Abstract
Traumatic brain injury (TBI) induces secondary injury mechanisms, including cell-cycle activation (CCA), which lead to neuronal cell death, microglial activation, and neurologic dysfunction. Here, we show progressive neurodegeneration associated with microglial activation after TBI induced by controlled cortical impact (CCI), and also show that delayed treatment with the selective cyclin-dependent kinase inhibitor roscovitine attenuates posttraumatic neurodegeneration and neuroinflammation. CCI resulted in increased cyclin A and D1 expressions and fodrin cleavage in the injured cortex at 6 hours after injury and significant neurodegeneration by 24 hours after injury. Progressive neuronal loss occurred in the injured hippocampus through 21 days after injury and correlated with a decline in cognitive function. Microglial activation associated with a reactive microglial phenotype peaked at 7 days after injury with sustained increases at 21 days. Central administration of roscovitine at 3 hours after CCI reduced subsequent cyclin A and D1 expressions and fodrin cleavage, improved functional recovery, decreased lesion volume, and attenuated hippocampal and cortical neuronal cell loss and cortical microglial activation. Furthermore, delayed systemic administration of roscovitine improved motor recovery and attenuated microglial activation after CCI. These findings suggest that CCA contributes to progressive neurodegeneration and related neurologic dysfunction after TBI, likely in part related to its induction of microglial activation.
Keywords: brain trauma, cell cycle, cyclin-dependent kinases, microglial activation, neurodegeneration
Introduction
Traumatic brain injury (TBI) represents a major public health problem, with >1.7 million new cases annually in the United States alone (Faul et al, 2010), accounting for 60% of all emergency department admissions and 50% of all trauma deaths (Dutton et al, 2010). Traumatic brain injury causes cell death and neurologic dysfunction through both direct physical disruption of tissue (primary injury), as well as through delayed and potentially reversible molecular and cellular pathophysiological mechanisms, which cause progressive white and gray matter damage (secondary injury) (Bramlett and Dietrich, 2007; Panter and Faden, 1992). Such delayed injury begins within seconds to minutes after the insult and may continue for days, weeks, or potentially months to years (Bramlett and Dietrich, 2007) and may be responsible for a significant component of neurodegeneration and neurologic impairment after TBI (Loane and Faden, 2010). Some of the more important secondary injury mechanisms involve intrinsic neuronal cell death pathways, microglial activation, and secondary neurotoxicity.
Previous studies have linked cell-cycle events and neuronal cell death. Historically, postmitotic cells such as neurons were believed to have permanently entered the G0 phase and were incapable of entering the cell cycle. However, recent studies have indicated that cell-cycle reentry of mature differentiated postmitotic neurons occurs, but once activated, it results in apoptosis rather than neuronal proliferation (Herrup and Yang, 2007; Kranenburg et al, 1996). The normal cell cycle is controlled by complex molecular mechanisms and progression through cell-cycle phases that require sequential activation of a large group of Ser/Thr kinases called cyclin-dependent kinases (CDKs) and their positive regulators (cyclins) (Arendt, 2003). The G1 phase is initiated sequentially by increased levels of members of the cyclin D family, activation of cyclin D-dependent kinase activity, phosphorylation of the Rb family, and activation of E2F (E2 promoter-binding factor) family of transcription factors. Active E2F induces transcription of various genes involved in cell cycle, such as cyclin A, which are required for later phases of the cell cycle (Stoica et al, 2009). The cyclin A partner, CDK2, can also phosphorylate Rb (Lundberg and Weinberg, 1998).
Increased levels of cyclin D and A were shown to be associated with increased phosphorylation of Rb (Freeman et al, 1994). This results in activation of E2F members; in neurons, active E2Fs can contribute to increased transcription of proapoptotic molecules such as caspases 3, 9, and 8 and Apaf-1 or anti-apoptotic Bcl-2 family members (Greene et al, 2004; Nguyen et al, 2003). Central nervous system injuries such as TBI induce cell-cycle activation (CCA) not only in neurons but also in glial cells; this can result in apoptosis of postmitotic cells (mature oligodendroglia) and in the proliferation and activation of mitotic cells such as astroglia and microglia. Microglial activation for certain phenotypes is associated with the release of proinflammatory molecules that can cause neurotoxicity (Byrnes and Faden, 2007; Giovanni et al, 2005). Therefore, CCA following TBI may initiate multiple secondary injury mechanisms that contribute to neuronal apoptosis and delayed neurotoxicity.
In this study, we present a comprehensive quantitative assessment of neuronal cell loss and microglial activation in the injured cortex and hippocampus, using a well-established murine CCI model. We also use a delayed treatment paradigm with the selective CDK inhibitor roscovitine to assess the role of CCA in progressive cell loss and chronic neurologic dysfunction after TBI.
Materials and methods
Controlled Cortical Impact Injury
All surgical procedures and experiments were carried out in accordance with protocols approved by the Georgetown University Animal Care and Use Committee. Our custom-designed CCI injury device (Fox et al, 1998; Loane et al, 2009) consists of a microprocessor-controlled pneumatic impactor with a 3.5-mm diameter tip. C57BL/6J mice (weighing 20 to 25 g) were anesthetized with isoflurane (induction at 4% and maintenance at 2%) evaporated in a gas mixture containing 70% N2O and 30% O2 and administered through a nose mask. The depth of anesthesia was assessed by monitoring respiration rate and pedal withdrawal reflexes. The mouse was placed on a heated pad, and a core body temperature was maintained at 37°C. The head was mounted in a stereotaxic frame, and the surgical site was clipped and cleaned with Nolvasan and ethanol scrubs. A 10-mm midline incision was made over the skull, the skin and fascia were reflected, and a 4-mm craniotomy was made on the central aspect of the left parietal bone. The impounder tip of the injury device was then extended to its full stroke distance (44 mm), positioned to the surface of the exposed dura, and reset to impact the cortical surface. Moderate injury was induced using an impactor velocity of 6 m/sec and a deformation depth of 2 mm, as detailed previously (Loane et al, 2009). After injury, the incision was closed with interrupted 6-0 silk sutures, anesthesia was terminated, and the animal was placed into a heated cage to maintain normal core temperature for 45 minutes after injury. All animals were monitored carefully for at least 4 hours after surgery and then daily. The systemic temperature of mice was controlled during injury and drug administration. Sham animals underwent the same procedure as injured mice, except for impact.
Drug Administration
Groups of mice (n=11 to 12 per group) received an intracerebroventricular injection of roscovitine, also referred to as CYC202 and seliciclib (R-1234, LC Laboratories, Woburn, MA, USA) or equal volume vehicle (saline with 1% dimethyl sulfoxide) 3 hours after injury. A 30 mmol/L (30 nmol/μL or 10.62 μg/μL) solution in saline with 1% dimethyl sulfoxide was injected into the left ventricle (coordinates from the bregma=A: −0.5, L: −1.0, V: −2.0) using a 30-G needle attached to a Hamilton syringe at a rate of 0.5 μL/min, with a final volume of 5 μL of roscovitine. The dose of drug used in this study was based on our previous data showing increased neuroprotection after central administration of roscovitine following brain injury in rats (Hilton et al, 2008), taking into account the cerebrospinal fluid volume differences across species. Sham-operated mice (each n=5 per group) received an intracerebroventricular injection of either roscovitine or vehicle, 3 hours after surgery. Groups of mice (n=3 to 4 per group) were subjected to the same magnitude of injury, followed by intracerebroventricular treatment of roscovitine or vehicle and killed for histology after 24 hours or 7 days.
Separate groups of mice (each n=6 per group) received intraperitoneal injection of roscovitine (50 mg/kg) or equal volume of vehicle, 3 hours after injury. This intraperitoneal dose of roscovitine was based on the results obtained from our pilot studies, and previously described favorable pharmacokinetic and metabolic profiles of systemic roscovitine administration in mice (Nutley et al, 2005). Other groups of mice (n=3 to 4 per group) were subjected to the same magnitude of injury, followed by intraperitoneal treatment of roscovitine or vehicle and killed for histology after 24 hours or 7 days.
Western Immunoblot
At 6 hours after injury, other mice (n=3 per group) treated with roscovitine or vehicle (intracerebroventricular) were anesthetized (100 mg/kg sodium pentobarbital, intraperitoneal), transcardially perfused with ice-cold saline, and decapitated. A 5-mm area surrounding the lesion epicenter on the ipsilateral cortex was rapidly dissected and immediately frozen on dry ice. Cortical tissue was homogenized in RIPA buffer and centrifuged at 15,000 r.p.m. for 15 minutes at 4°C to isolate proteins, and protein concentration was determined using the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). In all, 25 μg of protein was run on SDS-PAGE and transferred onto nitrocellulose membrane. The blots were then probed with antibodies against cyclin A (1:2,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), cyclin D1 (1:2,000, Lab Vision Products, Fremont, CA, USA), and fodrin (1:2,000, Enzo Life Sciences International Inc., Plymouth Meeting, PA, USA). Glyceraldehyde 3-phosphate dehydrogenase (1:2,000; Enzo Life Sciences International Inc.) was used as an endogenous control. Immune complexes were detected with the appropriate horseradish peroxidase-conjugated secondary antibodies (KPL Inc., Gaithersburg, MD, USA) and visualized using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific). Chemiluminescence was captured on a Kodak Image Station 4000R station (Kodak, Carestream Health Inc., Rochester, NY, USA), and protein bands were quantified by densitometric analysis using Carestream molecular imaging software (Carestream Health, Rochester, NY, USA). The data presented reflect the intensity of the target protein band compared with control and normalized based on the intensity of the endogenous control for each sample (expressed in arbitrary units).
Functional Assessment
Chronic motor recovery was assessed using a beam walk test as described previously (Fox et al, 1998; Loane et al, 2009). The beam walking task is particularly good at discriminating fine-motor coordination differences between injured and sham-operated animals. The test consists of a narrow wooden beam 5-mm wide and 120 mm in length, which is suspended 300 mm above a tabletop. Mice were placed on one end of the beam, and the number of foot faults for the right hindlimb recorded >50 steps. Mice were trained on the beam walk for 3 days before TBI and tested at 1, 3, 7, 14, and 21 days after injury.
Spatial learning and working memory was assessed using apparatus and acquisition paradigm of the Morris water maze (MWM) test on postinjury days 14, 15, 16, and 17, as described previously (Fox et al, 1998; Loane et al, 2009). The white circular pool was divided into four quadrants using a computer-based AnyMaze video tracking system (Stoelting Co., Wood Dale, IL, USA), and the platform was hidden in one quadrant 14 inches from the side wall. The cognitive outcomes were determined in terms of latency (seconds) to locate the hidden platform with a 90-second limit per trial. A visual cue test was performed on postinjury day 18 using a flagged platform placed in one of the quadrants to ensure visual acuity of all animals with a 90-second limit per trial and latency (seconds) to locate the flagged platform was recorded. All studies were performed by an investigator blinded to the treatment group.
Immunocytochemistry
At 24 hours after injury, mice (n=3 per group) treated with roscovitine or vehicle (intracerebroventricular) were anesthetized and transcardially perfused with saline and 10% buffered formalin phosphate solution. The brains were removed, postfixed in paraformaldehyde for 24 hours, and protected in 30% sucrose. Frozen brain sections (60 and 20 μm) were cut on a cryostat and mounted onto glass slides. Selected slides were with stained with Fluoro-Jade B (Chemicon, Temecula, CA, USA) to identify degenerating neurons, following the manufacturer's protocol.
Histology
Mice (n=3 to 4 per group) were anesthetized and transcardially perfused with saline and 10% buffered formalin phosphate solution (containing 4% paraformaldehyde; Fisher Scientific, Pittsburg, PA, USA) at 24 hours, 7 or 21 days after injury. The brains were removed, postfixed in paraformaldehyde for 24 hours, and protected in 30% sucrose. Frozen brain sections (60 and 20 μm) were cut on a cryostat and mounted onto glass slides.
Immunohistochemistry
Every fourth 60-μm section was processed for immunohistochemical analysis beginning from a random start point. Microglia were immunostained with anti-Iba-1 (1:1,000; Wako Chemicals, Richmond, VA, USA) overnight, washed in phosphate-buffered saline, and incubated with biotinylated anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA, USA) for 2 hours at room temperature. Sections were placed in avidin–biotin–horseradish peroxidase solution, diluted according to the manufacturer's instructions for 1 h (Vectastain elite ABC kit, Vector Laboratories), and then reacted with 3,3′-diaminobenzidine (Vector Laboratories) for color development.
Lesion Volume Assessment
Sections stained for Iba-1 were further counterstained with cresyl violet (FD NeuroTechnologies, Baltimore, MD, USA), dehydrated, and mounted for analysis. Lesion volume was estimated based on Cavalieri method of unbiased stereology using the Stereologer 2000 program software (Systems Planning and Analysis, Alexandria, VA, USA) as described previously (Loane et al, 2009).
Assessment of Neuronal Cell Loss in the Cortex and Hippocampal Subregions
Stereoinvestigator software (MBF Biosciences, Williston, VT, USA) was used to count the total number of surviving neurons in the cortex and cornu ammonis (CA) 1, CA2/3, and dentate gyrus (DG) subregions of the hippocampus using the optical fractionator method of unbiased stereology. The sampled region for each subfield was demarcated in the injured hemisphere and cresyl-violet neuronal cell bodies were counted. The volume of the cortical or hippocampal subfield was measured using a Cavalieri estimator method. The estimated number of surviving neurons in each field was divided by the volume of the region of interest to obtain cellular density expressed in cells/mm3.
Assessment of Microglial Morphology in the Cortex
Stereoinvestigator software (MBF Biosciences) was used to count the number of cortical microglia in each of the three microglial morphologic phenotypes (namely ramified, hypertrophic, and bushy) using the optical fractionator method of unbiased stereology. The sampled region was the ipsilateral cortex between −1.22 and −2.54 mm from the bregma, and dorsal to a depth of 2.0 mm from the surface. Every fourth 60-μm section was analyzed beginning from a random start point. Sections were analyzed using a Leica DM4000B microscope (Leica, Leica Microsystems Inc., Buffalo Grove, IL, USA). The optical dissector had a size of 50 × 50 μm2 in the x and y axes with a height of 10 μm and guard zone of 4 μm from the top of the section. Dissectors were positioned every 150 μm in the x and y axes. Microglial phenotypic classification was based on the length and thickness of the projections, the number of branches, and the size of the cell body as described previously (Soltys et al, 2001). The volume of the region of interest was measured using the Cavalieri estimator method. The estimated number of microglia in each phenotypic class was divided by the volume of the region of interest to obtain cellular density expressed in counts/mm3. Neurolucida software (MBF Biosciences) was used to create reconstructions of microglia at different stages of activation after injury by tracing the cell bodies and dendrites. Microglia were outlined using the live image setting, so that the width of the dendrites could be traced while focusing on the section. The cell bodies were outlined using the contour tool followed by tracing of the individual dendrites, using the dendrite line tool.
Statistical Analysis
The number of animals per group for every assessment was based on our previous studies using the CCI model and satisfied power requirements. The number of mice used per group per time point in each study is outlined in Table 1. Lesion volume, functional data, and unbiased stereological analysis were performed by an investigator blinded to the treatment group. Quantitative data were expressed as mean±s.e.m. Lesion volume was analyzed by one-tailed paired Student's t-test. Functional data were analyzed by repeated-measures one-way analysis of variance, followed by post hoc adjustments using Tukey's or Student–Newman–Keuls test across groups. The analysis of time course-based stereological data was performed by one-way analysis of variance, followed by post hoc adjustments using Tukey's tests for comparisons across different time points. In addition, one-tailed paired Student's t-test was performed versus vehicle-treated groups at each time point. Regression analysis between behavioral improvement (motor and cognition) and stereological assessment (lesion volume and DG neuronal cell loss, respectively) was performed by linear regression and a correlation coefficient (r2) was determined. The data for expression of biochemical markers using western blotting were analyzed by one-tailed paired Student's t-test. The functional data were analyzed using Sigma Stat Program, version 3.5 (Systat Software, San Jose, CA, USA). All other statistical tests were performed using the GraphPad Prism Program, version 3.02 for Windows (GraphPad Software, San Diego, CA, USA). P<0.05 was considered statistically significant.
Table 1. Use of animals.
| Study | Time after injury | Number of injured animals per group |
|---|---|---|
| Cyclin A, cyclin D1, and fodrin expression. Figures 1A to 1C | 6 hours | N=3 |
| Fine-motor coordination (beam walk) and cognitive (Morris water maze) assessment. Figures 2A and 2B | 21 days | N=11–12 |
| Lesion volume (unbiased stereology—cresyl-violet staining). Figure 2A to 2C | 21 days | N=4–5 |
| Neuronal degeneration (Fluoro-Jade B staining). Figure 4A | 24 hours | N=3 |
| Neuronal loss quantification in the cortex and hippocampus (unbiased stereology—cresyl-violet staining). Figure 4C, 4D to 4F | 24 hours 7 days 21 days | N=3–4 N=3–4 N=3–4 |
| Microglial activation quantification and classification in the cortex (unbiased stereology—Iba-1 staining). Figure 5A to 5D | 24 hours 7 days 21 days | N=3–4 N=3–4 N=3–4 |
| Fine-motor coordination (beam walk). Figure 6A | 21 days | N=6 |
| Microglial activation quantification and classification in the cortex (unbiased stereology—Iba-1 staining). Figure 6B and 6C | 24 hours 7 days 21 days | N=3–4 N=3–4 N=3–4 |
Results
Central Administration of Roscovitine Inhibits Cell-Cycle Activation and Apoptosis in the Cortical Tissue After Traumatic Brain Injury
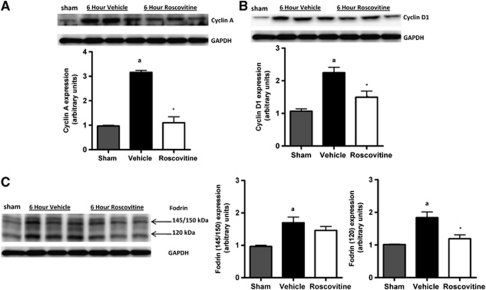
To evaluate the effects of cell-cycle inhibition in our model, western immunoblotting for key cell-cycle markers was performed in cortical extracts from vehicle- and roscovitine-treated injured mice. The data showed that cyclin A expression was significantly induced at 6 hours after TBI (Figure 1A; P<0.05 versus sham), and was strongly inhibited by roscovitine treatment (P=0.003 versus vehicle). Similarly, injury significantly increased the expression of cyclin D1 (Figure 1B; P<0.05 versus sham), and this was significantly attenuated in the roscovitine-treated group (P=0.02 versus vehicle). To assess the effects of roscovitine on apoptosis, the presence of cleaved fragments of fodrin (also known as spectrin) (Siman et al, 1984, 2004) was assessed in these samples. TBI significantly increased the cleavage of fodrin (Figure 1C; P<0.05 versus sham), as shown by increased levels of the 140/150-kDa and 120-kDa cleavage products of fodrin. Roscovitine treatment significantly decreased the level of the 120-kDa product of fodrin when compared with the vehicle-treated group (P=0.02 versus vehicle).
Figure 1.
Central administration of roscovitine inhibits cell-cycle activation and apoptosis in cortical tissue after TBI. (A, B) Western blot analysis of cortical tissue for markers of cell-cycle activation after TBI. Roscovitine treatment attenuated TBI-induced increases (aP<0.05 versus sham) in cyclin A (panel A; *P=0.003 versus vehicle) and in cyclin D1 (panel B; *P=0.02 versus vehicle) expression at 6 hours after injury (C) Western blot analysis of caspase-dependent fodrin cleavage after TBI. Roscovitine treatment failed to reduce TBI-induced increases (aP<0.05 versus sham) in the 140/150-kDa cleavage product, but significantly attenuated levels of 120-kDa fodrin cleavage products after TBI (*P=0.02 versus vehicle). Representative western immunoblots are shown. Analysis by one-tailed paired Student's t-test, versus vehicle, and sham groups. Mean±s.e.m.; n=3 per group. TBI, traumatic brain injury.
Roscovitine Treatment Improves Functional Recovery and Reduces Lesion Size After Traumatic Brain Injury
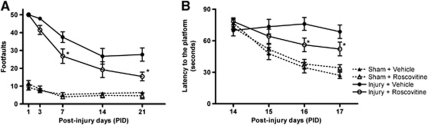
Functional assessment of fine-motor coordination was performed at various time points after injury using a beam walk test. Traumatic brain injury induced significant sensorimotor impairments at all time points when compared with sham-injured mice (Figure 2A; P<0.001 versus sham). Roscovitine-treated mice exhibited significant improvements in sensorimotor performance at 7 (P=0.01 versus vehicle) and 21 (P=0.0025 versus vehicle) days after injury when compared with vehicle-treated mice. There were no differences between sham-operated, vehicle-, and roscovitine-treated mice at any time point of motor assessment. Spatial learning was tested using the acquisition phase of the MWM test, and TBI resulted in learning impairments on 15, 16, and 17 days after injury (Figure 2B; P<0.001 versus sham). Roscovitine-treated mice showed improvements in cognitive performance with significantly reduced latency to find the submerged hidden platform on postinjury days 16 (P=0.007 versus vehicle) and 17 (P=0.02 versus vehicle) when compared with vehicle-treated mice. All TBI-injured mice performed well in the visual cue test, and swim speeds did not differ across groups (data not shown). There were no differences between sham-operated, vehicle-, and roscovitine-treated mice in cognitive outcomes.
Figure 2.
Roscovitine treatment improves functional recovery after TBI. (A) Fine-motor coordination deficits were quantified using a beam walk test. Hindlimb foot placement was recorded and the number of mistakes (foot faults) was recorded from 50 steps. Roscovitine treatment significantly improved fine-motor coordination at 7 (*P=0.01 versus vehicle) and 21 days (*P=0.0025 versus vehicle) after TBI. Analysis by repeated-measures one-way ANOVA, followed by post hoc adjustments using Tukey's test (interaction: F=4.58; groups: F=104.82; time: F=19.17). (B) Spatial learning and memory was assessed using a Morris water maze test. Roscovitine-treated mice had reduced latency to locate the submerged platform at days 16 (*P=0.005 versus vehicle) and 17 after TBI (*P=0.02 versus vehicle). Analysis by repeated-measures one-way ANOVA, followed by post hoc adjustments using Tukey's test. (interaction: F=4.63; groups: F=20.99; time: F=12.89). Mean±s.e.m.; n=11 to 12 per group. ANOVA, analysis of variance; TBI, traumatic brain injury.
Traumatic brain injury-induced lesion volume was measured by unbiased stereological techniques. Histologic assessment showed that vehicle-treated mice developed a large lesion after TBI (Figures 3A and 3B; 22.7±1.5 mm3), whereas roscovitine treatment resulted in a significant reduction in lesion size (P=0.003 versus vehicle; 11.9±1.4 mm3). There was a positive correlation between improved sensorimotor performance in the beam walk task and reduced lesion size owing to roscovitine treatment after TBI (Figure 3C; P<0.0001, r2=0.94).
Figure 3.
Roscovitine treatment reduces lesion size after TBI. (A) Unbiased stereological assessment of lesion volume at 21 days after TBI was performed on cresyl violet-stained brain sections. (B) Lesion quantification. Roscovitine treatment significantly reduced the lesion size at 21 days after TBI. (C) Linear regression analysis comparing TBI-induced lesion size with foot faults in the beam walk test at 21 days after TBI. Analysis by one-tailed paired Student's t-test in panel B (*P=0.003 versus vehicle) and Pearson's correlation (P<0.0001; r2=0.94) in panel C. Mean±s.e.m.; n=4 to 5 per group. TBI, traumatic brain injury.
Roscovitine Treatment Reduces Neuronal Degeneration and Progressive Neuronal Cell Loss in the Cortex and Dentate Gyrus After Traumatic Brain Injury
To determine the effects of central administration of roscovitine on TBI-induced neurodegeneration, we performed Fluoro-Jade B staining at 24 hours after TBI (Figure 4A). Representative confocal merged (Figure 4A1) and higher magnification images (Figure 4A1a and b) from the injured hemisphere in vehicle-treated mice indicate a large number of degenerating (Fluoro-Jade B positive) neurons, located around the lesion site (Figure 4A1b) and in subcortical regions (Figure 4A1a). In contrast, roscovitine-treated mice had less intense Fluoro-Jade B staining overall, around the lesion (Figure 4A2b) and subcortical regions (Figure 4A2a), suggesting fewer degenerating neurons.
Figure 4.
Roscovitine treatment attenuates neuronal degeneration and progressive neuronal cell loss in the cortex and dentate gyrus after TBI. (A) Qualitative assessment of neuronal degeneration at 24 hours after TBI using Fluoro-Jade B staining. Representative confocal images from vehicle-treated mice show degenerating neurons (Fluoro-Jade B positive) around the lesion injury site (1b) and in subcortical regions (1a), whereas roscovitine-treated mice had fewer Fluoro-Jade B-positive neurons at the injury site (2b) and in subcortical areas (2a), indicating attenuated neuronal degeneration. Higher magnification images from the indicated regions are shown. n=3 per group. (B) Representative images of cresyl violet-stained brain sections illustrate morphologic features of neurons and glial cells. Neurons have large stained cell bodies and processes and are indicated by white arrowheads. (C) Unbiased stereological quantification of neuronal cell loss in the cortex at 24 hours, 7 and 21 days after TBI. Progressive neuronal loss was observed in the cortex, and roscovitine treatment attenuated neuronal loss at 7 (*P=0.03 versus vehicle) and 21 days (*P=0.005 versus vehicle) after TBI. (D to F) Unbiased stereological quantification of neuronal cell loss in the CA1 (panel D), CA2/3 (panel E), and dentate gyrus (DG; panel F) subregions of the hippocampus at 24 hours, 7 and 21 days after TBI. Progressive neuronal loss was observed in the dentate gyrus and roscovitine treatment attenuated neuronal loss at 7 (*P=0.03 versus vehicle) and 21 days (*P=0.02 versus vehicle) after TBI. (G) Linear regression analysis comparing TBI-induced neuronal loss in the dentate gyrus with latency to reach the submerged platform on day 17 of the Morris water maze. Analysis by one-way ANOVA with Tukey's post hoc corrections in panels C to F for comparisons across different time points. One-tailed paired Student's t-test for comparison versus vehicle-treated groups at each time point. Counts/mm3; mean±s.e.m.; aP<0.01 versus sham, bP<0.05 versus 24 hours groups, *P<0.05 versus vehicle.; n=3 to 4 per group. Pearson's correlation (P<0.0001; r2=0.93) in panel G. ANOVA, analysis of variance; TBI, traumatic brain injury.
Stereological assessment of surviving neurons was performed in the cortex at 24 hours, 7 and 21 days after TBI (Figures 4B and 4C). Traumatic brain injury resulted in significant and progressive neuronal cell loss in the cortex over time (Figure 4C; P<0.01 versus sham). Central administration of roscovitine significantly improved neuronal survival in the cortex at both 7 (P=0.03 versus vehicle) and 21 (P=0.005 versus vehicle) days after injury, when compared with vehicle-treated samples (7 days: 119,947.6±13,410.2 counts/mm3 versus 68,737.1±4,723.5 counts/mm3 and 21 days: 159,706.6±7,820.6 counts/mm3 versus 74,072.6±10,260.6 counts/mm3, for roscovitine- and vehicle-treated samples, respectively).
Stereological assessment of surviving neurons was performed in CA1, CA2/3, and DG subregions of the hippocampus at 24 hours, 7 and 21 days after TBI. Traumatic brain injury resulted in rapid and significant neuronal loss in the CA1 subregion within 24 hours after injury (Figure 4D; P<0.001 versus sham), but additional neuronal loss was not apparent at later time points. A similar pattern of neuronal loss was observed in the CA2/3 region after TBI with a nonsignificant trend towards progressive neuronal loss over time (Figure 4E; P<0.01 versus sham). Roscovitine treatment failed to protect CA1 or CA2/3 neurons at any time point. In contrast, TBI resulted in significant and progressive neuronal loss in the DG over time (Figure 4F; P<0.001 versus sham). Furthermore, roscovitine treatment resulted in significantly improved neuronal survival in the DG at both 7 (P=0.03 versus vehicle) and 21 (P=0.02 versus vehicle) days after injury when compared with vehicle-treated samples (7 days: 563,034.8±18,486.7 counts/mm3 versus 364,984.0±18,979.8 counts/mm3 and 21 days: 480,858.7±11,347.3 counts/mm3 versus 347,507.9±16,396.1 counts/mm3, for roscovitine- and vehicle-treated samples, respectively). In addition, there was a strong correlation between improved cognitive performance in the MWM test and reduced neuronal cell loss in the DG owing to roscovitine treatment after TBI (Figure 4G; P<0.0001, r2=0.93).
Microglial Activation is Modulated by Roscovitine Treatment After Traumatic Brain Injury
Brain injury results in a switch in the microglial phenotype from a resting form displaying ramified cellular morphologies to more activated forms displaying hypertrophic or bushy morphologies, with the latter being the most reactive (Figure 5A; Davis et al, 1994; Soltys et al, 2001; Zhan et al, 2008). Stereological assessment of microglial cell number and activation phenotype was performed in the cortex at 24 hours, 7 and 21 days after TBI. There was a significant decrease in ramified microglia in vehicle- and roscovitine-treated samples at 21 days after injury when compared with sham controls (Figure 5B; P<0.05 versus sham). TBI resulted in a significant increase in hypertrophic microglia at 7 days after injury, followed by a return to control levels by 21 days (Figure 5C; P<0.05 versus sham). Roscovitine treatment had no effect on the levels of hypertrophic microglia. In contrast, TBI resulted in a marked and more sustained increase in bushy microglia through 21 days (Figure 5D; P<0.05 versus sham). Roscovitine treatment significantly reduced bushy microglia at both 7 (P=0.049 versus vehicle) and 21 days (P=0.02 versus vehicle) when compared with vehicle-treated samples (7 days: 4,612.4±560.6 counts/mm3 versus 9,494.5±1,184.7 counts/mm3 and 21 days: 3,584.5±767.8 counts/mm3 versus 6,578.1±709.2 counts/mm3, for roscovitine- and vehicle-treated samples, respectively).
Figure 5.
Microglial activation is modulated by roscovitine treatment after TBI. (A) Representative immunohistochemical images and Neurolucida reconstructions of ramified, hypertrophic, and bushy microglia illustrate the different morphologic features of each microglial phenotype. (B to D) Unbiased stereological quantification of microglial cell number and activation status in the cortex at 24 hours, 7 and 21 days after TBI. Ramified (panel B), hypertrophic (panel C), and bushy (panel D) microglial activation phenotypes were analyzed. Roscovitine treatment attenuated bushy microglia at 7 (*P=0.049 versus vehicle) and 21 days (*P=0.02 versus vehicle) after TBI. Analysis by ANOVA with Tukey's post hoc corrections for comparisons across different time points. One-tailed paired Student's t-test for comparison versus vehicle-treated groups at each time point. Counts/mm3; mean±s.e.m.; aP<0.05 versus sham, bP<0.05 versus 24 h groups, cP<0.05 versus 7 day groups; n=3 to 4 per group. ANOVA, analysis of variance; TBI, traumatic brain injury.
Systemic Administration of Roscovitine Improves Functional Recovery and Inhibits Microglial Activation After Traumatic Brain Injury
A separate group of animals was used to investigate the therapeutic potential of delayed systemic administration of roscovitine after TBI. Systemic administration of roscovitine resulted in significant improvements in sensorimotor performance at 14 (P=0.01 versus vehicle) and 21 (P=0.024 versus vehicle) days after injury when compared with vehicle-treated mice (Figure 6A). Similar to the central administration study, TBI resulted in a significant increase in hypertrophic microglia at 7 days after injury, followed by a return to control levels by 21 days (Figure 6B; P<0.05 versus sham). Systemic roscovitine treatment significantly limited these changes at 7 (P=0.04 versus vehicle) and 21 days (P=0.02 versus vehicle) when compared with vehicle-treated samples (7 days: 4,852.6±1,088.9 counts/mm3 versus 8,782.6±1,298.1 counts/mm3 and 21 days: 2,248.4±278.1 counts/mm3 versus 3,839.6±393.9 counts/mm3, for roscovitine- and vehicle-treated samples, respectively). Similarly, TBI caused a marked and sustained increase in the number of bushy microglia through 21 days (Figure 6C; P<0.05 versus sham) and systemic roscovitine treatment significantly reduced these changes at 21 days (P=0.048 versus vehicle) when compared with vehicle treatment (2,668.6±780.1 versus 6,950.6±946.9 counts/mm3).
Figure 6.
Systemic administration of roscovitine improves functional recovery and inhibits microglial activation after TBI. (A) Fine-motor coordination deficits were quantified using a beam walk test. Systemic roscovitine treatment significantly improved fine-motor coordination at days 14 (*P<0.01 versus vehicle) and 21 (*P<0.02 versus vehicle) after TBI. Analysis by repeated-measures one-way ANOVA, followed by post hoc adjustments using Student–Newman–Keuls test (interaction: F=6.12; groups: F=197.40; time: F=23.10). Mean±s.e.m.; n=6 per group. (B, C) Unbiased stereological quantification of microglial cell number and activation status in the cortex at 24 hours, 7 and 21 days after TBI. Hypertrophic (panel C) and bushy (panel D) microglial activation phenotypes were analyzed. Roscovitine treatment attenuated hypertrophic microglia at 7 (*P=0.04 versus vehicle) and 21 days (*P=0.02 versus vehicle), and bushy microglia at 21 days (*P=0.048 versus vehicle) after TBI. Analysis by ANOVA with Tukey's post hoc corrections for comparisons across different time points. One-tailed paired Student's t-test for comparison versus vehicle-treated groups at each time point. Counts/mm3; mean±s.e.m.; aP<0.05 versus sham, *P<0.05 versus vehicle, bP<0.05 versus 24 h groups, cP<0.05 versus 7 day groups; n=3 to 4 per group. ANOVA, analysis of variance; TBI, traumatic brain injury.
Discussion
Our data confirm the important role for CCA in secondary injury after TBI, and we show the neuroprotective potential of delayed central and systemic administration of a selective CDK inhibitor, roscovitine, after experimental TBI. Furthermore, the use of quantitative unbiased stereological assessment to address TBI-induced neuronal loss over time and its temporal/anatomic associations with microglial phenotype are also noteworthy.
The involvement of CCA in the pathophysiology of both brain and spinal cord trauma has been suggested previously (Byrnes and Faden, 2007; Byrnes et al, 2007; Cernak et al, 2005; Giovanni et al, 2005; Hilton et al, 2008). Previously, we have shown the neuroprotective effects of flavopiridol, a powerful although nonselective CDK inhibitor with other actions, after experimental TBI (Cernak et al, 2005; Giovanni et al, 2005). Roscovitine is a more selective CDK inhibitor, which acts specifically on CDKs 1, 2, and 5, and possibly on CDKs 7 and 9 (Meijer et al, 1997). Several studies have indicated that roscovitine is one of the most specific CDK inhibitors (Bach et al, 2005; Bain et al, 2007; Meijer et al, 1997). Central administration of roscovitine 30 minutes after injury decreased lesion volume and improved behavioral outcomes in a rat lateral fluid percussion model (Hilton et al, 2008). A recent study reported that intravenous administration of roscovitine was also neuroprotective in a focal cerebral ischemia model (Menn et al, 2010).
Compared with lateral fluid percussion, CCI injury is a pathobiologically distinct model that produces a focal injury, resulting in significant pathophysiological alterations, such as vascular disruption, cerebral edema, elevated intracerebral pressure, as well as significant long-term neurologic deficits (Cernak, 2005; Dixon et al, 1991). The CCI model has been extensively used to investigate the molecular and cellular events that occur during secondary injury and to evaluate novel therapeutic approaches (Faden et al, 2003; Loane et al, 2009; Mori et al, 1998).
Here, we show that increased expression of cyclin D1 and A, previously associated with neuronal death, is detected at 6 hours after injury. Central administration of roscovitine significantly reduced expression of these two key cyclins, showing the capacity of roscovitine to inhibit cell-cycle pathways in this brain injury model. Furthermore, our data show that TBI induced elevated levels of fodrin cleavage products; fodrin is a high molecular-weight (240 kDa) cytoskeletal protein that undergoes degradation catalyzed by activated caspases and other proteases during apoptosis, generating N-terminal 150-kDa and C-terminal 120-kDa products (Siman et al, 1984, 2004). The 120-kDa fragment primarily indicates a caspase-mediated cleavage, whereas the release of the 145/150-kDa fragment is both calpain (145 kDa) and caspase (150 kDa) mediated (Saatman et al, 2010). Roscovitine significantly decreases the levels of the 120-kDa cleavage product, in a manner that was temporally correlated with inhibition of the CCA, suggesting a connection between cell cycle and caspase-dependent apoptosis in the brain after TBI.
To assess neuronal survival after TBI, we performed Fluoro-Jade B staining at 24 hours. Traumatic brain injury caused extensive neurodegeneration, as indicated by dense Fluoro-Jade B-positive neurons not only around the lesion site but also within the subcortical regions. Our observation is in agreement with previous studies that have suggested that ischemia- and CCI-induced damage to the cortex may cause retrograde degeneration of neurons in subcortical regions (Hall et al, 2005; Iizuka et al, 1990). In contrast, roscovitine-treated mice showed fewer degenerating neurons, suggesting neuronal survival caused by this pharmacological intervention.
To quantify TBI-induced neurodegeneration in the cortex, we estimated neuronal cell loss in the cortex using unbiased stereological techniques. Cresyl violet staining can be used to distinguish morphologic features of neurons and glial cells (Martin, 1996; Nauta and Feirtag, 1986). Cresyl violet staining of neuroglial cells is confined to their nuclei, whereas neurons stain within the cell body, thereby enabling easy identification of neurons (Figure 6B). We observed a progressive neuronal loss in the cortex over time, beginning at 24 hours and continuing until 21 days, which provided further evidence of activated secondary injury processes initiated by TBI. Central administration of roscovitine after injury caused a significant recovery in neuronal cortical loss at 7 and 21 days.
To further understand TBI-induced neurodegeneration, we quantified neuronal cellular densities (number of neurons per unit volume) as a measure of neuronal loss in CA1, CA2/3, and DG subregions of the hippocampus using unbiased stereological techniques. This is to our knowledge the first detailed and unbiased analysis of progressive neuronal loss in specific regions of the hippocampus after CCI. Neuronal loss in the CA1 and CA2/3 regions was rapid with considerable loss by 24 hours after injury and nonsignificant further loss at later time points, suggesting the limited duration of secondary injury in these regions. In contrast, neuronal loss in the DG was more modest in the first 24 hours after injury, but was followed by a significant and progressive neuronal loss at later time points, indicating robust and continuing secondary injury processes in this region. Importantly, animals that received central administration of roscovitine showed significantly less neuronal loss at later time points in the DG region; in contrast, roscovitine treatment had no impact on the CA1 and CA2/3 regions. Roscovitine also significantly attenuated cognitive deficits in acquisition trials of the MWM task of spatial learning and memory. Improved cognitive performance in the MWM serves as a potential indicator of reduced hippocampal damage (Redish and Touretzky, 1998). The relative contribution and significance of different subregions in encoding and retrieval of learning and memory function can be quantified by counting neuronal cells in these regions (Gilbert and Brushfield, 2009; Lee and Kesner, 2004; Lee et al, 2005). Our data show a strong positive correlation between improvements in cognitive function and increased neuronal survival in the DG region of the hippocampus in roscovitine-treated animals (Figure 4D). The close correlation between improved cognition and reduced DG neuronal cell loss underscores the importance of the DG subregion of the hippocampus in the formation of spatial learning and memory (Lee et al, 2005) and indicates that trauma-induced loss of neurons in this region contributes to cognitive impairment. Importantly, the region (DG) showing the most persistent secondary injury is also the most susceptible to roscovitine therapeutic effects.
The size of the cortical lesion provides another good marker for secondary injury-mediated tissue loss and neurodegeneration. Unbiased stereology was used to quantify lesion volume after TBI, and the data showed a significant reduction in lesion size in mice that received central administration of a single dose of roscovitine 3 hours after injury compared with the vehicle-treated group. These roscovitine-treated animals showed markedly improved sensorimotor outcomes, a measure of cortical function, compared with vehicle-treated groups. Regression analysis showed a strong correlation between improved motor function and reduced lesion size. Traumatic brain injury-induced gait and hindlimb stride deficits that result in foot faults in the beam walk test are indicators of lesions in the hindlimb sensorimotor cortex (Goldstein, 2003). Thus, the extent of locomotor impairment after injury to the sensorimotor cortex could be directly correlated with the size of the lesion and both parameters are improved by roscovitine treatment.
Chronic inflammation after central nervous system trauma may provide a mechanistic link between early and chronic neurodegeneration (Nandoe, 2002). To better assess the neuroinflammatory response after brain trauma, we performed a quantitative assessment of microglial activation after TBI. Microglial activation status was based on morphologic features using unbiased stereology, and we determined microglia cell density (number of cells of each phenotype per unit of volume) in the injured cortex at various time points after injury. Although both neuroprotective and neurotoxic microglial phenotypes have been described (Davis et al, 1994; Loane and Byrnes, 2010; Soltys et al, 2001), microglial activation and the release of associated inflammatory factors have been proposed as an important contributing factor in chronic neurodegenerative disorders, including Alzheimer's disease (Eikelenboom et al, 2002). Our previous studies have indicated that sustained microglial activation after central nervous system trauma may have a role in neuronal cell loss after the release of neurotoxic molecules such as nitric oxide (Byrnes and Faden, 2007; Byrnes et al, 2007). On the basis of morphology, microglia can be classified into three categories corresponding to increasing activation status: ramified (resting), hypertrophic, and bushy (Davis et al, 1994; Soltys et al, 2001; Zhan et al, 2008). Ramified microglia (Figure 5A) have small cell bodies, thin and highly branched processes. In contrast, the hypertrophic microglia (Figure 5A) have larger cell bodies with thicker and shorter processes (Zhan et al, 2008), whereas bushy microglia (Figure 5A) have multiple short processes that form thick bundles around enlarged cell bodies (Zhan et al, 2008). Central nervous system injury causes transformation of ramified microglia into more active phenotypes, such as hypertrophic and bushy forms (Soltys et al, 2001). The relative proportion of each microglial phenotype determines the extent of microglial activation and inflammation. This is to our knowledge the first detailed analysis of progressive microglial activation in the cortex after CCI. In this study, we observed significant increases in activated hypertrophic and bushy microglial phenotypes after brain trauma. No significant changes were detected at 24 hours after injury in any phenotype, suggesting that this process is relatively slow to start. The numbers of hypertrophic microglia decreased 21 days after injury, but the highly activated bushy microglia continued to be significantly elevated as compared with sham controls at this time, underscoring the chronic nature of such microglia activation. We recorded a trend towards decreasing numbers of ramified microglia 21 days after TBI, suggesting that these cells are displaced or converted to the more active phenotypes. Previous studies suggested that CDK inhibitors reduce glial scar formation and microglial activation after TBI (Cernak et al, 2005; Giovanni et al, 2005; Hilton et al, 2008). Here, we take advantage of the quantitative and activation-dependent microglial classification to show that central administration of roscovitine significantly and consistently attenuated microglial activation as indicated by the reduced numbers of activated bushy microglia at both 7 and 21 days after TBI. The robust effect of roscovitine on reactive microglia might reflect an important component of the overall roscovitine-induced neuroprotection.
We also performed a proof-of-principle study to examine the effects of systemic administration of roscovitine because it is a more clinically relevant route of administration. Systemic administration of roscovitine did not cause any effects on physiologic variables, such as heart rate, oxygen saturation levels, and breathing rate (data not shown), but limited motor dysfunction and reduced the number of activated microglia at 7 and 21 days after TBI. However, it did not significantly improve trauma-induced cognitive impairment or lesion volume (data not shown); this may reflect the small number of animals used in this proof-of-principle study or possibly the inability of systemic roscovitine at the dose used to reach optimal therapeutic concentrations in the injured brain. The robust effect of systemic roscovitine treatment on microglial activation may indicate an effect on circulating macrophages, which are believed to significantly contribute to the activated microglia populations after trauma. However, systemic roscovitine administration after TBI did not affect the number of peripheral circulating cells, such as monocytes, lymphocytes, neurotrophils, basophils, and eosinophils (data not shown). Further studies are required to clarify these questions and to better delineate the penetration of the drug through the blood brain barrier after systemic delivery.
In conclusion, our findings show the progressive and sustained nature of neurodegeneration and neuroinflammation after TBI. Administration of the CDK inhibitor roscovitine reduced progressive neuronal cell loss/neurodegeneration in the hippocampus and cortex, and attenuated microglial activation/neuroinflammation in the cortex. Roscovitine also improved functional recovery and reduced lesion size after TBI. The therapeutic effects of roscovitine likely reflect its multipotential neuroprotective activities owing to direct inhibitory effects on CCA-dependent neuronal cell death, as well as microglial-mediated neuroinflammation. Our study provides further evidence that CDK inhibitors may be promising therapeutic agents for the treatment for TBI.
Acknowledgments
The authors thank Shihong Li, Kelly Wilson, Nicole Hockenbury, Michael Dinizo, Michael Murray, and Rainier Cabatbat for their expert technical assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by a grant from the National Institutes of Health, R01 NS052568.
References
- Arendt T. Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the ‘Dr Jekyll and Mr Hyde concept' of Alzheimer's disease or disease or the yin and yang of neuroplasticity. Prog Neurobiol. 2003;71:83–248. doi: 10.1016/j.pneurobio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bach S, Knockaert M, Reinhardt J, Lozach O, Schmitt S, Baratte B, Koken M, Coburn S, Tang L, Jiang T, Liang D, Galons H, Dierick J, Pinna L, Meggio F, Totzke F, Schächtele C, Lerman A, Carnero A, Wan Y, Gray N, Meijer L. Roscovitine targets, protein kinases and pyridoxal kinase. J Biol Chem. 2005;280:31208–31219. doi: 10.1074/jbc.M500806200. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shapiro N, Hastie C, McLauchlan H, Klevernic I, Arthur J, Alessi D, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett H, Dietrich W. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Byrnes K, Faden A. Role of cell cycle proteins in CNS injury. Neurochem Res. 2007;32:1799–1807. doi: 10.1007/s11064-007-9312-2. [DOI] [PubMed] [Google Scholar]
- Byrnes K, Stoica B, Fricke S, Di Giovanni S, Faden A. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain. 2007;130:2977–2992. doi: 10.1093/brain/awm179. [DOI] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Stoica B, Byrnes K, Di Giovanni S, Faden A. Role of the cell cycle in the pathophysiology of central nervous system trauma. Cell Cycle. 2005;4:1286–1293. doi: 10.4161/cc.4.9.1996. [DOI] [PubMed] [Google Scholar]
- Davis E, Foster T, Thomas W. Cellular forms and functions of brain microglia. Brain Res Bull. 1994;34:73–78. doi: 10.1016/0361-9230(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Dixon C, Clifton G, Lighthall J, Yaghmai A, Hayes R. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dutton R, Stansbury L, Leone S, Kramer E, Hess J, Scalea T. Trauma mortality in mature trauma systems: are we doing better? An analysis trauma mortality patterns, 1997–2008. J Trauma. 2010;69:620–626. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Bate C, Van Gool W, Hoozemans J, Rozemuller J, Veerhuis R, Williams A. Neuroinflammation in Alzheimer's disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- Faden AI, Fox GB, Di X, Knoblach SM, Cernak I, Mullins P, Nikolaeva M, Kozikowski AP. Neuroprotective and nootropic actions of a novel cyclized dipeptide after controlled cortical impact injury in mice. J Cereb Blood Flow Metab. 2003;23:355–363. doi: 10.1097/01.WCB.0000046144.31247.33. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald M, Coronado V. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- Freeman RS, Estus S, Johnson EM., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanni SD, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden A. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LB. Model of recovery of locomotor ability after sensorimotor cortex injury in rats. ILAR J. 2003;44:125–129. doi: 10.1093/ilar.44.2.125. [DOI] [PubMed] [Google Scholar]
- Greene L, Biswas S, Liu D. Cell cycle molecules and vertebrate neuron death: E2F at the hub. Cell Death Differ. 2004;11:49–60. doi: 10.1038/sj.cdd.4401341. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology. Nat Rev Neurosci. 2007;8:368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- Hilton G, Stoica B, Byrnes K, Faden A. Roscovitine reduces neuronal cell loss, glial activation, and neurological deficits after brain trauma. J Cereb Blood Flow Metab. 2008;28:1845–1859. doi: 10.1038/jcbfm.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke. 1990;21:790–794. doi: 10.1161/01.str.21.5.790. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, vanderEb A, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner R. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Weinberg R. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-CDK complexes. Mol Cell Biol. 1998;23:1044–1053. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH.(ed). (1996Neuroanatomy, Text and Atlas Appleton & Lange: Stamford, CT [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Menn B, Bach S, Blevins TL, Campbell M, Meijer L, Timsit S. Delayed treatment with systemic (S)-roscovitine provides neuroprotection and inhibits in vivo CDK5 activity increase in animal stroke models. PLoS One. 2010;5:e12117. doi: 10.1371/journal.pone.0012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kawamata T, Katayama Y, Maeda T, Aoyama N, Kikuchi T, Uwahodo Y. Antioxidant, OPC-14117, attenuates edema formation, and subsequent tissue damage following cortical contusion in rats. Acta Neurochir Suppl. 1998;71:120–122. doi: 10.1007/978-3-7091-6475-4_36. [DOI] [PubMed] [Google Scholar]
- Nandoe R. Head trauma and Alzheimer's disease. J Alzheimer's Dis. 2002;4:303–308. doi: 10.3233/jad-2002-4405. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Feirtag M.(eds). (1986Fundamental Neuroanatomy New York: WH Freeman and Company [Google Scholar]
- Nguyen M, Boudreau M, Kriz J, Couillard-Despres S, Kaplan D, Julien J. Cell cycle regulators in the neuron death pathway of amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J Neurosci. 2003;23:2131–2140. doi: 10.1523/JNEUROSCI.23-06-02131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutley BP, Raynaud FI, Wilson SC, Fischer PM, Hayes A, Goddard PM, McClue SJ, Jarman M, Lane DP, Workman P. Metabolism and pharmacokinetics of the cyclin-dependent kinase inhibitor R-roscovitine in the mouse. Mol Cancer Ther. 2005;4:125–139. [PubMed] [Google Scholar]
- Panter SS, Faden AI. Pretreatment with NMDA antagonists limits release of excitatory amino acids following traumatic brain injury. Neurosci Lett. 1992;136:165–168. doi: 10.1016/0304-3940(92)90040-e. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Comput. 1998;10:73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Baudry M, Lynch G. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci USA. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL. Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiol Dis. 2004;16:311–320. doi: 10.1016/j.nbd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Soltys Z, Ziaja M, Pawlinski R, Setkowicz Z, Janeczko K. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J Neurosci Res. 2001;63:90–97. doi: 10.1002/1097-4547(20010101)63:1<90::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Byrnes KR, Faden AI. Cell cycle activation and CNS injury. Neurotox Res. 2009;16:221–237. doi: 10.1007/s12640-009-9050-0. [DOI] [PubMed] [Google Scholar]
- Zhan X, Kim C, Sharp F. Very brief focal ischemia simulating transient ischemic attacks TIAs) can injure brain and induce Hsp70 protein. Brain Res. 2008;1234:184–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]