Abstract
Chronic, high-frequency electrical stimulation of the subthalamic nuclei (STNs) has become an effective and widely used therapy in Parkinson's disease (PD), but the therapeutic mechanism is not understood. Stimulation of the STN is believed to reorganize neurophysiological activity patterns within the basal ganglia, whereas local field effects extending to tracts adjacent to the STN are viewed as sources of nontherapeutic side effects. This study is part of a larger project investigating the effects of STN stimulation on speech and regional cerebral blood flow (CBF) in human subjects with PD. While generating measures of global CBF (gCBF) to normalize regional CBF values for a subsequent combined analysis of regional CBF and speech data, we observed a third effect of this therapy: a gCBF increase. This effect was present across three estimates of gCBF ranging from values based on the highest activity voxels to those based on all voxels. The magnitude of the gCBF increase was related to the subject's duration of PD. It is not clear whether this CBF effect has a therapeutic role, but the impact of deep brain stimulation on cerebrovascular control warrants study from neuroscience, pathophysiological, and therapeutic perspectives.
Keywords: deep brain stimulation, movement disorder, Parkinson's disease, positron emission tomography, subthalamic nucleus
Introduction
Chronic electrical stimulation of deep brain structures has become widely used in the treatment of movement disorders (Vitek, 2008; Benabid et al, 2009). Tens of thousands of individuals with Parkinson's disease (PD) are treated with high-frequency, deep brain stimulation of the subthalamic nucleus (STN-DBS) worldwide (Volkmann, 2007). In PD, STN-DBS significantly improves tremor, rigidity, and bradykinesia, often allows a significant reduction in the therapeutic doses of medication thereby reducing dyskinesias, and is associated with improvements in activities of daily living and quality of life. The most common cognitive sequelae of STN-DBS seem to be a period of postoperative confusion (Benabid et al, 2009) in some individuals and reduced performance on tests of verbal fluency, although this is not a consistent finding (Witt et al, 2004; Parsons et al, 2006).
While improving the motor impairments in PD that are responsive to levodopa, STN-DBS does not typically provide therapeutic benefit to speech, and in some individuals, it can make speech worse (Kleiner-Fisman et al, 2006; Klostermann et al, 2008), except for improving voice quality (Sidtis et al, 2010). This study is part of a larger project directed at characterizing STN-DBS changes in brain activity during speech using positron emission tomography (PET), a technique well suited to mapping brain function during speech production, especially with individuals receiving DBS.
In spite of the widespread successful application of STN-DBS in PD, the therapeutic mechanisms are not fully understood (Vitek, 2008; Benabid et al, 2009). Originally conceived as a ‘reversible lesion' that controlled an overly active STN, STN-DBS is now viewed as producing a more complex neurophysiological response (Vitek, 2008). Several possible effects have been suggested: interference with neural signals, desynchronization of abnormal oscillations, inhibition, excitation, and modulation of neurotransmitter and hormonal signaling (Benabid et al, 2009). In addition to direct effects on the STN target, DBS also produces an indirect effect in the form of local field of stimulation (Maks et al, 2009), and its influence on surrounding tissue, particularly the corticobulbar tract (Tommasi et al, 2008), is seen as responsible for the most common side effects of STN-DBS, which can involve sensory changes, muscle contractions in facial areas, and dysarthria (Tommasi et al, 2008; Benabid et al, 2009). In the process of determining global cerebral blood flow (gCBF) values to normalize regional CBF data for a combined analysis with speech production data (Sidtis, 2007), a third effect of STN-DBS was observed: a global increase in the rate of CBF.
Materials and methods
Subjects
Subjects were recruited from a clinical population of individuals with PD who were being treated with bilateral STN-DBS. A total of 166 whole-brain CBF scans were obtained from 7 right-handed male subjects with idiopathic PD who participated in a study of the effects of STN-DBS on CBF during speech production using PET. In all, 12 whole-brain scans were obtained with STN-DBS on and 12 were obtained with STN-DBS off (1 STN-DBS off session was terminated after 10 scans at the subject's request). The STN-DBS ‘on' and ‘off' PET scans were performed on different days, separated by at least 1 week. The order of the on and off scan days was randomized, with four subjects being scanned off first and three subjects being scanned on first. All subjects were studied at least 12 hours after their last dose of levodopa, taken the evening before each scan day. This washout period for levodopa is routinely used in studies on the effects of DBS (Ceballos-Baumann et al, 1999; Payoux et al, 2004; Karimi et al, 2008). Subject characteristics are described in Table 1. Surgical implantation and programming were not part of the study, but these procedures are briefly described as part of the subject characterization. All subjects provided informed consent for the speech (Nathan Kline Institute) and PET (Feinstein Research Institute) components of this study.
Table 1. Demographic characteristics of subjects.
| SID | Age (years) | PD duration (years) | Levodopa (mg) | DBS duration (months) | Left amp (volts) | Right amp (volts) | H and Y (off) | H and Y (on) | UPDRS III (off) | UPDRS III (on) |
|---|---|---|---|---|---|---|---|---|---|---|
| 103 | 54 | 14 | 250 | 44 | 3.2 | 2.8 | 3.0 | 2.0 | 51.0 | 25.0 |
| 104 | 57 | 16 | 400 | 27 | 3.0 | 3.0 | 5.0 | 4.0 | 57.0 | 55.5 |
| 106 | 59 | 10 | 600 | 09 | 3.0 | 3.0 | 2.5 | 2.5 | 27.5 | 19.0 |
| 107 | 62 | 15 | 600 | 02 | 2.5 | 2.6 | 2.5 | 2.0 | 26.0 | 23.0 |
| 109 | 49 | 09 | 300 | 04 | 2.6 | 2.5 | 2.5 | 2.0 | 23.5 | 21.5 |
| 110 | 62 | 11 | 600 | 56 | 3.0 | 3.3 | 4.0 | 3.0 | 52.5 | 31.0 |
| 111 | 56 | 11 | 400 | 37 | 3.0 | 3.0 | 2.0 | 1.0 | 11.5 | 4.0 |
PD, Parkinson's disease; STN-DBS, deep brain stimulation of the subthalamic nucleus; UPDRS III, United Parkinson's Disease Rating Scale III.
All subjects were right-handed males and all but one subject were native speakers of English (106 was a native speaker of Italian who immigrated to the United States as an adolescent). In all cases, the stimulation frequency was 185 Hz, pulse width 60 μ seconds. The medical indications for STN-DBS were advanced, medically refractory PD with marked clinical swings between medication doses (i.e., on/off effects), as well as levodopa-induced dyskinesias. Both on and off evaluations were performed at least 12 hours after the last dose of levodopa, which was taken the evening before the study. On and off studies were performed on different days separated by at least 1 week. Subject 104 had maximum scores on the rigidity items on the UPDRS III, which were not responsive to DBS. Given the unknown washout time of DBS effects, the UPDRS on–off differences may underestimate the therapeutic effects.
Deep Brain Stimulation Stimulating Electrode Positioning
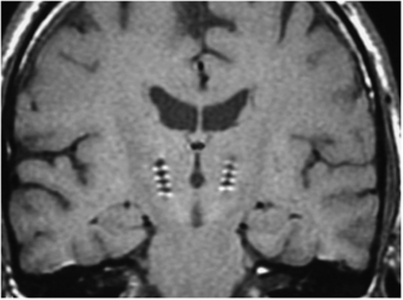
For surgical placement of the stimulating electrodes, subjects were fitted with a Leksell stereotactic frame with magnetic resonance imaging-compatible pins under sterile conditions. A magnetic resonance image was obtained and images were transferred to a computer workstation for operative planning. The initial target coordinates were determined and safe trajectories established. The patient was returned to the operating room and head position was locked using a Mayfield adaptor (Integra, Plainsboro, NJ, USA). The target coordinates were set on the frame, the operating arc was attached, and the surgical trajectory was aligned. Patients were awake during surgery. Microelectrode recordings were made to refine targeting. Once the target position was established, the stimulating electrodes (Medtronic Inc., Model 3387, Medtronic, Minneapolis, MN, USA) were inserted. Test stimulations were performed in a paired bipolar sequence (185 Hz, 60 μseconds, 0 to 4 Volts) to insure that no adverse motor effects were present. Leads were then fixed in position and serial fluoroscopic images were obtained to confirm that the leads had not migrated during fixation. Two impulse generators (Soletra, Medtronic Inc.) were implanted subcutaneously in the chest in a subsequent surgery. Typical positioning of the stimulating electrodes is depicted in Figure 1.
Figure 1.
Coronal section of the brain showing the typical bilateral placement of the high-frequency stimulating leads in the subthalamic nuclei.
Impulse Generator Programming
Initial programming of the impulse generators occurred 2 to 4 weeks after surgical implantation with the patient off PD medication. Starting with contact 0 in monopolar configuration (i.e., case set to positive), the voltage was slowly titrated up from 0 Volt in 0.1 to 0.2 Volt increments until beneficial effects were noted or the patient reported a nontransient side effect. This procedure was repeated for each of the four contacts. The contact that yielded the greatest benefit and/or exhibited the greatest therapeutic window was selected for chronic stimulation and the lowest effective voltage was used initially. The contralateral stimulator was programmed independently using the same protocol. Additional adjustments were made and contacts added as necessary with bilateral stimulation in effect.
Study Procedures
Subjects were brought to the General Clinical Research Center at the Feinstein Research Institute of the North Shore-Long Island Jewish Medical Center around 0800 hours to be consented, interviewed, and given instructions regarding the procedures. They were then given a tour of the PET suite and briefed again about the study procedures after being able to see the apparatus. The current impulse generator settings were recorded and hard copy produced using a Medtronic programming device. Before the scanning sessions, all of the subjects were being stimulated 24 hours/day, with the average duration of treatment being 25.6±21.2 months. The individual durations are listed in Table 1. The stimulators were typically turned off at 0900 hours. Approximately 30 minutes later, subjects were positioned in the PET scanner (GE Advance Tomograph, General Electrics, Milwaukee, WI, USA) and an intravenous line placed in the subject's left arm for H215O injection at 1000 hours. A stereotactic headholder and three-dimensional laser alignment were used for stable and reproducible head positioning. Lightweight headphones were attached to the headholder to facilitate communication with the subject. A 10-minute transmission scan was performed for attenuation correction, followed by a two-dimensional PET scan to establish the delay time between H215O injection and the detection of brain activity by the scanner. This was followed by a series of 12 whole-brain three-dimensional PET scans. In the DBS off studies, stimulation was off for an average of 61 minutes (minimum 40 minutes, maximum 86 minutes) before the first scan. There is no standard off period in DBS studies with off times often unreported. When reported, off times can range from 10 to 42 minutes (Ceballos-Baumann et al, 1999; Payoux et al, 2004; Karimi et al, 2008) to as long as 12 hours (Hilker et al, 2003). When STN-DBS is turned off, tremor worsens within minutes and bradykinesia and rigidity worsen over a period of 30 to 60 minutes (Temperli et al, 2003). The mean total duration of scanning was 85.1±8.5 minutes in the off condition and 90.9±10.8 minutes in the on condition. These durations were not significantly different. The average interscan intervals for on and off conditions did not differ either, at 8.3 and 7.6 minutes, respectively. Several intervals for scans occurring later in both the on and off series were longer to accommodate subjects' needs.
The scanning sequence began and ended with a resting scan. The remaining 10 scans consisted of 2 repetitions of 5 pseudorandomized speech repetition tasks (lip closure, /pa/, /pa-ta-ka/, /pop-the-top-cop/) and a spontaneous speech task in which subjects produced a monologue on a topic of their choice. These tasks were produced in random order once in the first half of the study and repeated in reverse order in the second half. On the basis of the observed brain delay time, each speech task was initiated 15 seconds before detection of H215O in the brain. Tasks were performed for 60 seconds using the procedure reported previously (Sidtis et al, 2006). Blood flow was measured using a modified slow bolus injection of H215O (Sidtis et al, 2006; Silbersweig et al, 1993). Performance on the speech tasks was recorded on digital audio and video media for subsequent analyses. After the PET study, all subjects underwent a protocol motor speech examination and were evaluated using the disability scale of Hoehn and Yahr (1967) and the United Parkinson's Disease Rating Scale III (UPDRS III) to rate motor function (Fahn and Elton, 1987). The Hoehn and Yahr scale ranges from stage 0 (no signs of disease) to stage 5 (wheelchair bound or bedridden unless aided). The UPDRS III rates disability in a wide range of motor function. After speech and PD examinations, medication and STN-DBS were resumed.
Image Processing and Data Extraction
All images were aligned and spatially normalized using SPM99 software (SPM99, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). Raw images were aligned for each subject using rigid body registration. Aligned images were then spatially normalized to the PET template using a nonlinear transformation. Large, irregular anatomic regions of interest were drawn for each slice using SCANVP software (Feinstein Institute for Medical Research, Manhasset, NY, USA; http://www.feinsteinneuroscience.org/) in MATLAB (Mathworks, Sherborn, MA, USA). A composite image of all 166 scans was created to generate template regions of interest for each slice, which were drawn to include only the brain area, analogous to a brain mask. These whole-brain templates were then applied to individual images. For each subject, values from these regions of interest were averaged to obtain an estimate of gCBF. Three such estimates were calculated using a thresholding method (Rottenberg et al, 1991). Owing to the resolution of functional imaging techniques, estimates of blood flow or metabolism contain partial volume error caused by mixtures of gray and white matter and cerebrospinal fluid. The thresholding approach was developed to improve the accuracy of measurements of gray matter responses in neurobehavioral studies, but it also has the advantage of tolerating individual anatomic differences (Sidtis, 2007). One estimate of gCBF was based on the average CBF across all voxels in each slice for each individual. A second gCBF estimate was based on an average of the voxels that fell in the upper 10% of values for an individual, based on the individual's whole-brain data. A third gCBF estimate was based on an average of the maximum voxel values on each slice. These three estimates of gCBF provide an indication of whether global effects were driven by flow rates combining that of gray and white matter, flow rates approximating that of gray matter, or the highest flow rates (Iida et al, 2000; Ingvar et al, 1965). For each subject, averages across all slices were then calculated for each scan in each condition for each gCBF measure. These average values were then normalized across subjects using the by-administered H215O dose.
Administered H215O Dose and Dose Normalization
H215O was collected and activity counted (Coincidence Technologies, GE, Milwaukee, WI, USA) in a reservoir during the interscan interval before injection. A dose normalization was computed to minimize the effects of changes in 15O dose over the 12 sequential scans, to normalize all subjects to a single dose (the grand mean of all conditions), and to account for any differences in the relationships between 15O dose and gCBF across DBS states.
A ratio was used to generate the normalization factors for each scan (Arnt et al, 1996; Sidtis et al, 2006). The normalization factor for an individual scan was the ratio of the grand mean of the 15O doses across scans and DBS conditions, and the 15O dose for the individual scan, weighted by the proportion of variance (r2) accounted for by the correlation between dose and CBF measure for each DBS condition. The normalization factor was as follows:
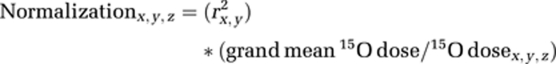 |
where:
x=DBS (on, off)
y=CBF measure (max, 10%, 100%)
z=Sequence (1 to 12)
grand mean 15O dose was calculated across DBS states and scan sequences.
r2=proportion of variance accounted for by the relationship between 15O dose and each gCBF measure in DBS on and off conditions. The correlations between 15O dose and each gCBF measure were calculated separately for DBS on and off conditions using all scans (both between and within subjects) in each condition.
Each gCBF value was multiplied by the appropriate normalization factor, and the product was added to the original gCBF value. This increased all gCBF values, but the increases were greater for scans obtained with 15O doses below the grand mean, and fractional for scans obtained with 15O doses above the grand mean. All normalizations were proportional to the relationship between 15O dose and gCBF in each condition for each measure.
Results
15O Dose
There were no significant differences in the doses of 15O administered to the DBS on and off groups nor was there an interaction between DBS status and scan sequence. However, there was a significant effect of temporal sequence (F(11, 66)=2.28; P=0.02) with the administered dose declining over the 12 scan sequence. To determine the appropriate weighting to be applied to the dose normalization across scans described above, correlations between administered 15O dose and each CBF measure were determined separately for the DBS on and off groups. For the DBS on group, 15O dose was positively correlated for the max CBF (r=0.726; P<0.001), upper 10% CBF (r=0.643; P<0.001), and 100% CBF (r=0.416; P<0.001). For the DBS off group, 15O dose was positively correlated for the max CBF (r=0.358; P=0.001), upper 10% CBF (r=0.343; P=0.002), but not for 100% CBF (r=0.187; P=0.09). These correlations between 15O dose and gCBF measures differed between the on and off groups for max CBF (Fisher's Z-transformation: z=3.449; P<0.002) and upper 10% CBF (z=2.557; P=0.01), but not for 100% CBF (z=1.614; P=0.11) measures.
Deep Brain Stimulation Effects on Cerebral Blood Flow
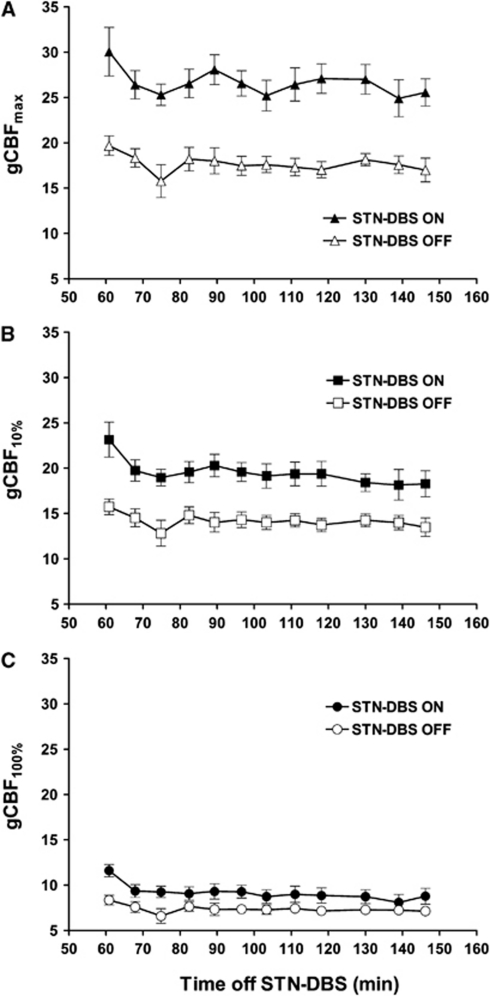
Separate analyses of variance were performed for each CBF measure with DBS status (on, off) and sequence (1 to 12) as repeated measures. For the CBFmax measure, there was a significant DBS effect (F(1, 6)=162.74; P<0.001) with consistently higher flow values in the DBS on condition. There was an effect of sequence (F(11, 66)=2.24; P=0.022), owing to some fluctuation in the initial scans, but the DBS state did not interact with sequence (Figure 2). The same pattern of DBS effect was found for the CBF10 (F(1, 6)=61.079; P<0.001) and CBF100 measures (F(1, 6)=25.069; P=0.002). All seven subjects showed higher CBF in the DBS on condition. The average increase for CBFmax was 50.9%, which was greater than the 37.1% for CBF10 (t(7)=−5.566; P=0.001), which was greater than the 23.7% for CBF100 (t(7)=−8.709; P<0.001).
Figure 2.
Mean global CBF (gCBF) values (±1 s.e.) for 3 measures of flow for each of 12 serial whole-brain PET scans as a function of temporal sequence and STN-DBS status. The gCBFmax (A) represents the highest voxel values, gCBF10% (B) represents the average of the upper 10% of voxels in each slice, and the gCBF100% (C) represents the average of all of the voxels in each slice. Global CBF was higher with STN-DBS on and STN-DBS status interacted with time with each global CBF measure. CBF, cerebral blood flow; PET, positron emission tomography; STN-DBS, deep brain stimulation of the subthalamic nucleus.
Correlates of Deep Brain Stimulation Induced Global Cerebral Blood Flow Changes
Relationships between demographic, clinical, and gCBF10 measures were examined. In the on condition, gCBF10 increased with the total amplitude (left plus right side) of stimulation (r=0.222; P=0.04). Increased age was associated with decreased gCBF10 in both the off (r=−0.229; P=0.037) and on (r=−0.359; P=0.001) conditions. With regard to clinical status, the on–off difference in gCBF10, expressed as a percentage, indicated that gCBF10 increases in the on condition were greater in subjects with a longer duration of PD when age was taken into account (r=0.444; P=0.002). The percentage change in gCBF10 was not correlated with any other performance measures expressed as on–off change scores.
Although change scores did not reveal relationships between gCBF10 and functional status, the relationships between gCBF10 and functional status measures differed in the off and on conditions. In the off condition, gCBF was not associated with either the Hoehn and Yahr disability scale or the UPDRS motor performance scale. However, increased gCBF10 was associated with an increased rate of pausing on the speech tasks during scanning with STN-DBS off (r=0.334; P=0.019). In contrast, with STN-DBS on, subjects with higher gCBF10 values were those with greater disability on their Hoehn and Yahr (r=0.259; P=0.017) and UPDRS III scores (r=0.249; P=0.023). In this condition, gCBF10 was not associated with the pausing rate, but increased gCBF10 was associated with longer pause durations (r=0.402; P=0.004) and greater variability in pause durations (r=0.502; P<0.001).
The relationship between gCBF10 and clinical status was also addressed by examining the effects of STN-DBS on the dynamic range of gCBF10. Expressed as a percentage of the minimum value across the 12 scans for each subject in each condition, the mean gCBF10 range was slightly larger in the on condition (62.3%) than in the off condition (54.1%), but this difference was not significant. Three of the seven subjects had a larger gCBF10 range in the on condition and these three subjects had greater improvements in their Hoehn and Yahr (52.7% improvement) and UPDRS III (80.1% improvement) scores with STN-DBS on than did the four subjects with larger gCBF10 ranges in the off condition (Hoen and Yahr: 25% improvement; UPDRS III: 38% improvement). These differences did not correspond to the order in which the on and off scans were performed.
Deep Brain Stimulation Effects: Rest Versus Speech
To assess whether the DBS effects on gCBF were influenced by the speech tasks or rest, the percentage increases during DBS on for the two resting scans, which occurred at the beginning and end of the 12 scan sequence, were compared with increases on the two adjacent speech scans (scans 2 and 11). As the order of the different speech tasks was randomized across sequences, the speech condition represented a range of tasks. There was no significant difference between the increases during DBS on for any of the gCBF measures.
Discussion
These results reveal a previously unappreciated effect of the therapeutic high-frequency stimulation of the STN: a significant increase in gCBF. This effect was present with all three estimates of CBF and in all seven subjects. The increases in gCBF were estimated at 37% for gray matter (gCBF10) and a 24% for the combination of gray and white matter (gCBF100). The increase averaged 51% for the maximum blood flow values (gCBFmax). Global CBF decreased with age, regardless of DBS status, but the magnitude of the gCBF effect increased with the duration of PD, when age was taken into account. The results of this study suggest that high-frequency stimulation of the STN produces a global increase in blood flow, and further, that the properties of CBF differ in the presence or absence of STN-DBS.
The reduction of CBF with age has been repeatedly observed (Kety, 1956; Shaw et al, 1984; Pantano et al, 1984; Gur et al, 1987), and the relationship between gCBF and age does not seem to be affected by STN-DBS. More significantly, STN-DBS produced a significant increase in gCBF. It is not clear whether this CBF effect is the direct result of stimulating the STN or an indirect local field effect on adjacent tissue. A region near the STN in rats, which included Forel's field, the medial pole of the zona incerta, and part of the prerubral area, was found to have a significant influence on the control of cerebral circulation by Golanov et al (2001), who named the site the ‘subthalamic cerebrovasodilator area' (SVA). Considered more of a functional than an anatomic area, stimulation of this region produced a diffuse and bilateral CBF increase that appeared within seconds of the onset of stimulation but persisted for minutes after its cessation. The magnitude of the gCBF increase was related to the intensity of SVA stimulation. Independent of metabolism, the CBF increase for a 10-second stimulation train at 50 Hz (20 μA) peaked at 25%, but could be as high as 70% to 100% depending on frequency and amperage of the stimulation (Golanov et al, 2001). The SVA was characterized as part of a network that includes the rostral ventrolateral medulla, which contains neurons that are excited by hypoxia (Sun and Reis, 1993), and the medullary cerebrovasodilator area, which increases CBF when stimulated (Golanov et al, 2000). This network is believed to mediate a pattern of responses that protect the brain from hypoxia by redirecting blood from systemic circulation to cerebral circulation.
An earlier study by Manrique et al (1977) stimulated the STN directly in conscious, behaving goats and reported 80% to 100% increases in CBF after brief periods (10 seconds) of stimulation (100 Hz, 3 mA). Although they did not measure CBF, Angyan and Angyan (1999) reported voltage-related increases in blood pressure, measured at the carotid artery, respiration rate, and transient increases in heart rate as a result of STN stimulation in behaving cats (10 seconds, 100 Hz, 0.1 to 1.0 mA). They concluded that the STN is involved in adjusting cardiorespiratory function to motor activity. The gCBF changes in this study are consistent with the magnitudes of CBF changes associated with stimulation of the SVA and STN reported in these animal models.
The recognized indirect, local field effects of STN-DBS on the corticobulbar tract adjacent to the STN have been associated with motor signs and are not likely the cause of the observed gCBF changes. If the global increase in CBF is an indirect rather than a direct effect of STN stimulation, the SVA (i.e., neurons in the zona incerta or Forel's field), may be the source. Recently, Gradinaru et al (2009) used a novel optogenetic technique in conjunction with hemi-Parkinsonian rats and transgenic mice to study the therapeutic mechanisms of STN-DBS. They found that neither optogenetic inhibition nor excitation of intrinsic STN cells improved hemi-Parkinsonian symptoms. However, high-frequency optogenetic stimulation of afferent fibers entering the STN had a strong and reversible effect on hemi-Parkinsonian symptoms. This stimulation of afferent fibers was found to antidromically drive activity in the motor cortex. Antidromic influences on afferents from other areas, including the zona incerta, although not studied, were acknowledged as other potential sources of therapeutic benefits. Although Gradinaru et al (2009) did not study CBF, the mechanism that they described is reminiscent of the antidromic influence on the rostral ventrolateral medulla that produces elevated CBF when the fastigial nucleus of the cerebellum is stimulated (Reis and Golanov, 1997).
The mechanism of the observed gCBF increases is unknown, but the therapeutic similarities between STN-DBS and levodopa are notable and the effects of dopamine on CBF require some comment. Dopamine has been implicated as a factor in the control cerebral circulation (Leenders et al, 1985; Iadecola, 1998; Krimer et al, 1998). The administration of clinically effective doses of levodopa to PD patients and normal controls has been shown to produce diffuse increases in CBF without changes in oxygen utilization, suggesting cerebrovascular dilation (Leenders et al, 1985). A similar dissociation between CBF and glucose metabolism has been found on a regional basis after levodopa administration in PD subjects (Hirano et al, 2008). In general, stimulation of the dopamine system increases CBF (Leenders et al, 1985).
Both the electrophysiological and dopamine studies show that these interventions can produce increased CBF uncoupled from metabolism. This may account for the significant difference in the relationships between isotope dose and CBF in the STN-DBS on and off conditions in this study. The stronger association between dose and CBF in the on condition may reflect cerebral vasodilation, which would be expected from animal studies. This possibility will require further study. The on and off gCBF values also reflect different aspects of the subjects' clinical status. In the off condition, gCBF did not correlate with either clinical status measure, but the percentage change in gCBF between off and on increased with the duration of PD. As cerebral perfusion is decreased in PD (Melzer et al, 2011), this suggests that STN-DBS may have a greater effect on gCBF as the disease progresses. The relationships between gCBF and the clinical status scales in the on but not off conditions also suggest that gCBF is an indirect measure of clinical status: greater on effects in gCBF reflect more advanced PD. With regard to the measures of pausing during speech, higher gCBF in the off condition may be associated with more normal speech, whereas higher gCBF appears to be associated with more abnormal speech.
These results indicate that the effects of high-frequency stimulation of the subthalamic region on CBF warrant further attention with regard to the therapeutic action of STN-DBS and its associated behavioral changes. They also suggest that the clinical application of STN-DBS may provide an opportunity to study the mechanisms of cerebrovascular control. Existing animal studies and human PET results suggest that stimulation in the region of the STN can produce both global and regional increases in CBF (Pinto et al, 2004; Karimi et al, 2008; Hershey et al, 2003; Hirano et al, 2008), and some of these effects are independent of cerebral metabolism. It should be noted that this study did not use absolute quantitation; hence, the actual magnitude of the effects of STN-DBS on gCBF requires additional study. A recent semi-quantitative PET study of the effects of STN-DBS on glucose metabolism showed an 11% increase in metabolic rate with stimulation (Garraux et al, 2011). Increases in gCBF exceeding those found for metabolism would be anticipated from the uncoupling showed in the animal studies. The source of this gCBF effect may be the anatomic STN or the functional SVA. Global CBF increases produced by STN-DBS may have a role in its therapeutic effectiveness in PD, or they may be an indicator of the degree to which cerebrovascular control has been impacted by PD. The potential for chronically inducing increased CBF may be a consideration in the selection of candidates for STN-DBS.
Finally, with the expansion of DBS to more sites in the brain to treat a wider range of neurobehavioral conditions (Rabins et al, 2009) from depression (Giacobbe et al, 2009; Mayberg, 2009) and obsessive compulsive disorder (Mallet et al, 2008) to epilepsy (Hamani et al, 2009) and disorders of consciousness (Schiff, 2009), the possibility that long-term and short-term responses may be triggered globally or in multiple systems should be seriously considered as part of the mechanism of action of these therapeutic approaches. The presence of temporally persistent, widespread brain changes after focal stimulation may account for the some of the apparent contradictions in models of DBS that invoke a single mechanism (Kopell et al, 2006) and may be a factor in the observation that stimulation of different sites in the basal ganglia produce comparable results (Benabid et al, 2009).
Acknowledgments
The assistance of C Margouleff and D Bjelke with PET scanning, T Chaly and R Matacchieri with cyclotron support, P Spetsieris with Scanvp, and V Godier and K Cameron with speech analysis is gratefully acknowledged. The thoughtful comments of the anonymous reviewers contributed to the quality of this paper.
The authors declare no conflict of interest.
Footnotes
This work was supported by NIH R01 DC 007658, the Parkinson's Disease Foundation, and the Bachmann-Strauss Foundation.
References
- Angyan L, Angyan Z. Subthalamic influences on the cardiorespiratory functions in the cat. Brain Res. 1999;847:130–133. doi: 10.1016/s0006-8993(99)02011-9. [DOI] [PubMed] [Google Scholar]
- Arnt S, Cizadlo T, O'Leary D, Gold S, Andreasen NC. Normalizing counts and cerebral blood flow intensity in functional imaging studies of the human brain. Neuroimage. 1996;3:175–184. doi: 10.1006/nimg.1996.0019. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, Moringlane JR, Alesch F. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease. Arch Neurol. 1999;56:997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, UPDRS program members 1987Unified Parkinson's disease rating scale Recent Developments in Parkinson's Disease(Fahn S, Marsden CD, Goldstein M, Calne DB, eds), Vol. 2,Florham Park NJ: Macmillan Healthcare Information [Google Scholar]
- Garraux G, Bahri MA, Lemaire C, Degueldre C, Salmon E, Kaschten B. Brain energization in response to deep brain stimulation of subthalamic nuclei in Parkinson's disease. J Cereb Blood Flow Metab. 2011;31:1612–1622. doi: 10.1038/jcbfm.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe P, Mayberg HS, Lozano AM. Treatment resistant depression as a failure of brain homeostatic mechanisms: implications for deep brain stimulation. Exp Neurol. 2009;219:44–52. doi: 10.1016/j.expneurol.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Christensen JRC, Reis DJ. Neurons of a limited subthalamic area mediate elevations in cortical cerebral blood flow evoked by hypoxia and excitation of neurons of the rostral ventrolateral medulla. J Neurosci. 2001;21:4032–4041. doi: 10.1523/JNEUROSCI.21-11-04032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanov EV, Ruggiero DA, Reis DJA. Brainstem area mediating cerebrovascular and EEG responses to hypoxic excitation of rostral ventrolateral medulla in rat. J Physiol (Lond) 2000;529:413–429. doi: 10.1111/j.1469-7793.2000.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of Parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M. Age and regional cerebral blood flow at rest and during cognitive activity. Arch Gen Psychiat. 1987;44:617–621. doi: 10.1001/archpsyc.1987.01800190037006. [DOI] [PubMed] [Google Scholar]
- Hamani C, Andrade D, Hodaie M, Wennberg R, Lozano A. Deep brain stimulation for the treatment of epilepsy. Int J Neural Syst. 2009;19:213–226. doi: 10.1142/S0129065709001975. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, Lehrke R, Koulousakis A, Herholz K, Sturm V, Heiss W-D. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab. 2003;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- Hirano S, Asanuma K, Ma Y, Tang C, Feigin A, Dhawan V, Carbon M, Eidelberg D. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson's disease. J Neurosci. 2008;28:4201–4209. doi: 10.1523/JNEUROSCI.0582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurogenic control of the cerebral microcirculation: is dopamine minding the store. Nat Neurosci. 1998;1:263–265. doi: 10.1038/1074. [DOI] [PubMed] [Google Scholar]
- Iida H, Law I, Pakkenberg B, Karup-Hansen A, Eberl S, Holm S, Hansen AK, Gundersen HJG, Thomsen C, Svarer C, Ring P, Friberg L, Paulson OB. Quantitation of regional cerebral blood flow corrected for partial volume effect using O-15 water and PET: I. Theory, error analysis, and stereologic comparison. J Cereb Blood Flow Metab. 2000;20:1237–1251. doi: 10.1097/00004647-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Ingvar DH, Cronqvist S, Ekberg R, Risberg J, Hoedt-Rasmussen K. Normal values of regional cerebral blood flow in man, including flow and weight estimates of gray and white matter. Acta Neurol Scand Suppl. 1965;14:72–78. doi: 10.1111/j.1600-0404.1965.tb01958.x. [DOI] [PubMed] [Google Scholar]
- Karimi M, Golchin N, Tabbal D, Hershey T, Videen TO, Wa J, Usche JWM, Revilla FJ, Hartlein JM, Wernle AR, Mink JW, Perlmutter JS. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson's disease. Brain. 2008;131:2710–2719. doi: 10.1093/brain/awn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. J Chronic Dis. 1956;3:478–486. doi: 10.1016/0021-9681(56)90146-1. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, Lang AE, Deuschl G. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21:S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, Marzinzik F, Curio G, Sappok T. Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson's disease. J Neurol Neurosurg Psychiatr. 2008;79:522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- Kopell BH, Rezai AR, Chang JW, Vitek JL. Anatomy and physiology of the basal ganglia: implications for deep brain stimulation for Parkinson's disease. Mov Disord. 2006;21:S238–S246. doi: 10.1002/mds.20958. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Mully EC, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Wolfson L, Gibbs JM, Wise RJS, Causon R, Jones T, Legg NJ. The effects of L-dopa on regional cerebral blood flow and oxygen metabolism in patients with Parkinson's disease. Brain. 1985;108:171–191. doi: 10.1093/brain/108.1.171. [DOI] [PubMed] [Google Scholar]
- Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatr. 2009;80:659–666. doi: 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chereau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardes S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Verin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B, Pelissolo A, STOC Study Group Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- Manrique M, Alborch E, Delgado JMR. Cerebral blood flow and behavior during brain stimulation in the goat. Am J Physiol. 1977;232:H495–H499. doi: 10.1152/ajpheart.1977.232.5.H495. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pearson JF, Rüeger S, Pitcher TL, Livingston L, Graham C, Keenan R, Shankaranarayanan A, Alsop DC, Dalrymple-Alford JC, Anderson TJ. Arterial spin labeling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain. 2011;134:845–855. doi: 10.1093/brain/awq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15:635–641. doi: 10.1161/01.str.15.4.635. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Rogers SA, Braaten AJ, Woods SP, Tröster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation: a critical review. Lancet Neurol. 2006;5:578–580. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, Gaura V, Rascol O, Samson Y, Agid Y. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol. 2004;61:1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- Pinto S, Thobois S, Costes N, Le Bars D, Benabid A-L, Broussolle E, Pollak P, Gentil M. Subthalamic nucleus stimulation and dysarthria in Parkinson's disease: a PET study. Brain. 2004;127:602–615. doi: 10.1093/brain/awh074. [DOI] [PubMed] [Google Scholar]
- Rabins P, Appleby BS, Brandt J, DeLong MR, Dunn LB, Gabriels L, Greenberg BD, Haber SN, Holtzheimer PE, Mari Z, Mayberg HS, McCann E, Mink SP, Rasmussen S, Schlaepfer TE, Vanter DE, Vitek JL, Walkup J, Mathews DJH. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Arch Gen Psychiatry. 2009;66:931–937. doi: 10.1001/archgenpsychiatry.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, Golanov EV. Autonomic and vasomotor regulation. Int Rev Neurobiol. 1997;41:121–149. doi: 10.1016/s0074-7742(08)60350-5. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Moeller JR, Strother SC, Dhawan V, Sergi M. Effects of percent thresholding on the extraction of [18F]fluorodeoxyglucose positron emission tomographic region-of-interest data. J Cereb Blood Flow Metab. 1991;11:A83–A88. doi: 10.1038/jcbfm.1991.42. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic deep-brain stimulation in the severely brain injured: rationale and proposed mechanisms of action. Ann N Y Acad Sci. 2009;1157:101–116. doi: 10.1111/j.1749-6632.2008.04123.x. [DOI] [PubMed] [Google Scholar]
- Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34:855–862. doi: 10.1212/wnl.34.7.855. [DOI] [PubMed] [Google Scholar]
- Sidtis D, Rogers T, Godier V, Tagliati M, Sidtis JJ. Voice and fluency changes as a function of speech task and deep brain stimulation. J Speech Lang Hear Res. 2010;53:1–11. doi: 10.1044/1092-4388(2010/09-0154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ. Some problems for representations of brain organization based on activation. Brain Lang. 2007;102:130–140. doi: 10.1016/j.bandl.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ, Gomez C, Naoum A, Strother SC, Rottenberg DA. Mapping cerebral blood flow during speech production in hereditary ataxia. Neuroimage. 2006;3:246–254. doi: 10.1016/j.neuroimage.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith CD, Cahill C, Schnorr L, Grootoonk S, Spinks T, Clark J, Frackowiak K, Jones T. Detection of thirty-second cognitive interactions in single subjects with positron emission tomography: a new low-dose H215O regional imaging technique. J Cereb Blood Flow Metab. 1993;13:617–629. doi: 10.1038/jcbfm.1993.80. [DOI] [PubMed] [Google Scholar]
- Sun M-K, Reis DJ. Hypoxic excitation of medullary vasomotor neurons in rats are not mediated by glutamate or nitric oxide. Neurosci Lett. 1993;157:219–222. doi: 10.1016/0304-3940(93)90741-3. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure J-G, Burkhard PR, Bogousslavsky J, Vingerhoets FJG. How do parkinsonian signs return after discontinuation of subthalamic DBS. Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Tommasi G, Krack P, Fraix F, Le Bas J-F, Chabardes S, Benabid A-L, Pollak P. Pyramidal tract side effects induced by deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatr. 2008;79:813–819. doi: 10.1136/jnnp.2007.117507. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Deep brain stimulation: how does it work. Cleve Clin J Med. 2008;75:S59–S65. doi: 10.3949/ccjm.75.suppl_2.s59. [DOI] [PubMed] [Google Scholar]
- Volkmann J. Deep brain stimulation for Parkinson's disease. Parkinsonism Relat Disord. 2007;13:S462–S465. doi: 10.1016/S1353-8020(08)70050-6. [DOI] [PubMed] [Google Scholar]
- Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, Krack P. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol. 2004;61:697–700. doi: 10.1001/archneur.61.5.697. [DOI] [PubMed] [Google Scholar]