Abstract
Alcohol consumption is a risk factor for breast cancer in humans. Experimental studies indicate that alcohol exposure promotes malignant progression of mammary tumors. However, the underlying cellular and molecular mechanisms remain unclear. Alcohol induces a pro-inflammatory response by modulating the expression of cytokines and chemokines. Monocyte chemoattractant protein-1 (MCP-1), also known as chemokine (C-C motif) ligand 2 (CCL2), is a pro-inflammatory chemokine implicated in breast cancer development/malignancy. We investigated the role of MCP-1 in alcohol-promoted mammary tumor progression. Using a xenograft model, we demonstrated that alcohol increased tumor angiogenesis and promoted growth/metastasis of breast cancer cells in C57BL/6 mice. Alcohol up-regulated the expression of MCP-1 and its receptor CCR2 in breast cancer cells in vitro and in vivo. Using a three-dimensional (3-D) tumor/endothelial cell co-culture system, we demonstrated MCP-1 regulated tumor/endothelial cell interaction and promoted tumor angiogenesis. More importantly, MCP-1 mediated alcohol-promoted angiogenesis; an antagonist of the MCP-1 receptor CCR2 significantly inhibited alcohol-stimulated tumor angiogenesis. The CCR2 antagonist abolished ethanol-stimulated growth of mammary tumors in mice. We further demonstrated that MCP-1 enhanced the migration, but not the proliferation of endothelial cells as well as breast cancer cells. These results suggest that MCP-1 plays an important role in ethanol-stimulated tumor angiogenesis and tumor progression.
Keywords: Alcohol, angiogenesis, chemokines, metastasis, migration
Introduction
One in eight American women will be stricken with breast cancer, the second leading cause of cancer-related mortality among American women [1]. Although the exact etiology of breast cancer remains unclear, environmental factors play an important role. Epidemiological studies indicate that alcohol consumption increases breast cancer risk in a dose-dependent manner [2–7]. Alcohol may also enhance the growth of existing breast tumors and increase the aggressiveness of breast cancer cells to invade and metastasize [8–10]. Nonetheless, the mechanism by which alcohol contributes to breast tumor initiation or progression has yet to be established.
A causal role was recently attributed to inflammation in many malignant diseases, including breast cancer. Cytokines and chemokines are important inflammatory mediators involved in carcinogenesis and malignant transformation. Particularly chemokines have substantial effects as chemotactic factors on normal development, inflammation, atherosclerosis, and angiogenesis [11]. Chemokines have been implicated in many aspects of tumorigenesis cell biology, including roles in the regulation of cancer cell growth, angiogenesis, metastasis, and host immune response [12]. Monocyte chemoattractant protein-1 (MCP-1) also known as chemokine (C-C motif) ligand 2 (CCL2), is a pro-inflammatory chemokine and recruits and activates monocytes during the inflammatory response. While MCP-1 is minimally expressed by normal breast epithelial duct cells, it is highly expressed by breast tumor cells at primary tumor sites; this indicates MCP-1 is acquired in the course of malignant transformation and suggests it plays a role in breast cancer development and/or progression [13].
It has been demonstrated that alcohol induces pro-inflammatory mediators, and enhanced inflammation may underlie many diseases or disorders caused by alcohol abuse [14,15]. Alcohol up-regulates the expression of MCP-1 in the brain of humans and animals [16,17]. In this study, we sought to determine whether alcohol induces the expression of MCP-1 in breast cancer cells and to investigate the role of MCP-1 in alcohol-promoted malignant progression of mammary tumors. Our results indicate that MCP-1 is responsive to alcohol exposure and mediates ethanol-promoted angiogenesis and tumor growth in vitro and in vivo.
Materials and Methods
Materials
Ethanol, fibrinogen, aprotinin, and thrombin were purchased from Sigma Chemical Co. (St. Louis, MO). CCR2 antagonist was purchased from Calbiochem (San Diego, CA). Anti-CD31 antibody was obtained from BD Pharmingen (San Diego, CA). Anti-MCP1 and anti-CCR2 antibodies were obtained from BD Biosciences (Franklin Lakes, NJ). Cytodex 3 beads were obtained from Amersham Pharmacia Biotech (Piscataway, NJ).
Cell culture
Mice mammary adenocarcinoma cell line E0771 was provided by Dr. Enrico Mihich (Roswell Park Cancer Institute, Buffalo, NY) and maintained in DMEM media supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml)/streptomycin (100 U/ml) and 0.25 mg/ml amphotericin B at 37°C in humidified air containing 5% CO2. Human MDA-MB231 breast cancer cells and SVEC4-10EE2 mouse vascular endothelial cells were grown in DMEM medium containing 10% FBS and 100 U/ml penicillin and streptomycin at 37°C with 5% CO2. MDA-MB231 breast cancer cells are aggressive mammary tumor cells and responsive to ethanol exposure. Human umbilical vein endothelial cells (HUVEC) were isolated from fresh human placentas with 1 mg/ml of type I collagenase and grown in Clonetics Endothelial Cell Growth Medium-2 (EGM-2; Lonza, Walkersville, MD). HUVECs were used between passages 3 and 10.
Animals and ethanol exposure
Female C57BL/6 mice age 5–6 weeks were purchased from Harlan Laboratories (Indianapolis, IN) and allowed to acclimate for 1 week with standard chaw diet and water before beginning the experiment. Mice were divided into two groups and fed with standard chaw ad libitum. The mice in the ethanol-exposed group (n = 22) were given 2% ethanol in drinking water for a 12 hour-period during the night starting at 8:00 pm, and then replaced with water without ethanol at 8:00 am for the remaining 12 hours each day for 3 weeks. The mice in the control group (n = 20) were provided with regular drinking water only. The consumption of regular water versus ethanol-containing water was monitored daily; no significant difference in the liquid intake between the control and ethanol group was found. The average consumption of water or ethanol-containing water for each mouse was approximately 4 ml/day. The blood ethanol concentration (BEC) was determined at 6:00 am using an Analox AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA) as previously described [18]. The BEC was 42.5 ± 14.1 mg/dl. All procedures were carried out according to the guidelines for the care and use of laboratory animals implemented by the National Institutes of Health and the Guidelines of the Animal Welfare Act approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Mouse model of tumor xenograft
E0771 mouse breast cells are syngeneic to C57BL/6 mice. E0771 cells, implanted subcutaneously in C57BL/6 mice, are immunosuppressive and highly aggressive, invading dermal layers and the peritoneum locally as well as the lung distantly and have characteristics that closely mirror human disease [19]. Briefly, three days after ethanol exposure, E0771 cells (2.5 × 105 in 100 μl PBS) were injected into the secondary mammary pads of mice using a 23-gauge needle. The mice were continually provided with 2% ethanol in drinking water or regular drinking water without ethanol. The size of the tumors was monitored every two days; two perpendicular dimensions of tumors were measured with a dial caliper. The volume was calculated based on the formula: V = 0.25 a × b2; a is the longest and b is the shortest dimension. At the end of experiment, animals were sacrificed and the tumors were removed. Some of the tumor tissues were fixed with 10% neutral formalin for immunohistochemical studies.
To evaluate the effect of a CCR2 antagonist on ethanol-mediated tumor growth, CCR2 antagonist (10 μg/kg in 50 μl of DMSO) or DMSO was injected intraperitoneally one day following ethanol exposure. CCR2 antagonist was administered every other day. The dosage of CCR2 antagonist was selected based on previous studies [20]. At specified times after the treatment, the size of the tumors was measured as described above. There were twelve animals for each treatment group.
Immunohistochemistry
Immunohistochemical procedure was performed as described [21]. Briefly, tumor tissues were sectioned at a thickness of 4 μm and incubated in 0.3% H2O2 in methanol for 30 min at room temperature and then treated with 0.1% TritonX-100 for 10 min in PBS. The sections were washed with PBS three times and then blocked with 1% BSA and 0.01% TritonX-100 for 1 hour at room temperature. The sections were incubated with primary antibodies: anti-MCP-1 (1:100), anti-CCR2 (1:100) or anti-CD31 (1:50) overnight at 4°C. Negative controls were performed by either omitting the primary antibody or incubating sections with nonspecific mouse or rat serum IgG at the same dilution as the primary antibodies.
After rinsing in PBS, sections were incubated with biotinylated secondary antibodies (Vector Laboratories Inc., Burlingame, CA) for 1 hour at room temperature. The sections were washed 3 times with PBS, then incubated in avidin–biotin–peroxidase complex (Vector Laboratories Inc. 1:100 in PBS) for 1 hour and developed in 0.05% 3,3′-diaminobenzidine (DAB) (Sigma) containing 0.003% H2O2 in PBS.
To determine the average micrcovessel density (AMVD), the microvessels detected by CD31 immunohistochemistry were counted at 10 randomly chosen visual fields under microscopy. The AMVD of 10 selected microscopic fields was calculated and expressed as the number of microvessels per mm2 area.
Analysis of tumor metastasis
After 24 days of ethanol exposure, mice (n = 22 for ethanol-exposed group and n = 20 for control group) were sacrificed and lungs were removed and fixed with 10% formalin. Tissues were paraffin-embedded and sectioned at a thickness of 5 μm. The sections were stained with Hematoxylin–Eosin (H&E), examined, and photographed under a microscope.
Three-dimensional endothelial cell and tumor co-culture system
To investigate the effect of ethanol on tumor angiogenesis, we utilized a three dimensional (3-D) model of endothelial cell and tumor cell co-culture adapted from a previous study with some modifications [22]. In this model, endothelial cells were induced to form a 3-D capillary tube-like network on a fibrin gel bead system in the presence or absence of tumor cells. Briefly, HUVEC or SVEC cells were trypsinized and cells (1×106) were mixed with cytodex beads (3×103) in 4 ml medium (EGM™-2 medium for HUVEC and DMEM for SVEC) in 50 ml centrifuge tubes. The mixtures were incubated at 5% CO2 at 37°C and gently shaken every 20 minutes for 4 hours. At the fourth hour of incubation, an additional 4 ml medium was added and the incubation continued for 4 more hours. The mixtures of cells/cytodex beads were then transferred to 25 ml tissue culture flasks and incubated overnight to allow the cells not attached to the beads to attach to the flask. After incubation, the mixtures of cells/cytodex beads were transferred to 50 ml centrifuge tubes and washed 3 times with 20 ml Ca2+- and Mg2+-free PBS. Then the beads with adherent cells were re-suspended in medium containing 2.5 mg/ml fibrinogen and 0.15 U/ml aprotinin (Sigma) at a pH of 7.4. A volume of 0.5 ml fibrinogen/bead solution was added to 24-well cell culture plates that were pre-coated with 0.625 U of thrombin (Sigma). The fibrinogen/bead solution was allowed to coagulate for 5 minutes at room temperature and then placed in 37°C and 5% CO2 for 20 minutes. The resulting fibrin gels contained endothelial cells (HUVEC or SVEC) adhering to the beads. One milliliter of medium with 0.15 U/ml aprotinin was added to each well and allowed to equilibrate with the fibrin clot for 30 minutes at 37°C and 5% CO2. The medium was removed and replaced with 1 ml fresh medium containing 0.15 U/ml aprotinin. For co-culture of endothelial cells/breast tumor cells, E0771 or MDA-MB231 cells (2 × 104 or 4 × 104) were layered on top of the fibrin gels. The medium was changed every other day.
Ethanol exposure protocol in vitro
A method utilizing sealed containers was used to maintain ethanol concentrations in the culture medium [23]. Briefly, the appropriate amount of ethanol (using 95% ethanol stock) was added to the culture medium to bring the ethanol concentration to the desired level (0.2%). Cell culture plates were placed on a rack inside a plastic container sealed with a tight-fitting lid. A 200-ml water bath was present in the bottom of each container; the bath consisted of ethanol at the same concentration present in the culture media. Just before sealing each container, CO2 (60 ml) was injected. The containers were placed in a humidified environment and maintained at 37°C with 5% CO2. With this method, ethanol concentrations in the culture medium can be accurately maintained [23].
Immunoblotting
The procedure for immunoblotting has been previously described [24]. Briefly, aliquots of protein samples (30 μg) were separated on a SDS-polyacrylamide gel by electrophoresis. The separated proteins were transferred to nitrocellulose membranes. The membranes were blocked with either 5% BSA or 5% nonfat milk in 0.01 M PBS (pH 7.4) and 0.05% Tween-20 (TPBS) at room temperature for 1 hour. Subsequently, the membranes were probed with primary antibodies directed against target proteins overnight at 4°C. After three quick washes in TPBS, the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (Amersham, Arlington Hts. IL). The immune complexes were detected by the enhanced chemiluminescence method (Amersham). In some cases, the blots were stripped and re-probed with an anti-actin antibody.
Cell migration assay
Cell migration was analyzed using a Transwell Migration System (Costar Corp., Acton, MA) as previously described [24]. The transwell insert consists of an upper chamber and a lower chamber separated by a membrane with 8 μm pore size. SVEC cells (3×104) were plated into the upper chamber and maintained in DMEM containing 2% FBS. The lower compartment of the chamber was filled with medium containing 2% FBS and MCP-1 (0, 5 or 10 ng/ml). The chambers were incubated at 37°C in 5% CO2 for 12 hours. The membrane was fixed with methanol and the cells remaining on the upper chamber were removed. The migrated cells were stained with Giemsa and counted. For each well, five random microscopic fields were counted and the mean of triplicate wells was obtained.
Statistical analyses
The data were expressed as the mean ± SEM. Differences among treatment groups were tested using analysis of variance (ANOVA). Differences in which p was less than 0.05 were considered statistically significant. In cases where significant differences were detected, specific post-hoc comparisons between treatment groups were examined with Student-Newman-Keuls tests. In some experiments, results were analyzed by an unpaired Student’s t-test. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL).
Results
Ethanol promotes mammary tumor growth and metastasis in mice
We investigated the effect of ethanol on the growth and metastasis of mammary tumors using a tumor xenograft model. Mouse mammary adenocarcinoma cells (E0771) are syngeneic to C57BL/6 mice and implantation of E0771 cells into mammary pads resulted in mammary tumor formation [19]. Mammary tumors were detected approximately one week after initial implantation (Fig. 1). Ethanol exposure enhanced tumor growth. As shown in Fig.1A, the growth rate of mammary tumors in the ethanol-exposed group was significantly higher than that in the control group. Mammary tumors were removed after 24 days of ethanol exposure at the end of the experiment for further measurement. As illustrated in Figs. 1B and 1C, ethanol consumption significantly increased the weight of mammary tumors. We further determined whether ethanol consumption promoted mammary tumor metastasis. Lung metastases were detected by histologic analysis. As shown in Fig. 2, lung metastatic carcinoma nodes were detected in 14 out of 22 mice in the ethanol-exposed group, while they were identified in only 6 out of 20 mice in the control group.
Figure 1.
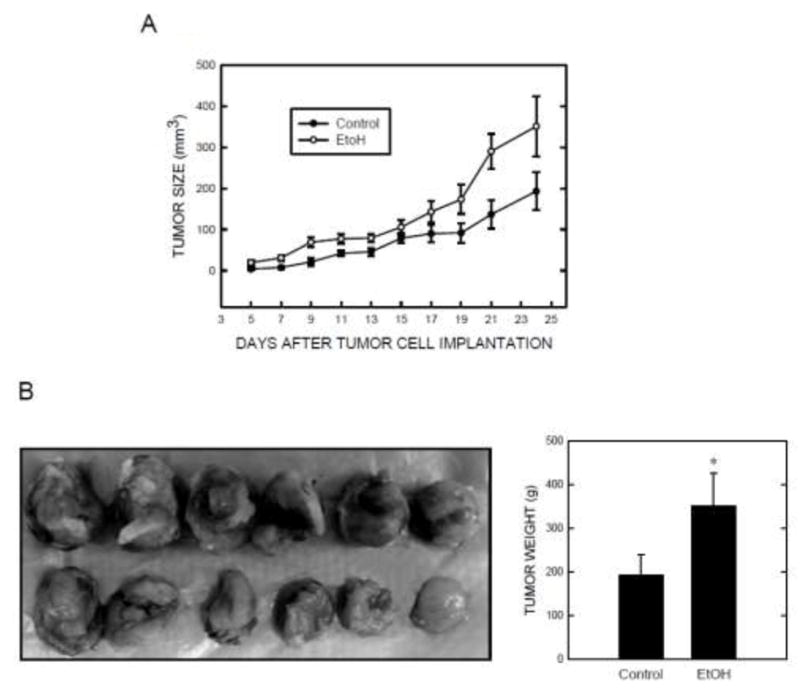
Effect of ethanol on the growth of mammary tumors. A: Female C57BL/6 mice were exposed to either 0% (control) or 2% ethanol (EtOH) in drinking water. Three days after ethanol exposure, syngeneic mouse breast cancer cells (E0771) were injected into the secondary mammary pads of both control and ethanol-exposed mice. The mice continually received 2% ethanol in drinking water or regular drinking water alone. The size of the mammary tumors was measured every two days up to twenty four days as described under the Materials and Methods. For the ethanol-exposed group, each data point was the mean ± SEM of 22 mice (n = 22). For the control group, each data point was the mean ± SEM of 20 mice (n = 20). B: At the end of experiment, the tumors were removed for subsequent analyses. A representative image shows mammary tumors removed from control and ethanol-exposed mice. C: The average weight of tumors in ethanol-exposed and control groups was measured. * denotes a significant difference from the control.
Figure 2.
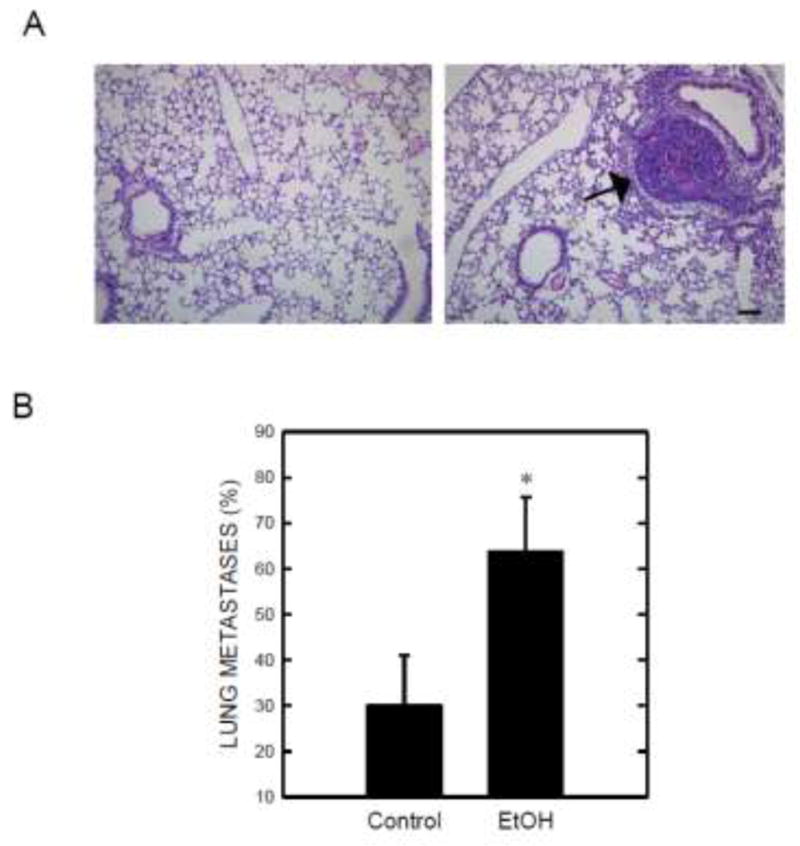
Effect of ethanol on tumor metastasis. Mice received implantation of E0771 cells and ethanol exposure as described above. Twenty four days following ethanol exposure, the mice were sacrificed and lungs were removed for histological analysis. Lung tissues were fixed, sectioned, and stained with H&E as described under the Materials and Methods. A: A representative microphotograph shows metastatic carcinoma nodes in the lungs of ethanol-exposed mice. Bar = 50 μm B: The percentage of mice with metastatic carcinoma nodes in the lungs is presented. * denotes a significant difference from the control.
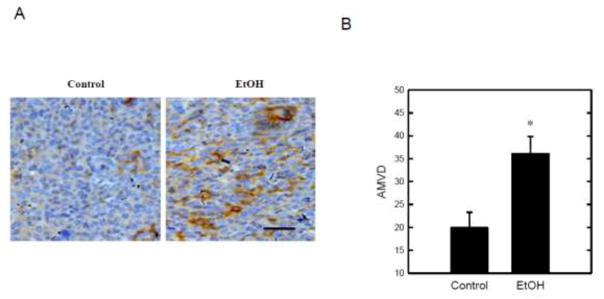
Ethanol enhances tumor angiogenesis in mice
Since tumor growth and metastasis are dependent on the sustained formation of new blood vessels, we determined the effect of ethanol on tumor angiogenesis in a tumor xenograft model. CD31 is a marker of endothelial cells. We used a morphometric analysis of CD31 immunohistochemistry to evaluate tumor angiogenesis (Fig. 3A). As shown in Fig. 3B, there was an approximately 2-fold increase of average micrcovessel density (AMVD) in the ethanol-treated mammary tumor tissues compared to the control tissues (Fig. 3B). We also performed H&E staining on both ethanol-treated and control samples; more microvessels were observed in tumor tissues of ethanol-exposed mice compared to the samples obtained from the control group (data not shown). These results suggested that ethanol consumption may enhance tumor growth by promoting angiogenesis.
Figure 3.
Effect of ethanol on tumor angiogenesis. Mice received implantation of E0771 cells and ethanol exposure as described above. Twenty four days following ethanol exposure, the mice were sacrificed and mammary tumors were removed. A: Mammary tumor tissues were fixed and sectioned. The sections were processed for CD31 immunohistochemistry (IHC) for the detection of microvessels as described under the Materials and Methods. Bar = 50 μm. B: Microvessels in tumor tissues were detected by CD31 IHC and the average microvessel density (AMVD) was determined by counting the number of microvessels per mm2 area. At least 6 areas from each group were examined. * denotes a significant difference from the control.
Ethanol promotes MCP-1 and CCR2 expression
Cytokines and chemokines play an important role in tumor angiogenesis and progression. Chemokine MCP-1 or CCL2 has been implicated in tumor development and angiogenesis [13]. It has been reported that ethanol up-regulated the expression of MCP-1 in the brain of humans and animals [16,17]. We sought to determine whether ethanol induced the expression of MCP-1 and its receptor CCR2 in mammary tumor tissues. As shown in Fig. 4A, a higher expression of MCP-1 and CCR2 was observed in tumor tissues of ethanol exposed animals compared to control animals. Ethanol-induced expression of MCP-1 and CCR2 was confirmed in vitro; ethanol increased the expression of MCP-1 and CCR2 in cultured E0771 cells (Fig. 4B).
Figure 4.
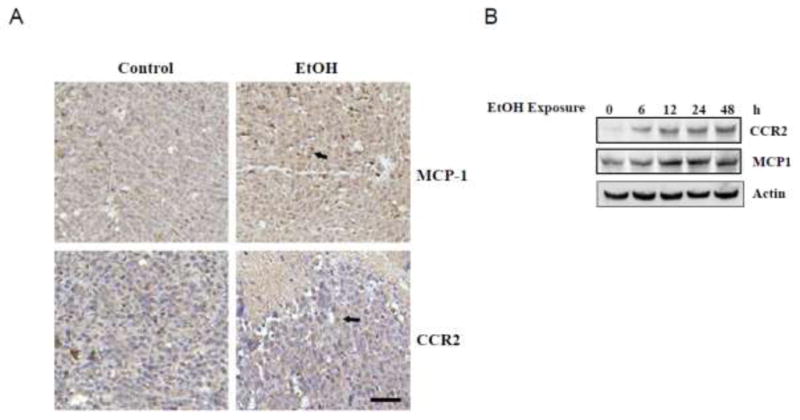
Effect of ethanol on the expression of MCP-1 and CCR2 in mammary tumors and cultured breast cancer cells. Twenty four days following ethanol exposure, the mice were sacrificed and mammary tumors were removed. A: Tumor tissues were fixed and sectioned. The sections were processed for MCP-1 or CCR2 IHC as described under the Materials and Methods. Bar = 50 μm. B: E0771 cells in culture were exposed to ethanol (0.2%) for specified times. The expression of MCP-1 and CCR2 was determined with immunoblotting analysis as described under the Materials and Methods. The experiment was replicated three times.
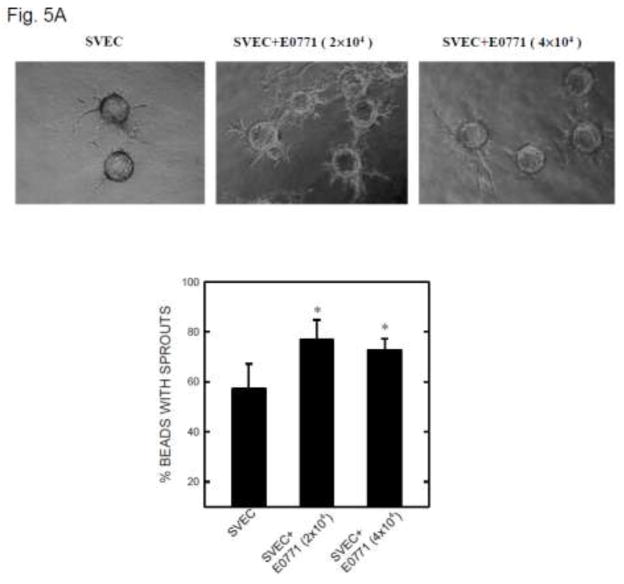
Involvement of MCP-1 in tumor angiogenesis
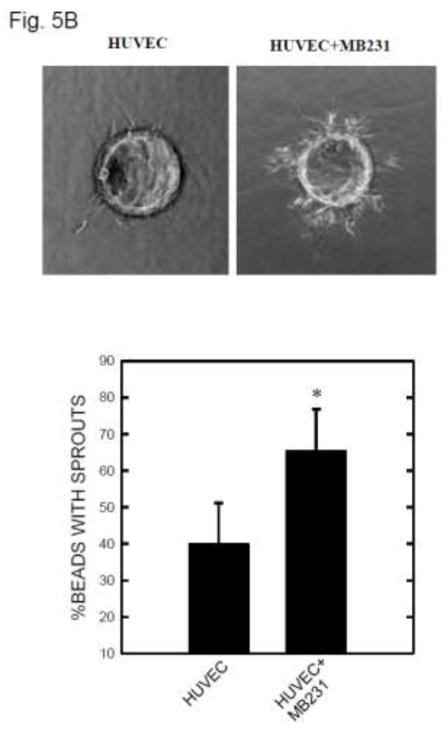
To test the hypothesis that MCP-1 may mediate ethanol-induced tumor angiogenesis, we used a three-dimensional (3-D) angiogenic model in which endothelial cells and breast cancer cells were cultured together, but without direct contact. In this system, endothelial cells attached to cytodex beads are able to form a 3-D capillary tube-like network indicative of angiogenesis [22]. As shown in Fig. 5A, SVEC cells attached to cytodex beads sprouted from the beads and formed short, narrow cordlike structures. The inclusion of mouse breast cancer cells (2×104 or 4×104 E0771 cells) in this system significantly increased the number and the length of sprouts from SVEC cells. The percentage of spouts was 57.2% in SVEC culture alone and 76.8% in SVEC co-cultured with E0771 cells. A higher density of E0771 cells (4×104), however, did not further increase the sprouting. Similarly, the addition of human breast cancer cells (MDA-MB231) promoted sprouting of HUVEC cells (Fig. 5B).
Figure 5.
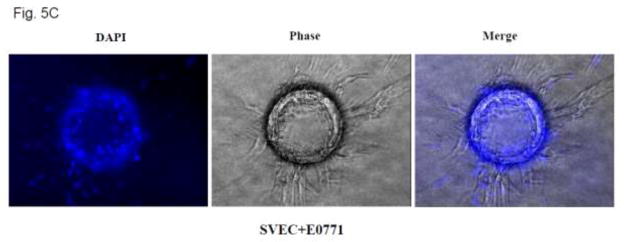
Tumor angiogenesis in a 3-D model. A: SVEC cells attached to cytodex beads were suspended in fibrin gel, and E0771 breast cancer cells (2 × 104 or 4 × 104) were placed on top of the gel. The images of SVEC cells with sprouts were captured at 12 hours after culture (top panel). Five hundred beads in three culture wells were examined for each treatment group. The percentage of beads with sprouts was determined (bottom panel). * denotes a significant difference from the control. B: HUVEC cells attached to cytodex beads were suspended in fibrin gel and MDA-MB231 breast cancer cells (2 × 104) were placed on top of the gel. The images of HUVEC cells with sprouts were captured at 12 hours after culture (top panel). The percentage of beads with sprouts was determined. * denotes a significant difference from the control. C: Fibrin gel containing SVEC cells/beads was fixed with 4% paraformaldehyde and stained with DAPI. The images of beads with cells were captured under a fluorescence microscope.
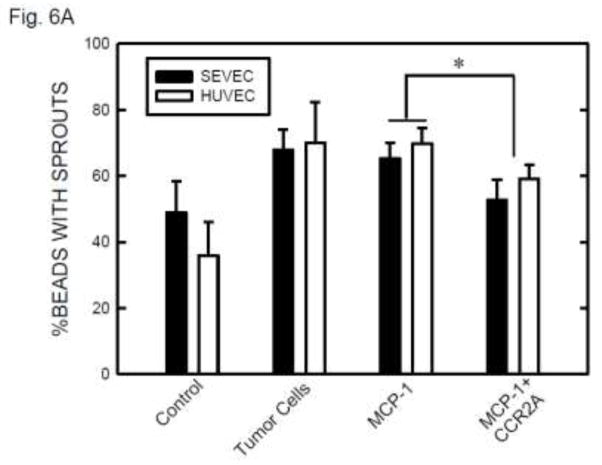
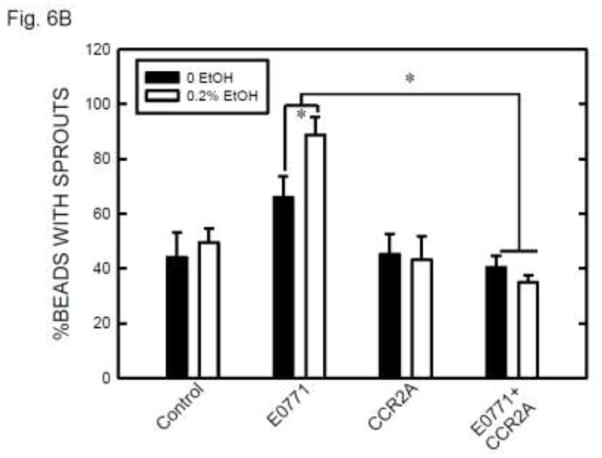
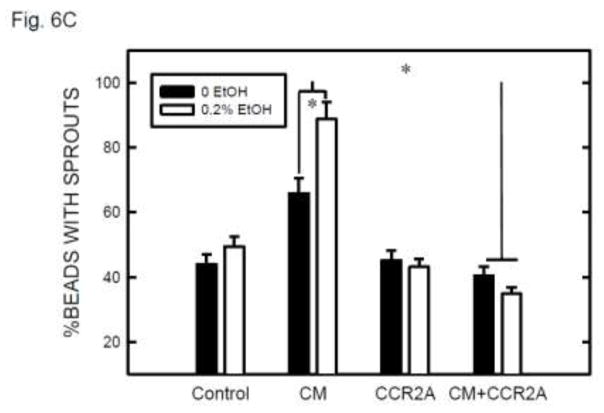
DAPI fluorescence staining of nuclei confirmed that the vascular sprouts and capillary network were formed by multi-cells (Figure 5C). The involvement of MCP-1 in angiogenesis was indicated by exogenous MCP-1-mediated enhancement of formation of vascular sprouts (Fig. 6A). Furthermore, CCR2 antagonist blocked MCP-1-mediated sprouting. Ethanol exposure increased angiogenesis in SVEC/E0771 co-cultures (Fig. 6B). Treatment with CCR2 antagonist abolished an ethanol-induced increase in the angiogenesis in SVEC/E0771 co-cultures (Fig. 6B). We further examined the effect of conditioned medium collected from ethanol-treated E0771 cells on angiogenesis. As shown in Figs. 6C, conditioned medium collected from ethanol-treated E0771 cells significantly enhanced angiogenesis and CCR2 antagonist blocked this enhancement. Together, these results suggested that MCP-1 was involved in ethanol-enhanced tumor angiogenesis.
Figure 6.
The role of MCP-1 in ethanol-induced angiogenesis. A: Endothelial cells (SVEC or HUVEC) attached to cytodex beads were suspended in fibrin gel containing MCP-1 (0 or 10 ng) with/without CCR2 antagonist (20 nM). At 12 hours after incubation, the images of endothelial cells with sprouts were captured and the percentage of beads with sprouts was determined as described above. Values were the mean ± SEM of three independent experiments. * denotes a significant difference. B: SVEC cells attached to cytodex beads were suspended in fibrin gel containing CCR2 antagonist (0 or 20 nM). One milliliter of medium containing E0771 cells (2 × 104) and ethanol (0 or 0.2%) was layered on top of the gel. Ethanol exposure was carried out using a sealed container system as described under the Materials and Methods. At 12 hours after incubation, the images of endothelial cells with sprouts were captured and the percentage of beads with sprouts was determined as described above. Values were the mean ± SEM of three independent experiments. * denotes a significant difference. C: E0771 cells were maintained in medium containing 1% FBS and exposed to ethanol (0 or 0.2%) for 24 hours. The conditioned media (CM) were collected and 1 ml of medium was added to fibrin gels containing SVEC cells/beads with/without CCR2 antagonist (CCR2A, 20 nM). At 12 hours after incubation, the images of endothelial cells with sprouts were captured and the percentage of beads with sprouts was determined as described above. Values were the mean ± SEM of three independent experiments. * denotes a significant difference.
MCP-1 regulates the migration of endothelial cells and breast cancer cells
Angiogenesis is regulated by the activation of endothelial cells. The activation usually refers to an increased proliferation or enhanced migratory ability of endothelial cells. As shown in Fig. 7A, MCP-1 stimulated the migration of SVEC cells and this stimulation was blocked by CCR2 antagonist. On the other hand, MCP-1 had little effect on the viability and proliferation of SVEC cells (data not shown). Similarly, ethanol stimulated the migration of E0771 breast cancer cells and CCR2 antagonist eliminated the stimulation (Fig. 7B). MCP-1 did not affect the proliferation of E0771 cells (data not shown).
Figure 7.
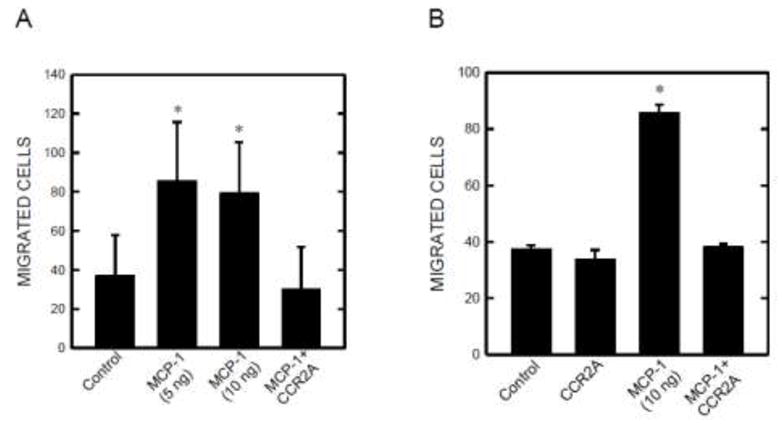
Effect of MCP-1 on the migration of endothelial cells and breast cancer cells. SVEC cells (A) and E0771 breast cancer cells (B) were treated with MCP-1 (5 or 10 ng/ml) with/without CCR2 antagonist (20 nM) for 12 hours. The migration of these cells was evaluated using a transwell system as described under the Materials and Methods. The experiment was replicated three times. * denotes a significant difference from the control and MCP-1 plus CCR2A group.
CCR2 antagonist inhibits ethanol-promoted tumor growth in a mouse tumor xenograft model
To further confirm the role of MCP-1 in ethanol-induced tumor promotion, we evaluated the effect of CCR2 antagonist on ethanol-promoted mammary tumor growth in a tumor xenograft model. As shown in Fig. 8, ethanol consistently promoted mammary tumor growth in mice. Injection of CCR2 antagonist blocked ethanol-stimulated tumor growth.
Figure 8.
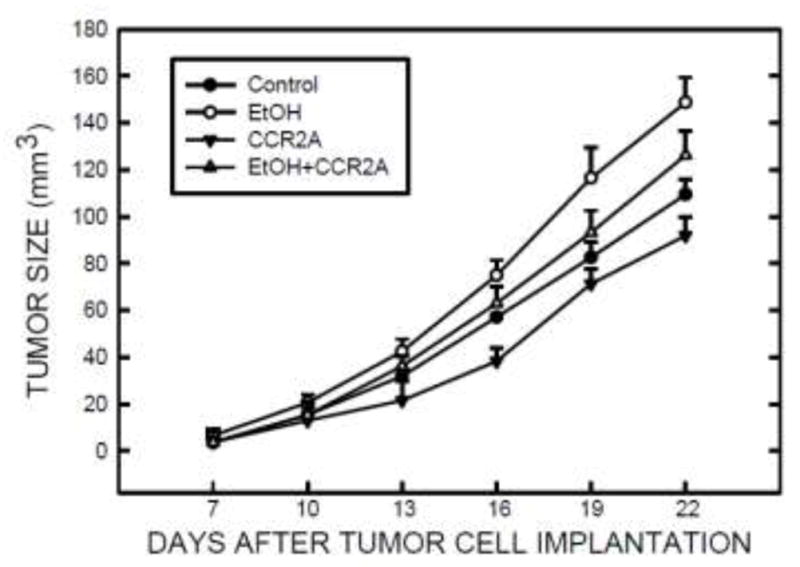
The role of MCP-1 in ethanol-mediated tumor growth in mice. The implantation of E0771 cells and ethanol exposure were performed as described under the Materials and Methods. One day after ethanol exposure, C57BL/6 mice received intraperitoneal injection of CCR2 antagonist (10 μg/kg) every other day. Mice continually received ethanol exposure and CCR2 antagonist injection up to 22 days. The size of the mammary tumors was measured every three days as described under the Materials and Methods. n = 12 for each treatment group.
Discussions
Using a mammary tumor xenograft model, we demonstrate that exposure to ethanol at a modest concentration (2% in drinking water) promotes tumor growth and metastasis in C57BL/6 mice. Our ethanol feeding regimen results in a BEC of 42 mg/dl which is equivalent to approximately two drinks of alcohol in humans. This is a physiologically relevant concentration. A previous study using a similar ethanol feeding regimen (1% ethanol in drinking water during the dark cycle) also results in a BEC around 40 mg/dl in C57BL/6 mice [25]. In that study, ethanol exposure for three weeks significantly increased the growth of melanoma in mice. A recent study demonstrates that chronic administration of Lieber-DeCarli liquid diet containing 3.5% ethanol increases the incidence of liver tumor in mice [26]. In our study, seventeen days of ethanol exposure resulted in a significant increase in mammary tumor size and by twenty four days, the size of the tumors in the ethanol-exposure group was almost two times larger than the control group (Fig. 1). In addition, ethanol exposure promotes tumor metastasis to lungs; the metastatic rate increased from 30% in the control group to 63.6% in the ethanol-exposed group (Fig. 2). Thus, we established a valuable mouse model for studying ethanol-induced promotion of mammary tumors.
A recent study also uses a tumor xenograft model to investigate the effect of mammary tumor growth [27]. In that study, Met-1 mouse breast cancer cells were injected subcutaneously to syngeneic female FVB/N mice. The mice were provided drinking water containing 20% ethanol. Although a high concentration of ethanol was provided, the resulting BEC was approximately 80 mg/dl which is a physiologically relevant concentration. A significant tumor growth promoting effect was observed following 20 days of ethanol exposure and the maximal effect is shown following 44 days of ethanol exposure [27]. On the other hand, information regarding ethanol’s effect on tumor metastasis is limited. Only one study by Yirmiya et al. [28] evaluated the relationship between ethanol exposure and tumor metastasis. They show that consumption of a liquid diet containing ethanol for two weeks before and three weeks after mammary tumor inoculation significantly increased lung metastases. Thus, these animal studies support epidemiological findings and indicate that ethanol promotes the malignant progression of breast cancers.
Angiogenesis plays a critical role in tumor growth and metastasis. The concept that ethanol may enhance angiogenesis has been previously proposed not only by us, but by others as well [25,29,30]. The current study supports this notion and demonstrates that ethanol enhances angiogenesis in vitro and in vivo. The mechanisms of ethanol-promoted angiogenesis are complex and may be mediated at multiple levels. It may be caused by 1) a direct effect on endothelial cells, such as enhancement of proliferation or migration of endothelial cells; 2) alterations in the tumor microenvironment, such as cell/extracellular matrix interaction, stromal cell functions, and recruitment of pro-inflammatory mediators; and 3) targeting tumor cells which promote endothelial/tumor cell interaction. Previously we showed that at a relatively high concentration (0.4%), ethanol can directly enhance tube formation of SVEC cells in culture, supporting that ethanol may directly affect endothelial cells [30]. In the current study, we evaluate whether ethanol can target tumor cells and alter tumor/endothelial cell interaction using a 3-D model of tumor/endothelial cell co-cultures. Our results show the inclusion of breast cancer cells in this 3-D system enhances angiogenesis, indicating it is a good system to study tumor/endothelial cell interaction (Fig. 5). Since there is not direct contact between tumor cells and endothelial cells in this system, the effect must be mediated by secreted molecules from tumor cells. Ethanol promotes angiogenesis in this tumor/endothelial co-culture system (Fig. 6). The conditioned medium collected from ethanol treated E0771 cells enhances angiogenesis. Since these conditioned media were collected and stored for an extended period, the ethanol concentration was negligible. Thus, their pro-angiogenic effect must be mediated by the molecules secreted by tumor cells rather than the ethanol itself.
MCP-1 is a potent chemoattractant for monocytes and macrophages to areas of inflammation and is implicated in multiple inflammatory diseases [31]. MCP-1 emerges as a pivotal regulator of tumor growth, progression, and metastasis [12,13,32,33]. MCP-1 is minimally expressed by normal breast epithelial duct cells, but extensively in breast cancer cells; the expression of MCP-1 is highly associated with advanced disease course and progression [13]. The pro-malignancy activities of MCP-1 in breast cancer may be mediated by 1) an increase in deleterious tumor-associated macrophages (TAM) and an inhibition of anti-tumor T cell activities; 2) the modulation of tumor-promoting interactions between tumor cells and cells of the tumor microenvironment; 3) a direct increase in migratory and invasive properties of breast cancer cells [13]. MCP-1 is also a potent angiogenic chemokine that induces angiogenesis in two complementing levels: 1) by increasing TAM presence at breast cancer sites; TAM can produce angiogenic factors; 2) by acting directly on endothelial cells to promote angiogenesis. MCP-1 is an apparent ethanol-responsive protein and its expression is induced by ethanol exposure in the brain [16,17] and breast cancer cells (current study). It is therefore important to determine the role of MCP-1 in ethanol-promoted angiogenesis and tumor growth.
In our in vitro system, exogenous MCP-1 is sufficient to promote angiogenesis (Fig. 6). The pro-angiogenic effect of MCP-1 may be mediated by its promotion of endothelial cell migration (Fig. 7). CCR2 antagonist effectively inhibits ethanol-promoted angiogenesis and abolishes the pro-angiogenic effect of conditioned medium collected from ethanol-treated breast cancer cells; the evidence strongly supports that MCP-1 produced by breast cancer cells in response to ethanol exposure acts in a paracrine fashion to stimulate angiogenesis (Fig. 6). MCP-1 may also act in an autocrine fashion as it regulates the migration of breast cancer cells (Fig. 7B). The involvement of MCP-1 in tumor progression is further supported by animal studies showing CCR2 antagonist significantly inhibits ethanol-promoted tumor growth.
The mechanisms underlying ethanol-mediated angiogenesis and tumor promotion are very complex and likely multiple contributors/mediators are involved. For example, we have found that VEGF is also involved in ethanol-mediated angiogenesis and mammary tumor growth (data not shown). A recent study shows that the up-regulation of RNA polymerase III-dependent transcription may be involved in ethanol-induced tumor promotion [26]. Regardless of other potential pathways, our finding clearly establishes a role of MCP-1 in ethanol-induced angiogenesis and tumor growth, providing a novel insight into the mechanisms of ethanol-mediated tumor promotion.
Acknowledgments
This research is supported by grants from National Institute of Health (AA017226 and AA015407).
Abbreviations
- AMVD
average micrcovessel density
- CCL2
chemokine (C-C motif) ligand 2
- CCR2
CC chemokine receptor 2
- CCR2A
CCR2 antagonist
- MCP-1
Monocyte chemoattractant protein-1
Footnotes
Conflict of interests:
The authors declare that they have no conflict of interests.
References
- 1.ACS. Breast Cancer Facts & Figures 2009–2010. American Cancer Society; Atlanta: 2009. pp. 1–36. [Google Scholar]
- 2.Key J, Hodgson S, Omar RZ, Jensen TK, Thompson SG, Boobis AR, Davies DS, Elliott P. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control. 2006;17:759–770. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 3.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30:38–37. [PMC free article] [PubMed] [Google Scholar]
- 4.Seitz HK, Maurer B. The relationship between alcohol metabolism, estrogen levels, and breast cancer risk. Alcohol Res Health. 2007;30:42–43. [PubMed] [Google Scholar]
- 5.Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- 6.Tjonneland A, Christensen J, Olsen A, Stripp C, Thomsen BL, Overvad K, et al. Alcohol intake and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2007;18:361–373. doi: 10.1007/s10552-006-0112-9. [DOI] [PubMed] [Google Scholar]
- 7.Visvanathan K, Crum RM, Strickland PT, You X, Ruczinski I, Berndt SI, Alberg AJ, Hoffman SC, Comstock GW, Bell DA, Helzlsouer KJ. Alcohol dehydrogenase genetic polymorphisms, low-to-moderate alcohol consumption, and risk of breast cancer. Alcohol Clin Exp Res. 2007;31:467–476. doi: 10.1111/j.1530-0277.2006.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoll BA. Alcohol intake and late-stage promotion of breast cancer. Eur J Cancer. 1999;35:1653–1658. doi: 10.1016/s0959-8049(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 9.Vaeth PA, Satariano WA. Alcohol consumption and breast cancer stage at diagnosis. Alcohol Clin Exp Res. 1998;22:928–934. [PubMed] [Google Scholar]
- 10.Weiss HA, Brinton LA, Brogan D, Coates RJ, Gammon MD, Malone KE, Schoenberg JB, Swanson CA. Epidemiology of in situ and invasive breast cancer in women aged under 45. Br J Cancer. 1996;73:1298–1305. doi: 10.1038/bjc.1996.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Front Biosci. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 12.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 13.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke Z, Wang X, Liu Y, Fan Z, Chen G, Xu M, Bower KA, Frank JA, Li M, Fang S, Shi X, Luo J. Ethanol induces endoplasmic reticulum stress in the developing brain. Alcohol Clin Exp Res. 2011;35:1574–1583. doi: 10.1111/j.1530-0277.2011.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewens A, Luo L, Berleth E, Alderfer J, Wollman R, Hafeez BB, Kanter P, Mihich E, Ehrke MJ. Doxorubicin plus interleukin-2 chemoimmunotherapy against breast cancer in mice. Cancer Res. 2006;66:5419–5426. doi: 10.1158/0008-5472.CAN-05-3963. [DOI] [PubMed] [Google Scholar]
- 20.Major TC, Olszewski B, Rosebury-Smith WS. A CCR2/CCR5 antagonist attenuates an increase in angiotensin II-induced CD11b+ monocytes from atherogenic ApoE−/− mice. Cardiovasc Drugs Ther. 2009;23:113–120. doi: 10.1007/s10557-008-6157-0. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z, Shi X, Ke ZJ, Luo J. Overexpression of glycogen synthase kinase 3beta sensitizes neuronal cells to ethanol toxicity. J Neurosci Res. 2009;87:2793–2802. doi: 10.1002/jnr.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Htay A, Dos Santos W, Gillies GT, Fillmore HL, Sholley MM, Broaddus WC. In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. J Neurooncol. 2009;92:121–128. doi: 10.1007/s11060-008-9742-y. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Miller MW. Differential sensitivity of human neuroblastoma cell lines to ethanol: correlations with their proliferative responses to mitogenic growth factors and expression of growth factor receptors. Alcohol Clin Exp Res. 1997;21:1186–1194. [PubMed] [Google Scholar]
- 24.Xu M, Bower KA, Wang S, Frank JA, Chen G, Ding M, Wang S, Shi X, Ke Z, Luo J. Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol Cancer. 2010;9:285. doi: 10.1186/1476-4598-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan W, Bailey AP, Shparago M, Busby B, Covington J, Johnson JW, Young E, Gu JW. Chronic alcohol consumption stimulates VEGF expression, tumor angiogenesis and progression of melanoma in mice. Cancer Biol Ther. 2007;6:1211–1217. doi: 10.4161/cbt.6.8.4406. [DOI] [PubMed] [Google Scholar]
- 26.Zhong S, Machida K, Tsukamoto H, Johnson DL. Alcohol induces RNA polymerase III-dependent transcription through c-Jun by co-regulating TATA-binding protein (TBP) and Brf1 expression. J Biol Chem. 2011;286:2393–2401. doi: 10.1074/jbc.M110.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong J, Holcomb VB, Tekle SA, Fan B, Núñez NP. Alcohol consumption promotes mammary tumor growth and insulin sensitivity. Cancer Lett. 2010;294:229–235. doi: 10.1016/j.canlet.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yirmiya R, Ben-Eliyahu S, Gale RP, Shavit Y, Liebeskind JC, Taylor AN. Ethanol increases tumor progression in rats: possible involvement of natural killer cells. Brain Behav Immun. 1992;6:74–86. doi: 10.1016/0889-1591(92)90061-r. [DOI] [PubMed] [Google Scholar]
- 29.Gu JW, Elam J, Sartin A, Li W, Roach R, Adair TH. Moderate levels of ethanol induce expression of vascular endothelial growth factor and stimulate angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R365–372. doi: 10.1152/ajpregu.2001.281.1.R365. [DOI] [PubMed] [Google Scholar]
- 30.Qian Y, Luo J, Leonard SS, Harris GK, Millecchia L, Flynn DC, Shi X. Hydrogen peroxide formation and actin filament reorganization by Cdc42 are essential for ethanol-induced in vitro angiogenesis. J Biol Chem. 2003;278:16189–16197. doi: 10.1074/jbc.M207517200. [DOI] [PubMed] [Google Scholar]
- 31.Melgarejo E, Medina MA, Sánchez-Jiménez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41:998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Raffaghello L, Cocco C, Corrias MV, Airoldi I, Pistoia V. Chemokines in neuroectodermal tumour progression and metastasis. Semin Cancer Biol. 2009;19:97–102. doi: 10.1016/j.semcancer.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]