Abstract
To develop a less-stressful and simple method for measurement of the cerebral metabolic rate of oxygen (CMRO2) in small animals, the steady-state method was applied to injectable 15O2-PET (15O2-positron emission tomography) using hemoglobin-containing vesicles (15O2-HbV). Ten normal rats and 10 with middle cerebral arterial occlusion (MCAO) were studied using a small animal PET scanner. A series of 15O-PET scans with C15O-labeled HbV, H215O, and 15O2-HbV were performed with 10 to 15 minutes intervals to measure cerebral blood volume (CBV), cerebral blood flow (CBF), and CMRO2. Positron emission tomography scans were started with a tracer injection using a multiprogramming syringe pump, which provides a slowly increasing injection volume to achieve steady-state radioactivity for H215O and 15O2-HbV scans. The radioactivity concentration of 15O rapidly achieved equilibrium in the blood and whole brain at about 2 minutes after H215O and 15O2-HbV administration, which was stable during the scans. The whole brain mean values of CBF, CBV, and CMRO2 were 54.3±2.0 mL per 100 g per minute, 4.9±0.4 mL/100 g, and 2.8±0.2 μmoL per g per minute (6.2±0.4 mL per 100 g per minute) in the normal rats, respectively. In the MCAO model rats, all hemodynamic parameters of the infarction area on the occlusion side significantly decreased. The steady-state method with 15O-labeled HbV is simple and useful to analyze hemodynamic changes in studies with model animals.
Keywords: 15O-gas PET, cerebral oxygen metabolism, hemoglobin-containing vesicles, injectable 15O2, steady-state method
Introduction
15O-gas positron emission tomography (PET) has been used to clarify the mechanisms of cerebrovascular diseases (CVDs) using PET (Temma et al, 2006, 2008). An intravenous injectable 15O2 method was proposed using a special device and the blood was collected from another rat because radioactivity of 15O-gas from the airways affects measurement of cerebral radioactivity counts (Magata et al, 2003; Temma et al, 2006). Our previous study reported the feasibility of another injectable 15O2 method using artificial hemoglobin-containing vesicles (HbV), which does not require killing of other rats for blood collection and the special device (Tiwari et al, 2010). Since these injectable 15O2 methods applied bolus injection of tracers to measure cerebral hemodynamic parameters, multipoint arterial blood samplings to obtain arterial input functions were required, which may be stressful for small animals and may change physical conditions. Therefore, we developed a new steady-state method for cerebral blood flow (CBF) measurement using H215O and a multiprogramming syringe pump that provides a slowly increasing injection volume to reduce rats' stress (Kobayashi et al, 2011). The steady-state method is ideal for small animal studies because the injection volumes and blood samplings are limited.
The purpose of this study was to apply the steady-state method to 15O2-labeled HbV (15O2-HbV) PET scans to measure the cerebral metabolic rate of oxygen (CMRO2). C15O-labeled HbV (C15O-HbV) PET was also performed to obtain the cerebral blood volume (CBV), which is usually used for correction of the oxygen extraction fraction (OEF).
Materials and methods
Labeling Procedure of Hemoglobin-Containing Vesicles
The synthesis and purification processes of HbV (Oxygenix Co. Ltd., Tokyo, Japan) and rheological properties of HbV were described previously in detail (Phillips et al, 1997; Ogata, 2000; Takeoka et al, 2002; Tiwari et al, 2010). -cysteine (2.8 mM) was added to HbV vials, which were kept under anoxic conditions. The HbV was labeled with C15O and 15O2 by the method reported previously (Tiwari et al, 2010). The HbV solution of 2 mL for C15O labeling and 8 mL for 15O2 labeling was prepared in a 50-mL volume vial. Target gas containing C15O or 15O2 was delivered into the HbV solution through a bubbling needle. The bubbling time of the radioactive gas was 2 minutes, and the vial was vortexed during 15O2 bubbling. Labeled HbV was then sampled using a syringe and radioactivity was measured using a dose meter (Capintec Inc., Ramsey, NJ, USA).
Animal Positron Emission Tomography Studies
Animal studies were approved by the Animal Care Committee at the University of Fukui and conducted in accordance with the international standards for animal welfare and institutional guidelines. Adequate measures were taken to minimize pain and discomfort. Male Sprague-Dawley rats from Japan SLC Inc. (Hamamatsu, Japan) were housed for 1 week under a 12-hour light/12-hour dark cycle with free access to food and water. The rats were fasted with no food overnight with water supplied ad libitum before the PET experiments.
Twenty rats (298.5±8.8 g) were anesthetized with an intraperitoneal injection of chloral hydrate (0.4 mg/g body weight, intraperitoneally). A PE-50 catheter was inserted into the femoral artery for blood sampling and the femoral vein for 15O-radiotracers administration. In 10 rats, the left middle cerebral artery (MCA) was occluded intraluminally using a nylon 4-0 surgical monofilament (Ethilon; Ethicon, Inc., Somerville, NJ, USA) for 1 hour before the PET scans (Longa et al, 1989; Kuge et al, 1995). The PET studies were performed with a list-mode scan protocol using a small animal PET scanner (SHR-41000; Hamamatsu Photonics, Hamamatsu, Japan) (Yamada et al, 2008). The scanner acquires 213 slices covering an axial length of 160 mm, with a three-dimension mode, achieving a resolution of ∼2.0 mm full width at half maximum in the transaxial direction and 2.8 mm full width at half maximum in the axial direction. The rats were placed in a supine position on the scanner bed, and the limbs were fixed using surgical tape. The orientation of the cranial position was determined using a laser beam on the scanner. During the PET scan, body temperature was maintained at about 37°C using a controller (TC-1, Brainscience idea, Osaka, Japan). Before emission scans, a transmission scan was performed for attenuation correction using a 68Ge/68Ga external source. A 3-minute PET scan was started with an intravenous bolus administration of about 20 MBq C15O-HbV in a 0.4-mL injection volume. Approximately 10 μL of arterial blood was sampled every 1 minute, and radioactivity in the blood samples was immediately measured with a well scintillation counter (ARC380; Aloka, Tokyo, Japan). After the decay of C15O, a 5-minute scan was started with intravenous administration of ∼185 MBq H215O. The 3-mL tracer was injected using a 10-mL syringe set up on a multiprogramming syringe pump (FP-2000; Melquest, Toyama, Japan). Details of this steady-state method were described in our previous report (Kobayashi et al, 2011). About 50 μL arterial blood was sampled every 1 minute during the H215O scan, and radioactivity in the blood samples was immediately measured with the well scintillation counter. After the decay of H215O, a 5-minute PET data acquisition was started with intravenous administration of ∼185 MBq 15O2-HbV in a 3-mL volume using the same injection method as for the H215O scan. Briefly, the injection speed was controlled rapidly for the first 5 seconds to fill the dead volume of the venous line with a small amount of overshoot, the rate was then changed moderately for 40 seconds to increase blood radioactivity, followed by a slow but gradually increasing administration rate to compensate for the decay in blood 15O radioactivity. To compensate for the decay in blood 15O radioactivity in the later phase, the injection rate was continuously changed under the assumption of an inverse decay function as expressed by the following equation:
here v (μL/min) is the injection velocity for the syringe pump, λ (/min) is the decay constant of 15O, and t (minutes) is the time after the injection of 15O-radiotracers. The same constants were used in the equation for both 15O2-HbV and H215O administration. About 200 μL arterial blood was sampled every 1 minute during the 15O2-HbV scan. In all, 50 μL of the arterial blood was put into a microtube to measure whole blood radioactivity. In all, 50 μL acetonitrile, a deproteinization agent, was added into the remaining arterial blood and the sample was centrifuged at 3000 g for 2 minutes. About 100 μL of supernatant fluid including acetonitrile was obtained, and radioactivity was immediately measured with a well scintillation counter. Arterial blood gases and other physiological parameters were measured using a blood gas analyzer (ABL555; Radiometer, Copenhagen, Denmark) and a hemooximeter (OSM3; Radiometer) after the transmission scan, and before and after the 15O2-HbV PET scan.
Magnetic Resonance Imaging Scan and 2,3,5-Triphenyl-Tetrazolium Chloride Staining
After PET scans, the normal rats and 3 of the 10 MCA occlusion (MCAO) model rats were scanned by magnetic resonance imaging (MRI) using a 3.0-T MRI scanner (Signa Horizon, GE Medical Systems, Milwaukee, WI, USA). In the MRI scans, the rats were placed and fixed between a pair of surface coils. T2-weighted images of each rat's brain were acquired using a fast spin echo method with repetition time/echo time of 5050/85 milliseconds, 256 × 256 matrix, slice thickness 1.0 mm, spacing 0 mm, field of view 10 cm, phase-field of view 8 cm and excitation number of 3. The rat brains were removed immediately after the MRI scans, sliced and stained with 2,3,5-triphenyl-tetrazolium chloride (TTC). The remaining seven rats with MCAO were killed immediately after the PET scans, and the brains were sliced and stained with TTC.
Data Analysis
The PET images were reconstructed using the Fourier rebinning-filtered back projection method with an attenuation correction, calibrated by the crosscalibration factor. The CBV image was calculated using PET data averaged for the last 2 minutes of the 3-minute C15O-HbV scan because the time–activity curve of the brain achieved a plateau at about 1 minute after the tracer administration. The radioactivity concentration of the arterial blood sampled every 1 minute after the C15O-HbV injection was averaged and used for the CBV calculation. The CBF was calculated from H215O images using the steady-state method as reported previously (Kobayashi et al, 2011). The average image of the last 3 minutes data of the 5-minute H215O-PET scan was used for the CBF calculation.
Since the radioactivity concentrations of the brain and arterial blood reached a plateau about 2 minutes after 15O2-HbV administration (see Results), the average of the last 3 minutes data of the 5-minute 15O2-HbV scan were used for OEF and CMRO2 calculations. The OEF image was calculated from the averaged images of H215O and 15O2-HbV scans based on the established steady-state method with the following equations (Frackowiak et al, 1980; Lammertsma et al, 1982);
 |
where OEF' is uncorrected OEF, CbO2, and CbH2O (Bq/mL) are the average brain radioactivity concentration of the 15O2 and H215O images. CaO2, CpO2, CaH2O, and CpH2O (Bq/mL) are the mean arterial and plasma concentrations in the 15O2 and H215O scans, respectively. Hta is arterial hematocrit. The CBV correction method was applied to the calculation of OEF from OEF' as follows (Lammertsma et al, 1983; Lammertsma and Jones, 1983).
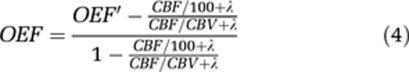 |
In the equation (4), λ is the decay constant of 15O. The CMRO2 was calculated by the following equation using the CBF, OEF images, and arterial O2 content (O2c), which was obtained from the blood gas and hemoglobin data of each rat.
The parametric PET images of normal rats were coregistered with each MRI image using Dr View software (AJS, Tokyo, Japan). In the analysis of normal rats, MRI coronal slices were used to draw ROIs (regions of interest) at three slice levels of the whole brain, frontal cortex, visual cortex, striatum, thalamus, and sensorimotor cortex referring to the rat brain atlas (Kobayashi et al, 2011). The same ROIs were applied to all parametric PET images to obtain cerebral hemodynamic parameters in each normal rat. In the MCAO model rats, multiple circle ROIs (2.4 mm in diameter) were placed on the infarction area and the corresponding contralateral regions with reference to the TTC staining brain slices, as well as the MRI images in the three MCAO model rats. The hemodynamic parameters of the affected cortex in the MCAO model rats were compared with the corresponding contralateral region and those of the sensorimotor cortex in the normal rats using one-way analysis of variance with a post hoc Sheffe's F-test. Blood gas data in the normal and MCAO model rats were also compared using repeated-measures analysis of variance and a post hoc paired t-test. A P value of <0.05 was considered statistically significant.
Results
Labeling Efficiency and Physiological Parameters
Labeling efficiency values of H215O, C15O-HbV, and 15O2-HbV were 123.4±3.4, 65.7±12.2, and 95.7±4.1 MBq/mL, respectively.
Table 1 shows arterial blood gas data after the transmission scan, and before and after the 15O2-HbV PET in the normal and MCAO model rats. Only PCO2 values of the MCAO model rats decreased significantly for all conditions compared with normal rats (P<0.05), although no rat group showed any difference among conditions (repeated-measures analysis of variance). The injection volume of the 15O2-HbV and H215O was 1.65 mL during the 5-minute PET scan. The sampled blood volumes were about 0.65 and 1.40 mL for the H215O and 15O2-HbV scans, respectively.
Table 1. Arterial blood gas data in normal and MCAO model rats.
|
After transmission |
Before
15O2-HbV |
After
15O2-HbV |
||||
|---|---|---|---|---|---|---|
| Normal | MCAO | Normal | MCAO | Normal | MCAO | |
| pH | 7.31±0.04 | 7.31±0.03 | 7.32±0.06 | 7.32±0.06 | 7.32±0.05 | 7.32±0.06 |
| PCO2 (mm Hg) | 47.8±2.2 | 44.0±2.6* | 48.6±1.5 | 39.9±3.8* | 49.0±1.1 | 42.2±3.7* |
| PO2 (mm Hg) | 88.2±4.0 | 87.0±6.0 | 88.1±5.8 | 88.0±5.6 | 90.5±6.5 | 87.1±7.9 |
| Hct (%) | 48.0±2.3 | 49.2±2.0 | 47.3±2.3 | 49.5±1.8 | 47.3±2.0 | 48.3±1.7 |
| O2 Sat (%) | 96.7±1.2 | 95.8±2.3 | 95.4±2.1 | 94.7±2.3 | 97.0±1.3 | 94.9±3.9 |
| Hb (g/dL) | 15.1±0.5 | 15.5±0.8 | 15.1±0.4 | 15.5±0.6 | 15.1±0.6 | 15.6±0.6 |
| O2c (mLO2/g) | 0.20±0.01 | 0.21±0.01 | 0.20±0.01 | 0.21±0.01 | 0.20±0.01 | 0.21±0.01 |
ANOVA, analysis of variance; Hb, hemoglobin; Hct, hematocrit; MCAO, middle cerebral arterial occlusion; O2 Sat, arterial oxygen saturation; O2c, O2 content; PCO2, arterial carbon dioxide tension; PO2, arterial oxygen tension.
*P<0.05 compared between normal rats and MCAO rats for each condition by ANOVA. The three different conditions did not affect blood gas data in any group (repeated-measures ANOVA).
Normal Rat Study
The 15O2-HbV radioactivity concentration rapidly achieved equilibrium in the whole blood, plasma, and the brain at about 2 minutes after radiotracer administration using the same injection program as the H215O-PET (Figure 1), and the curves were similar to those of H215O. Representative PET images of a normal rat are given in Figure 2. Table 2 shows hemodynamic parameters of the whole brain mean and the regional cerebral values in the normal rats. The parameters were not significantly different among the brain regions.
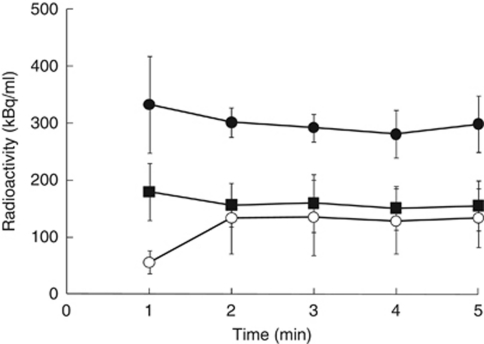
Figure 1.
Time–activity curves of the whole blood, plasma, and the whole brain using 15O2-HbV with the steady-state method. The radioactivity reached equilibrium in the whole blood (•), plasma, (▪) and the whole brain (○) rapidly at ∼2 minutes after administration. HbV, hemoglobin-containing vesicles.
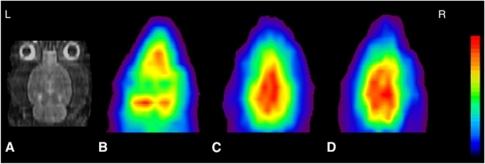
Figure 2.
Coronal magnetic resonance imaging (MRI) (A), C15O-HbV (B), H215O (C), and 15O2-HbV (D) positron emission tomography (PET) images of a normal rat. Average PET images of 2 minutes for C15O-HbV, and 3 minutes for H215O and 15O2-HbV after reaching equilibrium were used to calculate the hemodynamic parameters. Each PET image was coregistered to the individual MRI, and sliced at the same level. The upper values for the color scale were 50, 300, and 300 (kBq/mL) for (B), (C), and (D), respectively. HbV, hemoglobin-containing vesicles.
Table 2. Hemodynamic parameters of normal rats.
| Whole brain | Frontal cortex | Visual cortex | Striatum | Thalamus | |
|---|---|---|---|---|---|
| CBF (mL per minute 100 g) | 54.3±2.0 | 54.1±1.8 | 53.8±2.1 | 55.1±2.2 | 56.2±1.8 |
| OEF | 0.56±0.04 | 0.53±0.05 | 0.56±0.05 | 0.55±0.04 | 0.56±0.05 |
| CMRO2 (μmoL per minute per g) | 2.78±0.19 | 2.60±0.21 | 2.73±0.20 | 2.72±0.19 | 2.82±0.21 |
| (mL per minute per 100 g) | (6.2±0.4) | (5.8±0.5) | (6.1±0.4) | (6.1±0.4) | (6.3±0.5) |
| CBV (mL/100 g) | 4.9±0.4 | 5.1±0.5 | 5.1±0.4 | 4.8±0.4 | 5.0±0.5 |
CBF, cerebral blood flow; CBV, cerebral blood volume; CMRO2, cerebral metabolic rate for oxygen; OEF, oxygen extraction fraction.
See values for the sensorimotor cortex in Table 3.
Middle Cerebral Arterial Occlusion Model Rat Study
The MCAO model rats also showed rapid radioactivity equilibrium of H215O and 15O2-HbV in the brain and blood within 2 minutes using the steady-state method. Figure 3 shows MRI images, TTC staining, and 15O-PET images in a MCAO model rat. The TTC staining brain slice revealed an infarction area in the left cerebral hemisphere after MCAO. Cerebral hemodynamic parameters of the normal rats and the MCAO model rats are given in Table 3. The hemodynamic parameters in the contralateral side were not significantly different from those in the sensorimotor cortex of the normal rats. All hemodynamic parameters in the ipsilateral hemisphere decreased significantly compared with the contralateral side (P<0.01).
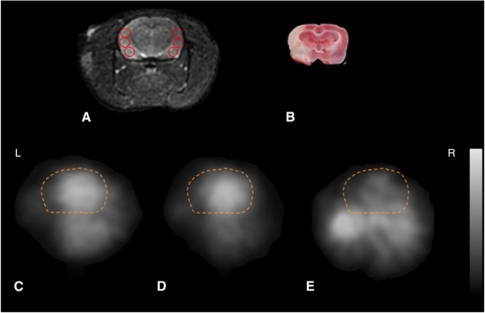
Figure 3.
Transaxial magnetic resonance imaging (MRI) (A), 2,3,5-triphenyl-tetrazolium chloride (TTC) staining (B), H215O (C), 15O2-HbV (D), and C15O-HbV (E), positron emission tomography (PET) images on a left middle cerebral arterial occlusion (MCAO) model rat. All images are shown at the same slice level, including the sensorimotor cortex, etc. The regions of interest (ROIs) were placed on the infarction in the ipsilateral side and on the normal cortex in the contralateral side (A). Dashed lines are contours of the brain. The upper values for the gray scale were 300, 300, and 50 (kBq/mL) for (C), (D), and (E), respectively. HbV, hemoglobin-containing vesicles.
Table 3. Hemodynamic parameters of the rats under normal and MCAO conditions.
|
Normal (n=20) |
MCAO (n=10) |
||
|---|---|---|---|
| Sensorimotor cortex | Contralateral | Ipsilateral | |
| CBF (mL per minute per 100 g) | 55.8±1.8 | 56.2±1.9 | 23.8±2.6* |
| OEF | 0.56±0.04 | 0.54±0.03 | 0.24±0.04* |
| CMRO2 (μmol per minute per g) | 2.86±0.21 | 2.88±0.19 | 0.55±0.13* |
| (mL per minute per 100 g) | (6.4±0.5) | (6.4±0.4) | (1.2±0.3*) |
| CBV (mL/100 g) | 5.0±0.4 | 5.3±1.0 | 2.8±0.5* |
ANOVA, analysis of variance; CBF, cerebral blood flow; CBV, cerebral blood volume; CMRO2, cerebral metabolic rate for oxygen; MCAO, middle cerebral artery occlusion; OEF, oxygen extraction fraction.
*P<0.0001 compared among the three groups (20 hemispheres of normal rats and 10 of contra- and ipsi-lateral hemispheres of MCAO rats) for each hemodynamic parameter using ANOVA and post hoc Sheffe's F-test.
Discussion
In this study, the steady-state method was applied to 15O2-HbV PET to measure the cerebral oxygen metabolism of rats under normal and MCAO conditions, providing rapid radioactivity equilibrium in both the blood and the brain. The physical condition of rats was stable during PET experiments because of the small volumes of injection as well as arterial blood samplings. Injectable 15O2-HbV is ideal for measuring the cerebral oxygen metabolism with small animals because 15O2-gas inhalation affects cerebral PET counts due to its high nasal radioactivity. Although cerebral oxygen metabolism of normal rats was estimated using the 15O2-HbV with a single bolus injection method (Tiwari et al, 2010), a small animal may be stressed from frequent multipoint arterial blood samplings in each 15O experiment. In our previous study, the steady-state method was applied to measure CBF using H215O and provided stable values. The method was considered suitable for repeated CBF measurements because it requires only a few arterial blood samplings during the steady state of the PET scan (Kobayashi et al, 2011).
The same injection program was applied to 15O2-HbV administration in the present study. Although the labeling efficiency of the 15O-radiotracers was slightly different between H215O and 15O2-HbV (123.4 and 95.7 MBq/mL), the difference did not affect the time to achieve equilibrium of radioactivity in the blood and the brain because the same level of radioactivity of 61.7 MBq/mL for both radiotracers (185 MBq in 3 mL volume) was injected at the scan start. The injection volume of the 15O-radiotracer did not affect the physical condition of the rats during the PET experiments (Table 1) because of the small injection volume (1.0 and 0.25 mL net injection volumes for H215O and 15O2-HbV, respectively, after subtraction of the sampled blood volumes) and slowly increasing injection velocity (Morton et al, 1997). In addition, HbV injection did not change the rats' physical condition. Although the PCO2 values in the MCAO model rats were significantly decreased compared with those in the normal rats, probably due to hyperventilation in a few rats during PET experiments, the CBF was not affected significantly because other blood gas data were not changed compared with those of normal rats. This effect of MCAO operation on the arterial blood gas data was similar to a previous report (Temma et al, 2006). The PCO2 values seem slightly higher and the PO2 values seem slightly lower in normal rats compared with those in humans probably due to the tendency of hypoventilation under anesthesia during experiments. Similar results were observed in a previous study with normal rats (Tiwari et al, 2010).
In the present study, OEF images were corrected using CBV images obtained from the C15O-HbV, providing accurate cerebral oxygen metabolism. This blood volume correction has never been applied in small animal studies. The CBV correction is necessary because the CBV is usually changed in the affected area of a CVD model animal. The CBV images were obtained using data from 1 to 3 minutes after C15O-HbV administration because cerebral counts reached a constant level at about 1 minute after administration. The hemodynamic parameters in the contralateral side of the MCAO model rats were not different from those in the normal rats. However, the parameters of the ipsilateral infarction area showed significant decreases (Table 3), because the MCAO model in the present study caused complete infarction in the brain territory of the occluded MCA. Since complete occlusion and infarction would prevent the ability of the tracers to reach the lesion, the MCAO model in this study may affect the time to achieve equilibrium and oxygen membrane permeability in the ischemic tissue, which may influence on quantitative values obtained by the steady-state method. This significant reduction of parameters in the region of cerebral infarction was similar to a previous report (Temma et al, 2006). A single-compartment analysis used in the steady-state method may overestimate cerebral water clearance and tissue counts (Ohta et al, 1992, 1996); however, this method was established in human PET studies and many studies with CVD patients were reported (Yamauchi et al, 1996, 1998, 1999; Okazawa et al, 2001; Ouchi et al, 2001). Although slightly different values were observed between the steady-state method and the bolus administration method in a previous study with CVD, the values were not significantly different (Okazawa et al, 2001).
The 15O2-HbV injection yields metabolic 15O-water, which may affect arterial blood and cerebral PET counts. However, the radioactivity of the metabolic water was considered to reach equilibrium in the early phase after 15O2-HbV administration because the plasma radioactivity in the 15O2-HbV scan reached a plateau at 2 minutes after administration in the present study using the steady-state method (Figure 1). The cerebral radioactivity of H215O-PET also achieved equilibrium at 2 minutes after administration as reported previously. The average images of H215O and 15O2-HbV were obtained using PET data from 2 to 5 minutes after administration because cerebral PET counts and arterial blood radioactivity were constant during that time. Stable hemodynamic parameters were obtained with the same quality and noiseless PET images, as well as stable radioactivity of the arterial blood and plasma (Table 2). Although the steady-state method may induce systemic underestimation of CBF because of tissue heterogeneity between gray and white matter as compared with the bolus injection method in the human study (Kanno et al, 1984); however, the structure of the brain cortex in rats is not so complicated (Zilles and Wree, 1995), and the effect of brain tissue heterogeneity is not considered so significant as compared with the human brain because we placed ROIs on the cortices (Figure 3A) and basal ganglia. The cerebral hemodynamic parameters of the normal rats using the present steady-state method were similar to the results reported previously using the bolus method (Magata et al, 2003; Temma et al, 2006; Tiwari et al, 2010), in which slightly higher values of OEF and CMRO2 in normal rats were observed compared with those of normal human subjects. Differences in species and effects of anesthesia during the experiments are considered to provide these differences. Higher CBV values may also be caused by anesthesia, and many previous reports showed various rage of CBV from 2.5 to 4.7 mL/100 g (Sandor et al, 1986; Todd et al, 1993; Perles-Barbacaru and Lahrech, 2007). A smaller size of HbV than normal RBC may also have affected the CBV value because smaller vessels might be delineated by C15O-HbV. The blood pool correction using CBV was applied in this steady-state method, and thus, the influence of slightly greater vascular radioactivity on OEF values would be negligible.
A small amount of 15O2-gas may be detached from 15O2-HbV in the lungs and exhaled, which may affect radioactivity in the head and airways of rats during PET scans. However, it has already been reported that the 15O2-gas released from the nose and mouth showed little influence on quantitative values of cerebral oxygen metabolism in previous studies using injectable 15O2 (Magata et al, 2003; Temma et al, 2006). Although the radioactivity of 15O2 released from the nose and mouth of the rats in 15O2-HbV studies has never been measured, the influence of detached 15O2 on quantitative hemodynamic values would be similarly small, as in previous studies. The penumbra regions were not examined in this study because a reperfusion model after MCAO was not applied and most of the ischemic regions showed infarction. A reperfusion model after MCAO would be appropriate to evaluate the pathophysiology of the penumbra area. In the rats with MRI images, the brains were excluded about 15 to 20 minutes after the PET scans. There was a slight time difference from the procedure without MRI in the timing of the TTC staining; however, the infarction area in the TTC staining was not significantly different from that in the PET and MRI images (Figure 3).
In conclusion, 15O2-HbV with the steady-state method is stable and useful, and may be ideal to measure the cerebral oxygen metabolism of small animals with less stress. The CBV correction with C15O-HbV provides precise cerebral oxygen metabolism, especially in CVD model animals. Basic research with CVD models will be accelerated by injectable 15O-HbV tracers with the steady-state method.
Acknowledgments
The author thanks Satonao Nakakoji, Akira Ito, Hiroshi Oikawa, and other staff of the Biological Imaging Research Center, University of Fukui for their technical support.
The authors declare no conflict of interest.
Footnotes
This study was partly funded by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (19790861, 22791180) and the Fukui Brain Project, Research and Education Program for Life Science, University of Fukui.
References
- Frackowiak RS, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Kanno I, Lammertsma AA, Heather JD, Gribbs JM, Rhodes CG, Clark JC, Jones T. Measurement of cerebral blood flow using bolus inhalation of C15O2 and positron emission tomography: description of the method and its comparison with the C15O2 continuous inhalation method. J Cereb Blood Flow Metab. 1984;4:224–234. doi: 10.1038/jcbfm.1984.31. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kiyono Y, Maruyama R, Mori T, Kawai K, Okazawa H. Development of an H215O steady-state method combining a bolus and slow increasing injection with a multiprogramming syringe pump. J Cereb Blood Flow Metab. 2011;31:527–534. doi: 10.1038/jcbfm.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge Y, Minematsu K, Yamaguchi T, Miyake Y. Nylon monofilament for intraluminal middle cerebral artery occlusion in rats. Stroke. 1995;26:1655–1657. doi: 10.1161/01.str.26.9.1655. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Heather JD, Jones T, Frackowiak RS, Lenzi GL. A statistical study of the steady state technique for measuring regional cerebral blood flow and oxygen utilisation using 15O. J Comput Assist Tomogr. 1982;6:566–573. doi: 10.1097/00004728-198206000-00022. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. Description of the method. J Cereb Blood Flow Metab. 1983;3:416–424. doi: 10.1038/jcbfm.1983.67. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Wise RJ, Heather JD, Gibbs JM, Leenders KL, Frackowiak RS, Rhodes CG, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 2. Results in normal subjects and brain tumour and stroke patients. J Cereb Blood Flow Metab. 1983;3:425–431. doi: 10.1038/jcbfm.1983.68. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Magata Y, Temma T, Iida H, Ogawa M, Mukai T, Iida Y, Morimoto T, Konishi J, Saji H. Development of injectable O-15 oxygen and estimation of rat OEF. J Cereb Blood Flow Metab. 2003;23:671–676. doi: 10.1097/01.WCB.0000066792.97069.B3. [DOI] [PubMed] [Google Scholar]
- Morton D, Safron JA, Rice DW, Wilson DM, White RD. Effects of infusion rates in rats receiving repeated large volumes of saline solution intravenously. Lab Anim Sci. 1997;47:656–659. [PubMed] [Google Scholar]
- Ogata Y. Characteristics and function of human hemoglobin vesicles as an oxygen carrier. Polym Adv Technol. 2000;11:205–209. [Google Scholar]
- Ohta S, Meyer E, Thompson CJ, Gjedde A. Oxygen consumption of the living human brain measured after a single inhalation of positron emitting oxygen. J Cereb Blood Flow Metab. 1992;12:179–192. doi: 10.1038/jcbfm.1992.28. [DOI] [PubMed] [Google Scholar]
- Ohta S, Meyer E, Fujita H, Reutens DC, Evans A, Gjedde A. Cerebral water clearance in humans determined by PET: I. Theory and normal values. J Cereb Blood Flow Metab. 1996;16:765–780. doi: 10.1097/00004647-199609000-00002. [DOI] [PubMed] [Google Scholar]
- Okazawa H, Yamauchi H, Sugimoto K, Takahashi M, Toyoda H, Kishibe Y, Shio H. Quantitative comparison of the bolus and steady-state methods for measurement of cerebral perfusion and oxygen metabolism: PET study using 15O-gas and water. J Cereb Blood Flow Metab. 2001;21:793–803. doi: 10.1097/00004647-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Nobezawa S, Yoshikawa E, Futatsubashi M, Kanno T, Okada H, Torizuka T, Nakayama T, Tanaka K. Postural effects on brain hemodynamics in unilateral cerebral artery occlusive disease: a positron emission tomography study. J Cereb Blood Flow Metab. 2001;21:1058–1066. doi: 10.1097/00004647-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Perles-Barbacaru AT, Lahrech H. A new magnetic resonance imaging method for mapping the cerebral blood volume fraction: the rapid steady-state T1 method J Cereb. Blood Flow Metab. 2007;27:618–631. doi: 10.1038/sj.jcbfm.9600366. [DOI] [PubMed] [Google Scholar]
- Phillips WT, Lemen L, Goins B, Rudolph AS, Klipper R, Fresne D, Jerabek PA, Emch ME, Martin C, Fox PT, McMahan CA. Use of oxygen-15 to measure oxygen-carrying capacity of blood substitutes in vivo. Am J Physiol. 1997;272:2492–2499. doi: 10.1152/ajpheart.1997.272.5.H2492. [DOI] [PubMed] [Google Scholar]
- Sandor P, Put JC, de Jong W, de Wied D. Continuous measurement of cerebral blood volume in rats with the photoelectric technique: effect of morphine and naloxone. Life Sci. 1986;39:1657–1665. doi: 10.1016/0024-3205(86)90163-3. [DOI] [PubMed] [Google Scholar]
- Takeoka S, Teramura Y, Atoji T, Tsuchida E. Effect of Hb-encapsulation with vesicles on H2O2 reaction and lipid peroxidation. Bioconjug Chem. 2002;13:1302–1308. doi: 10.1021/bc025546k. [DOI] [PubMed] [Google Scholar]
- Temma T, Magata Y, Kuge Y, Shimonaka S, Sano K, Katada Y, Kawashima H, Mukai T, Watabe H, Iida H, Saji H. Estimation of oxygen metabolism in a rat model of permanent ischemia using positron emission tomography with injectable 15O-O2. J Cereb Blood Flow Metab. 2006;26:1577–1583. doi: 10.1038/sj.jcbfm.9600302. [DOI] [PubMed] [Google Scholar]
- Temma T, Kuge Y, Sano K, Kamihashi J, Obokata N, Kawashima H, Magata Y, Saji H. PET O-15 cerebral blood flow and metabolism after acute stroke in spontaneously hypertensive rats. Brain Res. 2008;1212:18–24. doi: 10.1016/j.brainres.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Tiwari VN, Kiyono Y, Kobayashi M, Mori T, Kudo T, Okazawa H, Fujibayashi Y. Automatic labeling method for injectable 15O-oxygen using hemoglobin-containing liposome vesicles and its application for measurement of brain oxygen consumption by PET. Nucl Med Biol. 2010;37:77–83. doi: 10.1016/j.nucmedbio.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Todd MM, Weeks JB, Warner DS. Microwave fixation for the determination of cerebral blood volume in rats. J Cereb Blood Flow Metab. 1993;13:328–336. doi: 10.1038/jcbfm.1993.41. [DOI] [PubMed] [Google Scholar]
- Yamada R, Watanabe M, Omura T, Sato N, Shimizu K, Takahashi M, Ote K, Katabe A, Moriya T, Sakai K, Yamashita T, Tanaka E. Development of a small animal PET scanner using DOI detectors. IEEE Trans Nucl Sci. 2008;55:906–911. [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Nakamura K, Yamamoto Y, Yonekura Y, Konishi J, Kimura J. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. 1996;61:18–25. doi: 10.1136/jnnp.61.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Okazawa H. Cerebral hematocrit decreases with hemodynamic compromise in carotid artery occlusion. A PET study. Stroke. 1998;29:98–103. doi: 10.1161/01.str.29.1.98. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Ueno M, Nishizawa S, Konishi J, Shio H. Significance of increased oxygen extraction fraction in 5-year prognosis of major cerebral arterial occlusive diseases. J Nucl Med. 1999;40:1992–1998. [PubMed] [Google Scholar]
- Zilles K, Wree A.1995Cortex: areal and laminar structure The Rat Nervous System(Paxinos G, eds), 2nd ed,San Diego, CA: Academic Press, Inc.649–685. [Google Scholar]