Abstract
Recent reports based on a chemiluminescent enzymatic assay for detection of adenosine conclude that cultured astrocytes release adenosine during mildly hypoxic conditions. If so, astrocytes may suppress neural activity in early stages of hypoxia. The aim of this study was to reevaluate the observation using high-performance liquid chromatography (HPLC). The HPLC analysis showed that exposure to 20 or 120 minutes of mild hypoxia failed to increase release of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine from cultured astrocytes. Similar results were obtained using a chemiluminescent enzymatic assay. Moreover, since the chemiluminescent enzymatic assay relies on hydrogen peroxide generation, release of free-radical scavengers from hypoxic cells can interfere with the assay. Accordingly, adenosine added to samples collected from hypoxic cultures could not be detected using the chemiluminescent enzymatic assay. Furthermore, addition of free-radical scavengers sharply reduced the sensitivity of adenosine detection. Conversely, use of a single-step assay inflated measured values due to the inability of the assay to distinguish adenosine and its metabolite inosine. These results show that cultured astrocytes do not release adenosine during mild hypoxia, an observation consistent with their high resistance to hypoxia.
Keywords: adenosine, astrocytes, brain ischemia, cell culture, energy metabolism
Introduction
Adenosine is a potent inhibitory modulator that acts via presynaptic and postsynaptic A1 receptors to suppress excitatory, but not inhibitory, transmission (Dunwiddie and Masino, 2001). Adenosine is released in response to metabolic disturbances, such as hypoxia or ischemia, and during periods of intense neuronal activity (Higgins et al, 1994). Efflux of adenosine is facilitated by equilibrative adenosine transporters and is traditionally viewed as an autocrine protective mechanism that reduces neural activity when the cytosolic adenosine concentration is rising (Brundege and Dunwiddie, 1997). A study showed that ischemic conditions induced adenosine release from neurons but not from astrocytes (Parkinson et al, 2002). However, more recent observations suggest that cultured astrocytes are also sensitive to hypoxia and release adenosine in response to mild hypoxia. These observations are of potential importance, because astrocytic adenosine release may control excitatory transmission (Martin et al, 2007). Nevertheless, it is a concern that data on adenosine release from astrocytes have been collected using a chemiluminescent assay that heavily depends on serial enzymatic reactions and nonlinear generation of photons (Kather et al, 1987; Martin et al, 2007). In the present study, we directly compared astrocytic adenosine release using the chemiluminescent enzymatic assay and high-performance liquid chromatography (HPLC; Goldman et al, 2010). We found that the chemiluminescent assay could lead to inaccurate measurements due to the presence of compound(s) that interfered with the enzymatic steps or due to the reactions to the adenosine metabolites, rendering the detection of adenosine using this method unreliable. Furthermore, our HPLC results showed that mild hypoxia failed to increase the release of adenosine and adenine nucleotides from cultured astrocytes. Based on these observations, we conclude that astrocytes are not a source of adenosine during mildly hypoxic conditions.
Materials and methods
Cell Culture Conditions
Primary neocortical astrocyte cultures were prepared from P0-2 Wistar rat pups (Taconics) as previously described (Lin et al, 1998). Briefly, dissociated cells were plated on culture flasks and maintained in DMEM/F12 containing 10% fetal bovine serum, 100 IU/mL penicillin and 100 μg/mL streptomycin (5% CO2/95% air, 37°C). Astrocytes were subcultured on 12-well or 24-well plates for subsequent chemiluminescence assay and HPLC analysis, respectively. A C6 glioma cell line was kept in identical conditions and used for a subset of experiments. For experiments, cultures underwent five half-washes with artificial cerebrospinal fluid (in mmol/L: NaCl 124, KCl 2.5, NaH2PO4 1.25, CaCl2 2, MgSO4 1, NaHCO3 26) either with 10 mmol/L glucose or without glucose. For the chemiluminescent assay, those with glucose were placed back in their home incubator. Those without glucose were placed in a hypoxic incubator at 37°C with 95% N2 and 5% CO2 for either 20 or 60 minutes. After the allotted time, the supernatants from the wells were removed and plated in white, opaque 96-well plates for chemiluminescent assay. For HPLC analysis, the cells were incubated in normoxic (95% air, 5% CO2) or hypoxic (95% N2, 5% CO2) chamber for either 20 or 120 minutes. All experiments were approved by the Institution of Animal Care and Use Committee of University of Rochester.
Chemiluminescent Adenosine Assay
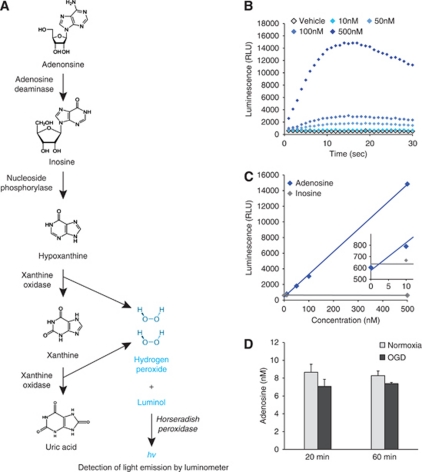
The chemiluminescent adenosine assay depends on the degradation of adenosine into uric acid and hydrogen peroxide (Figure 1A). Specificity for adenosine depends on a two-step principle. A luminol buffer was mixed with 0.2 mol/L Na2HPO4, 0.2 mol/L KH2PO4, 2 mmol/L EDTA, and 2.5 mmol/L luminol (all from Sigma-Aldrich, St Louis, MO, USA). The buffer pH was adjusted to 8.4 because pilot studies indicated this was an optimal pH for assay function. All enzyme mixes were made in this buffer. The first enzyme mix contained 3 U/mL nucleoside phosphorylase (Sigma-Aldrich, catalog # N8264), 10 U/mL xanthine oxidase (Roche Applied Sciences, Indianapolis, IN, USA, catalog # 10110434001) and 3 U/mL horseradish peroxidase (Sigma-Aldrich, catalog # P6140) final concentrations. This enzyme mix was added to samples in a 1:1 ratio and the luminescence was allowed to stabilize at near-baseline levels, which usually required ∼30 minutes. This step was taken to represent the degradation of endogenous inosine, xanthine, hypoxanthine, and hydrogen peroxide. The second enzyme mix was the same as the first, but with the addition of adenosine deaminase (Roche Applied Sciences, catalog # 10102121001) to yield a final adenosine deaminase concentration of 5 U/mL. This second enzyme mix was added to the existing volume in the wells at a 1:2 ratio, the plate was shaken and luminescent readings were collected every second after enzyme addition for up to 60 seconds. The enzyme concentrations used were determined through pilot experiments to optimize for assay sensitivity and kinetics. All enzyme mix additions and luminescence readings were performed using a luminometer (Perkin-Elmer, Victor2, Wallac, Gaithersburg, MD, USA).
Figure 1.
Establishment of a chemiluminescent adenosine assay. (A) Diagrammatic depiction of the enzymatic steps used in the assay. (B) Luminescent readings over time of different concentrations of adenosine show concentration-dependent differences in luminescence. (C) A plot of the maximum luminescence reading (0 to 30 seconds) versus adenosine concentration in the sample generates linear standard curves. Insert, a magnified view of 0 to 10 nmol/L adenosine. (D) Use of the assay failed to detect adenosine increases in 20 or 60 minutes oxygen-glucose deprivation (OGD)-treated cultures (n=4).
High-Performance Liquid Chromatography Analysis of Purines
The analyses were performed using CoulArray 5600A HPLC system (ESA Inc., Sunnyvale, CA, USA) and a model 526 UV detector (ESA Inc.) as previously described (Cui et al, 2009; Goldman et al, 2010). Chromatographic separation was achieved by using a Lichrospher 100 RP-18 column (5 μm, 250 mm × 3 mm; Merck, KGaA, Darmstadt, Germany). For measurements of samples, we used the mobile phase, which consisted of 215 mmol/L KH2PO4, 2.3 mmol/L tetrabutylammonium bisulfate, 3.2% (v/v) acetonitrile (HPLC grade) and HPLC grade water, pH 6.2. The flow rate was maintained at 0.25 mL/min. Daily calibration curves were prepared by a four point standard (3, 1, 0.3, or 0.1 μmol/L) of adenosine triphosphate (ATP), adenosine triphosphate (ADP), adenosine monophosphate (AMP), adenosine, inosine and IMP in 0.4 mol/L perchloric acid, respectively. Eluted purines were detected at 260 nm, and the chromatographic peaks were integrated using CoulArray software (ESA Inc.).
Statistics
All data are expressed as mean values±standard error of the mean. Significance was determined using a Student's t-test.
Results
Establishment of the Chemiluminescent Adenosine Assay
Evaluation of the potential of this assay was performed using adenosine standards dissolved in artificial cerebrospinal fluid. The hydrogen peroxide formed by sequential catabolism of purines to uric acid is detected by the oxidation of luminal in the presence of peroxidase (Figure 1A). We used a two-step procedure to determine adenosine concentration, selectively. In step 1, any purine lower in the catabolic sequence than adenosine was converted to uric acid by addition of enzymes except adenosine deaminase. After addition of the second enzyme mix, luminescent readings peaked in wells containing adenosine within 20 seconds, and the assay was sensitive enough to distinguish concentrations of adenosine down to 10 nmol/L (Figure 1B). The maximum luminescence detected from samples correlated strongly and linearly with adenosine concentrations (Figure 1C), so this measurement was used to generate daily standard curves from which sample adenosine concentrations could be calculated. Furthermore, when the same assay was performed using inosine standards, no signal was detected (Figure 1C), further supporting the conclusion that this two-step assay was specific for adenosine and not for its metabolites. This sensitivity and specificity of the assay was promising and we next proceeded to analyze samples collected from cultures.
Inherent Problem with Adenosine Detection from Hypoxic Cultures
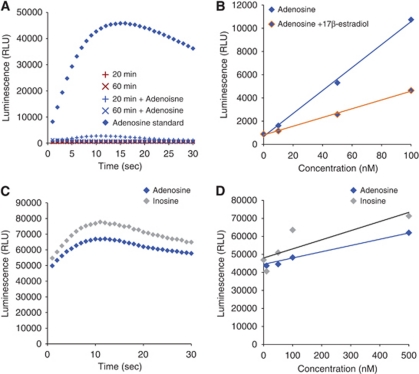
Oxygen-glucose deprivation (OGD), a model of ischemia, is known to induce ATP (measured by luciferase ATP assay; Liu et al, 2008) and adenosine (measured by thin layer chromatography; Parkinson and Xiong, 2004) release from cultured astrocytes. To confirm these observations by using this assay, astrocyte cultures were exposed to 20 and 60 minutes of OGD. Unexpectedly, OGD failed to induce an increase in adenosine concentrations (Figure 1D). Given the very low signal obtained and the slight decrease in luminescence from OGD samples, we suspected the assay was not functioning properly with samples collected from cultured cells. In confirmation, the assay was unable to detect 500 nmol/L adenosine spiked into samples drawn from 20 and 60 minutes of OGD, whereas 500 nmol/L adenosine in calibration solutions was clearly within the sensitivity of this assay (Figure 2A).
Figure 2.
Anomalies in use of the chemiluminescent adenosine assay. (A) Spiking of samples drawn from hypoxic cells shows an inconsistent ability to detect the presence of normally detectable adenosine concentrations. The ability of the assay to function normally and detect adenosine (500 nmol/L) is abolished after cells are exposed to 20 or 60 minutes mild hypoxia. (B) Addition of known free-radical scavengers at low concentrations dampens assay luminescence. (C) Lack of the sensitivity in single-step procedure. The luminescence intensity detected from inosine (500 nmol/L) was similar to adenosine (500 nmol/L) by using single-step procedure. (D) Single-step procedure failed to distinguish inosine (10 to 500 nmol/L) from adenosine (10 to 500 nmol/L).
One possibility is that free-radical scavengers produced by cells under stress (Griffin et al, 2005; Schroeter et al, 1999) could interfere with the hydrogen peroxide generated by the necessary assay degradation of adenosine. In support of this idea, we observed that luminescent readings from standards dissolved in buffers containing known free-radical scavengers were reduced. Addition of 10 nmol/L 17β-estradiol (Sigma-Aldrich) resulted in luminescent readings half those of scavenger-free buffers (Figure 2B). Other manipulations of the assay buffer including low calcium concentrations or addition of nucleotides (UTP) did not have any effect on the assay (data not shown). After these experiments, we concluded that this chemiluminescent assay is susceptible to cellular products released from hypoxic cells and unsuitable for detection of adenosine collected from biological samples.
Mild Hypoxia Did Not Induce Adenosine Release from Astrocytes
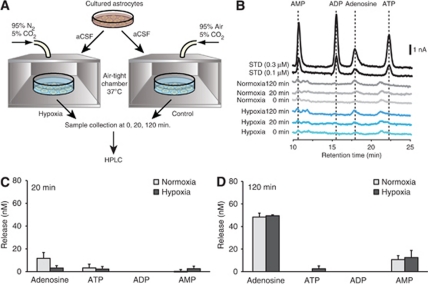
Surprisingly, studies based on the chemiluminescent assay have shown that mildly hypoxic conditions increase adenosine concentrations in astrocyte cultures (Martin et al, 2007). In these studies, a single-step method was used in which all enzymes were applied at once. To evaluate whether the single-step procedure accurately detected adenosine, we next evaluated this modification of the assay. Unfortunately, use of a single-step assay failed to distinguish adenosine and inosine signals (Figures 2C and 2D) and produced distinct results as compared with the two-step procedure (Figures 1B and 1C). We further evaluated whether astrocytes could release adenosine in response to mildly hypoxic conditions. Figure 3A illustrates the procedure for a mildly hypoxic condition replicating the method described by Martin et al (2007). Cells were washed with artificial cerebrospinal fluid, pH 7.4, and 24-well plates were placed into special chambers equipped with thermostat housing. Chambers were incubated at 37°C under hypoxic conditions by gassing the special chamber with a gas mixture consisting of 95% N2, 5% CO2, for 0 to 120 minutes. For controls, the cultures were incubated at 37°C under normoxic conditions (95% O2, 5% CO2) for the same length of time (Figure 3A). Adenine nucleotides and adenosine were quantified using HPLC with ultraviolet absorbance to examine both direct adenosine release and adenosine produced by degradation of ATP in the extracellular space (Figure 3B). Twenty minutes of incubation in the hypoxia chamber did not trigger adenosine release (normoxia 11.69±5.11 versus hypoxia 3.15±2.07, respectively, P=0.17). In addition, none of the adenine nucleotide concentrations were affected by 20 minutes of mild hypoxia exposure (Figure 3C). Consistently, no differences were found between 120 minutes normoxia and hypoxia in both adenine nucleotides and adenosine concentrations (Figure 3D). These results clearly indicate that cultured astrocytes do not release adenosine during mildly hypoxic conditions.
Figure 3.
Mildly hypoxic conditions fail to increase extracellular concentration of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adeosine monophosphate (AMP), and adenosine. (A) Schematic of experimental procedure. Cells were incubated in normoxic (95% air, 5% CO2) or hypoxic (95% N2, 5% CO2) chamber for 20 or 120 minutes. Samples were measured by high-performance liquid chromatography (HPLC). (B) Representative HPLC chromatograms of normoxia and hypoxia samples. Standards of adenosine, AMP, ADP and ATP (0.1 and 0.3 μmol/L each) are shown on top. (C, D) Histogram summarizing the mean concentrations of adenosine, AMP, ADP and ATP after 20 minutes (C) or 120 minutes (D) normoxia (n=4) and hypoxia (n=4). Concentrations of ADP were below detection level in all experimental condition.
Discussion
Adenosine triphosphate serves as a universal energy currency in all living organisms. Adenosine is the backbone on which the three high-energy phosphate bonds of ATP store and transport energy. When the supply of O2 is lower than the demand, the cytosolic concentration of adenosine increases in direct proportion to a decline in ATP. Adenosine rapidly exits hypoxic cells via an equilibrative nucleoside transporter, resulting in a steady increase in the extracellular concentration of adenosine (Dunwiddie and Masino, 2001). Essentially all cell types express adenosine receptors, which generally suppress activity and thereby preserve viability of metabolically stressed cells. The dual function of adenosine as an energy metabolite and as a transmitter provides an elegantly simple and powerful mechanism of endogenous protection. In the brain, active firing neurons will release adenosine, which binds to A1 receptors and counteracts additional neuronal firing primarily through the inhibition of presynaptic Ca2+ channels. This effect is concomitant with hyperpolarization of the resting membrane potential by the opening of K+ channels (Higgins et al, 1994).
This study was prompted by the report that cultured astrocytes release adenosine during mildly hypoxic conditions. This observation was surprising, because cultured astrocytes are extraordinarily resistant to hypoxia and can survive for days without a significant loss of cells in oxygen-depleted conditions (Chesler, 2005). However, if astrocytes release adenosine during mildly hypoxic condition, this mechanism could have an important neuroprotective role by suppressing the activity of nearby neurons. We have here compared adenosine release from cultured astrocytes using a chemiluminescence assay (Kather et al, 1987; Martin et al, 2007) with the more traditional HPLC detection of purines (Cui et al, 2009; Goldman et al, 2010). Our analysis show that cultured astrocytes do not release adenosine during mildly hypoxic conditions and that the chemiluminescence assay is unreliable. Consistent with our findings, a study using thin layer chromatography showed that tritium-labeled adenine nucleotides and adenosine released from astrocytes were not significantly affected by OGD treatment (Parkinson et al, 2002). Similarly, adenosine release from astrocytes was not observed during OGD, but was enhanced after reintroduction of oxygen and glucose (Ciccarelli et al, 1999). Furthermore, OGD significantly increased the release of purines from cultured neurons, but not from cultured astrocytes (Parkinson et al, 2002). In agreement with this observation, OGD evoked tetrodotoxin-sensitive neuronal adenosine release in hippocampal slices (Pedata et al, 1993). Neurons are more susceptible to hypoxia-induced cell death than astrocytes (Litsky et al, 1999), which may correlate with a faster depletion of cellular ATP and thereby an increase in adenosine (Parkinson and Xiong, 2004). Astrocytes contain large quantities of glycogen and can survive for long periods without oxygen, meaning that they are less reliant on glucose for ATP levels maintenance (Fillenz et al, 1999; Shulman et al, 2001). One study has, however, been able to detect adenosine release from astrocytes in response to hypoxic conditions by flushing the spinner flask with 95% N2/5% CO2. In this study, astrocytes were cultured at high density on microcarrier beads and it is possible that the cells experienced more severe hypoxia during these conditions as a consequence of increased competition for O2 (Kulik et al, 2010). Based on the literature and the observations reported here, we conclude that it is more likely that highly metabolically active neurons are the source of adenosine released during hypoxic conditions, in accordance with earlier reports.
The chemiluminescent adenosine assay is based on the principle that enzymatic degradation of adenosine into hydrogen peroxide (and uric acid) serves as a substrate for generation of photons in presence of horseradish peroxidase and luminol (Figure 1A). Since the metabolic products of adenosine also are present in biological samples, the specificity for adenosine is obtained by first adding the enzymes that degrade inosine, hypoxanthine, and xanthine. Adenosine deaminase is added in a second step after photon production in the first step has stabilized (Kather et al, 1987). Our study was unable to reproduce the mildly hypoxic condition-induced adenosine release (Martin et al, 2007). It is possible that the difference in results reflect the use of serial two-step instead of single-step procedure. Our analysis indicated that it is critical to use the two-step principle, since it is not otherwise possible to discriminate between inosine and adenosine (Figures 2C and 2D). Notably, inability to discriminate adenosine from its assay metabolites would likely be confounding; it has previously been reported that metabolic disturbances, including inhibition of oxidative phosphorylation or glycolysis, induced ∼2- to 4-fold more inosine and hypoxanthine release than adenosine release from astrocytes (Parkinson et al, 2002). In addition, a study using spinal cord tissue preparations showed that hypoxia induces the release of intracellularly produced inosine. The hypoxia-induced increase of extracellular inosine was markedly higher than that of adenosine (Takahashi et al, 2010). Moreover, although we found that the chemiluminescence assay accurately detected adenosine when the two-step procedure was followed, it was not possible to quantify adenosine in samples collected from hypoxic cultures even when the two-step procedure was used. During hypoxic conditions, most cell types, including astrocytes, release free-radical scavengers (Griffin et al, 2005; Schroeter et al, 1999). Since the last step of the assay relies on hydrogen peroxide generated by xanthine oxidase, it is likely that the free-radical scavengers released from hypoxic cells will interfere with this last enzymatic step. In support of this mechanism, addition of low concentrations of an exogenous free-radical scavenger (17β-estradiol) potently reduced the luminescence readout (Figure 2B). Thus, cultured astrocytes do not release adenosine during mildly hypoxic conditions, and the chemiluminescence assay cannot be used for measurement of adenosine released in response to hypoxia.
The authors declare no conflict of interest.
Footnotes
This work was supported by NINDS/NIH.
References
- Brundege JM, Dunwiddie TV. Role of adenosine as a modulator of synaptic activity in the central nervous system. Adv Pharmacol. 1997;39:353–391. doi: 10.1016/s1054-3589(08)60076-9. [DOI] [PubMed] [Google Scholar]
- Chesler M. Failure and function of intracellular pH regulation in acute hypoxic-ischemic injury of astrocytes. Glia. 2005;50:398–406. doi: 10.1002/glia.20141. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Giuliani P, D'Alimonte I, Ballerini P, Caciagli F, Rathbone MP. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25:93–98. [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci USA. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fillenz M, Lowry JP, Boutelle MG, Fray AE. The role of astrocytes and noradrenaline in neuronal glucose metabolism. Acta Physiol Scand. 1999;167:275–284. doi: 10.1046/j.1365-201x.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin S, Clark JB, Canevari L. Astrocyte-neurone communication following oxygen-glucose deprivation. J Neurochem. 2005;95:1015–1022. doi: 10.1111/j.1471-4159.2005.03418.x. [DOI] [PubMed] [Google Scholar]
- Higgins MJ, Hosseinzadeh H, MacGregor DG, Ogilvy H, Stone TW. Release and actions of adenosine in the central nervous system. Pharm World Sci. 1994;16:62–68. doi: 10.1007/BF01880657. [DOI] [PubMed] [Google Scholar]
- Kather H, Wieland E, Waas W. Chemiluminescent determination of adenosine, inosine, and hypoxanthine/xanthine. Anal Biochem. 1987;163:45–51. doi: 10.1016/0003-2697(87)90091-1. [DOI] [PubMed] [Google Scholar]
- Kulik TB, Aronhime SN, Echeverry G, Beylin A, Winn HR. The relationship between oxygen and adenosine in astrocytic cultures. Glia. 2010;58:1335–1344. doi: 10.1002/glia.21011. [DOI] [PubMed] [Google Scholar]
- Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- Litsky ML, Hohl CM, Lucas JH, Jurkowitz MS. Inosine and guanosine preserve neuronal and glial cell viability in mouse spinal cord cultures during chemical hypoxia. Brain Res. 1999;821:426–432. doi: 10.1016/s0006-8993(99)01086-0. [DOI] [PubMed] [Google Scholar]
- Liu HT, Sabirov RZ, Okada Y. Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal. 2008;4:147–154. doi: 10.1007/s11302-007-9077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Sinclair CJ, Othman T, Haughey NJ, Geiger JD. Differences between rat primary cortical neurons and astrocytes in purine release evoked by ischemic conditions. Neuropharmacology. 2002;43:836–846. doi: 10.1016/s0028-3908(02)00083-7. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J Neurochem. 2004;88:1305–1312. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- Pedata F, Latini S, Pugliese AM, Pepeu G. Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J Neurochem. 1993;61:284–289. doi: 10.1111/j.1471-4159.1993.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Mertsch K, Giese H, Muller S, Sporbert A, Hickel B, Blasig IE. Astrocytes enhance radical defence in capillary endothelial cells constituting the blood-brain barrier. FEBS Lett. 1999;449:241–244. doi: 10.1016/s0014-5793(99)00451-2. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Otsuguro K, Ohta T, Ito S. Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br J Pharmacol. 2010;161:1806–1816. doi: 10.1111/j.1476-5381.2010.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]