonists (+)-PHNO and NPA was greater than that on the binding of the antagonist raclopride, in rats anaesthetised with isoflurane, but of similar magnitude in conscious rats (McCormick et al, 2011). Although these data implicate the effects of anaesthesia in the increased sensitivity of agonist radioligands, we do not believe them to be a major factor, because the increased sensitivities of both [11C]-(+)-PHNO and [11C]NPA have been shown in awake humans (Narendran et al, 2010; Willeit et al, 2008).
Finally, it is possible that increased sensitivity of the agonist ligands is a consequence of receptor internalisation on their affinity. It has been proposed that D2/3 dopamine receptor internalisation could have a role in the change of the observed PET signal, rather than solely the direct blockade of the receptor by dopamine (Laruelle, 2000). The intracellular environment has lower pH and sodium ion concentration than does the extracellular environment, and the affinity of benzamides (such as raclopride and IBZM) for D2 receptors is reduced by decreasing pH and sodium ion concentration. The effect of receptor internalisation in decreasing the affinity of radioligands for the D2 receptor has been shown in a cell line expressing D2 receptors (Guo et al, 2010). This theory is supported by the finding that after amphetamine, the time course of the changes in binding of PET ligands is considerably longer than that of the increase in extracellular dopamine levels as measured by microdialysis (Houston et al, 2004; Laruelle et al, 1997). The discrepancy in the time course of the two measurements may be explained by dopamine-induced receptor internalisation. An elegant experiment showed a significantly faster recovery of baseline [11C]MNPA binding levels after amphetamine in arrestin3 knockout mice (unable to internalise the D2 receptor) than in wild-type controls, although the magnitude of the reduction was similar (Skinbjerg et al, 2010). If sensitivity to the ionic environment for affinity for agonist ligands, such as [11C]-(+)-PHNO and [11C]NPA, is greater in magnitude than that for the antagonists, greater changes in binding after an amphetamine challenge may be observed. This theory is not supported by the evidence from assessments of a cell line expressing D2 receptors, in which the decrease in affinity for internalised receptors was of similar magnitude for both agonists and antagonists (Guo et al, 2010).
The aim of our study was to compare the sensitivity of [11C]-(+)-PHNO to changes in endogenous dopamine with that of [11C]raclopride, using a within-subject design. We used amphetamine, the best-validated tool available, to induce an acute increase in extracellular dopamine levels. If [11C]-(+)-PHNO can be shown to be more sensitive to dopamine fluctuations than [11C]raclopride, it can be used to detect smaller changes in endogenous dopamine than currently possible. [11C]-(+)-PHNO could then allow for examination of the effects of challenges such as nicotine or bupropion, which increase dopamine by smaller amounts than amphetamine, and for which the use of [11C]raclopride is not practical. As [11C]-(+)-PHNO enables the examination of extrastriatal D2 and D3 receptors, its use will also allow the examination of dopamine fluctuations in extrastriatal brain regions, such as the substantia nigra and the hypothalamus.
Materials and methods
Human Subjects
This study was approved by the Essex 1 Research Ethics Committee and the ARSAC (Administration of Radioactive Substances Advisory Committee) and was conducted at the GlaxoSmithKline Clinical Imaging Centre (Hammersmith Hospital, London, UK).
This was an open-label study in healthy male volunteers aged 25 to 55 years, who were free from clinically significant illness or disease as determined by their medical history and standard laboratory tests. Each subject received four PET scans, two with [11C]-(+)-PHNO and two with [11C]-raclopride in a counterbalanced order. For each ligand, a baseline scan was followed by a second postamphetamine scan, in which 3 hours before the start of the scan an oral dose of 0.3 mg/kg of amphetamine was administered on an empty stomach. Venous blood samples were collected to measure amphetamine levels at the start and at the end of scan 2 (3 and 4.5 hours after dosing). The two administrations of amphetamine were separated by a minimum of 6 days. A total of 13 healthy volunteers were enrolled into the study, 10 of whom completed 4 PET scans and received 2 doses of amphetamine. A further three subjects received only two [11C]-(+)-PHNO scans and one dose of amphetamine each (two of these dropped out of the study, and the third was withdrawn because of an incidental finding).
During the course of the study, emerging data suggested a very low Kd for [11C]-(+)-PHNO at the D3 receptor in vivo (KdND=0.2 to 0.6 nmol/L and an estimated ED50 of 0.03 ng/kg (Gallezot et al, 2009)). This raised concerns about the possibility of (+)-PHNO occupying a significant proportion of the D3 receptor, and a consequent ‘carry-over' effect from the first to the second scan if conducted on the same day. Such a ‘carry over' could potentially bias our results towards recording a greater [11C]-(+)-PHNO reduction than can be attributed to dopamine effects. As a precaution, we changed the study design, and subsequent subjects received their second [11C]-(+)-PHNO scans with a separation of at least 24 hours from their baseline scan. Subjects 1 to 6 received both [11C]-(+)-PHNO scans on the same day (‘same-day subjects'), whereas subjects 7 to 13 received their [11C]-(+)-PHNO scans on separate days (‘different-day subjects').
Positron Emission Tomography Data Acquisition
In total, 46 PET scans were acquired on a Siemens Biograph HiRez XVI PET scanner (Siemens Healthcare, Erlangen, Germany). A low-dose computed tomography scan (effective dose=0.2 mSv) was acquired for attenuation and model-based scatter correction. Subjects were injected with a single intravenous bolus of [11C]-(+)-PHNO or [11C]raclopride. [11C]-(+)-PHNO was prepared from N-despropyl PHNO, (+)-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol, hydrochloride, by [11C]propionyl chloride acylation, followed by reduction of the [11C]amide intermediate (Wilson et al, 2005, with minor modifications). The reaction, purification, and formulation steps were fully automated to reliably produce high-purity [11C]-(+)-PHNO in a radiochemical decay-corrected yield of 16%±5% after a synthesis time of 35 minutes. [11C]raclopride was prepared as described previously (Ehrin et al, 1987).
After bolus administration of the radioligand, dynamic emission data were acquired continuously for 90 minutes. The dynamic images were subsequently reconstructed, using a filtered back-projection algorithm (direct inversion Fourier transform) with a 128 matrix, a zoom of 2.6, a transaxial Gaussian filter of 5 mm, into 26 frames (8 × 15 seconds, 3 × 1, 5 × 2, 5 × 5, and 5 × 10 minutes).
Magnetic Resonance Imaging Data Acquisition
High-resolution T1-weighted magnetic resonance images were acquired to aid in the definition of the anatomic ROIs using a Siemens Tim Trio, 3-T magnetic resonance imaging scanner at the GSK Clinical Imaging Centre. The structural T1-weighted images were acquired with an inversion recovery prepared spoiled gradient echo sequence with repetition time=3,000 milliseconds, echo time=3.66 milliseconds, flip angle=9°, inversion time=1,100 milliseconds, slice thickness=1 × 1 × 1 mm3, and matrix =256 × 192.
Image Analysis
Dynamic PET images were registered to each individual subject's MR image, and corrected for motion using a frame-to-frame registration process with a mutual information cost function. Regional analysis was facilitated by manual delineation of ROIs on the T1-MR image according to strict anatomic criteria (Tziortzi et al, 2011). The ROIs included the hypothalamus, substantia nigra and ventral tegmental area (SN&VTA), ventral pallidum substantia innominata, globus pallidus, VST, thalamus, caudate, and putamen. The cerebellum was defined through nonlinear registration (using SPM5; Wellcome Trust Centre for Neuroimaging, London, UK) of a template MR image and corresponding cerebellar brain atlas to each individual's MR image. The derived subcortical and cerebellum ROIs were applied to the motion-corrected dynamic image data to generate regional time-activity curves. A basis function implementation of the simplified reference tissue model (Gunn et al, 1997; Lammertsma and Hume, 1996), with the cerebellum as the reference tissue, was applied to the time-activity curves to derive regional estimates of nondisplaceable BP (BPND) estimates. BPND is proportional to the available concentration of receptor sites (Innis et al, 2007).
Estimation of the Effects of Amphetamine
The relative change in BPND elicited by amphetamine (ΔBPND) was calculated for each ROI as the difference between BPND measured in the baseline condition (BPNDBase) and that measured in the postamphetamine condition (BPNDAmph), expressed as a percentage of BPNDBase:
 |
Statistical Analysis
Within-subjects comparison of ΔBPND between radioligands was performed using repeated-measures analysis of variance (RM ANOVA), with ΔBPND as the dependent variable, the tracer as the repeated condition, and the ROI as a cofactor. The significance levels of the condition and the condition by region interaction are reported. A significance level of P=0.05 was selected for all statistical tests. Post hoc comparisons of ΔBPND for both ligands in each individual ROIs were evaluated using a paired t-test (one-tailed tests were used because we hypothesise that ΔBPND will be greater for [11C]-(+)-PHNO). No adjustments were made for multiple comparisons in post hoc tests.
Results
Demographic and scan parameter data for all subjects are shown in Table 1. There were no significant differences between the baseline and the postamphetamine scans in injected dose or injected mass for either ligand. No significant difference in amphetamine plasma level was observed between the postamphetamine [11C]raclopride and [11C]-(+)-PHNO scans (RM ANOVA, P=0.7). Amphetamine plasma levels were relatively stable throughout the scans for both ligands and consistent with the published literature (Narendran et al, 2010; Willeit et al, 2008).
Table 1. Demographic, scan, and plasma data.
|
[11C]raclopride (n=10) |
[11C]-(+)PHNO (n=13) |
|||
|---|---|---|---|---|
| Baseline | Postamphetamine | Baseline | Postamphetamine | |
| Injected dose (MBq) | 189±77 | 228±82 | 169±68 | 184±73 |
| Injected mass (μg) | 2.77±1.73 | 2.24±1.23 | 1.62±1.15 | 1.51±0.8 |
| Plasma amphetamine (0 minutesa, ng/mL) | — | 55.6±5.8 | — | 59.2±7.8 |
| Plasma amphetamine (90 minutesa, ng/mL) | — | 52.8±8.6 | — | 54.7±7.5 |
Refers to time after injection of radioligand in the second scan.
Baseline and postamphetamine BPND values of [11C]raclopride (n=10) and [11C]-(+)-PHNO (n=13) are presented in Figure 1 and Table 2, respectively. Summary parametric images derived from all subjects are shown in Figure 1. [11C]raclopride BPND at baseline in the SN&VTA and hypothalamus were <0.4, and hence were not considered sufficiently robust to be used for the quantitation of amphetamine-induced change. Our concerns about the ‘carry-over' effects of (+)-PHNO have led us to compare the ΔBPNDPHNO of ‘same-day subjects' versus that of ‘different-day subjects' (Table 3). ΔBPNDPHNO for ‘same-day subjects' was higher in all regions except the thalamus and hypothalamus; however, this difference was not statistically significant (MANOVA, F(8,4)=1.35, P=0.41). As a result of this finding, we used [11C]-(+)-PHNO data from all subjects in our main analysis.
Figure 1.
Summary parametric images derived from all subjects. Images shown for baseline and reduction in BPND after amphetamine (relative change between scans 1 and 2) for [11C]-(+)-PHNO and [11C]raclopride in stereotaxic space overlaid onto the T1-weighted MNI template. MRI, magnetic resonance imaging.
Table 2. Mean BPND at baseline and after amphetamine.
|
[11C]raclopride BPND |
[11C]-(+)-PHNO BPND |
|||||||
|---|---|---|---|---|---|---|---|---|
| Region | Baseline | Postamphetamine | ΔBPNDRAC% | Baseline | Postamphetamine | ΔBPNDPHNO% | ΔBPNDPHNO/ΔBPNDRAC | P-value |
| HypoTHa | 0.20±0.06 (0.19±0.07) | 0.14±0.11 (0.16±0.11) | 1.36±0.35 (1.54±0.34) | 0.98±0.30 (1.09±0.33) | −28±11 (−29±12) | |||
| SN&VTAa | 0.32±0.05 (0.29±0.05) | 0.30±0.06 (0.30±0.07) | 1.42±0.29 (1.51±0.33) | 1.01±0.19 (1.12±0.17) | −27±16 (−22±21) | |||
| VPSI | 1.08±0.11 (1.07±0.12) | 0.92±0.11 (0.94±0.10) | −14±10 (−14±11) | 3.19±0.63 (3.22±0.72) | 2.38±0.48 (2.58±0.56) | −24±14 (−19±15) | 1.71 (1.35) | 0.029* (0.096) |
| GP | 1.46±0.17 (1.43±0.19) | 1.29±0.16 (1.28±0.21) | −11±7 (−11±7) | 3.03±0.38 (3.10±0.37) | 2.70±0.45 (2.98±0.37) | −11±11 (−3±8) | 0.95 (0.31) | 0.342 (0.005*) |
| VST | 1.83±0.21 (1.90±0.25) | 1.61±0.27 (1.70±0.28) | −13±7 (−12±4) | 2.46±0.31 (2.50±0.37) | 1.95±0.30 (2.01±0.37) | −21±6 (−20±5) | 1.65 (1.71) | 0.005* (0.0015*) |
| TH | 0.45±0.05 (0.43±0.06) | 0.42±0.05 (0.41±0.06) | −5±5 (−5±5) | 0.47±0.1 (0.52±0.08) | 0.41±0.08 (0.45±0.08) | −14±7 (−14±6) | 2.53 (3.04) | 0.01* (0.014*) |
| CD | 2.21±0.30 (2.13±0.33) | 2.05±0.28 (2.00±0.34) | −7±4 (−6±5) | 1.56±0.23 (1.46±0.26) | 1.36±0.19 (1.34±0.26) | −12±7 (−8±6) | 1.65 (1.28) | 0.0615 (0.29) |
| PU | 2.98±0.30 (2.99±0.36) | 2.67±0.34 (2.67±0.43) | −11±6 (−11±6) | 2.24±0.19 (2.22±0.22) | 1.89±0.17 (1.91±0.22) | −16±4 (−14±4) | 1.44 (1.23) | 0.035* (0.13) |
CD, caudate; GP, globus pallidus; HypoTH, hypothalamus; PU, putamen; SN&VTA, substantia nigra and ventral tegmental area; TH, thalamus; VPSI, ventral pallidum substantia innominata; VST, ventral striatum.
Values in parentheses are for different-day subjects only (n=7). Significance levels are of post hoc one-tailed paired t-tests comparing ΔBPNDPHNO and ΔBPNDRAC.
*Denotes significant result (P<0.05).
Comparisons between the ligands were not made in these regions (see text).
Table 3. Mean ΔBPND values for all subjects (n=13), same-day (n=6), and different-day (n=7) [11C]-(+)-PHNO scans.
| HypoTH | SN&VTA | VPSI | GP | VST | TH | CD | PU | |
|---|---|---|---|---|---|---|---|---|
| % Change | ||||||||
| [11C]-PHNO all subjects (n=13) | −28±11 | −27±16 | −24±14 | −11±11 | −21±6 | −14±7 | −12±7 | −16±4 |
| [11C]-PHNO same day (n=6) | −26±11 | −33±6 | −31±10 | −19±9 | −22±8 | −13±8 | −17±6 | −18±3 |
| [11C]-PHNO different day (n=7) | −29±12 | −22±21 | −19±15 | −3±8 | −20±5 | −14±6 | −8±6 | −14±4 |
CD, caudate; GP, globus pallidus; HypoTH, hypothalamus; PU, putamen; SN&VTA, substantia nigra and ventral tegmental area; TH, thalamus; VPSI, ventral pallidum substantia innominata; VST, ventral striatum.
MANOVA (same-day/different-day comparison) F(6, 6)=1.94, P=0.22.
Within-Subjects Comparison of ΔBPND Between Radioligands
Amphetamine-induced ΔBPNDPHNO was significantly higher than ΔBPNDRAC (RM ANOVA, F(1,9)=6.58, P=0.03; Figure 2). Post hoc one-tailed paired t-tests revealed that ΔBPNDPHNO was significantly higher than ΔBPNDRAC in the ventral pallidum, VST, thalamus, and putamen (Table 2). The largest displacement in [11C]-(+)-PHNO BPND was measured in regions with the highest D3 fraction (SN&VTA and hypothalamus, ΔBPND ∼30%) (Searle et al, 2010; Tziortzi et al, 2011), where ΔBPNDRAC values were unavailable for a direct comparison with ΔBPNDPHNO.
Figure 2.
Reduction in BPND for [11C]-(+)-PHNO and [11C]raclopride after amphetamine. Columns represent mean values with associated s.d. error bars.
To rule out any potential influence of mass ‘carry over' of (+)-PHNO, we repeated the within-subjects comparison of ΔBPND between radioligands, using only the seven subjects who were scanned with [11C]-(+)-PHNO on different days. The result remained significant (RM ANOVA: F(1,6)=8.043, P=0.03; Figure 2). Post hoc one-tailed paired t-tests revealed that [11C]-(+)-PHNO ΔBPND was significantly higher than [11C]raclopride ΔBPND in the VST and thalamus (Table 2).
Effect of Order of Ligand Administration on ΔBPND
To examine any potential effects of sensitisation by amphetamine, we examined the effect of the order of ligand administration on the ΔBPND within each ROI using independent samples t-tests. There were no significant differences in [11C]raclopride ΔBPND across all ROIs between those subjects who had their [11C]raclopride scans on the second study day (after a previous dose of amphetamine) and those who had their [11C]raclopride scans on the first study day. The same lack of effect was found for [11C]-(+)-PHNO. In addition, there was no significant effect of the order of ligand administration when it was included as a covariate in the RM ANOVA comparing ΔBPND between radioligands (P=0.75).
Discussion
An administration of amphetamine induced a significantly larger change in [11C]-(+)-PHNO binding as compared with [11C]raclopride, with the magnitude of ΔBPNDPHNO:ΔBPNDRAC similar to that found in preclinical studies involving anaesthesia (Ginovart et al, 2006; Narendran et al, 2006). Of the four factors identified earlier, which could explain the increased ΔBPND with [11C]-(+)-PHNO compared with [11C]raclopride after an amphetamine challenge, the effects of anaesthesia do not appear to be important. Recently published data in awake and anaesthetised rodents implied that the greater sensitivity of agonists to amphetamine-induced dopamine release is a side effect of anaesthesia, which may not be present in conscious humans (McCormick et al, 2011). In our study, a direct within-subject comparison of agonist and antagonist ligands contradicts this speculation. In this respect, our data are consistent with previous work showing the change in binding of the D2/3 agonist [11C]NPA to be higher than that of [11C]raclopride in awake humans (Narendran et al, 2010). Our data do not allow us to explore the potential greater decrease in affinity of (+)-PHNO as compared with raclopride for the internalised D2 and D3 receptors (caused by a change in the ionic environment of internalised receptors); hence, further discussion will focus on the role of D2high and D3 receptors.
Previous work has compared the effect of amphetamine on the binding of the D2/3 agonist [11C]NPA with that of the D2/3 antagonist [11C]raclopride, and found the ΔBPNDNPA:ΔBPNDRAC to be ∼1.4 in anaesthetised primates (Narendran et al, 2004) and ∼1.6 in awake humans (Narendran et al, 2010). As the contribution of D3 to both [11C]NPA and [11C]raclopride signals in the striatum is negligible, the greater sensitivity of [11C]NPA was attributed to the greater sensitivity of ligands binding at the D2high sites to fluctuations in dopamine levels. Although [11C]-(+)-PHNO binding is a mixture of D3 and D2 components, in the dorsal caudate and putamen, the contribution of D3 is negligible (Searle et al, 2010; Tziortzi et al, 2011). In our study, ΔBPNDPHNO:ΔBPNDRAC=1.53 in the dorsal caudate and putamen, consistent with ΔBPNDNPA:ΔBPNDRAC, and showing that the binding of [11C]-(+)-PHNO to the D2high receptor is a significant factor in its enhanced sensitivity to an amphetamine challenge.
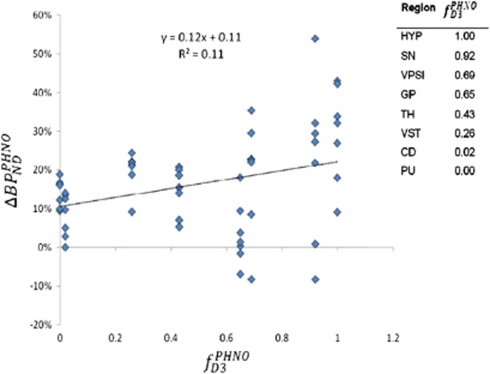
In regions other than the caudate and putamen, the contribution of D3 to [11C]-(+)-PHNO binding is significant, and any effects of amphetamine will depend on the affinity of dopamine for D3 and for D2high receptors. We performed a regression of regional ΔBPNDPHNO against regional fD3PHNO (the fraction of total [11C]-(+)-PHNO binding that can be attributed to D3) obtained from data reported by Tziortzi et al (Table 3, Method A1). To eliminate any potential bias caused by (+)-PHNO mass carry over in regions rich in D3 (such as the SN&VTA and the hypothalamus), we examined only the data obtained from seven subjects who were scanned with [11C]-(+)-PHNO on separate days. We found a significant effect of fD3PHNO on ΔBPNDPHNO (r=0.325, P=0.014, Figure 3), indicating that the interaction of dopamine with the D3 receptor has a significant role in the effect of amphetamine on [11C]-(+)-PHNO binding. In our analysis, we make the assumption that the amount of dopamine released by amphetamine is similar in all basal ganglia regions, as well as in the thalamus, hypothalamus, and SN&VTA. Microdialysis literature comparing the effects of amphetamine on extracellular dopamine levels in these regions is not extensive, but the differences between SN and striatal dopamine release are minimal in one study (Robertson et al, 1991), although not in another (Kalivas et al, 1989). In the striatum itself, the literature does suggest that amphetamine-induced dopamine release in the VST is approximately twice that in the dorsal striatum (Drevets et al, 1999). We stress that our assumption of homogeneous regional dopamine release may not be valid, in which case our subsequent analysis will only be an approximation.
Figure 3.
Regression of ΔBPNDPHNO against fD3PHNO.
In ROIs where a direct comparison of [11C]-(+)-PHNO and [11C]raclopride could be conducted, ΔBPNDPHNO/ΔBPNDRAC in regions such as the globus pallidus and VST was not significantly different from that in the caudate and putamen, where D3 contribution to the [11C]-(+)-PHNO signal is negligible. Hence, a conclusive statement on the role of D3 in the sensitivity of [11C]-(+)-PHNO to an amphetamine challenge requires comparative data in the SN&VTA using a suitable high-affinity D2 radioligand such as [11C]FLB-457 or [18F]fallypride.
Our data allowed us to derive two incidental findings, which bear on the physiology of dopaminergic neurotransmission. First, because [11C]-(+)-PHNO binds to both D2high and D3, our data allow us to comment on the relative affinity of dopamine for the two sites. This estimation assumes that the baseline concentrations of dopamine and the amounts of dopamine released by amphetamine are similar in all regions. A regression of ΔBPNDPHNO against fD3PHNO provides an estimate of  (95% confidence interval=1.34 to 4.63) (Figure 3). The positive value suggests that D3 has the higher affinity, and the value of the slope suggests that the two dissociation constants have the same order of magnitude. Although in vitro assessments often found higher selectivity of dopamine for D3 over D2 (Sokoloff et al, 1992), most of these were conducted in receptors configured to a mixture of D2high and D2low states under nonphysiologic conditions—which may lead to an underestimation of the affinity of dopamine for D2high. As mentioned above, this analysis assumes a similar magnitude of dopamine release in the various ROIs, and in addition, the baseline occupancies of the D2 and D3 receptors by dopamine are disregarded.
(95% confidence interval=1.34 to 4.63) (Figure 3). The positive value suggests that D3 has the higher affinity, and the value of the slope suggests that the two dissociation constants have the same order of magnitude. Although in vitro assessments often found higher selectivity of dopamine for D3 over D2 (Sokoloff et al, 1992), most of these were conducted in receptors configured to a mixture of D2high and D2low states under nonphysiologic conditions—which may lead to an underestimation of the affinity of dopamine for D2high. As mentioned above, this analysis assumes a similar magnitude of dopamine release in the various ROIs, and in addition, the baseline occupancies of the D2 and D3 receptors by dopamine are disregarded.
Second, we estimated the proportion of the D2 receptors configured in the D2high state  in the dorsal striatum, using the method developed by Narendran et al (2004) for [11C]NPA and [11C]raclopride.
in the dorsal striatum, using the method developed by Narendran et al (2004) for [11C]NPA and [11C]raclopride.
 |
In anaesthetised primates fD2high was estimated at ∼80%, whereas a subsequent study in humans found a somewhat lower estimate of ∼60% (Narendran et al, 2010). As the D3 contribution to the [11C]-(+)-PHNO signal in the dorsal striatum is negligible, we estimated the fD2high here, and found it to be very consistent with the previous estimate, at ∼65%.
Several methodological caveats of our study need to be mentioned. The first six subjects examined received two [11C]-(+)-PHNO injections ∼5 hours apart, and thus their ΔBPNDPHNO estimates could be biased owing to a ‘mass carry-over' effect of nontracer amounts of (+)-PHNO remaining bound to D3 during the postamphetamine scan. This could lead to an overestimation of the occupancy attributed to endogenous dopamine. We do not think that this potential bias explains the difference between ΔBPNDPHNO and ΔBPNDRAC for several reasons. First, a separate comparison of data obtained from the last seven subjects (who received their postamphetamine [11C]-(+)-PHNO injection at least 7 days after their baseline scan) reveals a notably higher ΔBPNDPHNO than ΔBPNDRAC. A post hoc analysis using only the seven subjects who were scanned with [11C]-(+)-PHNO on different days indicated that the within-subject difference between ΔBPNDPHNO and ΔBPNDRAC remained significant. The ratio of mean ΔBPNDPHNO/ΔBPNDRAC for ‘same-day' subjects was similar to that of that for the whole group at ∼1.3 in most ROIs (Table 2). Second, any carry-over effects of (+)-PHNO from the baseline scan would be manifested only at D3 and not at D2. However, ΔBPNDPHNO was higher than ΔBPNDRAC even in ROIs such as the dorsal caudate and putamen, where the contribution of D3 is negligible. However, our data did show that ΔBPNDPHNO values for ‘same-day subjects' were higher than for ‘different-day' subjects in all regions, except the thalamus and hypothalamus. Although the difference did not reach statistical significance with the subject numbers in this study, when considered with what is known about the Kd of [11C]-(+)-PHNO at the D3 receptor (Gallezot et al, 2009), it is apparent that extreme caution should be exercised. If consecutive [11C]-(+)-PHNO scans are performed on the same day, accounts of possible carry-over effects must be included in the quantification of [11C]-(+)-PHNO binding.
Nontracer conditions during [11C]-(+)-PHNO scans could also lead to an underestimation of the occupancy by endogenous dopamine, owing to the effects of competition between dopamine and (+)-PHNO. The balance between this effect and ‘carry over' may be difficult to estimate, but examples of the possible effects are presented in Supplementary Information, Appendix. On the basis of these simulations, we do not believe that any nontracer binding of [11C]-(+)-PHNO substantially changes the conclusions of our study. However, it is essential that, in all studies using [11C]-(+)-PHNO, the consequences of nontracer conditions at the D3 receptor are considered in quantification. The best estimate we have for the in vivo Kd of [11C]-(+)-PHNO at the D3 receptor is ∼30 ng/kg (Gallezot et al, 2009). With this in mind, tracer conditions (i.e., occupancy of <5% of the target) would require an injection of <1.5 ng/kg, or ∼0.1 μg total dose in a 70 kg subject. The specific activity to achieve this is not attainable using the Grignard chemistry required to synthesise [11C]-(+)-PHNO. Future studies with this ligand will need to recognise that, outside the tracer range, [11C]-(+)-PHNO receptor binding is dependent on injected ligand mass, as well as receptor density and endogenous dopamine levels.
Our study design required that each subject receives two doses of amphetamine; therefore, sensitisation to amphetamine was a potential problem. However, the evidence for a sensitisation effect with amphetamine in the literature is conflicting. Sensitisation to a single administration of intravenous amphetamine was examined in the course of a test–retest assessment of amphetamine-induced striatal dopamine release in humans using [123I]IBZM single photon emission computed tomography (Kegeles et al, 1999). Subjects underwent measurement of striatal dopamine release on two occasions, 16±10 days apart, after administration of 0.3 mg/kg amphetamine. No significant differences were found between the second and first administration of amphetamine. In contrast, both greater psychomotor response and increased dopamine release were seen at both 14 and 365 days after the first exposure to amphetamine in a [11C]raclopride study (Boileau et al, 2006). It is noteworthy that in this study, subjects received oral amphetamine at the same dose (0.3 mg/kg) on three separate occasions (as opposed to only one previous dose in the Kegeles study above). To control for possible effects of sensitisation, we counterbalanced the order of radioligand administration in our study. We found no evidence of an amphetamine sensitisation effect, as for both ligands, the ΔBPND did not depend on the order of administration.
The effect sizes for ΔBPNDPHNO in the VST and putamen were greater than seen in a previous human study (see Table 4) (Willeit et al, 2008). In addition, the effect sizes in these ROIs were comparable to that of the agonist radioligand [11C]NPA (Narendran et al, 2010), even though our study used a lower dose of amphetamine (0.3 versus 0.5 mg/kg). Thus, [11C]-(+)-PHNO has at least an equal sensitivity to that of [11C]NPA to detect extracellular dopamine fluctuations. An added advantage of [11C]-(+)-PHNO is that it can be used to measure binding in extrastriatal regions such as the SN and hypothalamus, which is not possible with [11C]NPA. Against this greater sensitivity, we must acknowledge the methodological complications required to acquire well-quantified data with [11C]-(+)-PHNO. These include complicated radiosynthesis, requirement for high specific activity to achieve tracer conditions and to avoid nausea in volunteers, and the need to separate consecutive scans by a sufficient time gap to avoid potential carry-over effects at the D3 receptor. With these factors in mind, the choice of radioligand to image endogenous dopamine release will be conditioned by the requirements of each particular study.
Table 4. Effect sizes for [11C]-(+)-PHNO and [11C]NPA ΔBPND after amphetamine challenge.
| Amphetamine (ng/L)a | VST | CD | PU | |
|---|---|---|---|---|
| [11C]raclopride (this study, n=10) | 55.6±5.8 | 1.9 | 1.8 | 1.8 |
| [11C]PHNO (this study, n=13) | 59.2±7.8 | 3.7 | 1.5 | 3.8 |
| [11C]PHNO (Willeit et al, 2008, n=11) | 53.2±24.4 | 1.9 | 1.9 | 2.3 |
| [11C]NPA (Narendran et al, 2010, n=10) | 73.9±5.4 | 2.3 | 2.6 | 4.5 |
CD, caudate; [11C]NPA, [11C]-N-propyl-norapomorphine; PU, putamen; VST, ventral striatum.
Amphetamine values refer to plasma concentration measured at the start of the post-dose scan.
In summary, we used a within-subject design to provide evidence that [11C]-(+)-PHNO is more sensitive than [11C]raclopride in detecting the fluctuations of extracellular dopamine. Our data suggest that both the agonist nature of [11C]-(+)-PHNO and its selectivity for the D3 receptor contribute to its higher sensitivity compared with [11C]raclopride. We emphasise that mass effects are a potential confounding factor in imaging with [11C]-(+)-PHNO, and therefore same-day scans should be approached cautiously to take account of possible carry-over effects.
Infrastructure support for this study was provided by the Imperial College and GSK. Dr Paul Shotbolt is supported by a Wellcome Translational Medicine Training Fellowship.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was funded by GlaxoSmithKline (GSK).
Supplementary Material
References
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Prosser T, Synder SH. Dopamine receptor binding: specificity, localization and regulation by ions and guanyl nucleotides. Life Sci. 1978;23:495–499. doi: 10.1016/0024-3205(78)90160-1. [DOI] [PubMed] [Google Scholar]
- Creese I, Usdin TB, Snyder SH. Dopamine receptor binding regulated by guanine nucleotides. Mol Pharmacol. 1979;16:69–76. [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, Mathis CA. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;21:694–709. doi: 10.1016/S0893-133X(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Ehrin E, Gawell L, Högberg T, de Paulis T, Ström P. Synthesis of [methoxy-3H]- and [methoxy-11C]- labelled raclopride. J Labelled Compounds Radiopharm. 1987;24:931–940. [Google Scholar]
- Finnema SJ, Halldin C, Bang-Andersen B, Gulyas B, Bundgaard C, Wikstrom HV, Farde L. Dopamine D(2/3) receptor occupancy of apomorphine in the nonhuman primate brain—a comparative PET study with [11C]raclopride and [11C]MNPA. Synapse. 2009;63:378–389. doi: 10.1002/syn.20615. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Beaver J, Nabulsi N, Weinzimmer D, Slifstein M, Gunn R, Ding YS, Huang Y, Carson RE, Rabiner E. [11C]PHNO studies in rhesus monkey: in vivo affinity for D2 and D3 receptors and dosimetry. J Nucl Med Meeting Abstracts: 2009;50:601. [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R, Slifstein M, bi-Dargham A, Laruelle M, Javitch JA, Rayport S. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartvig P, Torstenson R, Tedroff J, Watanabe Y, Fasth KJ, Bjurling P, Langstrom B. Amphetamine effects on dopamine release and synthesis rate studied in the Rhesus monkey brain by positron emission tomography. J Neural Transm. 1997;104:329–339. doi: 10.1007/BF01277655. [DOI] [PubMed] [Google Scholar]
- Houston GC, Hume SP, Hirani E, Goggi JL, Grasby PM. Temporal characterisation of amphetamine-induced dopamine release assessed with [11C]raclopride in anaesthetised rodents. Synapse. 2004;51:206–212. doi: 10.1002/syn.10296. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Bourdelais A, Abhold R, Abbott L. Somatodendritic release of endogenous dopamine: in vivo dialysis in the A10 dopamine region. Neurosci Lett. 1989;100:215–220. doi: 10.1016/0304-3940(89)90687-3. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Zea-Ponce Y, bi-Dargham A, Rodenhiser J, Wang T, Weiss R, Van Heertum RL, Mann JJ, Laruelle M. Stability of [123I]IBZM SPECT measurement of amphetamine-induced striatal dopamine release in humans. Synapse. 1999;31:302–308. doi: 10.1002/(SICI)1098-2396(19990315)31:4<302::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- McCormick PN, Ginovart N, Wilson AA. Isoflurane anaesthesia differentially affects the amphetamine sensitivity of agonist and antagonist D2/D3 positron emission tomography radiotracers: implications for in vivo imaging of dopamine release. Mol Imaging Biol. 2011;13:737–746. doi: 10.1007/s11307-010-0380-3. [DOI] [PubMed] [Google Scholar]
- McCormick PN, Kapur S, Seeman P, Wilson AA. Dopamine D2 receptor radiotracers [(11)C](+)-PHNO and [(3)H]raclopride are indistinguishably inhibited by D2 agonists and antagonists ex vivo. Nucl Med Biol. 2008;35:11–17. doi: 10.1016/j.nucmedbio.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, Cooper TB, Martinez D, Kegeles LS, Abi-Dargham A, Laruelle M. In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse. 2004;52:188–208. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- Narendran R, Mason S, Laymon C, Lopresti B, Velasquez N, May M, Kendro S, Martinez D, Mathis C, Frankle G. A comparative evaluation of the dopamine D2/3 agonist radiotracer [11C]NPA and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. J Pharmacol Exp Ther. 2010;333:533–539. doi: 10.1124/jpet.109.163501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, Gunn RN, Laruelle MA. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Damsma G, Fibiger HC. Characterization of dopamine release in the substantia nigra by in vivo microdialysis in freely moving rats. J Neurosci. 1991;11:2209–2216. doi: 10.1523/JNEUROSCI.11-07-02209.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Bonaventure P, Leysen JE. Endogenous dopamine limits the binding of antipsychotic drugs to D3 receptors in the rat brain: a quantitative autoradiographic study. Histochem J. 1996;28:791–799. doi: 10.1007/BF02272152. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Leysen JE. Autoradiographic evidence for the occlusion of rat brain dopamine D3 receptors in vivo. Eur J Pharmacol. 1992;218:373–375. doi: 10.1016/0014-2999(92)90196-b. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo-Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Sibley DR, De LA, Creese I. Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D-2 dopamine receptor. J Biol Chem. 1982;257:6351–6361. [PubMed] [Google Scholar]
- Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, Thorsell A, Heilig M, Pike VW, Halldin C, Sibley DR, Innis RB. D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: a PET study in a receptor internalization-deficient mouse model. Neuroimage. 2010;50:1402–1407. doi: 10.1016/j.neuroimage.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, Schwartz JC. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol. 1992;225:331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [(11)C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Seeman P, Wilson AA, Kapur S. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Molinoff PB. Effect of guanine nucleotides on striatal dopamine receptors. Nature. 1978;275:453–455. doi: 10.1038/275453a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.