Abstract
The relation of cortical microcirculation, oxygen metabolism, and underlying neuronal network activity remains poorly understood. Anatomical distribution of cortical microvasculature and its relationship to cortical functional domains suggests that functional organizations may be revealed by mapping cerebral blood flow responses. However, there is little direct experimental evidence and a lack of electrophysiological evaluation. In this study, we mapped ocular-dominance columns in primary visual cortex (V1) of anesthetized macaques with capillary flow-based laser speckle contrast imaging and deoxyhemoglobin-based intrinsic optical imaging. In parallel, the local field potentials (LFPs) and spikes were recorded from a linear array of eight microelectrodes, carefully positioned into left and right eye columns in V1. We found differential activation maps of blood flow, after masking large superficial draining vessels, exhibited a column-like pattern similar as the oximetric maps. Both the activated spikes and γ-band LFP demonstrated corresponding eye preference, consistent with the imaging maps. Our results present direct support in favor of previous proposals that the regulation of microcirculation can be as fine as the submillimeter scale, suggesting that cortical vasculature is functionally organized at the columnar level in a manner appropriate for supplying energy demands of functionally specific neuronal populations.
Keywords: cortical mapping, electrophysiology, functional MRI, neurovascular coupling, ocular dominance, optical imaging
Introduction
Local changes in cerebral blood flow (CBF) have a dynamic and intimate interaction in space and time with neural activity (Attwell et al, 2010; Iadecola, 2004; Lauritzen, 2005). The spatial specificity of the chain of physiological events elicited by neuronal circuitry at different peristimulus phases has been exploited to map functional architecture in the cerebral cortex (columnar structures) with optical imaging (Blasdel and Salama, 1986; Grinvald et al, 1986; Lu and Roe, 2008; Wang et al, 1996) and blood-oxygenation-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) methods (Chen et al, 2007; Cheng et al, 2001; Menon et al, 1997; Shmuel et al, 2010). However, the spatial scale of local neurovascular control in the cortical network is a major source of controversy in a number of BOLD studies (Op de Beeck, 2010; Shmuel et al, 2010). Since the BOLD contrast results from a complex interplay of neuronal, metabolic, hemodynamic, and MRI parameters (Logothetis, 2008), characterizing CBF regulation at the submillimeter scale may lead to potentially cleaner interpretation and ultimately better understanding of fMRI results.
There have been relatively few studies of CBF during the past decade due to the difficulties and limitations of perfusion-based fMRI (Zappe et al, 2008). However, a prevailing hypothesis is that the CBF response can exhibit spatial specificity at the columnar scale (Woolsey et al, 1996). Based on the notion that increased flow leads to increased cerebral blood volume (CBV), columnar specificity of blood volume responses have been demonstrated on rat barrel cortex and striate cortex of cats and monkeys with the intrinsic optical imaging (IOI) technique (Sheth et al, 2004; Vanzetta et al, 2004), as well as with contrast agent-enhanced CBV fMRI methods (Fukuda et al, 2006). Spatially specific regulation of CBF has been also observed in the vertical dimension of cortex in which laminar-specific regulation of CBF in deep cortical layers was independent of dilation of large surface vessels (Caesar et al, 2003; Fabricius et al, 1997; Tian et al, 2010). Thus, there are indications that blood flow can be regulated at a local scale. The size of this scale has been demonstrated by CBV-based hemodynamic signal to be <400 μm (Vanzetta et al, 2004) and, thus far, by CBF-based signal to be ∼1.1 mm (the spacing of orientation columns in cat visual cortex; Duong et al, 2001). There still remains a lack of electrophysiological evaluation of these findings.
Here, we examined the question of whether CBF is a measure that can be used to study neurovascular regulation at a submillimeter scale. To image changes in blood flow, we used the laser speckle contrast imaging (LSCI) technique to visualize ocular-dominance (OD) columns in primary visual cortex (V1) of anesthetized macaque monkeys. This is a technique with superior spatio-temporal resolution that monitors microcirculation via differential tracking of the movement of red blood cells (Cheng and Duong, 2007; Dunn et al, 2001; Durduran et al, 2004; Li et al, 2011; Wang et al, 2007; Weber et al, 2004). To directly relate these measures to other measures of submillimeter organization, we compared maps obtained by CBF with functional oximetry maps obtained by the IOI technique and further validated these with electrical measures (single-unit spiking and local field potentials, LFPs). We find that the CBF maps correlate highly with both IOI maps and with electrophysiological measures. Our results provide direct evidence that blood flow is regulated at a submillimeter scale and support the fundamental concept that cortical vasculature is highly functionally organized (Gardner, 2010; Shmuel et al, 2010). Our LFP recordings also provide further evidence for a coupling between hemodynamic responses and γ oscillations (Niessing et al, 2005). Portion of this work has been reported in abstract form briefly elsewhere (Wang and Roe, 2010).
Materials and methods
Animal Preparation
Four hemispheres in three Macaque monkeys (Macaca mulatta) were used for these experiments. Animals were inducted by ketamine (10 mg/kg intramuscularly) and atropine (0.05 mg/kg) and then anesthetized with thiopental sodium (1 to 2 mg/kg/h intravenously) and isoflurane (0.2% to 1.5%), paralyzed with vecuronium bromide (0.05 mg/kg/h intravenously) and artificially ventilated. Anesthetic depth was assessed continuously via electroencephalogram signal measured by implanted wires and end-tidal CO2, pulse oximetry, and heart rate were monitored throughout the surgery and experiments. Pupils were dilated with atropine and eyes were fitted with customized primate contact lenses (Danker Laboratories Inc., Sarasota, FL, USA) of appropriate curvature to focus on a computer screen at 57 cm distance. The details of surgical preparation were similar to the descriptions in our recent work (Lu and Roe, 2008). The craniotomy and durotomy were performed over a region around the lunate sulcus (centered about 15 mm anterior to occipital ridge and 10 mm lateral to midline) to expose visual areas V1 and V2 (near the V1/V2 border at an eccentricity of 2° to 5° from the fovea). In all cases, a chronic chamber was implanted afterwards (Chen et al, 2002). All surgical and experimental procedures conformed to the guidelines of the National Institute of Health and were approved by the Vanderbilt Animal Care and Use Committees.
Visual Stimulation
Full screen black–white gratings were generated by VSG 2/5 or ViSaGe (Cambridge Research System, Rochester, UK) and presented on a CRT monitor running at 100 Hz refresh rate. Typically, the high-contrast (90%) monochromatic sinusoidal gratings (with spatial frequency at 1.5 c/deg, temporal frequency at 2 Hz) were presented to reveal OD maps in the imaging session. Electromechanical shutters were placed in front of the eyes that randomly opened to left or right eye for monocular stimulation. During the stimulus intervals and the blank condition, eye shutters were closed. Screen extent spanned 24 × 18° of visual field. Mean luminance for all stimuli, including the blank stimulus (uniform gray screen), was kept ∼30 cd/m2. The synchronization between stimulus presentation (VSG System), eye shutters, and data acquisition (both Imager 3001 (Optical Imaging Inc., Germantown, NY, USA) and Plexon systems (Dallas, TX, USA)) was custom made on the basis of the NI LabView platform (National Instruments, Austin, TX, USA).
Intrinsic Optical Imaging and Data Analysis
Before electrophysiological recording, we first obtained functional oximetry maps of the columnar structure by the IOI technique. The brain was stabilized with agar and images were acquired through a glass coverslip. Raw reflectance images were captured by using Imager 3001, with 605- or 630-nm illumination (for details, see Lu and Roe, 2008). Signal-to-noise ratio was enhanced by trial averaging (25 trials per stimulus condition) and by synchronization of acquisition with heart rate and respiration. For the IOI session, the Imager 3001 system was set at 2 × 2 binning mode (512 × 512 pixels) and resulted in the pixel size of 24 μm. Each stimulus was presented for 2.5 seconds after the first 0.5-second baseline during which 12 consecutive image frames were taken. The interstimulus period was set at least 8 seconds that allowed the hemodynamic signal back to baseline. Stimuli were presented in blocks and one recording session usually consisted of 40 to 50 blocks.
To obtain reliable maps of activated domains, we used both the traditional differential method (Vanzetta et al, 2004) as well as a statistical analysis method (Lu and Roe, 2008; Tanigawa et al, 2010). Briefly, difference maps were calculated in the standard manner (e.g., sum of left minus sum of right eye conditions; Lu and Roe, 2008). In our statistical analysis, the images acquired during the first 500 milliseconds baseline and during monocular stimulation within one trial were averaged, respectively. Averaged images from each block (total 40 to 50 blocks) were pooled together and a two-tailed t-test was conducted on a pixel-by-pixel basis between the stimulation of left and right eye. Consequently, the functional maps contained only pixels passing the significance threshold. Cluster-size thresholding (activated areas >100 μm) was applied for correction for multiple comparisons (to remove activation due to random statistical fluctuations). For better visualization, the activation maps were smoothed by a Gaussian filter with 8 × 8 kernel. Low-frequency noise was reduced by convolving the maps with a 150-pixel diameter circular mean filter and then subtracting the result from the smoothed maps. Columnar spacing was determined by autocorrelation analysis of functional maps (Duong et al, 2001). For OD columns, the average spacing between neighboring stripes was measured as the distance between the center peak and the neighboring peak of the autocorrelation function. The point spread was estimated by taking the full width at half maximum of the autocorrelation center peak profile.
Laser Speckle Contrast Imaging and Data Analysis
After we obtained the OD map via IOI, using the same stimulus paradigm, we switched to LSCI imaging of CBF changes. The LSCI system is readily integrated with the IOI setup by changing the illumination source to the laser and the operational mode of the CCD camera (Dalsa 1M60, Imager 3001, Waterloo, ON, Canada). The laser illumination (785 nm) was set up similarly to that of our previous study (Wang et al, 2007). The Dalsa 1M60 camera was set to highly sensitive, nonbinning mode to record the raw speckle images at a frame rate of 60 Hz. Specific f-stop of the imaging lens (the f number was ∼4 to 6 in this study) was chosen to allow the speckles to be sampled appropriately in spatial domain (Wang et al, 2007; Zakharov et al, 2009).
For each trial (3-second duration), a total of 180 raw speckle image frames were recorded and then converted into contrast images using the standard LSCI procedure (Wang et al, 2007). To suppress random high-frequency noises, every 10 contrast frames within one trial were averaged, which resulted in 18 frames per trial. Since the speckle images suffered from substantial physiologic and instrumental noise (Durduran et al, 2004), we utilized pixel-based statistical t-tests to evaluate which pixels exhibited significantly different blood flow between the stimulation of left and right eyes. The blood flow images acquired during the first 0.5-second baseline (frames 1 to 3) and 0.5 to 2.5 seconds after the stimulus onset (frames 7 to 18) within one trial were averaged, respectively. To eliminate vessel artifacts, the pixels overlying large blood vessels were excluded from consideration using a blood vessel mask obtained with 540 nm illumination (green light). P<0.001 was used as the significance threshold for detecting the CBF activation and the cluster-size thresholding same as the above IOI analysis was applied.
Electrophysiological Recording and Signal Analysis
Following imaging, cortical electrical activity was recorded extracellularly with a custom-designed linear array of eight Epoxylite-coated tungsten microelectrodes (FHC Inc., Bowdoinham, ME, USA), each with exposed standard sharp tip (<3 μm) of ∼1 MΩ impedance (measured at 1 kHz). Guided by OD maps and blood vasculature pattern, the array (spacing between two electrodes is ∼410 μm) was carefully placed to target centers of left and right eye columns and then advanced very slowly through the artificial dura into cortex using a computerized micromanipulator (Nan Instruments, Nazareth, Israel). In all cases, voltages were measured against a local reference inside the chamber. The broadband neural signals were recorded using a Multichannel Acquisition Processor system (Plexon Inc.) at 40 kHz. Single units were isolated online (bandpass filtered between 300 Hz and 8 kHz) with Rasputin software (Plexon Inc.) and basic response profiles were characterized. Spike sorting was repeated offline using the Plexon Offline Sorter to ensure that all action potentials were well isolated throughout one recording session. Spiking responses to monocular stimulation were computed in peristimulus time histograms with 10 milliseconds bin width and normalized by the average spike rate and its standard deviation for individual recording sites. Spontaneous spiking activity during prestimulus phase was subtracted from the mean spike rate during the stimulus period. For the LFP, the signals were filtered between 0.7 and 300 Hz, amplified (1,000 × gain) and digitized at 1 kHz. Time-frequency analysis was conducted to illustrate the temporal structure of the LFP signals and their stimulus response preferences. Wavelet transform was performed on single trials and the resulting single-trial spectrograms of the absolute values were averaged. The number of cycles of the wavelet family we used here was set to 7 (Lakatos et al, 2005), with the central frequency ranging from 5 to 100 Hz in 1 Hz steps. At 5 Hz, this leads to a wavelet duration of 445.6 milliseconds and to a spectral bandwidth of 1.43 Hz, and at 100 Hz to a duration of 22.2 milliseconds and a bandwidth of 28.6 Hz. As seen, the wavelet decomposition is nonuniform in the time-frequency domain. These averaged spectrograms thus include both activity that is phase-locked to the stimulus (‘evoked') and activity that is only loosely time-locked (‘induced') (Katzner et al, 2009). The detailed description of the wavelet transform can be found in many previous studies (Lakatos et al, 2005) and our recent work (Wang and Roe, 2011). The averaged spectrograms were then normalized by the mean power of the prestimulus baseline frequency by frequency and was measured in dB unit (10 × (log10 (Pstim)−log10 (Pbase))).
Definition of Ocular-Dominance Index
We quantified OD preference for each of the IOI (oximetry), the LSCI (CBF), and extracellular recording (spikes and LFPs) data sets by using an ocular-dominance index (ODI) defined as follows (Berens et al, 2008):
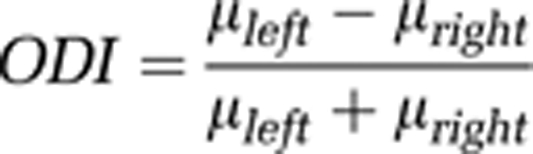 |
where μleft and μright denote the response value to stimuli presented to the left and right eye, respectively. This index varies in the range from −1 (preferred right eye) and 1 (preferred left eye). The mean values within distinctly monocular areas of oximetry and CBF column maps were taken to compute ODIOximetry and ODICBF for IOI and LSCI imaging results. The baseline-subtracted spike rate to monocular stimulation was used to calculate the ODIspikes. To obtain a ‘response value' for calculating the ODILFP, the increased power from all the frequency bins of the normalized spectrograms were averaged while the stimulus was presented. Since the dominant increase in relative power (compared with the baseline period) is concentrated within the 30 to 100 Hz range (mainly in the γ-band range), we did not attempt to differentiate the power changes of the LFP at other frequency bands.
To compute correlation coefficient between pairs of OD indices, we used a Spearman's ρ, a rank correlation measure appropriate for bounded data and a Wilcoxon rank sum test for comparing the strength of OD tuning between two indicators (a Kruskal–Wallis test for group comparison). The coefficient of determination (R2) was calculated to assess the goodness of linear fit between any pair of the OD indicators. All data processing and statistics were conducted using custom-written code for MATLAB.
Results
Imaging Ocular-Dominance Columns with Blood Flow and Oxygenation
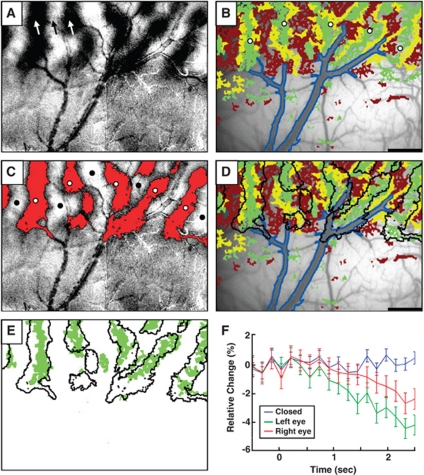
Functional maps of OD structures in all subjects were obtained by using both conventional differential (left–right subtraction) and statistical t-test analyses. Figures 1A and 1C show a representative example, which superimposes resulting maps from both methods. The red-colored pixels (Figure 1C) are those with statistically greater activation to left eye over right eye stimulus conditions (P<0.001). These pixels largely overlap with the dark (left eye preference) stripes obtained from the differential analysis. Overall, stripe-like organizations revealing preference for the left eye (in black/red colors) and right eye (white) stimuli are clearly segregated in the image. Another important feature of these maps is the V1/V2 boundary formed by the presence of monocular columns in V1 and the lack of OD organization in V2 (Ts'o et al, 1990). These two methods produced essentially the same maps in all repeated IOI imaging sessions (>95% of all the data set). Close inspection of the activated domains in Figure 1 reveals that the pixels within some big blood vessel regions failed to reach statistical threshold, suggesting that the statistical analysis is a robust method for rejecting potential spurious responses and leads to more conservative estimation of functionally specific activation regions. This finding is consistent with what we previously reported (Lu and Roe, 2008; Tanigawa et al, 2010) and also compatible with other fMRI data analysis (Fukuda et al, 2006). Thus, statistical analysis not only reduces the influence of large vascular fluctuations, but also provides quantitative assessment of activation maps. Such statistical evaluation is important for attaining the CBF maps from LSCI measurements.
Figure 1.
Comparison of oximetric (intrinsic) signal and cerebral blood flow (CBF) maps. (A) Differential ocular-dominance (OD) map. The dark and light stripes result from differential analysis of oxymetry signals (605 nm) in response to left (dark) and right (light) eye stimulation. Black and white arrows indicate locations of electrode penetrations for recordings shown in Figure 3. (B) Representative OD map of activated CBF responses. The map of statistically significant eye preference is overlaid on the blood vessel map. Pixels overlying large blood vessels (outlined in blue) were excluded from analysis. The green pixels exhibited greater CBF signal to stimulation of the left eye than to the right eye; those in orange exhibited greater signal to stimulation of the right eye than left eye (P<0.001). Those in yellow were equally activated by stimulation of either eye (P>0.05). (C) The red pixels are those with significant response (P<0.001) to left eye stimulation. As seen from the superposition, the activated columnar areas from the pixel-wise t-test largely overlap with the left eye preference OD columns, demonstrating a substantial consistency between the results of these two analysis methods. White dots: sampled locations of graph shown in panel F. (D) CBF map with oximetric OD outline overlain (black outlines). (E) Overlay of left eye preferring pixels from CBF map (green pixels) and left eye preferring OD outline from oximetry map (black outline). There is a high degree of overlap, as indicated by the large proportion (79.1%=9,055/11,595) of the green pixels falling inside the black outlines. (F) Time course of response to left eye stimulation (green line) and right eye stimulation (red line), and blank (blue line, both eyes closed) sampled from left eye columns (average of five locations indicated with white dots in panel B). Anterior is down and medial to the right. Scale bar: 1 mm, applied to panels A–E.
The monocularly presented gratings elicited pronounced changes in CBF activity in both V1 and V2. Figure 1B illustrates a CBF activation map obtained in this case superimposed on the vasculature map. The pixels in orange and green represent significantly stronger blood flow modulation by stimulation of the right eye and the left eye (P<0.001), respectively. In the map, large superficial blood vessels are outlined in blue, and changes of blood flow within these regions were excluded from the analysis. Pixels in yellow are locations where change in blood flow is not different for left versus right eye stimulation (P>0.05). Consistent with oximetry mapping, the boundary between V1 and V2 is clearly delineated by the differences of activated CBF responses: as almost all neurons in V2 are binocular, V2 exhibits no eye-specific (OD) modulation of microcirculation under the present stimulation condition. In V1, the distributions of these activated pixels appear a little more patchy than that seen in oximetry-based functional maps. However, the stripe-like patterns are evident and reproducible under repeated measurements from all the subjects. Superimposition of the left eye preference columns from the oximetry mapping (black outlines) onto the CBF map reveals good alignment of these two maps. This is more clearly illustrated in Figure 1E, which illustrates that roughly 80% of the left eye preferring CBF pixels (green pixels) fall within the left eye preferring oximetry columns (black outlines). Notethat, these two sets of data were acquired in separate sessions 3 months apart, resulting in less than perfect alignment, due to factors such as slightly different camera angle, differential distortion of cortex due to relative flatness of cortical imaging plane, and differences in illumination conditions. Despite this, the alignment is unmistakable. We also evaluated the time course of the CBF response (Figure 1F). In this analysis, we sampled from left eye columns (average of five locations indicated with white dots in Figure 1B). For each time point plotted, we averaged 10 frames (acquired at 60 Hz) to obtain better signal-to-noise ratio, reducing the temporal resolution to ∼167 milliseconds. This plot shows that, for each eye (left: green, right: red), the time course of response evolved gradually over a period of 2 to 3 seconds and reached an amplitude of up to ∼5% signal change. These signals were significantly different from blank (eyes closed: blue) and also exhibited appropriate eye preference (left eye signal significantly greater than right eye signal).
Validating Ocular-Dominance Columns with Local Field Potential and Spiking
To determine the size of these functionally specific domains, we measured OD spacings and sizes using autocorrelation analysis. A three-dimensional autocorrelation plot is shown in Figure 2A. Clear periodicity was observed (see radial projection of this asymmetrically organized map, red line). The periodic spacings of the OD structures measured from four hemispheres were 820±150 μm, consistent with the observations what we (Lu and Roe, 2008) and others have reported (Vanzetta et al, 2004). Based on the estimated distance between these OD columns, we made a custom-designed linear array of eight microelectrodes and positioned the array to record within the centers of targeted OD columns (Figure 2B). Thus, neighboring channels recorded neural responses from OD columns with distinct eye preferences.
Figure 2.
(A) Autocorrelation plot of the oximetric ocular-dominance (OD) map in Figure 1. The contour map of the autocorrelation function (blue) reveals periodic pattern in the OD map. The red contour shows the profile at zero lag. The columnar spacing (distance between the center and its neighboring peak) was 720 μm; and the full width at half maximum (of the center peak) was 274 μm. (B) Picture of electrophysiological recording setup with a custom-designed linear array of microelectrodes. The spacing of eight-channel array was 410 μm (for a total distance of 2.87 mm). The array was positioned orthogonal to the OD columns (and parallel to the lunate sulcus). Scale bar: 1 mm.
Our CBF maps indicated a clear structure related to eye-specific activation that paralleled preference revealed by intrinsic signal imaging (oximetry signals). Within the same imaging sessions, we then examined the eye preference of neuronal response in the left and right eye columns. We used custom made linear electrode arrays with 410 μm spacing. This electrode array was placed such that electrodes were centered on OD columns (see Figure 1A, arrows) and carefully advanced to penetrate the artificial dura. With these arrays, both subthreshold field potentials and single neuron spikes were simultaneously recorded.
With single-unit recording, individual visual cortical neurons showed robust and highly reproducible trial-by-trial responses to monocular stimuli. Figure 3A displays one set of the sorted single action potentials of three neurons sampled from three nearby microelectrodes in the same session while they were stimulated by the left or right eye input (top and bottom panels, respectively; recording locations indicated with arrows in Figure 1A). The drifting gratings were presented to one eye for 3.5 seconds (preceded by 0.5-second baseline and followed by 0.5-second poststimulus period). As expected, shown by the increased raster dot density and elevated peristimulus time histogram spike rates, responses were characterized by an onset response followed by a relatively sustained firing rate. As evident from the eye preference in responses, channels 1 and 3 fell in left eye columns and channel 2 in a right eye column. The firing rates of each neuron to left and right eye stimuli differed in the neighboring channels (P<0.001). This confirms that the eye preferences measured by the spiking responses of individual neurons in adjacent columns are consistent with the columnar maps obtained with both IOI and LSCI techniques.
Figure 3.
Single-unit and local field potential (LFP) recordings confirm ocular-dominance preferences. Ch1, 2, and 3 are recorded from three sites indicated with arrows in Figure 1A. (A) Raster and peristimulus time histograms (PSTHs) plots of typical single neuron spiking responses. Three columns represent three individual neurons recorded from three neighboring microelectrodes (Ch1, Ch2, and Ch3) that were carefully positioned on different eye columns. The top and bottom panels show the same neuron's response to the left and right eye stimuli, respectively. In each panel below the raster plot, the corresponding PSTHs are displayed (calculated with 10 milliseconds bin size). Each row in the raster stands for one stimulus period, 4.5-second long, and the colored dots represent the action potentials of the neurons. The spike rates of Ch1 and Ch3 neurons during left eye stimulation are significantly higher than that to right eye stimulation (P<0.0001); the firing of Ch2 neuron responds in an opposite way (P<0.0001). Thus, the eye preferences measured by the spiking responses are consistent with the columnar maps obtained by imaging. (B) Spectrograms of LFP signals recorded simultaneously with the spikes shown in panel A (Ch1, Ch2, and Ch3). The top and bottom panels of each column represent the LFP response to the left and right eye stimuli, respectively. Similar to the spiking responses in panel A, the increased power (mainly in the γ-band LFP) of Ch1 and Ch3 during left eye stimulation are significantly higher than those of right eye stimulation (P<0.0001), whereas the LFP recorded in Ch2 responds in an opposite way (P<0.0001). Color bar shows the relative changes in the LFP power compared with the baseline period in dB unit.
The spectrograms of LFP signals from the same channels exhibited consistent response patterns (Figure 3B). In all the panels in Figure 3B, the power of LFP, mainly in the γ-band range (30 to 100 Hz), increased noticeably in response to the external stimulus onset and remained sustained until the stimulus turned off (Katzner et al, 2009; Niessing et al, 2005). Consistent with eye preference, the increased powers of Ch1 and Ch3 compared with the baseline were significantly higher during the left eye stimulation than that to right eye stimulation (P<0.0001); in contrast, the LFP of Ch2 responded in an opposite preference. The calculated ODI for Ch1 and 3 were biased to left eye (0.53 and 0.64) and Ch2 was biased to right eye (−0.47). In all, 75.0% of all the penetration sites (24 out of 32 sites) that had well-isolated single units and 64.28% of all LFP sites (18 out of 28 sites) had significant OD preference (t-test, P<0.05). Importantly, this indicates the eye preferences of the recording sites in V1 can also be inferred by the measured LFP activation and further validate the imaged OD maps.
Comparison of Neural and Hemodynamic Responses
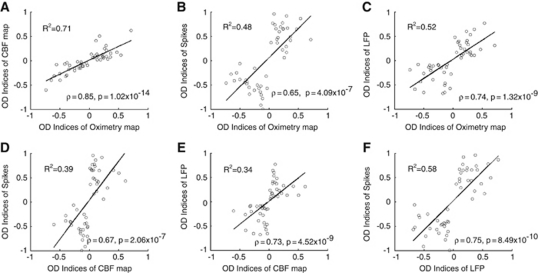
To examine the relationship between the different measures of ocular dominance, we computed the ODIs for each of our four measures: oximetry, CBF, spike rates, and LFP (see Materials and methods for details). We then conducted pairwise comparison between each pair of indicators (a total of six combinations), displayed in Figures 4A–4F. We found significant correlations between each pair of OD indicators (Spearman, P<10−6), although the linear trends varied (explained variances range from 34% to 71%). For instance, the relationship of the ODIs inferred from the oximetry and CBF signal (Figure 4A) from all the imaging sessions demonstrated a high correlation (Spearman's ρ=0.85; P=1.02 × 10−14). Similarly, the OD tuning indexed by the spike and LFP (Figure 4F) shows a strong correlation (Spearman's ρ=0.75; P=8.49 × 10−10).
Figure 4.
(A–F) Pairwise comparisons among the ocular-dominance (OD) indices of oximetry maps, cerebral blood flow (CBF) maps, spikes, and local field potentials (LFPs) from all the cases are displayed as scatter plot. The black line is the least square linear fit to the pooled data and the R2 values are given for each combination. Spearman's rank correlations in all combinations of four OD indicators with various degrees are found significant (confidence level included in each subplot), which suggests that the parameters of labeling neuronal and hemodynamic processes measured here in V1 all robustly show the ocular preference tuning.
Interestingly, a significant difference was found between the magnitudes of ODIOximetry (mean absolute value: 0.28) and ODICBF (mean absolute value: 0.17) (Wilcoxon rank sum test, P=4.24 × 10−4). This could indicate that the deoxyhemoglobin signal has significantly stronger ocular preference than the blood flow activity in V1, a finding consistent with previous studies comparing deoxyhemoglobin and CBV functional maps (Fukuda et al, 2006; Vanzetta et al, 2004). Also, the LFP responses of 30 to 100 Hz frequency band had a mean absolute ODILFP of 0.30, significantly lower than the spiking activity of 0.53 (Wilcoxon rank sum test, P=7.43 × 10−6), which is consistent with recent report (Berens et al, 2008). With a Kruskal–Wallis test, the single-unit spiking response has the best power to discriminate the eye preferences and the power of mapping the OD structure with CBF signal is significantly lower than that of other three indicators (P=6.18 × 10−13). However, this could in part be due to the fact that, because we targeted the centers of OD columns, the single-unit sample is biased toward highly monocular cells whereas the LFP recordings are averaging over a larger range of ocular preference.
Discussion
In the anesthetized primate visual cortex, we assessed OD organization using two different optical imaging techniques that detect the biophysical parameters of stimulus-evoked oxygen metabolic and hemodynamic processes, respectively. We then confirmed these maps using image-guided extracellular recording of single neuron spikes and LFPs. In all subjects under repeated measures, the column-specific spiking and LFP activity and associated changes in blood oxygenation and blood flow were spatially localized at the submillimeter level.
Spatial Specificity of Hemodynamic Regulation
Functional maps in neuroimaging rely on identifying areas that respond selectively to various aspects of cognitive and sensorimotor processing. In brain activation process, the early-phase of flow regulation will lose its spatial specificity with time, because the blood, although deoxygenated at the local level, spreads out quite rapidly as it flows away toward the draining veins (Lauritzen, 2005; Logothetis, 2008). The spatio-temporal dynamics of the underlying physiological processes must be properly taken into account to achieve higher spatial resolution. Previously, one major confounding source of the BOLD signal detected in the low field MR scanner is the draining veins emerging from the cortex perpendicular to the cortical surface (Menon et al, 1997; Shmuel et al, 2010). To minimize such nonspecific large-vessel contribution to CBF activation, improved methods of data acquisition and postprocessing are needed. As the imaging parameters of LSCI here were set to be sensitive to the movement of red blood cells in capillaries (Durduran et al, 2004; Wang et al, 2007), we were able to detect highly local changes in the microvasculature network and achieve high spatial resolution mapping. Statistical significance tests identified pixels with CBF responses with eye-specific preference. As shown in Figure 1, these tests revealed that CBF-derived eye preference maps were spatially colocalized with OD maps based on deoxygenation signals.
One caution in anesthetized animal experimental procedure should be noted here. Isoflurane, which is known for maintaining stable anesthesia level over hours, has been reported to cause a significant reduction in the systemic vascular resistance and a marked increase in basal CBF, compared with other anesthetics like α-chloralose (Duong et al, 2001; Sicard et al, 2003). In our experiment, we utilized a cocktail of thiopental sodium and isoflurane, in which isoflurane was used as supplement to minimize its impact onto the vessel dilation.
Spatial Specificity of Neural Computation
Electrophysiological recordings further validated these results. Not surprisingly, robust spiking responses preferentially activated by single eye stimulation were consistent with columnar structures revealed by the functional oximetry and blood flow maps (Figure 3). However, the spatial extent of LFP signal in cortical network, a measure that reflects a population response, still remains elusive (Berens et al, 2008; Katzner et al, 2009). To address this, we designed a linear electrode array with roughly 400 μm spacing, a spacing that matched the spacing of OD columns. Remarkably, guided by functional images, we showed for the first time eye-specific tuning of γ-band LFP signal between the adjacent OD columns (Figure 3). These findings are consistent with previous reports showing columnar (∼1 mm) localization of γ oscillations in the neocortex. Carandini and colleagues (Katzner et al, 2009) showed that amplitude of γ oscillations decreased rapidly with distance and reflected selectivity of single orientation columns in cat visual cortex. Our results confirm and extend conclusions from these studies (Berens et al, 2008). As the spacing distance between channels in our electrode array is 410 μm (Figure 2B), the eye selectivity evident from these LFP recordings indicate an integration range on the order of half a millimeter or less. Note that the calculated ODIs of spikes and LFP in this study were higher than what Berens et al (2008) reported, probably because the sites within different eye columns have been preferentially sampled in the functional image-guided penetrations. Emergence of these fast rhythms faithfully reflects columnar-specific computation in cortical network and in parallel with eye-specific spiking responses (Figure 4F).
Link Between Neural and Vascular Networks
The intriguing configuration of microvasculature in the cerebral cortex has been discovered by Hall (1831), who stated that ‘the number and distribution of the minute and capillary vessels is accurately proportioned and adapted to the object of the circulation.' The functional significance of this arrangement in nature still is largely unknown (Tian et al, 2010; Zheng et al, 1991; see Attwell et al, 2010 for review). Both the arterial supply and the capillary network in the cortex contain various flow-control structures sensitive to the fluctuation of energy demands (Iadecola, 2004), in a way that spatially ‘filters' (or blurs) neuronal activity patterns. The anatomical architecture of neural and vasculature networks may put spatial constraints on the fineness of local microcirculatory control. Given that capillaries can be found with ∼24 μm spacing in primary sensory cortex (Zheng et al, 1991), it implies that in principle the brain is physiologically capable of regulating hemodynamics at a very fine spatial scale (Tian et al, 2010). Our observation continues to support this point of view and suggests that cortical vasculature is functionally organized, although imaging blood flow at the single capillary level may be beyond the current spatial resolution of our imaging system.
In summary, the present study implemented a combination of electrophysiological and imaging measurements, targeting different perspectives of neuronal and hemodynamic processes during visual stimulation. Our results support previous fMRI studies that showed CBF response is relatively specific to orientation columns in cats (Duong et al, 2001) and previous reports that the γ-band LFP is well tuned to ocular preference (Berens et al, 2008). It sheds light on the spatial colocalization of blood flow, oxygenation, and γ-band LFP in response to visual stimulation, and is relevant to the proposed hierarchy of microcirculation control during neuro-hemodynamic process (cf. Attwell et al, 2010). Improved insights into the mechanistic aspects of neurovascular regulation will further help to clarify the fundamental interpretation of BOLD fMRI signal by isolating relationship within computational modules (columns) in cortical circuitry (Logothetis, 2008), and enable better localization in human functional imaging.
Acknowledgments
The authors thank Hisashi Tanigawa for support of programming visual stimulus presentation, Robert Friedman for providing the NI LabVIEW support, Gang Chen, Lisa Chu, and Alyssa Zuehl for their assistance of surgeries and experiments.
The authors declare no conflict of interest.
Footnotes
This work was funded by NIH EY11744 (AWR), DA023002 (AWR), Vanderbilt Vision Research Center, and Vanderbilt University Center for Integrative and Cognitive Neuroscience.
References
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P, Keliris GA, Ecker AS, Logothetis NK, Tolias AS. Comparing the feature selectivity of the gamma-band of the local field potential and the underlying spiking activity in primate visual cortex. Front Syst Neurosci. 2008;2:1–11. doi: 10.3389/neuro.06.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel GG, Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986;321:579–585. doi: 10.1038/321579a0. [DOI] [PubMed] [Google Scholar]
- Caesar K, Thomsen K, Lauritzen M. Dissociation of spikes, synaptic activity, and activity-dependent increments in rat cerebellar blood flow by tonic synaptic inhibition. Proc Natl Acad Sci USA. 2003;100:16000–16005. doi: 10.1073/pnas.2635195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Heider B, Williams GV, Healy FL, Ramsden BM, Roe AW. A chamber and artificial dura method for long-term optical imaging in the monkey. J Neurosci Methods. 2002;113:41–49. doi: 10.1016/s0165-0270(01)00475-7. [DOI] [PubMed] [Google Scholar]
- Chen LM, Turner GH, Friedman RM, Zhang N, Gore JC, Roe AW, Avison MJ. High-resolution maps of real and illusory tactile activation in primary somatosensory cortex in individual monkeys with functional magnetic resonance imaging and optical imaging. J Neurosci. 2007;27:9181–9191. doi: 10.1523/JNEUROSCI.1588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Duong TQ. Simplified laser-speckle-imaging analysis method and its application to retinal blood flow imaging. Opt Lett. 2007;32:2188–2190. doi: 10.1364/ol.32.002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Waggoner RA, Tanaka K. Human ocular dominance columns as revealed by high-field functional magnetic resonance imaging. Neuron. 2001;32:359–374. doi: 10.1016/s0896-6273(01)00477-9. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Kim DS, Ugurbil K, Kim SG. Localized cerebral blood flow response at submillimeter columnar resolution. Proc Natl Acad Sci USA. 2001;98:10904–10909. doi: 10.1073/pnas.191101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durduran T, Burnett MG, Yu G, Zhou C, Furuya D, Yodh AG, Detre JA, Greenberg JH. Spatiotemporal quantification of cerebral blood flow during functional activation in rat somatosensory cortex using laser-speckle flowmetry. J Cereb Blood Flow Metab. 2004;24:518–525. doi: 10.1097/00004647-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Akgoren N, Dirnagl U, Lauritzen M. Laminar analysis of cerebral blood flow in cortex of rats by laser-Doppler flowmetry: a pilot study. J Cereb Blood Flow Metab. 1997;17:1326–1336. doi: 10.1097/00004647-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Moon CH, Wang P, Kim SG. Mapping iso-orientation columns by contrast agent-enhanced functional magnetic resonance imaging: reproducibility, specificity, and evaluation by optical imaging of intrinsic signal. J Neurosci. 2006;26:11821–11832. doi: 10.1523/JNEUROSCI.3098-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL. Is cortical vasculature functionally organized. Neuroimage. 2010;49:4. doi: 10.1016/j.neuroimage.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Hall M. A Critical and Experimental Essay on the Circulation of the Blood. Seeley and Burnside: London; 1831. [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key. Nat Rev Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Li N, Downey JE, Bar-Shir A, Gilad AA, Walczak P, Kim H, Joel SE, Pekar JJ, Thakor NV, Pelled G. Optogenetic-guided cortical plasticity after nerve injury. Proc Natl Acad Sci USA. 2011;108:8838–8843. doi: 10.1073/pnas.1100815108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lu HD, Roe AW. Functional organization of color domains in V1 and V2 of macaque monkey revealed by optical imaging. Cereb Cortex. 2008;18:516–533. doi: 10.1093/cercor/bhm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Strupp JP, Ugurbil K. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol. 1997;77:2780–2787. doi: 10.1152/jn.1997.77.5.2780. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Op de Beeck HP. Against hyperacuity in brain reading: spatial smoothing does not hurt multivariate fMRI analyses. Neuroimage. 2010;49:1943–1948. doi: 10.1016/j.neuroimage.2009.02.047. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Hageman N, Toga AW. Columnar specificity of microvascular oxygenation and volume responses: implications for functional brain mapping. J Neurosci. 2004;24:634–641. doi: 10.1523/JNEUROSCI.4526-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Chaimow D, Raddatz G, Ugurbil K, Yacoub E. Mechanisms underlying decoding at 7 T: ocular dominance columns, broad structures, and macroscopic blood vessels in V1 convey information on the stimulated eye. Neuroimage. 2010;49:1957–1964. doi: 10.1016/j.neuroimage.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa H, Lu HD, Roe AW. Functional organization for color and orientation in macaque V4. Nat Neurosci. 2010;13:1542–1548. doi: 10.1038/nn.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EM, De Crespigny AJ, D'Arceuil HE, Mandeville JB, Marota JJ, Rosen BR, Liu TT, Boas DA, Buxton RB, Dale AM, Devor A. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci USA. 2010;107:15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts'o DY, Frostig RD, Lieke EE, Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990;249:417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Slovin H, Omer DB, Grinvald A. Columnar resolution of blood volume and oximetry functional maps in the behaving monkey; implications for FMRI. Neuron. 2004;42:843–854. doi: 10.1016/j.neuron.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Wang G, Tanaka K, Tanifuji M. Optical imaging of functional organization in the monkey inferotemporal cortex. Science. 1996;272:1665–1668. doi: 10.1126/science.272.5268.1665. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hughes S, Dayasundara S, Menon RS. Theoretical and experimental optimization of laser speckle contrast imaging for high specificity to brain microcirculation. J Cereb Blood Flow Metab. 2007;27:258–269. doi: 10.1038/sj.jcbfm.9600357. [DOI] [PubMed] [Google Scholar]
- Wang Z, Roe AW. Neuronal selectivity and microcirculation localized at columnar level in primate visual cortex. San Diego: CA; Soc Neurosci Abstr. 2010;483:1. [Google Scholar]
- Wang Z, Roe AW. Trial-to-trial noise cancellation of cortical field potentials in awake macaques by autoregression model with exogenous input (ARX) J Neurosci Methods. 2011;194:266–273. doi: 10.1016/j.jneumeth.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Burger C, Wyss MT, von Schulthess GK, Scheffold F, Buck A. Optical imaging of the spatiotemporal dynamics of cerebral blood flow and oxidative metabolism in the rat barrel cortex. Eur J Neurosci. 2004;20:2664–2670. doi: 10.1111/j.1460-9568.2004.03735.x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko YE, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex. 1996;6:647–660. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Zakharov P, Volker AC, Wyss MT, Haiss F, Calcinaghi N, Zunzunegui C, Buck A, Scheffold F, Weber B. Dynamic laser speckle imaging of cerebral blood flow. Opt Express. 2009;17:13904–13917. doi: 10.1364/oe.17.013904. [DOI] [PubMed] [Google Scholar]
- Zappe AC, Pfeuffer J, Merkle H, Logothetis NK, Goense JB. The effect of labeling parameters on perfusion-based fMRI in nonhuman primates. J Cereb Blood Flow Metab. 2008;28:640–652. doi: 10.1038/sj.jcbfm.9600564. [DOI] [PubMed] [Google Scholar]
- Zheng D, LaMantia AS, Purves D. Specialized vascularization of the primate visual cortex. J Neurosci. 1991;11:2622–2629. doi: 10.1523/JNEUROSCI.11-08-02622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]