Abstract
Rationale
Only a small percentage of individuals seeking treatment for their marijuana use achieves sustained abstinence, suggesting more treatment options are needed.
Objectives
To determine the effects of baclofen (Study 1) and mirtazapine (Study 2) in a human laboratory model of marijuana intoxication, withdrawal and relapse.
Methods
Study 1: Daily marijuana smokers (n=10), averaging 9.4 (± 3.9) marijuana cigarettes/day, were maintained on placebo and each baclofen dose (60, 90 mg/day) for 16 days. Study 2: Daily marijuana smokers (n=11), averaging 11.9 (± 5.3) marijuana cigarettes/day, were maintained on placebo and mirtazapine (30 mg/day) for 14 days each. Medication administration began outpatient prior to each 8-day inpatient phase. On the first inpatient day of each medication condition, participants smoked active marijuana (Study 1: 3.3% THC; Study 2: 6.2% THC). For the next 3 days, they could self-administer placebo marijuana (abstinence phase), followed by 4 days in which they could self-administer active marijuana (relapse phase); participants paid for self-administered marijuana using study earnings.
Results
Study 1: During active marijuana smoking, baclofen dose-dependently decreased craving for tobacco and marijuana, but had little effect on mood during abstinence and did not decrease relapse. Baclofen also worsened cognitive performance regardless of marijuana condition. Study 2: Mirtazapine improved sleep during abstinence, and robustly increased food intake, but had no effect on withdrawal symptoms and did not decrease marijuana relapse.
Conclusions
Overall, this human laboratory study did not find evidence to suggest that either baclofen or mirtazapine showed promise for the potential treatment of marijuana dependence.
Keywords: withdrawal, treatment, cannabinoids, GABA receptor, antidepressant, self-administration
In the United States, the number of individuals with disorders associated with marijuana use is twice that of any other illicit drug (SAMHSA, 2007), with approximately 4 million adults meeting criteria for a lifetime diagnosis of marijuana dependence (Stinson et al., 2006). A subset of these individuals seeks treatment for their marijuana use but repeatedly fails to remain abstinent. In fact, relapse rates for marijuana smokers are comparable to those found for other drugs of abuse (Copeland et al., 2001; Stephens et al., 1994, 2000; Moore and Budney, 2003). For example, in a large multi-site treatment study testing psychological interventions (n=450), the highest abstinence rates were 15% at the 9-month follow-up (MTPRG, 2004). Other treatment trials report similar rates of abstinence at follow-up (Stephens et al., 2000). The addition of contingency management procedures to motivational and cognitive therapy increased rates of to over 27-37% at one year follow-up (Budney et al., 2006; Kadden et al., 2007), but there remains a clear need for improved treatment options for cannabis dependence.
There is still relatively little known regarding the factors that contribute to the high rates of marijuana relapse, but one strategy for improving treatment outcome may be to target symptoms of withdrawal. Marijuana withdrawal, characterized by a time-dependent, pharmacologically-specific pattern of restlessness, irritability, sleep difficulty, and marijuana craving (Haney et al., 1999b, 2005; Budney et al., 2004; Kouri and Pope, 2000; Hart et al., 2002), is a commonly-reported syndrome among patients presenting for marijuana treatment (see Copeland and Swift, 2009; Levin et al., 2006; Teesson et al., 2002). A number of controlled laboratory and clinical studies have tested whether potential treatment medications (e.g., bupropion, nefazadone, divalproex, buspirone) decrease symptoms of withdrawal or improve clinical outcome. The results of these studies have been negative overall (Haney et al., 2001, 2003, 2004; Levin et al., 2004; Carpenter et al., 2009), although there was a trend for buspirone to increase abstinence rates relative to placebo (McRae-Clark et al., 2009). Dronabinol (tetrahydrocannabidiol; Marinol) has been shown to significantly decrease many symptoms of withdrawal, including anxiety, trouble sleeping, chills, and marijuana craving under controlled conditions (Haney et al., 2004; Budney et al., 2007), but its effects on treatment outcome are not yet known.
We have developed a human laboratory model to test the effects of potential treatment medications on behavioral targets relevant to marijuana dependence: intoxication, withdrawal and relapse. Daily marijuana smokers are maintained on placebo and active medication under conditions in which they smoke active marijuana (intoxication), undergo several days of marijuana abstinence (withdrawal) and then have the opportunity to resume marijuana smoking, but at a financial cost (relapse). Participants are not seeking treatment for their marijuana use, as it would not be ethical to administer marijuana to those attempting to stop their drug use. This laboratory model, which is designed to provide data on the interaction between medications and marijuana to guide treatment trials, is not attempting to mimic clinical conditions, but rather model behaviors in the laboratory that will be predictive clinically (see Haney and Spealman, 2008 and Epstein et al., 2006 for a discussion of predictive vs. construct validity for models of psychiatric disorders). Using this model, we have shown that the α2-receptor agonist, lofexidine (2.4 mg/day) improved sleep during marijuana abstinence and significantly decreased marijuana relapse compared to placebo. Lofexidine was sedating and did not robustly attenuate most mood symptoms of withdrawal, but combining this dose of lofexidine with dronabinol (60 mg/day) further improved sleep and decreased marijuana withdrawal, craving and relapse relative to placebo (Haney et al., 2008).
The present set of placebo-controlled studies utilized this laboratory model to determine the effects of baclofen, a GABAB receptor agonist and antispasmodic medication (Study 1), and mirtazapine (Study 2), an antidepressant that enhances noradrenergic and serotonergic transmission. Baclofen has been shown to attenuate the self-administration of a low dose of smoked cocaine in research volunteers (Haney et al., 2006), and to decrease mood symptoms of heroin withdrawal in patients (e.g., anxiety, agitation, irritability, craving) compared to clonidine (Akhondzadeh et al., 2000). Mirtazapine has been shown to decrease withdrawal symptoms in alcohol-dependent patients (Liappas et al., 2005), specifically treating agitation and insomnia (Yoon et al., 2006), which are also symptoms of marijuana withdrawal. Although acting through distinct mechanisms, both baclofen and mirtazapine have sedating properties hypothesized to improve the agitation and sleep disruption associated with marijuana withdrawal, and thereby decrease marijuana relapse. The results for these two medications are presented in a single manuscript as a more efficient means of communicating data from similarly-designed studies.
Methods
Procedure
Participants were told that the study investigated how FDA-approved medications influence the effects of two different strength marijuana cigarettes (“Dose A”and “Dose B”). All volunteers provided a detailed drug and medical history, received medical and psychiatric evaluations shortly before study onset, and gave written informed consent for all aspects of the study. None was seeking treatment for their drug use.
Prior to study onset, participants completed two, 3-4 hour training sessions on the tasks. Study 1 (baclofen) comprised three, 8-day inpatient phases, and Study 2 (mirtazapine) comprised two, 8-day inpatient phases, with each phase testing a different dose of medication. Inpatient phases were separated by a 7-day outpatient phase, during which time medication dose was switched. These outpatient phases also allowed participants to return to their normal pattern of marijuana use, and decreased the length of continuous time inpatient. Participants were instructed to abstain from illicit drugs during the outpatient phase (excluding marijuana, for which no instructions were given). Urine toxicology was conducted at each laboratory visit. While outpatient, participants in Study 1 came to the laboratory every weekday to take their afternoon capsules (1530), and were instructed to take the morning (0900) and evening capsules (2100) at home. In Study 2, participants were instructed to take the evening capsules at 2130 and to come to the laboratory the following day to monitor compliance. Capsules were packed with riboflavin (50 mg), which was measured in urine using ultraviolet detection. In Study 1, compliance was also verified by measuring plasma baclofen levels once per outpatient phase. Side effects from the capsules were recorded at each laboratory visit.
Immediately prior to each inpatient stay, participants came to the laboratory for two marijuana sample sessions. In one session, they smoked an active marijuana cigarette (labeled “Dose A”), and in the other session, they smoked a placebo marijuana cigarette (labeled “Dose B”) using the smoking procedures described below. They were told that the strength of Dose A and Dose B would not change throughout the study, and that they should pay attention to how each dose made them feel as they would later make decisions regarding their self-administration.
Participants moved into the laboratory after the second marijuana sample session. Beginning at 0815 each morning after move-in, participants completed a 7-item visual analog scale (VAS) sleep questionnaire (see Haney et al., 2004), and a 44-item subjective-effects VAS (described below), and were then weighed. The first of six, 30-min task batteries, comprising five performance measures (described below) and the VAS began at 0915. Participants completed two task batteries from 1030-1145. The recreation area was available from 1215-1245. Four task batteries were completed from 1330-1645, and then the recreation area became available again at 1700. Two films were shown each evening. At 2155, the recreation area was no longer available. At 2330, participants were given $50 in ‘play money’ representing a portion of their daily study earnings. They were told that they could use this money to purchase individual puffs of marijuana on self-administration days. Each participant stored his money in a locked cabinet in the vestibule. Lights were turned off by 2400. Prior to discharge, participants were fully informed about the experimental conditions. The New York State Psychiatric Institute's Institutional Review Board approved all procedures.
Laboratory
Participants, in groups of three or four, lived in a residential laboratory in the New York State Psychiatric Institute. The laboratory has four private participant rooms, a common recreational area, two single-occupancy bathrooms, two single-occupancy shower rooms, and two vestibules used for exchanging supplies (see Haney et al., 1999a). Output from a video- and audio-monitoring system terminating in an adjacent room allowed for continuous observation of participants (except while in the bathroom or in private dressing areas), but no recordings were made. Each participant's computer was linked with a computer in the control room, allowing for a continuous on-line interaction between participants and staff, but not between participants.
Capsule Administration
Medication administration was double-blind and counter-balanced across participants. The New York State Psychiatric Institute Research Pharmacy packaged medication in size 00 opaque capsules with riboflavin filler. In Study 1, baclofen (Lioresal®) capsules (0, 20, 30 mg) were administered 3 times per day (0900, 1530, 2200); each dose was administered for 16 days. Doses were titrated up over the first 5 days of each outpatient phase. In Study 2, mirtazapine (Remeron®) capsules (0, 30 mg/day) were administered once per day (2130); each dose was administered for 14 days. The active dose was titrated up over 3 days and down over 3 days.
Marijuana Administration
Participants each received a single marijuana cigarette (provided by the National Institute on Drug Abuse) at each smoking occasion. Marijuana was administered using a cued-smoking procedure, in which they inhaled and held smoke in their lungs for set time periods, with a 40-sec interval between each puff (Foltin et al., 1987). Marijuana cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 h prior to use; each cigarette was rolled at the ends and smoked through a cigarette holder so that the marijuana was not visible.
Marijuana was either experimenter-administered at no cost, or was available to purchase for self-administration; participants were not informed of the condition until 0950 each morning. During the first inpatient day, participants smoked experimenter-administered, active marijuana (Dose A) six times throughout the day. The purpose of this day was to standardize marijuana exposure prior to the onset of abstinence. On the subsequent three inpatient days, Dose B (placebo marijuana) was available for self-administration, followed by four days when Dose A was available for self-administration. The three days of Dose B availability enforced marijuana abstinence regardless of whether marijuana was self-administered or not. Self-administration of Dose A following these three days of abstinence was the measure of relapse.
During self-administration days, participants had six opportunities throughout the day to purchase 0, 1, 2, or 3 puffs of the available dose using their study earnings. The cost was $10 for the first puff of the day, and $3 for all subsequent puffs; if all puffs were purchased on a given day, the cost was $61. Individuals who chose to smoke marijuana went to a vestibule alone, took out the appropriate amount of money from their lockbox, then smoked the number of puffs purchased using the cued-puffing procedures. Participants who did not choose to smoke were still required to sit in the vestibule for 2 minutes so that other participants would not know if they had purchased marijuana.
Task Battery and Mood Scales
Each task battery consisted of a 3-min digit-symbol substitution task (DSST), a 3-min repeated acquisition task, a 10-min divided attention task (DAT), a 10-min rapid information task (RIT), and an immediate and delayed digit-recall task. The battery, measuring aspects of learning, memory, vigilance, and psychomotor ability, was completed 6 times per day (Foltin et al., 1996). Participants were told to complete each task as quickly and as accurately as possible.
A 44-item computerized subjective-effects questionnaire VAS, comprising a series of 100-mm visual analog scales (VAS) labeled “Not at all” (0 mm) at one end and “Extremely” at the other end, was completed 8 times per day. The VAS included mood, physical symptom and drug effect descriptors; participants were instructed to rate the extent to which each descriptor applied to them at that moment. Based on a cluster analyses, we employed arithmetic means of individual item scores to produce seven subscales: Irritable (example items: ‘irritable’, ‘angry’); Anxious (e.g. ‘anxious’, ‘on edge’); Bad Effect (e.g. ‘muscle pain’, ‘chills’); Sedated (e.g. ‘sleepy’, ‘tired’); Social (e.g. ‘friendly’, ‘talkative’); High (e.g. ‘high’, ‘good effect’); Confused (e.g., ‘forgetful,’ ‘confused’). We also analyzed ratings of craving for marijuana, alcohol and cigarettes, which were not included in any subscale. Craving was measured using VAS ratings of “I Want Marijuana,” “I Want Alcohol” and “I Want Cigaretttes.” Thus, a total of 10 VAS items were analyzed.
Food
Each morning, participants received a box of food containing a wide variety of meal items, snacks and beverages to be consumed at any time. Frozen meal items (n =20) and additional units of any item were available by request. Participants were instructed to scan custom-designed bar codes whenever they ate or drank, specifying substance and portion. Food was not available between 2330 and 0815.
Sleep
Subjective Data
Each morning, participants completed a 7-item VAS sleep questionnaire (Haney et al., 2004), consisting of 100-mm lines anchored with “not at all” at the left end and “extremely” at the right end, labeled with: “I slept well last night,” “I woke up early this morning,” “I fell asleep easily last night,” “I feel clear-headed this morning,” “I woke up often last night,” “I am satisfied with my sleep last night,” and a fill-in question estimating how many hours they slept the previous night.
Objective Data
In Study 1, participants wore the Nightcap® sleep monitoring system (Respironics, Bend OR), consisting of a portable amplifier with one lead that attached to the forehead to measure body movement, and another lead that attached to the eyelid to measure eye movement. Due to equipment malfunction, data from the Nightcap were not available for several participants in Study 1, so data were not analyzed. In Study 2, objective measures of sleep were obtained by tracking gross motor activity using the Actiwatch® Activity Monitoring System (Actiwatch®: Respironics Company, Bend OR). Participants wore the watches on their wrist.
Tobacco cigarette smoking
The number of tobacco cigarettes smoked was recorded by counting cigarette butts in each participant's ashtray each evening. Participants were instructed not to share cigarettes or to throw out cigarette butts, and were monitored to prevent these events from occurring.
Data Analysis
Repeated measures analyses of variance (ANOVA) with planned comparisons were used to determine the effect of each medication dose on marijuana's direct effects, withdrawal and relapse. Behavioral outcomes included: the amount of money spent to purchase marijuana, peak subjective-effects, drug craving, task performance, number of cigarettes smoked per day, objective and subjective sleep measures, food intake (total energy intake, percent macronutrient, number and caloric content of individual eating occasions, defined as beginning with onset of food consumption and ending at the first pause in food reporting > 10 minutes), and body weight. There were two within-group factors: medication dose (Study1: 0, 60, 90 mg/day; Study 2: 0, 30 mg/day) and inpatient day. One planned comparison assessed if there was a difference between active marijuana administration and marijuana abstinence (defined as an average of peak daily values on days 2 and 3 of abstinence). Planned comparisons were done to determine if there was a medication effect on: (1) marijuana intoxication: each medication dose was compared to the placebo dose when active marijuana was experimenter-administered, (2) marijuana withdrawal; each medication dose was compared to placebo, (3) relapse: each medication dose was compared to placebo capsules. Results were considered statistically significant at p values <0.05. Huynh-Feldt corrections were used, when appropriate.
An advantage of using a repeated-measures, within-subjects designs is that they are well powered, due to the substantial correlations between levels. Power calculations, using correlations and standard deviations from a similarly-designed study (n=8 participants; Haney et al., 2008), were accomplished using the nQuery Advisor® statistical package (Statsol Solutions, Ltd., Cork, Ireland). With a sample size of 10, the test of a single contrast at the 0.050 level in a two-way repeated measures analysis of variance has over 90% power to detect medication effects on ratings of mood, marijuana craving and the amount of money spent to purchase active marijuana, of a similar magnitude as seen in the earlier study.
Results: Study 1
Participants
Ten male research volunteers completed the experiment (see Table 1). Three additional volunteers (1 female, 2 male) dropped out of the study during the marijuana abstinence phase (two were maintained on baclofen and one was on placebo); participants who dropped out reported that they were feeling angry (n=1), depressed (n=1) or not able to commit to the study (n=1). All participants reported infrequent use of illicit drugs other than marijuana, and urine drug screens only tested positive for cannabinoids. Both plasma baclofen levels and riboflavin fluorescence confirmed that participants were compliant in taking medication while outpatient.
TABLE 1. Demographic characteristics of study participants.
| Study 1 | Study 2 | |
|---|---|---|
| Number of participants | 10 | 11 |
| Race (Black/White/Mixed/Pacific Islander) | 6/1/3/0 | 9/1/0/1 |
| Age (years) | 29 ± 6 | 27 ± 5 |
| Ethnicity (Hispanic/non-Hispanic) | 3/7 | 3/8 |
| Marijuana use (#days/wk) | 6.7 ± 0.9 | 6.9 ± 0.2 |
| Marijuana cigarettes/day | 9.4 ± 3.9 | 11.9 ± 5.3 |
| Years of marijuana use | 11.5 ± 6.9 | 8.9 ± 4.8 |
| Cigarettes Smokers (#) | 8 | 7 |
| Cigarettes/day | 7.3 ± 1.7 | 11.8 ± 4.4 |
| Alcohol Drinkers (#) | 6 | 4 |
| Alcohol: Drinks/week | 14.8 ± 19.3 | 3.7 ± 2.3 |
| Weight (kg) | 75.4 (± 9.4) | 75.2 (± 12.1) |
| Education (years) | 13.0 ± 1.6 | 12.6 ± 1.1 |
Note: Data are presented as means (± standard deviation) or as frequency
Subjective-Effects Ratings and Drug Craving
Figure 1 and Table 2 portray subjective ratings as a function of baclofen dose during marijuana administration (3.3% THC) and during marijuana abstinence (0.0% THC). Under placebo baclofen conditions, abstinence was associated with significantly lower ratings of high, social and confused clusters compared to active marijuana (Table 2). During active marijuana administration, the low dose of baclofen (60 mg) decreased ratings of ‘high’ (Table 2), while the high dose (90 mg) significantly decreased marijuana craving and cigarette craving (Fig. 1). Baclofen had no effect on other subjective-effects ratings during marijuana abstinence, except that the low dose (60 mg) more than doubled alcohol craving among the 6 participants who reported drinking at least one alcoholic beverage/week [F(1,14) =15.83, p < .01].
Figure 1.
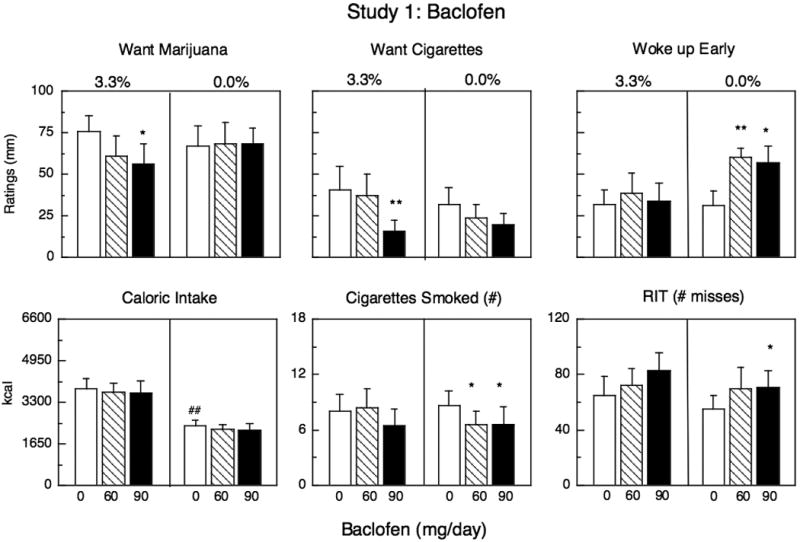
Mean peak effects during marijuana administration (3.3%) and during marijuana abstinence as a function of baclofen dose (Caloric Intake and Cigarettes Smoked reflect daily means not peak). Maximum score for ratings =100 mm. Each graph represents 10 participants except for the two middle panels, which represent the 8 participants who smoked cigarettes. Asterisks indicate a significant difference between active and placebo medication (*p <0.05; ** p <0.01). Number signs indicate a significant difference during marijuana administration and during marijuana abstinence under placebo medication conditions (#p <0.05; ## p <0.01). Error bars represent ± standard error of the mean (SEM). Cigarette craving and number of cigarettes smoked only included data from participants who smoked at least 5 cigarettes/day (n=8).
TABLE 2. Mean peak subjective effects during marijuana administration (3.3% THC) and during abstinence as a function of baclofen dose.
| Baclofen Dose (mg) | |||
|---|---|---|---|
| 0 | 60 | 90 | |
| High Cluster | |||
| 3.3% THC | 61 (9) | ↓44 (10)* | 57 (8) |
| 0.0% THC | ↓4 (1)## | 5 (3) | 5 (9) |
| Irritable Cluster | |||
| 3.3% THC | 9 (4) | 4 (2) | 8 (5) |
| 0.0% THC | 22 (8) | 17 (5) | 11 (5) |
| Anxious Cluster | |||
| 3.3% THC | 11 (5) | 7 (3) | 13 (5) |
| 0.0% THC | 11 (3) | 9 (2) | 9 (3) |
| Social Cluster | |||
| 3.3% THC | 55 (5) | 54 (6) | 51 (6) |
| 0.0% THC | ↓46 (7)# | 49 (6) | 49 (6) |
| Confused Cluster | |||
| 3.3% THC | 20 (11) | 10 (6) | 17 (7) |
| 0.0% THC | ↓9 (7)# | 5 (2) | 7 (3) |
| Tired Cluster | |||
| 3.3% THC | 27 (8) | 23 (6) | 32 (6) |
| 0.0% THC | 23 (6) | 23 (4) | 22 (5) |
|
| |||
| Eating Occasions (#/day) | |||
| 3.3% THC | 10.5 (0.9) | 10.2 (1.1) | 10.1 (1.0) |
| 0.0% THC | ↓6.4 (0.5)# | 6.7 (0.5) | 6.3 (0.7) |
Note Data in parentheses represent standard error of the mean; maximum mood score = 100 mm. Asterisks represent significant differences from placebo baclofen
p <0.05,
p <0.01, and the adjacent arrow signs indicate the direction of the significant effect. Number signs represent significant differences between active marijuana administration and marijuana abstinence under placebo baclofen conditions
p <0.05,
p <0.01, and the adjacent arrow signs indicate the direction of the significant effect.
Tobacco Cigarette Smoking
There were 8 cigarette smokers. Under placebo baclofen conditions, marijuana abstinence did not influence the number of cigarettes smoked relative to active marijuana administration. Baclofen had no significant effect on cigarette smoking during active marijuana administration, but during marijuana abstinence, baclofen (60, 90 mg) significantly decreased the number of cigarettes smoked (Fig. 1).
Food intake
Under placebo baclofen conditions, marijuana abstinence was associated with significantly less caloric intake than active marijuana administration (Fig. 1). The caloric content of each eating occasion did not vary, but participants had significantly fewer eating occasions throughout the day (Table 2). In addition, the proportion of calories derived from fat was significantly lower during abstinence relative to marijuana smoking: On days participants smoked active marijuana, 33% (± 2%) of the daily calories consumed came from fat, whereas during abstinence, 29% (± 2%) of daily calories consumed were derived from fat (p <0.05). Relative intake of proteins and carbohydrates increased during abstinence, but the effect was not significant. Baclofen had no effect on caloric intake during active marijuana administration or during marijuana abstinence.
Body Weight
Under placebo baclofen conditions, marijuana abstinence was associated with significantly lower body weight (1.4 kg; p <0.01) compared to active marijuana administration. Participants maintained on the highest baclofen dose weighed significantly more after smoking active marijuana than when maintained on placebo (1.1 kg; p <0.01). During abstinence, baclofen had no significant effect on body weight.
Task performance
Under placebo baclofen conditions, there was no significant difference between task performance during marijuana administration and marijuana abstinence. During active marijuana administration, baclofen (90 mg) significantly decreased accuracy in tracking the moving target on the Divided Attention Task [F(1,14) =12.00, p <0.01]. Baclofen also worsened performance on this task during marijuana abstinence: The lower baclofen dose (60 mg) increased latency to respond to the distracter symbol [F(1,14) =11.13, p <0.02] and decreased accuracy tracking the moving target [F(1,14) =9.98, p <0.02]. In the higher baclofen dose condition (90 mg), participants entered an average of 4 fewer patterns in the Digit Symbol Substitution Task (p <0.01), made 8 more errors entering a 10-digit sequence in the Repeated Acquisition Task (p <0.02), and missed more odd and even number sequences on the Rapid Information Task (Fig. 1) compared to placebo.
Subjective Sleep Measures
Under placebo baclofen conditions, there was no significant difference between sleep ratings during marijuana administration and abstinence. Baclofen also had no effect on sleep ratings during active marijuana administration. However, during marijuana abstinence, baclofen (60, 90 mg) increased ratings of ‘woke up early’ compared to placebo baclofen (Fig. 1).
Relapse
Baclofen had no significant effect on marijuana relapse, defined as the mean amount of money spent to purchase marijuana following the 3-day period of abstinence. Specifically, participants spent on average $9.77 ± 2.30, $7.15 ± 1.85 and $7.25 ± 2.10 for marijuana under 0, 60 and 90 mg/day baclofen, respectively. Fifty per cent of participants purchased at least one puff of marijuana under the 0 and 60 mg/day conditions, while 40% did so under the 90 mg/day condition.
Medication Side Effects
There were 8 outpatient visits during each medication phase. The most commonly reported side effect during these visits was fatigue, but the incidence (14-16 occasions) and number of participants (n=6-7) reporting this effect did not vary as a function of baclofen dose. Two participants reported dizziness and headache at least once, but these effects also did not vary as a function of baclofen dose.
Results: Study 2
Participants
Eleven male research volunteers completed the experiment (Table 1). One additional male volunteer (maintained on mirtazapine) dropped out of the study during the marijuana abstinence phase, stating that ‘when his body doesn't get weed he feels bad and angry.’ All participants reported infrequent use of illicit drugs other than marijuana, and urine drug screens only tested positive for cannabinoids. Riboflavin fluorescence confirmed that participants took the medication as instructed while outpatient.
Subjective-Effects Ratings and Drug Craving
Figures 2-3 and Table 3 portray effects as a function of mirtazapine dose during acute marijuana administration (6.2% THC) and during marijuana abstinence (0.0% THC). Under placebo mirtazapine conditions, marijuana abstinence was associated with significantly greater ratings of irritability, anxiety, marijuana craving, tired and confused, and significantly lower ratings of high and sociable compared to active marijuana administration (Fig. 2, Table 3). During active marijuana administration, mirtazapine had no significant effects on mood ratings or drug craving. During marijuana abstinence, mirtazapine significantly increased cigarette craving among the 7 participants who smoked (Fig. 2), but had no effect on abstinence-associated subjective effects (Table 3), marijuana craving (Fig. 2) or alcohol craving among the participants who drank weekly (n=4; data not shown).
Figure 2.
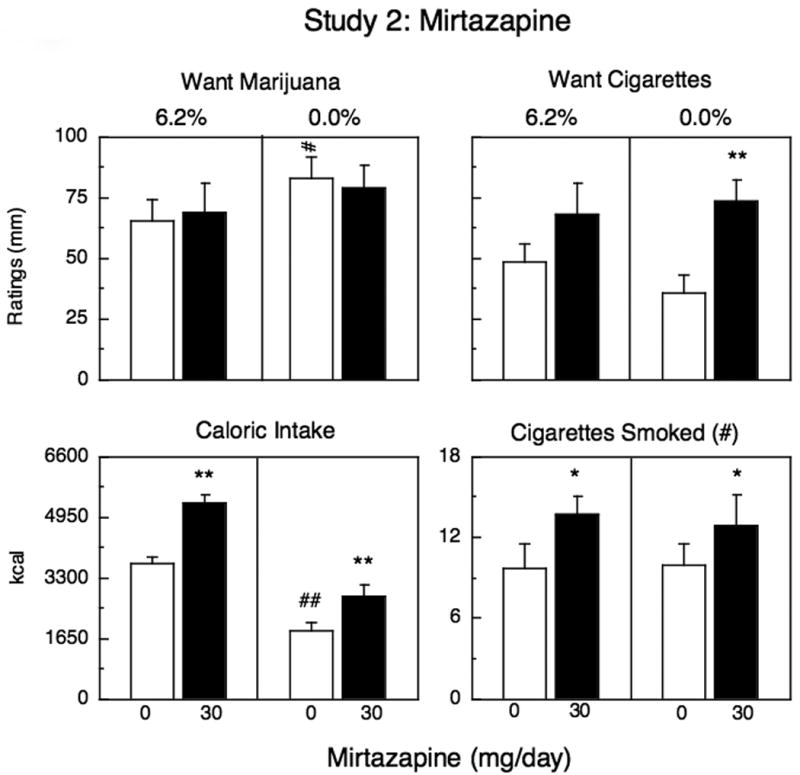
Mean peak effects (top panels) and mean effects (bottom panels) during marijuana administration (6.2%) and during marijuana abstinence as a function of mirtazapine dose. Each graph represents 11 participants except for the two panels on the right, which only represent the 7 participants who smoked at least 5 cigarettes/day. See Figure 1 for details.
Figure 3.
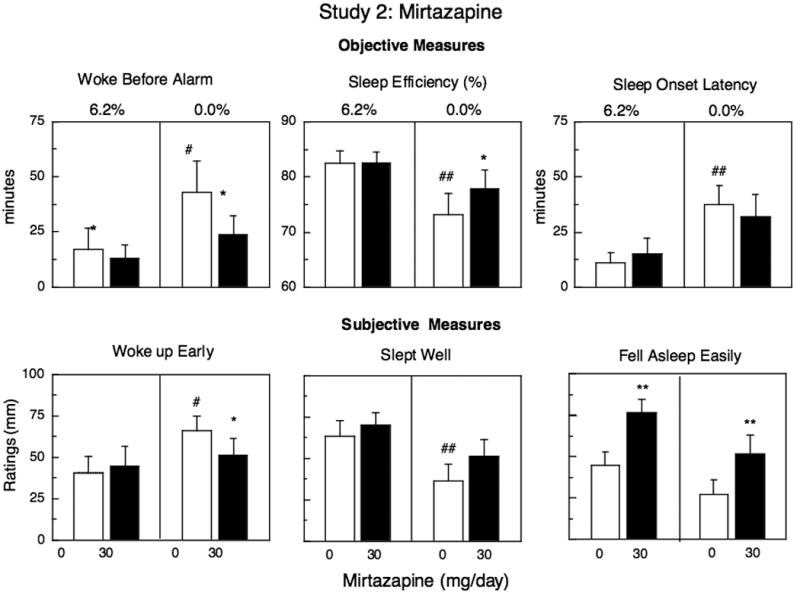
Mean effects on objective measures of sleep (top panels) and mean peak effects on subjective measures of sleep (bottom panels) during marijuana administration (6.2%) and during marijuana abstinence as a function of mirtazapine dose. See Figure 1 for details.
TABLE 3. Mean peak effects during marijuana administration (6.2% THC) and during abstinence as a function of mirtazapine dose.
| Mirtazapine Dose (mg) | ||
|---|---|---|
| 0 | 30 | |
| High Cluster | ||
| 6.2% THC | 68 (7) | 78 (7) |
| 0.0% THC | ↓4 (2)## | 4 (4) |
| Irritable Cluster | ||
| 6.2%THC | 9 (5) | 15 (8) |
| 0.0% THC | ↑31 (12)## | 38 (11) |
| Anxious Cluster | ||
| 6.2%THC | 8 (2) | 14 (4) |
| 0.0% THC | ↑17 (6) ## | 26 (8) |
| Social Cluster | ||
| 6.2%THC | 58 (8) | 61 (7) |
| 0.0% THC | ↓50 (8)# | 47 (7) |
| Confused Cluster | ||
| 6.2%THC | 8 (2) | 9 (3) |
| 0.0% THC | ↑17 (6)# | 19 (6) |
| Tired Cluster | ||
| 6.2% THC | 32 (5) | 31 (5) |
| 0.0% THC | ↑41 (7)# | 36 (7) |
|
| ||
| Eating Occasions (#/day) | ||
| 6.2% THC | 11.4 (0.7) | ↑14.5 (0.8)** |
| 0.0% THC | ↓5.6 (0.9) ## | ↑7.5 (0.9)** |
Note Data in parentheses represent standard error of the mean; maximum mood score = 100 mm. Asterisks represent significant differences from placebo mirtazapine
p <0.05,
p <0.01. Number signs represent significant differences between active marijuana administration and marijuana abstinence under placebo mirtazapine conditions
p <0.05,
p <0.01.
Tobacco Cigarette Smoking
There were 7 cigarette smokers. The number of cigarettes smoked did not vary as a function of marijuana administration or abstinence. Mirtazapine significantly increased the number of cigarettes smoked, both under conditions of active marijuana administration and under conditions of marijuana abstinence compared to placebo (Fig. 2).
Food intake
Under placebo mirtazapine conditions, marijuana abstinence was associated with significantly less caloric intake than active marijuana administration (Fig. 2). The caloric content of each eating occasion did not vary, but participants had significantly fewer eating occasions throughout the day (Table 3). In addition, the proportion of calories derived from fat was significantly less during abstinence relative to marijuana smoking. Specifically, on days participants smoked active marijuana, 34% ± 1 of the daily calories consumed came from fat, whereas during marijuana abstinence, 28% ± 2 of daily calories consumed were derived from fat (p <0.001). Relative intake of carbohydrates increased during abstinence compared to active marijuana administration [F(1,70) =4.03, p <0.05]. Mirtazapine significantly increased caloric intake during both active marijuana administration and marijuana abstinence relative to placebo (Fig. 3). Mirtazapine affected daily caloric intake by increasing the number of eating occasions under both conditions compared to placebo (Table 3); the proportion of fats, proteins and carbohydrates consumed was not altered by mirtazapine.
Body Weight
Under placebo mirtazapine conditions, participants weighed significantly less (1.7 kg; p <0.01) following 2-3 days of abstinence compared to the day after smoking active marijuana. Participants maintained on mirtazapine weighed significantly more the day after smoking active marijuana (1.1 kg; p <0.01) and after 2-3 days of marijuana abstinence (1.0 kg; p <0.01) than when maintained on placebo.
Task performance
Under placebo mirtazapine conditions, participants entered approximately 6 fewer patterns in the Digit Symbol Substitution Task when they smoked active marijuana compared to when they were abstinent (p <0.01). Mirtazapine had no effect on performance during active marijuana administration, but during marijuana abstinence, mirtazapine improved performance on the Rapid Information Task by increasing the number of odd and even sequences correctly identified (85.8%) compared to placebo (81.2%; p <0.01), and by tripling the percentage of number sequences recalled after a delay in the Digit Recall Task (54.5%) compared to placebo (18.2%; p <0.05).
Subjective and Objective Sleep Measures
Figure 3 portrays objective and subjective measures of sleep: Under placebo mirtazapine conditions, objective measures of sleep indicated that participants took longer to fall asleep, slept less efficiently, and woke up before the alarm during marijuana abstinence compared to the night when active marijuana was smoked. Subjective ratings confirmed that compared to active marijuana administration, abstinence was associated with significantly decreased ratings of ‘ slept well’ (p < 0.01) and increased ratings of ‘ woke early’ (p < 0.01), and ‘ woke often’ (Marijuana: 32 mm; Abstinence: 56 mm, p < 0.05). During active marijuana administration, mirtazapine significantly increased ratings of ‘fell asleep easily’ relative to placebo capsules. During marijuana abstinence, mirtazapine continued to increase ratings of ‘fell asleep easily,’ and reversed many of the abstinence-related decrements in sleep. Compared to placebo capsules, mirtazapine significantly decreased how early participants woke before the alarm, improved sleep efficiency and decreased ratings of ‘ woke up early.’
Relapse
Mirtazapine had no significant effect on marijuana relapse, defined as the mean amount of money spent to purchase marijuana following the 3-day period of abstinence Specifically, participants paid on average $16.92 ± 3.49 during placebo maintenance and $17.56 +3.37 under mirtazapine maintenance. The same number of participants purchased at least one puff of marijuana under each medication condition (64%).
Medication Side Effects
There were 4 outpatient visits during each medication phase. Participants reported few side effects, and their incidence and the number of participants reporting side effects did not vary as a function of medication dose: Two participants reported a single occurrence of ‘mild headache’ during the placebo phase and during the mirtazapine phase. Participants also reported feeling ‘tired’ during placebo maintenance (n=5) and during mirtazapine administration (n=6).
Discussion
This paper evaluated the influence of baclofen (Study 1) and mirtazapine (Study 2) on human laboratory measures of marijuana's direct behavioral effects, withdrawal and relapse in daily marijuana smokers. The results of Study 1 demonstrate that when participants smoked active marijuana, baclofen (90 mg) decreased marijuana craving. During marijuana abstinence, baclofen worsened one measure of sleep and worsened cognitive performance, but had few statistically significant effects on mood or behavior, and did not decrease measures of relapse. In Study 2, mirtazapine reversed abstinence-related disruptions in sleep and food intake, but also had little effect on mood overall, and did not decrease marijuana relapse.
It is important to note that there was minimal evidence of marijuana withdrawal in Study 1, even as defined liberally as a difference between marijuana intoxication and abstinence. Although participants ate approximately half as many calories during abstinence compared to active marijuana, the more essential withdrawal measures of disrupted sleep, irritability, anxiety and marijuana craving did not differ on days active marijuana was smoked compared to days of abstinence, thereby limiting any conclusions about baclofen's effects on this syndrome. The incidence of marijuana withdrawal symptoms among regular marijuana smokers is estimated to be at least 50% (Budney et al., 2004), and approximately half of the participants in Study 1 demonstrated withdrawal (e.g., a time-dependent doubling in ratings of irritability during abstinence relative to active marijuana administration), although the overall effect was not significant. Similarly, about half of the participants relapsed to marijuana (i.e., purchased at least 1 puff of marijuana), yet baclofen clearly did not alter this behavior.
One of baclofen's few behavioral effects, independent of marijuana condition, was to dose-dependently slow reaction time and worsen accuracy and performance on four out of the five tasks administered as part of the task battery. This performance decrement, consistent with case reports of baclofen-induced memory impairment (Sandyk and Gillman, 1985), occurred even in the absence of any reported increases in ratings of fatigue or sedation.
Baclofen also dose-dependently decreased tobacco cigarette craving, as well as the number of cigarettes smoked during marijuana abstinence, despite the fact that participants expressed no desire to decrease their cigarette use and there were no contingencies to decrease cigarette smoking. A pilot clinical trial similarly demonstrated that baclofen maintenance (60 mg/day) for 9 weeks decreased cigarette smoking compared to placebo in individuals contemplating smoking cessation (Franklin et al., 2009). Acute baclofen administration (20 mg) has been shown to increase how harsh cigarettes taste (Cousins et al., 2002), which may explain the present findings in nontreatment-seekers.
In contrast to Study 1, there was clear evidence of marijuana withdrawal in Study 2. During abstinence, participants reported more irritability, anxiety and marijuana craving compared to active marijuana smoking, and their sleep was significantly disrupted: both subjective ratings and objective measures of sleep efficiency and onset showed worsened sleep during abstinence, consistent with polysomnography studies of marijuana withdrawal (Schierenbeck et al., 2008; Bolla et al., 2008).
Although mirtazapine robustly improved almost all measures of sleep during marijuana abstinence, the medication did not improve withdrawal-associated mood symptoms, and did not decrease relapse measures. We had hypothesized that mirtazapine's sleep-enhancing effects, well documented in depressed patients (Schmid et al., 2006; Papakostas et al., 2008) and now demonstrated in marijuana-dependent volunteers, would decrease the amount of marijuana purchased after a bout of abstinence. This prediction stemmed from the finding that lofexidine improved sleep and decreased relapse without attenuating marijuana craving or mood symptoms of withdrawal (Haney et al., 2008). Both medications facilitate sleep, yet only lofexidine decreased marijuana relapse. Because mirtazapine increases synaptic norepinephrine by blocking presynaptic, inhibitory α2 autoreceptors, whereas lofexidine is an agonist at α 2 adrenergic receptors, thereby decreasing norepinephrine transmission, perhaps the mechanism by which lofexidine altered relapse was by decreasing noradrenergic hyperactivity during marijuana withdrawal (e.g., Lichtman et al., 2001) rather than by improving sleep per se.
In terms of other behavioral effects, participants in both studies consumed approximately half as many calories during abstinence compared to when marijuana was smoked. Baclofen had no effect on food intake, yet participants maintained on mirtazapine ate approximately 50% more calories per day and weighed more, regardless of marijuana condition, compared to placebo maintenance. These data are consistent with studies showing that mirtazapine (30-45 mg/day) is associated with weight gain and increased body fat (Laimer et al., 2006). Marijuana alone has robust effects on food intake (e.g., Haney et al., 2007), so it is notable that mirtazapine had these effects even under conditions of increased food intake.
Another behavior influenced by mirtazapine was tobacco cigarette smoking. In direct contrast with baclofen, mirtazapine increased both cigarette craving and the number of cigarettes smoked, independent of marijuana use or abstinence. To our knowledge, this is the first demonstration of mirtazapine's effects on cigarette smoking. More research is needed to confirm whether this finding is specific to marijuana smokers or also occurs in patients taking mirtazapine to treat depression.
There are several issues to consider with the present design. First, the marijuana tested in the laboratory was less potent than the average marijuana confiscated by US law enforcement agencies (> 9% THC; www.WhiteHouseDrugPolicy.gov). Nonetheless, even daily marijuana smokers demonstrated substantial intoxication from the relatively weak marijuana available from NIDA, likely because marijuana smokers titrate their smoking based on potency: Weaker marijuana is inhaled more forcefully than stronger marijuana (Heishman et al., 1989; Cooper and Haney, 2009). However, participants in Study 2, which tested marijuana almost twice as potent as that used in Study 1, had more robust symptoms of withdrawal and higher rates of relapse, suggesting that more potent marijuana should be used in studies focusing on marijuana withdrawal. Secondly, only one dose of mirtazapine was assessed, limiting our conclusions to the dose selected. Finally, the sample was not broadly diverse, as there were no women or non-Hispanic Caucasians enrolled, thereby limiting our conclusions to the population studied.
In summary, both baclofen (60, 90 mg/day) and mirtazapine (30 mg/day) were well tolerated in heavy marijuana smokers. Although we cannot comment on baclofen's potential for lessening marijuana withdrawal, the medication worsened cognitive performance and did not decrease our laboratory measure of relapse. Baclofen decreased marijuana craving when participants smoked active marijuana, but this single positive outcome does not suggest that baclofen would have a meaningful impact on marijuana treatment. The study does, however, support further testing of baclofen for tobacco cessation. Mirtazapine robustly improved most measures of sleep, yet increased food intake and cigarette craving and use, without decreasing either marijuana withdrawal or relapse. Although only a double-blind, placebo-controlled, clinical trial can definitively determine if a mediation is efficacious for the treatment of marijuana dependence, human laboratory studies can be a powerful means of guiding which medications should proceed to the more expensive clinical trials (Koob et al., 2009; Haney, 2009). The present data suggest that neither baclofen nor mirtazapine are candidates for further testing in a marijuana treatment trial.
Acknowledgments
The U.S. National Institute on Drug Abuse (NIDA) supported this research (DA19239, DA09236) supplied the marijuana cigarettes. The authors have no conflicts of interest. We are grateful to Brooke Roe, Diana Paksarian, Michael Rubin, Danielle Lion, and Matthew Pecht for their superb assistance in data collection.
References
- Akhondzadeh S, Ahmadi-Abhari SA, Assadi SM, Shabestari OL, Kashani AR, Farzanehgan ZM. Double-blind randomized controlled trial of baclofen vs. clonidine in the treatment of opiate withdrawal. J Clin Pharm Ther. 2000;25:347–353. doi: 10.1046/j.1365-2710.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, David PM, Verdejo-Garcia A, Benbrook AR. Sleep disturbance in heavy marijuana users. Sleep. 2008;31:901–908. doi: 10.1093/sleep/31.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancements improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiat. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9 tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng W, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18:53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103:107–113. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Swift W. Cannabis use disorder: epidemiology and management. Int Rev Psychiatry. 2009;21:96–103. doi: 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat. 2001;21:55–64. doi: 10.1016/s0740-5472(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Roberts DCS, de Wit H. GABAB receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Comer SD, Fischman MW. Effects of fenfluramine in food intake, mood, and performance of humans living in a residential laboratory. Physiol Behav. 1996;59:295–305. doi: 10.1016/0031-9384(95)02098-5. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O'Brien CP, Childress AR. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend. 2009;103:30–36. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson GW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. J Acquir Immune Defic Syndr. 2007;45:545–554. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opiod- and nonopioid-dependent volunteers. Neuropsychopharmacol. 2006;31:1814–1821. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacol. 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacol. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacol. 2003;165:157–65. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: Drug self-administration. Psychopharmacol. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacol. 1999a;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacol. 1999b;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacol. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Hart C, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral delta(9)-tetrahydrocannabinol in humans. Psychopharmacol. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stizer ML, Yingling JE. Effects of tetrahydrocannabidiol content on marijuana smoking behavior, subjective reports and performance. Pharmacol Biochem Behav. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: A Rosetta Stone approach. Nat Rev Drug Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8:483–92. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Laimer M, Kramer-Reinstadler K, Rauchenzauner M, Lechner-Schoner T, Strauss R, Engl J, Deisenhammer EA, Hinterhuber H, Patsch JR, Ebenbichler CF. Effect of mirtazapine treatment on body composition and metabolism. J Clin Psychiatry. 2006;67:421–424. doi: 10.4088/jcp.v67n0313. [DOI] [PubMed] [Google Scholar]
- Levin FR, Brooks DJ, Bisaga A, Raby W, Rubin E, Aharonovich E, Nunez E. Severity of dependence and motivation for treatment: comparison of marijuana- and cocaine-dependent treatment seekers. J Addict Dis. 2006;25:33–41. doi: 10.1300/J069v25n01_06. [DOI] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, Vosburg SK. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Prog Neuropsychopharmacol. 2005;29:55–60. doi: 10.1016/j.pnpbp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav. 2001;69:181–188. doi: 10.1016/s0091-3057(01)00514-7. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, Brady KT. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 2009;105:132–138. doi: 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. J Subst Abuse Treat. 2003;25:85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Homberger CH, Fava M. A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. J Psychopharmacol. 2008;22:843–848. doi: 10.1177/0269881107083808. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Gillman MA. Baclofen-induced memory impairment. Clin Neuropharmacol. 1985;8:294–295. doi: 10.1097/00002826-198509000-00011. [DOI] [PubMed] [Google Scholar]
- Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy, and marijuana. Sleep Med Rev. 2008;12:381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schmid DA, Wichniak A, Uhr M, Ising M, Brunner H, Held K, Weikel JC, Sonntag A, Steiger A. Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacol. 2006;31:832–844. doi: 10.1038/sj.npp.1300923. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Extended versus brief treatment for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH series h-32, DHHS Publication No SMA 07-4293) Rockville, MD: 2007. [Google Scholar]
- Teesson M, Lynskey M, Manor B, Baillie A. The structure of cannabis dependence in the community. Drug Alcohol Depend. 2002;68:255–262. doi: 10.1016/s0376-8716(02)00223-5. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Pae CU, Kim DJ, Namkoong K, Lee E, Oh DY, Lee YS, Shin DH, Jeong YC, Kim JH, Choi SB, Hwang IB, Shin YC, Cho SN, Lee HK, Lee CT. Mirtazapine for patients with alcohol dependence and comorbid depressive disorders: a multicentre, open label study. Prog Neuropsychopharmacol. 2006;30:1196–1201. doi: 10.1016/j.pnpbp.2006.02.018. [DOI] [PubMed] [Google Scholar]


