Abstract
Prevalence rates for leprosy have declined sharply over the past 20 y, with this decline generally attributed to the WHO multi-drug therapy (MDT) campaign to provide free-of-charge treatment to all diagnosed leprosy patients. The success of this program appears to have reached its nadir, however, as evidenced by the stalled decreases in both global prevalence and new case detection rates of leprosy. Mass BCG vaccination for the prevention of tuberculosis (TB) at national levels has had a positive effect on leprosy decline and is often overlooked as an important factor in current leprosy control programs. Because BCG provides incomplete protection against both TB and leprosy, newer more effective TB vaccines are being developed. The impact that application of these vaccines will have on current leprosy control programs is unclear. In this review, we assess the need for vaccines within leprosy control programs. We summarize and discuss leprosy vaccine strategies that have been deployed previously and discuss those strategies that are currently being developed to augment recent breakthroughs in leprosy control.
Keywords: leprosy, mycobacteria, T cell, vaccine
LEPROSY - THE CURRENT SITUATION
Leprosy (or Hansen’s disease) is one of the most renowned, but least understood, diseases of man. Although 16 countries reported more than 1000 new cases during 2009, all but Brazil, Democratic Republic of Congo and Mozambique have yet to achieve the goal of eliminating leprosy as a public health problem (a prevalence rate of < 1 case per 10,000 persons).1 Leprosy is not evenly spread across populations, however, with close contacts of patients known to be at significantly greater risk of contracting disease and smaller regions of higher incidence rates located within many countries.2-5
Leprosy is caused by infection with Mycobacterium leprae and presents across a bacteriologic, clinical, immunologic and pathologic spectrum. Infection of peripheral nerves is a hallmark of leprosy, but the mechanism(s) underlying nerve injury in leprosy is very poorly understood. Neuropathy begins as local anesthesia that, if left untreated, can develop into paralysis and crippling deformities. Neuropathy arises not only from the infection of peripheral nerves by M. leapre, a unique trait among bacteria, but also from the inflammatory and immunologic responses to the infection. Acute inflammatory complications called reactions often present during the course of treated or untreated leprosy. Up to half of all patients may be affected by the two major clinical types of leprosy reactions that occur. The inflammation associated with reactions is a medical emergency often requiring hospitalization, as severe nerve injury may rapidly develop, with subsequent loss of sensation, paralysis, and deformity.
Through the use of clinical, histopathological, and immunological diagnoses, the Ridley-Jopling scale characterizes five forms of leprosy: lepromatous leprosy (LL), borderline lepromatous (BL), mid-borderline (BB), borderline tuberculoid (BT), and tuberculoid leprosy (TT).6,7 In practice, because of the lack of available or dependable skin-smear or pathology services, most field programs use clinical criteria for classifying individual patients and selecting their treatment regimen. One such clinical system suggested by WHO uses the number of skin lesions and number of involved nerves to group leprosy patients into one of two simplified categories; MB leprosy (typically 5 or more lesions) and PB leprosy (less than 5 lesions). By more rigorous histological analyses, MB patients, encompassing the BB, BL and LL forms, are characterized as having multiple skin lesions largely devoid of functional lymphocytes. At the extreme MB pole, LL patients demonstrate high titers of anti-M. leprae antibodies but an absence of specific cell-mediated immunity.6 In the absence of a strong cellular immune response, LL patients do not control bacterial replication and have high bacterial indices (BI; a measure of the number of acid-fast bacilli in the dermis expressed in a logarithmic scale). In marked contrast, PB leprosy patients, encompassing the BT and TT forms, are characterized as having one or few skin lesions and granulomatous dermatopathology with a low or absent BI. At the extreme PB pole, TT patients demonstrate a specific cell-mediated immunity against M. leprae and have an absent, or low, BI.
Following diagnosis, patients are provided antibiotic treatments in the form of multidrug therapy (MDT). WHO has provided free MDT for all reported leprosy patients since 1995, initially through a drug fund provided by the Nippon Foundation and now through the MDT donation provided by Novartis and the Novartis Foundation for Sustainable Development. For MB patients, a combination employing rifampicin, dapsone and clofazimine is provided over the course of 12 mo, while for PB patients, only rifampicin and dapsone are provided over 6 mo.8 The MDT program has been the stimulus for markedly reduced leprosy case numbers, with prevalence rates reduced to the current level of ~250,000 cases per year from levels as high as 12 million cases per year only 20 y ago. Thus, at present the central tenets for leprosy control are the timely detection of new cases and the prompt treatment of patients with MDT.9
Need for a Leprosy Vaccine
Despite the positive impact that WHO-MDT has had on the global prevalence of leprosy, there are many indications that further effort is required to prevent the re-emergence of leprosy and continue efforts toward eradication. That effort should include an effective vaccine with potential for both prophylactic and therapeutic ude.
While overall MDT has been a success, complications can and do arise during its use. Both MB- and PB-MDT treatments are long (6 and 12 mo respectively) and there is widespread skepticism that efforts to provide truncated, standardized treatment regimen will be effective. This skepticism is rooted in the fact that MB patients who are misclassified receive the less rigorous drug regime designed to treat PB disease and are often skin smear positive at the end of treatment. Our own data indicate that anti-M. leprae antibodies remain elevated in patients receiving inappropriate treatment, either through misdiagnosis or poor treatment compliance, leaving these patients as potential sources for continued transmission in the community.10
While MDT remains effective in the majority of cases, relapse (or possibly re-infection) can occur. Although relapse rates are generally low (~1%), relapse rates in some areas are unacceptably high. The extent of relapse depends on several operational factors, on the duration of follow-up, and can occur long after treatment has ended.11,12 Relapse may be related to poor MDT compliance. For example, patients often neglect their treatments, because clofazimine causes stigmatizing skin discoloration or they become weary of the length of treatment.13,14 The largest reported study is a 6-y follow up of 47,276 patients conducted in the China, which revealed an overall relapse rate of 0.73/ 1,000 person-years [with the risk of PB patient relapse (1.04/1,000 person-years) significantly greater than that for MB patients (0.61/1,000 person-years)].15,16 A 10-y prospective study in the Philippines observed an overall relapse rate equivalent to 2.8/1,000 person-years.17 Significant differences were noted in the relapse rates of MB patients followed at a referral center (9%) vs. field clinics (3%). The rates observed in those studies are significantly better than those achieved in southern India, where a relapse rate equivalent to 20/1,000 person- years was observed among MB patients given 2 y MDT. This rate was reduced to 10/1,000 person-years in patients that were treated until they became smear negative.18 Importantly, in both the southern India and Philippine studies, higher rates of relapse have been observed in patients with a high BI at the time of diagnosis, indicating that these patients likely require longer treatment.11,19
MDT efficacy will also disappear with the emergence of drug resistance.20-24 Dapsone resistance is relatively widespread and, when coupled with clofazimine noncompliance, the net result is that patients are receiving rifampicin monotherapy. This situation is regarded as highly conducive for the emergence of resistance25 and several investigators have reported multidrug-resistant strains of M. leprae.24,26-29 The situation concerning enough that in 2008 WHO Global Leprosy Programme initiated a Sentinel Surveillance Network to monitor drug resistance in leprosy. In 2009, reports were received regarding 213 MB relapse cases with a BI of two and more who were recruited into the surveillance system from six countries (Brazil, China, Colombia, India, Myanmar and Vietnam).30 Of these 213 relapse cases tested for drug resistance, 12 were found to be dapsone resistant and 9 were rifampicin resistant. Two relapse cases from Brazil and Colombia were resistant to both dapsone and rifampicin. While ofloxacin and minocycline have been added to the drug arsenal for the treatment of leprosy in an attempt to attenuate the spread of drug resistance and to provide secondary treatments,21,31-36 ofloxacin resistance was found in two relapse cases from India.30 The widespread emergence of drug resistant leprosy could have catastrophic consequences, undoing the efforts of the WHO-MDT campaign and causing a rebound of leprosy incidence.
An additional concern with current control strategies is that they suffer from reliance on passive case detection. Such strategies have no provision for preventing leprosy other than attempting to reduce the number of cases identified M. leprae carriers. Although educational campaigns have reduced some of the stigma associated with leprosy, stigma remains a major obstacle to self-reporting and early treatment. It is estimated that the delay from the time of onset of the first discernible symptom to clinical diagnosis is anywhere from 1–3 y in more than 50% of patients.37-39 In countries where leprosy is extremely rare, patients are typically misdiagnosed and mistreated before eventually being properly identified.40 A recent study conducted in India found that inadequate monitoring of a policy of ‘new case validation,’ in which treatment was not initiated until primary diagnosis had been verified by a leprosy expert, may have led to approximately 26% of suspect cases awaiting confirmation of diagnosis 1–8 mo after their initial primary health care visit.41 Such delays can have a dramatic and negative impact on nerve function impairment and response to treatment.42-44
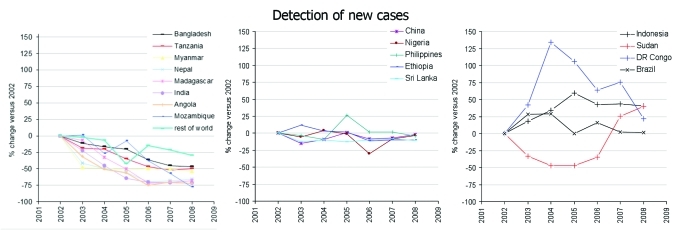
Finally, although integration into other aspects of the health care system has occurred in many countries, the success WHO-MDT has unfortunately led to a corresponding erosion of leprosy clinics, specialists and research. We would argue that this decline is premature, particularly because transmission of M. leprae still appears to be occurring at a relatively consistent rate in many countries (Fig. 1).9,45-47 Indeed, the level of leprosy control that can be attributed to MDT may have reached a plateau, with mathematical modeling suggesting that the disease will remain a major public health problem for at least several more decades.48,49 These concerns, along with the current limitations in control and treatment strategies, suggest that the development of additional tools and strategies, such as development of a sub-unit vaccine capable of promoting a long-lasting immune response, are necessary for the elimination of leprosy.
Figure 1.
New case detection rates in countries reporting over 1000 annual leprosy cases since 2002.
The Leprosy Vaccine Experience
While detect-and-treat or chemoprophylactic strategies do provide protection against leprosy, the nature of drug activity dictates that efficacy is limited to individuals who are already infected. It is our belief that control of leprosy by vaccination has significant advantages over control by drug treatment of patients. Foremost among these is that by promoting an immune memory response a vaccine, unlike drug treatment, could be used to provide active and sustained protection.
Evaluation of whole mycobacteria vaccines
So, if as we believe, a vaccine could have such a positive impact, why is one (other than BCG) not currently available? Several vaccines strategies centered on the use of whole mycobacteria have been evaluated (Table 1). As already mentioned, the most common vaccine strategy has been to immunize individuals with M. bovis BCG. The use of BCG is typically associated with tuberculosis, but it is worth noting that BCG was originally developed and widely implemented for the control of both leprosy and tuberculosis. The presence of a BCG scar has been recognized as a protective factor for leprosy.4 A huge benefit to the use of BCG has therefore been its efficiency, provided by its ability to confer protection against both diseases. The impact of BCG vaccination on leprosy is often overlooked as widespread vaccination campaigns have been coincident with the WHO-MDT campaign. The degree of protection against leprosy afforded by BCG vaccination, however, has varied dramatically between studies. Systematic meta-analyses indicate an overall protective efficacy of 26–41% in experimental studies vs. 61% in observational studies.50,51 The reason for the wide-ranging protection reported across various studies is unclear, but the use of different BCG strains may be a factor.52 As with tuberculosis, the protection afforded by BCG vaccination against leprosy is highest in younger individuals and wanes over time.53-55 Perhaps the best and clearest indication that BCG is not perfect is the indisputable fact that leprosy remains endemic in countries where BCG immunization is a widespread.
Table 1. Summary of clinical trials to identify novel vaccines for leprosy.
| vaccine | location | overall study size | study follow-up (years) |
% protection | reference | |
|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
| |
BCG BCG + killed M. leprae |
Venezuela |
29, 113 |
5 |
56 54 |
58
|
| |
|
|
|
|
|
|
| |
BCG BCG + killed M. leprae |
Malawi |
121, 020 |
6–9 |
49 49 |
59
|
| |
|
|
|
|
|
|
|
Whole bacteria |
BCG BCG + killed M. leprae M. w ICRC |
India |
171, 400 |
4–7 |
34 64 26 66 |
61
|
| |
|
|
|
|
|
|
| |
M. w (killed) |
India |
29, 420 |
8–10 |
39 |
64
|
| |
|
|
|
|
|
|
| |
BCG M. vaccae BCG + M. vaccae |
Vietnam |
432 |
8 |
58 55 66 |
65
|
| |
|
|
|
|
|
|
| |
BCG |
composite of multiple studies (meta-analysis) |
26–41a (experimental) 61 (observational) |
50
,
51
|
||
| |
|
|
|
|
|
|
| Sub-unit | None reported | |||||
a Setia and colleagues selected all the studies (experimental and observational) that described the efficacy of BCG with prevention of clinical leprosy (defined according to clinical and/or microscopic criteria) and excluded studies that measured the protective effect of BCG combined with other therapies (eg, chemotherapy, killed M leprae)/ Their meta-analysis was restricted to studies that measured the protective effect of BCG alone. Merle and colleagues selected only controlled trials with a clearly defined placebo (or nonintervention) group. To be included in their analysis, case-control studies had to define the criteria for selecting cases and controls, as well as the method for determining their BCG vaccination status.
In an attempt to identify a leprosy vaccine that gives greater and more consistent protection than BCG, the vaccine potential of killed M. leprae has been assessed in various trials. Many researchers have demonstrated that inoculation of heat-killed M. leprae provides a robust protection against subsequent infection in mice and this is commonly used as a positive control during lab experiments.56,57 Convit and colleagues compared the efficacy of BCG with and without killed M. leprae in human trials in Venezuela.58 Between 1983 and 1991, 29,113 people were vaccinated with BCG alone or BCG plus 6 x 108 irradiated, autoclaved M. leprae purified from the tissues of infected armadillos. By mid-1991, through annual clinical examinations of the trial population, leprosy cases had been confirmed in comparable numbers of individuals in the BCG arm and in the BCG/M. leprae arm during 150,026 person-years of follow-up. Thus, no evidence was found in the first 5 y of follow-up that BCG plus M. leprae offered better protection against leprosy than BCG alone. Retrospective analysis of the number of BCG scars found on each individual suggested that BCG alone conferred 56% protection against leprosy in this study population, and there was a suggestion that several doses of BCG offered additional protection.
Between 1986 and 1989, the Karonga Prevention Trial Group recruited individuals to a double-blind, randomized, controlled trial to evaluate the protection afforded by repeated BCG vaccination, or by BCG/M. leprae vaccination.59 This group had previously reported that in Karonga (northern Malawi), a single BCG vaccine administered by routine health services was found to afford greater than 50% protection against leprosy.60 Over 60,000 individuals lacking a BCG scar were randomly assigned treatment with BCG alone or BCG/killed M. leprae, while over 50,000 individuals with a BCG scar were randomly allocated placebo, a second BCG, or BCG/killed M. leprae. By mid-1995 (6–9 y after vaccination), 139 cases of leprosy had been identified, with 93 of those being diagnostically certain, definitely post-vaccination cases. The incidence rate of all new leprosy cases, across all ages, was significantly lower among the BCG recipients than among those who received placebo. This benefit was apparent in all subgroups, although the greatest effect was among children vaccinated before 15 y of age. As in the Venezuelan trial, the BCG/M. leprae vaccine was not found to improve the protection afforded by a primary BCG vaccination. Among BCG scar-positive individuals, a second BCG vaccination provided further protection against leprosy (about 50%) over a first BCG vaccination, again suggesting that a second BCG vaccination can add to the protection against leprosy.
A larger, but similar, trial was conducted by Gupte and colleagues in south India with enrollment of 171,400 volunteers between early 1991 and mid-1993.61 A double blind, randomized, prophylactic trial of four potential leprosy vaccines (BCG alone; BCG/killed M. leprae; and the alternative mycobacteria Mycobacterium w and ICRC, that have been demonstrated to provide protection in mice62,63) were compared with a placebo (saline) group. Although, in two surveys conducted in the 8 y following immunization, the observed leprosy incidence rates based on clinical examination of more than 70% of the original intake population were not sufficiently high to ascertain the protective efficacy of the candidate vaccines against the progressive and serious forms of leprosy, it was determined that BCG/M. leprae provided 64% protection, ICRC provided 65.5% protection, M. w provided 25.7% protection and BCG alone provided 34.1% protection. Thus, unlike the earlier trials in Venezuela and Malawi, the south India trial indicated that a BCG/M. leprae vaccine, and also the ICRC vaccine, met the requirements of public health utility and might be further deployed for control of leprosy.
Further development of a killed M. leprae-based strategy is, however, enormously constrained by the difficulties associated with scaling up production. Conditions with which to culture M. leprae have still not been discovered, so production would be limited to generating large numbers of bacteria in immune-compromised mice or armadillos. Reproducibly generating anything resembling a consistent product in these systems would appear to be extremely difficult, if not impossible, and this has motivated the evaluation of alternate, cultivatable mycobacteria as leprosy vaccines.
In 2005, Sharma and colleagues reported the results of a large scale, double blind immunoprophylactic trial of a M. w vaccine in Kanpur Dehat, Uttar Pradesh, India conducted between 1992 and 2001.64 The vaccine consisted of 1 x 109 heat killed M. w bacilli for the first dose and a second, half dose given 6 mo later. A total of 24,060 household contacts and index cases were immunized with M. w or placebo (20,194 received two doses and 3866 received a single dose). The vaccine recipients were followed by surveys conducted at 3, 6 and 9 y after the initial vaccination. As with other studies, the vaccine efficacy was highest in children when compared with adolescents and adults. When index cases, and not the contacts, received the M. w vaccine, surveys at the end of the first, second and third follow-up periods showed protective efficacies of 43%, 31% and 3%, respectively, were found. When only contacts received the vaccine, protective efficacies of 69%, 59% and 39% were observed. When both patients and contacts received the M. w vaccine, the protective efficacy was 68%, 60% and 28% at each follow-up time. Thus, the protective effect of the M. w vaccine was sustained for a period of about 7–8 y, following which it is suggested a booster vaccination may be needed. It is unclear, however, if the M. w vaccine can be boosted.
A trial was conducted in Vietnam involving vaccination with killed M. vaccae alone (108 bacteria), BCG alone or BCG plus 107 killed M. vaccae.65 Children living in close contact with leprosy were examined in the year before the vaccines were provided, and it was found that 14 of 446 (3.1%) examined had leprosy. Children were subsequently enrolled, vaccinated and incidence rates compared with pre-trial rates through follow-up. Among those children who were not vaccinated, 9 of 74 (12.2%) developed leprosy in the first 4 y of the study and 5 of 65 (7.7%) developed leprosy in the second 4 y. In comparison, among those vaccinated, 20 of 343 (5.8%) developed leprosy in the first 4 y and 5 of 323 (1.5%) developed leprosy in the second 4 y. This represents 53% protection in the first 4 y and 81% in the second 4 y. There were no significant differences in protection afforded by each of the three vaccines, indicating that both live BCG and killed M. vaccae provided protection in this trial, but the addition of killed preparation of M. vaccae to BCG did not enhance protection afforded by either alone.
Vaccination with M. habana has also been proposed on the basis of protection in mice and the induction of lepromin reactions in monkeys.66,67 M. habana vaccination induced stable lepromin conversion in 100% of LL cases and lepromin negative household contacts and augmentation of lepromin reactivity in 100% of lepromin positive household contacts.68 Overall, following M. habana vaccination, individuals without prior BCG vaccination scars showed higher augmentation of lepromin responses. The authors argued that M. habana vaccine appeared to be useful in stimulating specific cell-mediated immunity against M. leprae as evidenced by increased lepromin reactivity, and was suggested to be protective. There have, however, been no subsequent reports regarding the protective efficacy of M. habana vaccination.
To date, although variable in its protective efficacy, BCG is the best available vaccine for the prevention of leprosy. Although some of the studies outlined above indicate that multiple BCG vaccinations enhance protection58,59 and it is common practice in some countries to re-immunize leprosy patients with BCG, the efficacy of this secondary treatment is debated.50,69,70 Current WHO guidelines for tuberculosis do not support BCG revaccination for protection owing to limited efficacy in trials, and studies in Brazil indicate no substantial benefit of BCG revaccination against tuberculosis.71-73 It is possible that the live BCG vaccine is rapidly killed upon inoculation before it can adequately potentiate existing responses, and new methods may be required to allow boosting of the BCG response.
Advances toward defined vaccines
Experimental immunizations with crude antigens have been conducted, demonstrating that proteins within the M. leprae cell wall, cell membrane and cytosol all provide protection when administered with adjuvant before infection.74,75 As with the use of killed M. leprae, major constraints regarding the use of crude M. leprae antigens in a vaccine are the prohibitive nature of cultivating large enough numbers of M. leprae and the lack of consistency in production. A defined sub-unit vaccine produced by standard methods could circumvent the production and quality control issues surrounding live or purified whole cell or cell component vaccines, but such a vaccine for leprosy is still lacking.
Vaccination of mice with the Ag85 proteins purified from BCG culture filtrate, in conjunction with Freund’s incomplete adjuvant (FIA), provides protection by inhibiting M. leprae growth.76 Alternatively, recombinant Ag85A/B were reported as not providing protection when administered with either FIA or monophosphoryl lipid A, but in those experiments, interpretation was difficult because control mice did not exhibit good bacterial growth.77 It has been suggested that glycosylation may be important for antigenicity and this has been offered as a reason for the previous failure of recombinant antigens to confer protection. We consider this reason unlikely, however, because the 35kD, Ag85B and hsp65 antigens have all been shown to confer protection when expressed in a DNA vaccine.78-80 Both purified and/or recombinant 10kD, 25kD and 65kD proteins also provided protection when administered with FIA.81
In an attempt to make BCG more immunogenic and to extend its protective lifespan, several investigators have genetically-refined the bacteria. This is a major area of interest in the TB field, with several recombinant BCG (rBCG) being produced and advancing toward clinical trial (Areas Global TB Vaccine Foundation). The protection that these rBCG can afford against leprosy, and therefore the impact that these could have on leprosy control programs, is unclear.
Only some rBCG have been produced with leprosy in mind. Based upon data that BCG culture filtrate proteins, and the Ag85 series, protected mice against M. leprae challenge,76,79,82 Ohara and colleagues produced recombinant BCG strains that overexpress Ag85 complex components. Immunization of mice with rBCG which over-produce either the A or A/B components reduced the multiplication of M. leprae more than the vaccination with parental BCG.83,84 As with the human trials outlined above, provision of multiple doses of rBCG/85A to mice enhanced protection over that observed with a single dose.83 More recently, Maeda and colleagues have created a rBCG that secretes M. leprae major membrane protein (MMP)-II (also known as bacterioferritin; ML2038). This protein was identified as an immunodominant antigen using T cells from PB leprosy patients.85 Compared with parental BCG, the rBCG strain (BCG-SM) induces more potent Th1 immune responses86,87 and accordingly, this group recently reported that rBCG-SM inhibits the multiplication of M. leprae in the footpads of challenged mice more efficiently than control BCG.88
A major and potentially prohibitive influence over the use of any live rBCG vaccination is the apparent inability to boost the antigen-specific responses of individuals who have successfully been primed with current BCG strains. rBCG might therefore be best deployed in previously non-BCG immunized populations to prime the immune response, allowing heterologous boosting of the response at a later date if necessary. Concerns also abound regarding the use of live vaccines when the worldwide population living with immune suppression, through malnutrition or infection, is rising. HIV positive infants in South Africa have been found to be at a far higher risk of disseminated BCG infection than HIV negative counterparts and a high prevalence of BCG complications in children on HAART has also been reported.89-91
Toward a defined sub-unit vaccine for leprosy
The ideal vaccine against leprosy would induce strong, long-lasting T cell responses directed against M. leprae that would both prevent disease and reduce bacterial transmission. A defined sub-unit vaccine would appear well suited to provide a long-lasting line of protection but is still lacking. Selection and production of recombinant antigens has been simplified by the completion and publication of the M. leprae genome in 2001, and we believe a sub-unit vaccine for leprosy is attainable.92
Control of bacterial growth by PB patients indicates that these individuals mount a strong, but not necessarily curative, immune response against M. leprae. M. leprae infection does not always cause disease, and it is estimated that anywhere between 30–75% of infections are spontaneously cleared without causing significant symptoms.93,94 It is our belief that identifying antigens that are the targets of this response is the key to effective vaccination against leprosy. Within an American Leprosy Missions-supported project IDRI has identified numerous antigens that are recognized by PB leprosy patients and stimulate IFNγ secretion.95,96 We are currently in the process of identifying additional antigens and assessing the vaccine potential of several of these by experimental vaccination and infection of mice.
The transition from pre-clinical evaluation in animals to clinical trials in humans represents a hurdle for all vaccines. Armadillos can be naturally and experimentally infected with M. leprae, and develop a clinical and histopathological spectrum of leprosy similar to that observed in man. NHDP has pioneered the use of armadillos to investigate M. leprae-induced nerve damage, and with the ongoing description of the armadillo genome and immune system.97-101 Despite restrictions regarding reagent availability and time of infection, the armadillo infection model appears well suited for vaccine evaluations. The mouse footpad model supports M. leprae growth but does not replicate the nerve damage that is a common feature in leprosy patients.102 Regardless, the mouse footpad model has been used extensively to evaluate drug regimen and the emergence of drug resistance and does appear suitable for preliminary evaluation of vaccines. Thus, most vaccine testing has been performed using the mouse footpad model of M. leprae infection, using bacterial growth as the experimental endpoint. These experiments can take considerable time to yield results due to the remarkably slow growth of M. leprae. Over the last few years our groups have endeavored to find more rapid methods to identify potential vaccines (or exclude ineffective immunogens) before embarking on long-term mouse footpad studies.
Investigators at NHDP have employed a modified lepromin test to evaluate potential vaccines. Results demonstrate that, when compared with non-vaccinated mice, a significant influx of CD4+ cells occurs in the footpads of HKML vaccinated mice 4 weeks after M. leprae challenge. More recent studies have identified antigens that can also support this influx upon challenge (unpublished observations). When results from these short-term experiments are compared with results obtained from long-term M. leprae growth experiments involving a low dose challenge, antigens that support early cell infiltrations also reduce M. leprae growth. An additional breakthrough that could expedite vaccine testing in mice is the use of sensitive real-time PCR methods to determine bacterial burden rather than microscopic detection which has poor sensitivity.103
Investigators at IDRI have reduced the amount of time required to evaluate treatments by performing M. leprae infections in the ear and using the surrogate read out of draining lymph node (DLN) cellularity to determine the status of infection. We determined that following M. leprae infection there is a progressive infiltration of T cells at the infection site and that this infiltration is supported by a concomitant increase in DLN cellularity. These alterations did not occur when killed M. leprae was inoculated and were prevented by rifampicin treatment, indicating that the changes are driven by actual infection and can be used to assess vaccines.104 We were extremely surprised by our recent experiments to find that despite experimental vaccination inducing strong and transferable antigen-specific Th1 responses that could limit inflammation at the infection site, it did not reduce bacterial burden in the mouse footpad system.105
Although we do not currently know how to reconcile these differences, these data indicate that, despite the absence of control of bacterial burden, local inflammation can be controlled. This outcome would be highly beneficial to individual patients, as a common complication of leprosy is uncontrolled inflammatory reactions (reversal reactions and ENL) that cause significant distress and can occasionally result in hospitalization. At a population level, this outcome would likely not impact M. leprae transmission and would have a limited effect on new case numbers.
Where and How?
The largest number of leprosy cases are currently found in Brazil and India.9 With respective populations of 200 and 1150 million, it is difficult to perceive the implementation of widespread prophylactic vaccination campaigns solely for the prevention of leprosy. Leprosy is not evenly spread across populations and regions of higher incidence rates have been identified.5 It is conceivable that localized vaccination campaigns could be implemented. To date, most leprosy vaccine trials have been conducted in leprosy hyperendemic regions, have recruited large numbers (more 150,000 in the south India trail, for example) or have used contacts of recognized patients. Targeting of vaccination to at-risk individuals could be performed on the basis of known risk factors. It is well documented that contacts of MB patients have the highest risk of developing leprosy themselves.2-4 The selection of close contacts of patients for clinical trials appears to be the most tenable strategy to provide the power required to interpret incidence rates in vaccine vs. placebo groups.
Both experimental and observational studies generally find leprosy incidence rates above historic levels, an affect that can be attributed to active case finding and increased awareness of leprosy within the study population. For example, a recent large scale active case finding study involving clinical exam of 17,862 residents in northwest Bangladesh indicates that true prevalence rates in the region may be 6-fold higher than those being reported by traditional methods.106 Those findings are consistent with previous reports in which active case finding returned much higher prevalence rates than those being reported.5,107 Discrepancies in the degree of protection afforded by BCG vaccination between experimental and observational studies suggest that, due to variance in year to year leprosy incidence rates, experimental studies are better suited to distinguish protective vaccines.50 That said, observational studies yield results more quickly and do not deprive subjects of what could be protective measures, and chemoprophylaxis trials have been shown to reduce new leprosy incidence rates in the short-term.108-111
An alternative to current protracted MDT regimens would be to include a therapeutic vaccine, or immune therapy, in parallel with antibiotics. Provision of potent memory immune responses by a vaccine could confer active, long-term protection and reduce relapse rates even if short, non-sterilizing MDT regimen are provided. Applying a vaccine as part of a therapeutic treatment may represent an attractive development strategy, requiring fewer recruits and providing a well-defined population in which to track effects.
Potential complications due to vaccination
Approximately 50% of leprosy patients naturally develop acute inflammatory processes known as either type 1 (“reversal reactions”) or type 2 (“erythema nodosum leprosum”; ENL) reactions. Reactions often require hospitalization to prevent severe injury or in rare instances death.112 Although the factors that trigger type 1 reversal reactions remain unknown, some clinicians and researchers fear that immunization to boost inflammatory T cell responses will induce reactions. Various environmental factors may, but have not yet been confirmed to, be associated with the onset of type 1 reactions. Type 1 reversal reactions have, in some circumstances, been observed to follow immunization with other mycobacteria.113,114 Direct injection of IL-2 and IFNγ into leprosy lesions does not induce type 1 reactions.115,116 Reversal reactions are ameliorated using either corticosteroids or thalidomide.117 For type 2 reactions, other infections or viral illness, fever, immunization, and psychological stress have all been invoked, but no convincing evidence has supported any of these as definitive triggers. Prolonged intradermal IFNγ treatment of LL patients does increase the risk of type 2 reactions.115,116 The T cell inhibitor, cyclosporine, has been used to treat ENL with varied results and generally with success only in treatment of severe cases.118,119 These data suggest that T cell functions may not be a major feature of most type 2 reactions, and that immunization with T cell-based vaccines should not induce type 2 reactions. Taken together, these observations suggest that vaccination of uninfected individuals, individuals with sub-clinical infection or even patients with low BI, is likely to be safe and manageable. Indeed, it is common practice in some countries to re-immunize leprosy patients and their close contacts with BCG, even though the efficacy of this BCG re-immunization is debated.50,69,70 Vaccination of MB patients, particularly those with high BI, may be better considered as a dual therapy, with close observation, in conjunction with MDT.
The concept of using a vaccine in conjunction with chemotherapy for treatment of leprosy is not unique and has already been studied. Talwar and colleagues conducted a trial in which, in addition to chemotherapy of MB patients, immunotherapy was provided every 3 mo with an autoclaved M. w vaccine and contrasted with placebo injections.120 More rapid bacterial clearance occurred in the M. w group and was accompanied by distinct signs of clinical improvement. One hundred percent of BB, 85.7% of BL patients and 61.5% of LL patients converted to lepromin positivity after four doses of the vaccine and a significant number of vaccinated patients demonstrated an upgrading in skin lesions histopathologically.
Katoch and colleagues conducted a study involving 36 previously untreated, high BI BL/LL cases who were allocated to three treatment groups.121 All individuals received a modified MDT regimen, but in addition, one control group received distilled water, one group received BCG 0.1 mg per dose and one group received 2 x 108 killed M. w per dose, every 6 mo, until negative smears were obtained. The vaccines were well tolerated and the incidence of reactions was the same in all the groups during the first 2 y. Patients of the control group, however, continued to have reactions up to 3 y. While the patients in the control group took 5 y to become smear negative, all the patients in BCG group were smear negative by 3.5 y and those in the M. w group by 3 y. Viable bacteria (assessed by outgrowth in mouse footpads) were detected in patients on MDT alone up to 24 mo of therapy, whereas viable bacilli could not be detected in the two immunotherapy groups after 12 mo. Patients in both the immunotherapy groups showed accelerated granuloma clearance, histological upgrading and non-specific healing without granuloma formation compared with the control group. Thus, rather than causing reactions, the addition of immunotherapy actually reduced the time period of reactions by 33% and reduced the effective treatment period of achieving bacterial clearance by about 40%.
A similar study conducted was conducted in Chandigarh, India, with the distinction that MDT was provided for only 12 mo.122 Sixty untreated leprosy patients with a BI = 2 were randomly allocated to three treatment groups. Vaccine was either saline, intradermal BCG (105 live bacilli/per dose), or M. w (killed bacilli (1 x 108 as the first dose and 0.5 x 108 /dose in subsequent doses) and was administered at three monthly intervals for 4 total doses. By 12 and 24 mo, the patients in BCG group demonstrated a significantly greater reduction in clinical score compared with those in the M. w group, with both the BCG and M. w groups showing reduced clinical scores compared with the saline control group. BI declined by 2.40 units/year in patients receiving BCG, 2.05 units/year in the M. w group and 0.85 units/year in the control group. Although the incidence of type 1 reactions was marginally increased (although not significantly) in the BCG and M. w vaccinated groups, the incidence of type 2 reactions, neuritis and development of new deformities was decreased compared with the controls.
The safety of M. habana vaccination was assessed by Wakhlu and colleagues by carrying out intradermal vaccination of 31 LL leprosy cases (each received 1.5 mg = 6.27 x 108 M. habana bacilli) and 36 household contacts, who randomly received escalating doses of 1.5, 2.0, 2.5 mg vaccine.68 Despite stimulating specific cell-mediated immunity against M. leprae, as evidenced by increased lepromin reactivity, M. habana vaccination did not cause any additional adverse reactions in patients. Systemic side-effects were not observed at greater frequency than reported in other vaccine trials and systemic side-effects were easily controlled and were not accompanied by clinically detectable nerve or ocular damage.
These studies indicate that in leprosy patients with high BI cellular responses can be induced without exacerbating disease. While these trials provide some solace for the testing of new vaccines in MB patients, they do not provide any guarantees that sub-unit vaccines will not induce reactions. Pre-clinical evaluation of leprosy vaccines in animal models that present with inflammation at the infection site, such as the ear infection model, suggest that these vaccines may exacerbate inflammation.104 Unfortunately, mice do not develop nerve damage during experimental M. leprae infection and therefore do not lend themselves to resolving concerns over potential nerve damage. It may be that vaccine testing in M. leprae-infected armadillos, which can develop perturbations in nerve function and nerve damage, could resolve these ongoing safety concerns.
Assessing vaccine safety
A primary concern when establishing any vaccine is safety. If it is to be used as an adjunct to MDT, which appears to be the simplest way of developing and adopting any leprosy vaccine, it is possible that patients will undergo reversal reactions. The identification of surrogate biomarkers that could predict adverse events would be highly beneficial. Stefani recently assessed 27 plasma factors in multiplex assays to determine if any were indicators of onset of reactions.123 Compared with control patients who had no reactional episodes, in reversal reaction patients significant elevations of CXCL10 (IP-10) and IL-6 were observed, and in ENL patients significant elevations of IL-6, IL-7 and PDGF-BB were observed. Some of these markers were also found during an independent study conducted in India.124 Although it remains unclear how rapidly these cytokines become elevated before a reaction becomes clinically relevant, regular analysis of plasma for perturbations of these cytokines following immunotherapy may be prudent to provide optimal patient care.
How to interpret protective vaccination – correlates of protection?
Interpreting the protective efficacy of a vaccine against leprosy is likely to represent a further hurdle before widespread implementation. Although it should be relatively simple to demonstrate that vaccinated individuals have been primed and boosted, what this actually means in the context of infection/ disease is far more difficult to determine. Past studies have relied either on long-term follow-up and comparison of new case detection between unvaccinated and vaccinated groups, or on skin slit smears and biopsy to determine how vaccines have affected bacterial burden and histological responses. These techniques are invasive for the patient, decreasing willingness to participate. Furthermore, these techniques require a significant degree of skill and are time consuming for both the clinician and the pathologist. Changes in viable bacterial burden are most likely the best indicator of treatment outcome, but surrogate endpoints predictive of response could significantly shorten trials and expedite the adoption of new vaccines. The identification of simple biomarkers that could replace, reduce or negate the need for such invasive procedures would also make vaccine trials more tractable over larger populations.
The identification of potential correlates of protection for use in vaccine trials is hampered somewhat due to the relative paucity of publications regarding the immune response of leprosy patients during treatment. The IgM response against M. leprae PGL-I correlates with bacterial burden at time of diagnosis and the response declines during treatment.125-130 The IgG responses to the 35kD, Ag85A and Ag85B proteins similarly decline during treatment.131,132 We have endeavored to identify additional biomarkers that can be used objectively within trails to determine outcome. We have reported waxing and waning IgG responses against multiple protein antigens, with an indication that the anti-protein IgG responses may provide improved discrimination in comparison with anti-PGL-I antibodies.10,133 Circulating levels of the human enzyme β-glucuronidase were found to be significantly higher in BB/BL children compared with healthy children and children with other skin diseases, and MDT resulted in a significant fall in the leprosy cases.134 Similarly, nitrite concentrations, which are found at high serum concentrations in untreated MB leprosy patients, drop drastically with treatment.129 We have also identified multiple antigens that are recognized by leprosy patient T cells, although it is currently unclear how or if their responses are affected during treatment.96,123
While there has been relatively little effort to identify markers of cure for leprosy, this is an area of great interest for tuberculosis researchers in their quest for new and improved treatments.135-138 To date, the optimum markers for tuberculosis remain undefined, but several promising candidates have been indicated. It is noteworthy that as with leprosy patients having reversal reactions, tuberculosis patients have elevated circulating levels of CXCL10, with treatment leading to a reduction of CXCL10.139 While antibodies against ESAT-6, FdxA, LAM, Rv2626c and 38kD antigen were more abundant in untreated tuberculosis patients than in controls, serial plasma samples obtained after initiation of chemotherapy indicates that antibody levels against ESAT-6 and Rv2626c decrease during therapy whereas antibody levels to the 38kD antigen and LAM increase.140 Cell-based assays with short incubation periods (termed IFNγ release assays; IGRAs) have tended to demonstrate high antigen-specific IFNγ levels at diagnosis that decline with treatment. In one study, ESAT-6-specific IGRA responses were shown to have declined by 3 mo of treatment in all of 13 patients demonstrating an adequate clinical response to treatment, but to remain elevated in 5 patients with treatment failure.141 A more recent study demonstrated an overall decline in the IFNγ response to ESAT-6, but not typically a conversion to negativity, of latently infected tuberculosis patients treated with isoniazid.142 In another study, examining a group of recently tuberculosis-exposed schoolchildren, isoniazid preventive therapy reduced the frequency of IFNγ-producing T cells responding to ESAT-6 or CFP-10 by an average of 68% within a year.143 It seems likely that active tuberculosis gives rise to increased numbers of primed or partially activated T cells in the circulation, and that these cells are preferentially detected by short incubation IGRAs. As infection is cleared, these cells, and the response in IGRA, appear to decline.144 These studies indicate that assessment of T cell responses are used as a biomarker, quantitative assessments should be made on an individual basis. Thus, an improved understanding of how successful, and even unsuccessful, treatment affects antigen-specific responses of leprosy patients could help identify markers that could be used to expedite the introduction of a leprosy vaccine.
Summary
The current global leprosy control program, based on passive case detection and chemotherapy, suffers from common pitfalls inherent in controlling most infectious diseases. Passive case detection with slow, chronic diseases like leprosy allows for continued transmission during the preclinical stages of the disease and any treatment strategy short of directly observed chemotherapy is likely destined to yield incomplete treatment leaving it susceptible to the selection of drug resistant mutants. New tools are needed to close this gap in our strategy to continue to improve leprosy control and build upon recent breakthroughs. Among these are the deployment of simplified diagnostic tests that could improve and facilitate integration of leprosy control programs within general health services and the development of methods to track and evaluate drug resistance, but it is our belief that the introduction of a defined vaccine with the ability to limit the spread of M. leprae infection would have the greatest impact.
Acknowledgments
Leprosy vaccine research in the investigators laboratories is supported by the American Leprosy Missions.
Glossary
Abbreviations:
- BI
bacterial indices
- BB
borderline borderline
- BL
borderline lepromatous
- BT
borderline tuberculoid
- DLN
draining lymph node
- EC
endemic control
- FIA
Freund’s incomplete adjuvant
- HHC
healthy household contact
- LID
leprosy IDRI diagnostic
- LL
lepromatous leprosy
- MB
multibacillary
- MDT
multi-drug therapy
- PB
paucibacillary
- TB
tuberculosis
- TLR
Toll-like Receptor
- PGL
phenolic glycolipid
- TB
tuberculosis
- TT
tuberculoid
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/16848
References
- 1.WHO Global leprosy situation, 2010. Wkly Epidemiol Rec. 2010;85:337–48. [PubMed] [Google Scholar]
- 2.Bakker MI, Hatta M, Kwenang A, Van Mosseveld P, Faber WR, Klatser PR, et al. Risk factors for developing leprosy–a population-based cohort study in Indonesia. Lepr Rev. 2006;77:48–61. [PubMed] [Google Scholar]
- 3.Moet FJ, Pahan D, Schuring RP, Oskam L, Richardus JH. Physical distance, genetic relationship, age, and leprosy classification are independent risk factors for leprosy in contacts of patients with leprosy. J Infect Dis. 2006;193:346–53. doi: 10.1086/499278. [DOI] [PubMed] [Google Scholar]
- 4.Goulart IM, Bernardes Souza DO, Marques CR, Pimenta VL, Goncalves MA, Goulart LR. Risk and protective factors for leprosy development determined by epidemiological surveillance of household contacts. Clin Vaccine Immunol. 2008;15:101–5. doi: 10.1128/CVI.00372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker MI, Hatta M, Kwenang A, Klatser PR, Oskam L. Epidemiology of leprosy on five isolated islands in the Flores Sea, Indonesia. Trop Med Int Health. 2002;7:780–7. doi: 10.1046/j.1365-3156.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–73. [PubMed] [Google Scholar]
- 7.Scollard DM. Classification of leprosy: a full color spectrum, or black and white? Int J Lepr Other Mycobact Dis. 2004;72:166–8. doi: 10.1489/1544-581X(2004)072<0166:COLAFC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Joyce MP, Scollard DM. Leprosy (Hansen's Disease) In: Conn's Current Therapy. Edited by Bope ET: Saunders; 2004: 100-105. [Google Scholar]
- 9.WHO Global leprosy situation, 2009. Wkly Epidemiol Rec. 2009;84:333–40. [PubMed] [Google Scholar]
- 10.Duthie MS, Hay MN, Rada EM, Convit J, Ito L, Oyafuso LK, et al. Specific IgG antibody responses may be used to monitor leprosy treatment efficacy and as recurrence prognostic markers Eur J Clin Microbiol Infect Dis 2011. [DOI] [PubMed]
- 11.Gelber RH, Balagon VF, Cellona RV. The relapse rate in MB leprosy patients treated with 2-years of WHO-MDT is not low. Int J Lepr Other Mycobact Dis. 2004;72:493–500. doi: 10.1489/1544-581X(2004)72<493:TRRIML>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Honrado ER, Tallo V, Balis AC, Chan GP, Cho SN. Noncompliance with the world health organization-multidrug therapy among leprosy patients in Cebu, Philippines: its causes and implications on the leprosy control program. Dermatol Clin. 2008;26:221–9. doi: 10.1016/j.det.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Roche PW, Neupane KD, Failbus SS, Butlin CR. Dapsone drug resistance in the MDT era. Int J Lepr Other Mycobact Dis. 2000;68:323–5. [PubMed] [Google Scholar]
- 14.Ellard GA, Pannikar VK, Jesudasan K, Christian M. Clofazimine and dapsone compliance in leprosy. Lepr Rev. 1988;59:205–13. doi: 10.5935/0305-7518.19880026. [DOI] [PubMed] [Google Scholar]
- 15.Chen XS, Li WZ, Jiang C, Ye GY. Studies on risk of leprosy relapses in China: relapses after treatment with multidrug therapy. Int J Lepr Other Mycobact Dis. 1999;67:379–87. [PubMed] [Google Scholar]
- 16.Chen XS, Li WZ, Jiang C, Ye GY. Studies on risk of leprosy relapses in China: relapses after treatment with dapsone monotherapy. Int J Lepr Other Mycobact Dis. 1999;67:371–8. [PubMed] [Google Scholar]
- 17.Cellona RV, Balagon MF, dela Cruz EC, Burgos JA, Abalos RM, Walsh GP, et al. Long-term efficacy of 2 year WHO multiple drug therapy (MDT) in multibacillary (MB) leprosy patients. Int J Lepr Other Mycobact Dis. 2003;71:308–19. doi: 10.1489/1544-581X(2003)071<0308:LEOYWM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Girdhar BK, Girdhar A, Kumar A. Relapses in multibacillary leprosy patients: effect of length of therapy. Lepr Rev. 2000;71:144–53. doi: 10.5935/0305-7518.20000017. [DOI] [PubMed] [Google Scholar]
- 19.Norman G, Joseph G, Richard J. Relapses in multibacillary patients treated with multi-drug therapy until smear negativity: findings after twenty years. Int J Lepr Other Mycobact Dis. 2004;72:1–7. doi: 10.1489/1544-581X(2004)072<0001:RIMPTW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Ji B, Jamet P, Sow S, Perani EG, Traore I, Grosset JH. High relapse rate among lepromatous leprosy patients treated with rifampin plus ofloxacin daily for 4 weeks. Antimicrob Agents Chemother. 1997;41:1953–6. doi: 10.1128/aac.41.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cambau E, Bonnafous P, Perani E, Sougakoff W, Ji B, Jarlier V. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin Infect Dis. 2002;34:39–45. doi: 10.1086/324623. [DOI] [PubMed] [Google Scholar]
- 22.Maeda S, Matsuoka M, Nakata N, Kai M, Maeda Y, Hashimoto K, et al. Multidrug resistant Mycobacterium leprae from patients with leprosy. Antimicrob Agents Chemother. 2001;45:3635–9. doi: 10.1128/AAC.45.12.3635-3639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka M, Kashiwabara Y, Liangfen Z, Goto M, Kitajima S. A second case of multidrug-resistant Mycobacterium leprae isolated from a Japanese patient with relapsed lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2003;71:240–3. doi: 10.1489/1544-581X(2003)71<240:ASCOMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Matsuoka M, Kashiwabara Y, Namisato M. A Mycobacterium leprae isolate resistant to dapsone, rifampin, ofloxacin and sparfloxacin. Int J Lepr Other Mycobact Dis. 2000;68:452–5. [PubMed] [Google Scholar]
- 25.Grosset JH, Guelpa-Lauras CC, Bobin P, Brucker G, Cartel JL, Constant-Desportes M, et al. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int J Lepr Other Mycobact Dis. 1989;57:607–14. [PubMed] [Google Scholar]
- 26.Williams DL, Gillis TP. Molecular detection of drug resistance in Mycobacterium leprae. Lepr Rev. 2004;75:118–30. [PubMed] [Google Scholar]
- 27.Matsuoka M, Budiawan T, Aye KS, Kyaw K, Tan EV, Cruz ED, et al. The frequency of drug resistance mutations in Mycobacterium leprae isolates in untreated and relapsed leprosy patients from Myanmar, Indonesia and the Philippines. Lepr Rev. 2007;78:343–52. [PubMed] [Google Scholar]
- 28.Manjunatha UH, Lahiri R, Randhawa B, Dowd CS, Krahenbuhl JL, Barry CE. Mycobacterium leprae is naturally resistant to PA-824. Antimicrob Agents Chemother. 2006;50:3350–4. doi: 10.1128/AAC.00488-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You EY, Kang TJ, Kim SK, Lee SB, Chae GT. Mutations in genes related to drug resistance in Mycobacterium leprae isolates from leprosy patients in Korea. J Infect. 2005;50:6–11. doi: 10.1016/j.jinf.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Meeting on Sentinel Surveillance for Drug Resistance in Leprosy In: WHO Technical Report Series. Geneva: World Health Organization; 2011. [Google Scholar]
- 31.Gelber RH. Chemotherapy of lepromatous leprosy: recent developments and prospects for the future. Eur J Clin Microbiol Infect Dis. 1994;13:942–52. doi: 10.1007/BF02111496. [DOI] [PubMed] [Google Scholar]
- 32.Gelber RH, Murray LP, Siu P, Tsang M, Rea TH. Efficacy of minocycline in single dose and at 100 mg twice daily for lepromatous leprosy. Int J Lepr Other Mycobact Dis. 1994;62:568–73. [PubMed] [Google Scholar]
- 33.Gelber RH, Siu P, Tsang M, Richard V, Chehl SK, Murray LP. Activity of combinations of dapsone, rifampin, minocycline, clarithromycin, and sparfloxacin against M. leprae-infected mice. Int J Lepr Other Mycobact Dis. 1995;63:259–64. [PubMed] [Google Scholar]
- 34.Ji B, Grosset J. Ofloxacin for the treatment of leprosy. Acta Leprol. 1991;7:321–6. [PubMed] [Google Scholar]
- 35.Ji B, Grosset J. Combination of rifapentine-moxifloxacin-minocycline (PMM) for the treatment of leprosy. Lepr Rev. 2000;71(Suppl):S81–7. doi: 10.5935/0305-7518.20000074. [DOI] [PubMed] [Google Scholar]
- 36.Ji B, Perani EG, Petinom C, N'Deli L, Grosset JH. Clinical trial of ofloxacin alone and in combination with dapsone plus clofazimine for treatment of lepromatous leprosy. Antimicrob Agents Chemother. 1994;38:662–7. doi: 10.1128/aac.38.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Rojas V, Hernandez O, Gil R. Some factors influencing delay in leprosy diagnosis. Bull Pan Am Health Organ. 1994;28:156–62. [PubMed] [Google Scholar]
- 38.Chen XS, Li WZ, Jiang C, Ye GY. Leprosy in China: delay in the detection of cases. Ann Trop Med Parasitol. 2000;94:181–8. doi: 10.1080/00034980057527. [DOI] [PubMed] [Google Scholar]
- 39.Deps PD, Guedes BV, Bucker Filho J, Andreatta MK, Marcari RS, Rodrigues LC. Delay in the diagnosis of leprosy in the Metropolitan Region of Vitoria, Brazil. Lepr Rev. 2006;77:41–7. [PubMed] [Google Scholar]
- 40.Lockwood DN, Reid AJ. The diagnosis of leprosy is delayed in the United Kingdom. QJM. 2001;94:207–12. doi: 10.1093/qjmed/94.4.207. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui MR, Velidi NR, Pati S, Rath N, Kanungo AK, Bhanjadeo AK, et al. Integration of leprosy elimination into primary health care in orissa, India. PLoS ONE. 2009;4:e8351. doi: 10.1371/journal.pone.0008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira J, Mengue SS, Wagner MB, Duncan BB. Estimating hidden prevalence in Hansen's disease through diagnosis delay and grade of disability at time of diagnosis. Int J Lepr Other Mycobact Dis. 2000;68:464–73. [PubMed] [Google Scholar]
- 43.Nicholls PG, Croft RP, Richardus JH, Withington SG, Smith WC. Delay in presentation, an indicator for nerve function status at registration and for treatment outcome–the experience of the Bangladesh Acute Nerve Damage Study cohort. Lepr Rev. 2003;74:349–56. [PubMed] [Google Scholar]
- 44.Van Veen NH, Meima A, Richardus JH. The relationship between detection delay and impairment in leprosy control: a comparison of patient cohorts from Bangladesh and Ethiopia. Lepr Rev. 2006;77:356–65. [PubMed] [Google Scholar]
- 45.Lockwood DN, Suneetha S. Leprosy: too complex a disease for a simple elimination paradigm. Bull World Health Organ. 2005;83:230–5. [PMC free article] [PubMed] [Google Scholar]
- 46.WHO Global leprosy situation, 2005. Wkly Epidemiol Rec. 2005;80:289–95. [PubMed] [Google Scholar]
- 47.WHO Global leprosy situation, 2007. Wkly Epidemiol Rec. 2007;82:225–32. [PubMed] [Google Scholar]
- 48.Meima A, Richardus JH, Habbema JD. Trends in leprosy case detection worldwide since 1985. Lepr Rev. 2004;75:19–33. [PubMed] [Google Scholar]
- 49.Meima A, Smith WC, van Oortmarssen GJ, Richardus JH, Habbema JD. The future incidence of leprosy: a scenario analysis. Bull World Health Organ. 2004;82:373–80. [PMC free article] [PubMed] [Google Scholar]
- 50.Setia MS, Steinmaus C, Ho CS, Rutherford GW. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect Dis. 2006;6:162–70. doi: 10.1016/S1473-3099(06)70412-1. [DOI] [PubMed] [Google Scholar]
- 51.Merle CS, Cunha SS, Rodrigues LC. BCG vaccination and leprosy protection: review of current evidence and status of BCG in leprosy control. Expert Rev Vaccines. 2010;9:209–22. doi: 10.1586/erv.09.161. [DOI] [PubMed] [Google Scholar]
- 52.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 53.Zodpey SP, Bansod BS, Shrikhande SN, Maldhure BR, Kulkarni SW. Protective effect of Bacillus Calmette Guerin (BCG) against leprosy: a population-based case-control study in Nagpur, India. Lepr Rev. 1999;70:287–94. doi: 10.5935/0305-7518.19990032. [DOI] [PubMed] [Google Scholar]
- 54.Zodpey SP, Ambadekar NN, Thakur A. Effectiveness of Bacillus Calmette Guerin (BCG) vaccination in the prevention of leprosy: a population-based case-control study in Yavatmal District, India. Public Health. 2005;119:209–16. doi: 10.1016/j.puhe.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Rodrigues LC, Kerr-Pontes LR, Frietas MV, Barreto ML. Long lasting BCG protection against leprosy. Vaccine. 2007;25:6842–4. doi: 10.1016/j.vaccine.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 56.Shepard CC, van Landingham R, Walker LL. Searches among mycobacterial cultures for antileprosy vaccines. Infect Immun. 1980;29:1034–9. doi: 10.1128/iai.29.3.1034-1039.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shepard CC, van Landingham RM, Walker LL, Ye SZ. Comparison of the immunogenicity of vaccines prepared from viable Mycobacterium bovis BCG, heat-killed Mycobacterium leprae, and a mixture of the two for normal and M. leprae-tolerant mice. Infect Immun. 1983;40:1096–103. doi: 10.1128/iai.40.3.1096-1103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Convit J, Sampson C, Zuniga M, Smith PG, Plata J, Silva J, et al. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet. 1992;339:446–50. doi: 10.1016/0140-6736(92)91056-E. [DOI] [PubMed] [Google Scholar]
- 59.KarongaPreventionTrialGroup Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996;348:17–24. doi: 10.1016/S0140-6736(96)02166-6. [DOI] [PubMed] [Google Scholar]
- 60.Pönnighaus JM, Fine PE, Sterne JA, Wilson RJ, Msosa E, Gruer PJ, et al. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339:636–9. doi: 10.1016/0140-6736(92)90794-4. [DOI] [PubMed] [Google Scholar]
- 61.Gupte MD, Vallishayee RS, Anantharaman DS, Nagaraju B, Sreevatsa, Balasubramanyam S, et al. Comparative leprosy vaccine trial in south India. Indian J Lepr. 1998;70:369–88. [PubMed] [Google Scholar]
- 62.Sreevatsa, Desikan KV. Evaluation of the efficacy of candidate vaccines against M. leprae infection in mice. Indian J Lepr. 1988;60:252–9. [PubMed] [Google Scholar]
- 63.Bhide MB, Pradhan KS, Bapat CV. A vaccine from ICRC bacilli against M. leprae infection in mouse foot-pad. Lepr India. 1978;50:334–44. [PubMed] [Google Scholar]
- 64.Sharma P, Mukherjee R, Talwar GP, Sarathchandra KG, Walia R, Parida SK, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76:127–43. [PubMed] [Google Scholar]
- 65.Truoc LV, Ly HM, Thuy NK, Trach DD, Stanford CA, Stanford JL. Vaccination against leprosy at Ben San Leprosy Centre, Ho Chi Minh City, Vietnam. Vaccine. 2001;19:3451–8. doi: 10.1016/S0264-410X(01)00052-4. [DOI] [PubMed] [Google Scholar]
- 66.Singh NB, Lowe AC, Rees RJ, Colston MJ. Vaccination of mice against Mycobacterium leprae infection. Infect Immun. 1989;57:653–5. doi: 10.1128/iai.57.2.653-655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh NB, Srivastava A, Gupta HP, Kumar A, Srivastava S. Induction of lepromin positivity in monkeys by a candidate antileprosy vaccine: Mycobacterium habana. Int J Lepr Other Mycobact Dis. 1991;59:317–20. [PubMed] [Google Scholar]
- 68.Wakhlu A, Gaur SP, Kaushal GP, Misra A, Asthana P, Sircar AR. Response of Mycobacterium habana vaccine in patients with lepromatous leprosy and their household contacts. A pilot clinical study. Lepr Rev. 2001;72:179–91. [PubMed] [Google Scholar]
- 69.Düppre NC, Camacho LA, da Cunha SS, Struchiner CJ, Sales AM, Nery JA, et al. Effectiveness of BCG vaccination among leprosy contacts: a cohort study. Trans R Soc Trop Med Hyg. 2008;102:631–8. doi: 10.1016/j.trstmh.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 70.Cunha SS, Alexander N, Barreto ML, Pereira ES, Dourado I, de Fatima Maroja M, et al. BCG Revaccination Does Not Protect Against Leprosy in the Brazilian Amazon: A Cluster Randomised Trial. PLoS Negl Trop Dis. 2008;2:e167. doi: 10.1371/journal.pntd.0000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.WHO Global tuberculosis programme and global programme on vaccines. Statement on BCG revaccination for the prevention of tuberculosis. Wkly Epidemiol Rec. 1995;70:229–31. [PubMed] [Google Scholar]
- 72.Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005;366:1290–5. doi: 10.1016/S0140-6736(05)67145-0. [DOI] [PubMed] [Google Scholar]
- 73.Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr (Rio J) 2006;82(Suppl):S45–54. doi: 10.2223/JPED.1499. [DOI] [PubMed] [Google Scholar]
- 74.Gelber RH, Brennan PJ, Hunter SW, Munn MW, Monson JM, Murray LP, et al. Effective vaccination of mice against leprosy bacilli with subunits of Mycobacterium leprae. Infect Immun. 1990;58:711–8. doi: 10.1128/iai.58.3.711-718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ngamying M, Sawanpanyalert P, Butraporn R, Nikasri J, Cho SN, Levy L, et al. Effect of vaccination with refined components of the organism on infection of mice with Mycobacterium leprae. Infect Immun. 2003;71:1596–8. doi: 10.1128/IAI.71.3.1596-1598.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naito M, Matsuoka M, Ohara N, Nomaguchi H, Yamada T. The antigen 85 complex vaccine against experimental Mycobacterium leprae infection in mice. Vaccine. 1999;18:795–8. doi: 10.1016/S0264-410X(99)00327-8. [DOI] [PubMed] [Google Scholar]
- 77.Ngamying M, Varachit P, Phaknilrat P, Levy L, Brennan PJ, Cho SN. Effects of vaccination with several mycobacterial proteins and lipoproteins on Mycobacterium leprae infection of the mouse. Int J Lepr Other Mycobact Dis. 2001;69:43–5. [PubMed] [Google Scholar]
- 78.Britton WJ, Martin E, Kamath AT, Neupane KD, Roche PW. Immunoprophylaxis against Mycobacterium leprae infection with subunit vaccines. Lepr Rev. 2000;71(Suppl):S176–81. [PubMed] [Google Scholar]
- 79.Roche PW, Neupane KD, Failbus SS, Kamath A, Britton WJ. Vaccination with DNA of the Mycobacterium tuberculosis 85B antigen protects mouse foot pad against infection with M. leprae. Int J Lepr Other Mycobact Dis. 2001;69:93–8. [PubMed] [Google Scholar]
- 80.Nomaguchi H, Mukai T, Takeshita F, Matsuoka M, Maeda Y, Aye TM, et al. Effect of hsp65 DNA vaccination carrying immunostimulatory DNA sequences (CpG motifs) against Mycobacterium leprae multiplication in mice. Int J Lepr Other Mycobact Dis. 2002;70:182–90. [PubMed] [Google Scholar]
- 81.Gelber RH, Mehra V, Bloom B, Murray LP, Siu P, Tsang M, et al. Vaccination with pure Mycobacterium leprae proteins inhibits M. leprae multiplication in mouse footpads. Infect Immun. 1994;62:4250–5. doi: 10.1128/iai.62.10.4250-4255.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuoka M, Nomaguchi H, Yukitake H, Ohara N, Matsumoto S, Mise K, et al. Inhibition of multiplication of Mycobacterium leprae in mouse foot pads by immunization with ribosomal fraction and culture filtrate from Mycobacterium bovis BCG. Vaccine. 1997;15:1214–7. doi: 10.1016/S0264-410X(97)00018-2. [DOI] [PubMed] [Google Scholar]
- 83.Ohara N, Matsuoka M, Nomaguchi H, Naito M, Yamada T. Inhibition of multiplication of Mycobacterium leprae in mouse foot pads by recombinant Bacillus Catmette-Guerin (BCG) Vaccine. 2000;18:1294–7. doi: 10.1016/S0264-410X(99)00420-X. [DOI] [PubMed] [Google Scholar]
- 84.Ohara N, Matsuoka M, Nomaguchi H, Naito M, Yamada T. Protective responses against experimental Mycobacterium leprae infection in mice induced by recombinant Bacillus Calmette-Guerin over-producing three putative protective antigen candidates. Vaccine. 2001;19:1906–10. doi: 10.1016/S0264-410X(00)00439-4. [DOI] [PubMed] [Google Scholar]
- 85.Maeda Y, Mukai T, Spencer J, Makino M. Identification of an Immunomodulating Agent from Mycobacterium leprae. Infect Immun. 2005;73:2744–50. doi: 10.1128/IAI.73.5.2744-2750.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makino M, Maeda Y, Ishii N. Immunostimulatory activity of major membrane protein-II from Mycobacterium leprae. Cell Immunol. 2005;233:53–60. doi: 10.1016/j.cellimm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Makino M, Maeda Y, Inagaki K. Immunostimulatory activity of recombinant Mycobacterium bovis BCG that secretes major membrane protein II of Mycobacterium leprae. Infect Immun. 2006;74:6264–71. doi: 10.1128/IAI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maeda Y, Tamura T, Matsuoka M, Makino M. Inhibition of the Multiplication of Mycobacterium leprae by Vaccination with a Recombinant BCG that Secretes Major Membrane Protein-II in Mice Clin Vaccine Immunol 2009. [DOI] [PMC free article] [PubMed]
- 89.Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, et al. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007;25:14–8. doi: 10.1016/j.vaccine.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 90.Hesseling AC, Johnson LF, Jaspan H, Cotton MF, Whitelaw A, Schaaf HS, et al. Disseminated bacille Calmette-Guerin disease in HIV-infected South African infants. Bull World Health Organ. 2009;87:505–11. doi: 10.2471/BLT.08.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nuttall JJ, Davies MA, Hussey GD, Eley BS. Bacillus Calmette-Guerin (BCG) vaccine-induced complications in children treated with highly active antiretroviral therapy. Int J Infect Dis. 2008;12:e99–105. doi: 10.1016/j.ijid.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 92.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, et al. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–11. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 93.Browne SG. Self-healing leprosy: report on 2749 patients. Lepr Rev. 1974;45:104–11. doi: 10.5935/0305-7518.19740012. [DOI] [PubMed] [Google Scholar]
- 94.Ekambaram V, Sithambaram M. Self-healing in non-lepromatous leprosy in the area of the ELEP Leprosy Control Project Dharmapuri (Tamil Nadu) Lepr India. 1977;49:387–92. [PubMed] [Google Scholar]
- 95.Duthie MS, Goto W, Ireton GC, Reece ST, Sampaio LH, Grassi AB, et al. Antigen-specific T-cell responses of leprosy patients. Clin Vaccine Immunol. 2008;15:1659–65. doi: 10.1128/CVI.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sampaio LH, Stefani MM, Oliveira RM, Sousa AL, Ireton GC, Reed SG, et al. Immunologically reactive M. leprae antigens with relevance to diagnosis and vaccine development. BMC Infect Dis. 2011;11:26. doi: 10.1186/1471-2334-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Job CK, Sanchez RM, Hunt R, Truman RW, Hastings RC. Armadillos (Dasypus novemcinctus) as a model to test antileprosy vaccines; a preliminary report. Int J Lepr Other Mycobact Dis. 1993;61:394–7. [PubMed] [Google Scholar]
- 98.Scollard DM. Endothelial cells and the pathogenesis of lepromatous neuritis:insights from the armadillo model. Microbes Infect. 2000;2:1835–43. doi: 10.1016/S1286-4579(00)01335-6. [DOI] [PubMed] [Google Scholar]
- 99.Scollard DM, Lathrop GW, Truman RW. Infection of distal peripheral nerves by M. leprae in infected armadillos; an experimental model of nerve involvement in leprosy. Int J Lepr Other Mycobact Dis. 1996;64:146–51. [PubMed] [Google Scholar]
- 100.Adams JE, Pena MT, Gillis TP, Williams DL, Adams LB, Truman RW. Expression of nine-banded armadillo (Dasypus novemcinctus) interleukin-2 in E. coli. Cytokine. 2005;32:219–25. doi: 10.1016/j.cyto.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 101.Peña MT, Adams JE, Adams LB, Gillis TP, Williams DL, Spencer JS, et al. Expression and characterization of recombinant interferon gamma (IFN-gamma) from the nine-banded armadillo (Dasypus novemcinctus) and its effect on Mycobacterium leprae-infected macrophages. Cytokine. 2008;43:124–31. doi: 10.1016/j.cyto.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shepard CC. The experimental disease that follows the injection of human leprosy bacilli into foot-pads of mice. J Exp Med. 1960;112:445–54. doi: 10.1084/jem.112.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Truman RW, Andrews PK, Robbins NY, Adams LB, Krahenbuhl JL, Gillis TP. Enumeration of Mycobacterium leprae Using Real-Time PCR. PLoS Negl Trop Dis. 2008;2:e328. doi: 10.1371/journal.pntd.0000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duthie MS, Reece ST, Lahiri R, Goto W, Raman VS, Kaplan J, et al. Antigen-specific cellular and humoral responses are induced by intradermal Mycobacterium leprae infection of the mouse ear. Infect Immun. 2007;75:5290–7. doi: 10.1128/IAI.00564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raman VS, O'Donnell J, Bailor HR, Goto W, Lahiri R, Gillis TP, et al. Vaccination with the ML0276 antigen reduces local inflammation but not bacterial burden during experimental Mycobacterium leprae infection. Infect Immun. 2009;77:5623–30. doi: 10.1128/IAI.00508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moet FJ, Schuring RP, Pahan D, Oskam L, Richardus JH. The prevalence of previously undiagnosed leprosy in the general population of northwest bangladesh. PLoS Negl Trop Dis. 2008;2:e198. doi: 10.1371/journal.pntd.0000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schreuder PA, Liben DS, Wahjuni S, Van Den Broek J, De Soldenhoff R. A comparison of Rapid Village Survey and Leprosy Elimination Campaign, detection methods in two districts of East Java, Indonesia, 1997/1998 and 1999/2000. Lepr Rev. 2002;73:366–75. [PubMed] [Google Scholar]
- 108.Bakker MI, Hatta M, Kwenang A, Van Benthem BH, Van Beers SM, Klatser PR, et al. Prevention of leprosy using rifampicin as chemoprophylaxis. Am J Trop Med Hyg. 2005;72:443–8. [PubMed] [Google Scholar]
- 109.Nguyen LN, Cartel JL, Grosset JH. Chemoprophylaxis of leprosy in the southern Marquesas with a single 25 mg/kg dose of rifampicin. Results after 10 years Lepr Rev 2000, 71 Suppl:S33-35; discussion S35-36. [DOI] [PubMed]
- 110.Diletto C, Blanc L, Levy L. Leprosy chemoprophylaxis in Micronesia Lepr Rev 2000, 71 Suppl:S21-23; discussion S24-25. [DOI] [PubMed]
- 111.Smith CM, Smith WC. Chemoprophylaxis is effective in the prevention of leprosy in endemic countries: a systematic review and meta-analysis. MILEP2 Study Group. Mucosal Immunology of Leprosy. J Infect. 2000;41:137–42. doi: 10.1053/jinf.2000.0698. [DOI] [PubMed] [Google Scholar]
- 112.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338–81. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilkinson RJ, Lockwood DN. Antigenic trigger for type 1 reaction in leprosy. J Infect. 2005;50:242–3. doi: 10.1016/j.jinf.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 114.Zaheer SA, Mukherjee R, Ramkumar B, Misra RS, Sharma AK, Kar HK, et al. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J Infect Dis. 1993;167:401–10. doi: 10.1093/infdis/167.2.401. [DOI] [PubMed] [Google Scholar]
- 115.Kaplan G, Britton WJ, Hancock GE, Theuvenet WJ, Smith KA, Job CK, et al. The systemic influence of recombinant interleukin 2 on the manifestations of lepromatous leprosy. J Exp Med. 1991;173:993–1006. doi: 10.1084/jem.173.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992;175:1729–37. doi: 10.1084/jem.175.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Girdhar BK, Girdhar A, Chakma JK. Advances in the treatment of reactions in leprosy. Indian J Lepr. 2007;79:121–34. [PubMed] [Google Scholar]
- 118.van Gompel A, van den Enden E, van den Ende J. Cyclosporin A is not very effective in erythema nodosum leprosum (ENL) Trop Geogr Med. 1994;46:331. [PubMed] [Google Scholar]
- 119.Uyemura K, Dixon JF, Wong L, Rea TH, Modlin RL. Effect of cyclosporine A in erythema nodosum leprosum. J Immunol. 1986;137:3620–3. [PubMed] [Google Scholar]
- 120.Talwar GP, Zaheer SA, Mukherjee R, Walia R, Misra RS, Sharma AK, et al. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine. 1990;8:121–9. doi: 10.1016/0264-410X(90)90134-8. [DOI] [PubMed] [Google Scholar]
- 121.Katoch K, Katoch VM, Natrajan M, Sreevatsa, Gupta UD, Sharma VD, et al. 10-12 years follow-up of highly bacillated BL/LL leprosy patients on combined chemotherapy and immunotherapy. Vaccine. 2004;22:3649–57. doi: 10.1016/j.vaccine.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 122.Narang T, Kaur I, Kumar B, Radotra BD, Dogra S. Comparative evaluation of immunotherapeutic efficacy of BCG and mw vaccines in patients of borderline lepromatous and lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2005;73:105–14. [PubMed] [Google Scholar]
- 123.Stefani MM, Guerra JG, Sousa AL, Costa MB, Oliveira ML, Martelli CT, et al. Potential plasma markers of type 1 and type 2 leprosy reactions: a preliminary report. BMC Infect Dis. 2009;9:75. doi: 10.1186/1471-2334-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scollard DM, Chaduvula MV, Martinez A, Fowlkes N, Nath I, Stryjewska BM, et al. Increased CXCL10 Levels and Gene Expression in Type 1 Leprosy Reactions Clinical and vaccine immunology: CVI 2011. [DOI] [PMC free article] [PubMed]
- 125.Miller RA, Gorder D, Harnisch JP. Antibodies to phenolic glycolipid-I during long-term therapy: serial measurements in individual patients. Int J Lepr Other Mycobact Dis. 1987;55:633–6. [PubMed] [Google Scholar]
- 126.Chanteau S, Cartel JL, Celerier P, Plichart R, Desforges S, Roux J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int J Lepr Other Mycobact Dis. 1989;57:735–43. [PubMed] [Google Scholar]
- 127.Meeker HC, Schuller-Levis G, Fusco F, Giardina-Becket MA, Sersen E, Levis WR. Sequential monitoring of leprosy patients with serum antibody levels to phenolic glycolipid-I, a synthetic analog of phenolic glycolipid-I, and mycobacterial lipoarabinomannan. Int J Lepr Other Mycobact Dis. 1990;58:503–11. [PubMed] [Google Scholar]
- 128.Prakash K, Sehgal VN, Aggarwal R. Evaluation of phenolic glycolipid-I (PGL-I) antibody as a multidrug therapy (MDT) monitor. J Dermatol. 1993;20:16–20. doi: 10.1111/j.1346-8138.1993.tb03822.x. [DOI] [PubMed] [Google Scholar]
- 129.Rada E, Ulrich M, Aranzazu N, Rodriguez V, Centeno M, Gonzalez I, et al. A follow-up study of multibacillary Hansen's disease patients treated with multidrug therapy (MDT) or MDT + immunotherapy (IMT) Int J Lepr Other Mycobact Dis. 1997;65:320–7. [PubMed] [Google Scholar]
- 130.Cho SN, Cellona RV, Villahermosa LG, Fajardo TT, Jr., Balagon MV, Abalos RM, et al. Detection of phenolic glycolipid I of Mycobacterium leprae in sera from leprosy patients before and after start of multidrug therapy. Clin Diagn Lab Immunol. 2001;8:138–42. doi: 10.1128/CDLI.8.1.138-142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roche PW, Britton WJ, Failbus SS, Neupane KD, Theuvenet WJ. Serological monitoring of the response to chemotherapy in leprosy patients. Int J Lepr Other Mycobact Dis. 1993;61:35–43. [PubMed] [Google Scholar]
- 132.Drowart A, Chanteau S, Huygen K, De Cock M, Cartel JL, De Bruyn J, et al. Effects of chemotherapy on antibody levels directed against PGL-I and 85A and 85B protein antigens in lepromatous patients. Int J Lepr Other Mycobact Dis. 1993;61:29–34. [PubMed] [Google Scholar]
- 133.Duthie MS, Goto W, Ireton GC, Reece ST, Cardoso LP, Martelli CM, et al. Use of protein antigens for early serological diagnosis of leprosy. Clin Vaccine Immunol. 2007;14:1400–8. doi: 10.1128/CVI.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nandan D, Venkatesan K, Katoch K, Dayal RS. Serum beta-glucuronidase levels in children with leprosy. Lepr Rev. 2007;78:243–7. [PubMed] [Google Scholar]
- 135.Perrin FM, Lipman MC, McHugh TD, Gillespie SH. Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis. 2007;7:481–90. doi: 10.1016/S1473-3099(07)70112-3. [DOI] [PubMed] [Google Scholar]
- 136.Kaufmann SH, Parida SK. Tuberculosis in Africa: learning from pathogenesis for biomarker identification. Cell Host Microbe. 2008;4:219–28. doi: 10.1016/j.chom.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Walzl G, Ronacher K, Djoba Siawaya JF, Dockrell HM. Biomarkers for TB treatment response: challenges and future strategies. J Infect. 2008;57:103–9. doi: 10.1016/j.jinf.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 138.Wallis RS, Doherty TM, Onyebujoh P, Vahedi M, Laang H, Olesen O, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis. 2009;9:162–72. doi: 10.1016/S1473-3099(09)70042-8. [DOI] [PubMed] [Google Scholar]
- 139.Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, et al. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 140.Azzurri A, Kanaujia GV, Sow OY, Bah B, Diallo A, Del Prete G, et al. Serological markers of pulmonary tuberculosis and of response to anti-tuberculosis treatment in a patient population in Guinea. Int J Immunopathol Pharmacol. 2006;19:199–208. [PubMed] [Google Scholar]
- 141.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004;38:754–6. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- 142.Higuchi K, Harada N, Mori T. Interferon-gamma responses after isoniazid chemotherapy for latent tuberculosis. Respirology. 2008;13:468–72. doi: 10.1111/j.1440-1843.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 143.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2006;174:831–9. doi: 10.1164/rccm.200511-1783OC. [DOI] [PubMed] [Google Scholar]
- 144.Veenstra H, Crous I, Brahmbhatt S, Lukey P, Beyers N, van Helden PD, et al. Changes in the kinetics of intracellular IFN-gamma production in TB patients during treatment. Clin Immunol. 2007;124:336–44. doi: 10.1016/j.clim.2007.05.014. [DOI] [PubMed] [Google Scholar]