Abstract
Glaucoma is a neurodegenerative disease characterized by the apoptotic death of retinal ganglion cells (RGCs). The primary insult to RGCs in glaucoma is thought to occur to their axons as they exit the eye in the optic nerve head. However, pathological signaling pathways that exert central roles in triggering RGC death following axonal injury remain unidentified. It is likely that the first changes to occur following axonal injury are signal relay events that transduce the injury signal from the axon to the cell body. Here we focus on the c-Jun N-terminal kinase (JNK1-3) family, a signaling pathway implicated in axonal injury signaling and neurodegenerative apoptosis, and likely to function as a central node in axonal injury-induced RGC death. We show that JNK signaling is activated immediately after axonal injury in RGC axons at the site of injury. Following its early activation, sustained JNK signaling is observed in axonally-injured RGCs in the form of JUN phosphorylation and upregulation. Using mice lacking specific Jnk isoforms, we show that Jnk2 and Jnk3 are the isoforms activated in injured axons. Combined deficiency of Jnk2 and Jnk3 provides robust long-term protection against axonal injury-induced RGC death and prevents downregulation of the RGC marker, BRN3B, and phosphorylation of JUN. Finally, using Jun deficient mice, we show that JUN-dependent pathways are important for axonal injury-induced RGC death. Together these data demonstrate that JNK signaling is the major early pathway triggering RGC death after axonal injury and may directly link axon injury to transcriptional activity that controls RGC death.
Keywords: JNK, axonal injury, apoptosis, retinal ganglion cell, cJUN, mouse, neurodegeneration, neuroprotection, glaucoma
Introduction
Glaucoma is neurodegenerative disease characterized by optic nerve degeneration and retinal ganglion cell (RGC) death. Studies have shown that an important, initial site of injury to RGCs in glaucoma is to their axons as they exit the eye (Anderson and Hendrickson, 1974; Howell et al., 2007; Quigley et al., 1983; Schlamp et al., 2006). However, the pro-death signaling pathways activated by axonal injury are poorly defined. A main theory linking glaucomatous axon injury to RGC death is the neurotrophic deprivation hypothesis (Johnson et al., 2009). In other systems, withdrawal of neurotrophic factors triggers the activation of the canonical cJUN N-terminal kinase (JNK) pathway and downstream targets in the AP-1 family leading to neuronal death (Eilers et al., 2001; Ham et al., 2000). Also, JNK signaling in axons is known to be important in signaling to the neuronal cell body after axon injury (Cavalli et al., 2005; Hanz and Fainzilber, 2006; Lindwall and Kanje, 2005).
JNKs are important regulators of stress-induced apoptosis (Davis, 2000), and JNK signaling has been implicated in various neurodegenerations (Morishima et al., 2001; Perrin et al., 2009; Xia et al., 2001). In RGCs, the JNK pathway is activated by many apoptotic stimuli (Kwong and Caprioli, 2006; Lukas et al., 2009; Roth et al., 2003). The active form of JNK (phosphorylated; pJNK) is expressed in RGCs of human glaucoma eyes (Tezel et al., 2003). Inhibitors that block the activity of all three JNK isoforms provide a limited degree of neuroprotection to severely injured RGCs (Liu et al., 2011; Sun et al., 2011; Tezel et al., 2004; Yang et al., 2008), suggesting JNKs mediate pro-apoptotic signaling in RGCs. The canonical JNK signaling pathway acts by phosphorylating (activating) JUN, a transcription factor that, when activated, induces the expression of many genes known to be important in neurodegeneration (Ma et al., 2007; Whitfield et al., 2001). Phosphorylated JUN is expressed in glaucomatous RGCs (Levkovitch-Verbin et al., 2005). Decreasing JUN activation, by preventing phosphorylation, provides minor protection to RGCs after axotomy (Yoshida et al., 2002).
Despite the potential importance of JNK activation in RGC death, only a few studies have addressed the role of the JNK signaling in glaucoma or glaucoma-like insults. These studies have failed to address the role of individual JNK isoforms, identify pathways downstream of JNK activation that mediate RGC death, or determine whether JNK signaling is a major regulator of axonal injury-induced RGC death. Here, we show that JNK is phosphorylated immediately after axonal injury, in the axon near the site of injury. Notably, by characterizing the extent and duration of RGC survival following axonal injury, we show that perturbing JNK signaling provides robust protection to RGCs in comparison to other signaling pathways that have been implicated in RGC death. We further show that specific JNKs are activated and required to induce RGC cell death through a JUN-dependent mechanism. Together these data indicate that JNK-dependent signaling is a major pathway triggering RGC death after axonal injury and directly links the injury to transcriptional activity that controls RGC death.
Methods
Mice
Mice carrying null alleles for Jnk2 (Mapk9tm1Flv, Jackson Laboratory stock number 004321) and Jnk3 (Mapk10tm1Flv, Jackson Laboratory stock number 004322) were backcrossed onto DBA/2J for more than 10 generations and then intercrossed. In all experiments, only preglaucomatous DBA/2J mice , < 5 months of age, of each Jnk genotype were used. Mice with conditional deletion of Jun in the retina were generated by crossing mice carrying floxed alleles of Jun (Junfl/fl (Behrens et al., 2002) with mice expressing cre recombinase under the control of an early retinal promoter, six3 (Oliver et al., 1995). These mice were on a mixed genetic background of C57BL/6J and 129 origin. Note for control mice in the Junfl experiments, mice were either Jun+/+; Six3cre-, Jun+/+; Six3cre+, Jun+/fl; Six3cre-, or Junfl/fl; Six3cre- (no mice heterozygous for Jun deletion were used). No obvious differences were observed between these genotypes and were all used as controls. In the text these mice are collectively referred to as Jun+/?; Six3cre?. Mice were housed in a 12-hour light dark cycle and were fed chow and water ad libitum. All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology's statement on the use of animals in ophthalmic research and were approved by the University of Rochester's University Committee on Animal Resources.
Optic nerve injury
Controlled optic nerve crush (CONC) was performed as previously described (Harder and Libby, 2011; Libby et al., 2005). In brief, the optic nerve was exposed and crushed for 5 seconds approximately 0.5mm from the globe using self-closing forceps (Roboz RS-5027). Retinas were harvested at various time points after the procedure. Unmanipulated contralateral eyes or contralateral eyes that had a sham surgery performed (where the optic nerve was exposed but not crushed) were used as controls.
Immunohistochemistry and Cell Counts
Eyes were processed as previously described (Harder and Libby, 2011). Following fixation in 4% paraformaldehyde (PFA), the anterior segment of each eye was removed and the posterior eye cup was processed for cryosectioning or whole mount immunostaining. For immunohistochemistry on retinal sections, cryosections were blocked by incubating in 10% horse serum in 0.1% Triton X-100 in PBS (PBST) for 2-3 hours at room temperature. Sections were incubated with primary antibodies (rabbit anti-pJNK, Cell Signaling, 1:250; mouse anti-neurofilament, Abcam 1:1000) diluted in PBST containing 5% horse serum overnight at 4°C. Note, the pJNK antibody recognizes all three JNK isoforms phosphorylated at Thr183 and Tyr185 sites. The following day the sections were washed and incubated with Alexafluor-conjugated secondary antibodies (Invitrogen) diluted in PBST for a minimum of two hours. Sections were then counterstained with DAPI. For whole mount immunostaining, retinas were blocked in 0.3% Triton X-100 in PBS containing 10% horse serum for 3-4 hours. Retinas were then incubated in primary antibodies (rabbit anti-JUN, Abcam, 1:250; rabbit anti-cCASP3, RD, 1:1000; goat anti-BRN3B, Santacruz, 1:200; mouse anti-TUJ1, Covance, 1:1000) diluted in 0.3% Triton X-100 in PBS for 72 hours at 4°C. Following washes in PBS, the retinas were incubated with Alexafluor-conjugated secondary antibodies (Invitrogen) diluted in PBST for 24 hours at 4°C and then mounted on slides. RGC density varies greatly with respect to retinal location. Therefore, for each retina, images were obtained from eight 20× fields around the peripheral retina (two from each quandrant), each field approximately 220 μm from the peripheral edge of the retina (one half of a 20X field in from the peripheral margin). The numbers of neurons immunolabeled with cCASP3 or BRN3B in each image were quantified using the cell-counter tool in ImageJ.
Nissl counts
Following fixation in 4% PFA, retinas were flat-mounted and Nissl-stained with cresyl violet as previously described (Harder and Libby, 2011; Libby et al., 2005). For ganglion cell layer (GCL) cell counts, two 40X fields were obtained from each retinal quadrant, roughly equidistant from the peripheral edge of the retina (approximately one 40X field in from the peripheral margin of the retina). All GCL neurons within the field were counted with the exception of endothelial cells (which have an obvious elongated, non-neuronal morphology) using the cell counter tool in ImageJ. For each individual retina, the total count of surviving GCL neurons was obtained by averaging the eight counts for each retina.
Western Blotting
At least four retinas per genotype were processed for western blot analysis. Each retina was dissected in ice-cold PBS and transferred to 100μl of ice-cold lysis buffer [containing 10 μl phenylmethylsulfonly fluoride solution, 10 μl sodium orthovanadate solution, 10 μl of sodium fluoride and 10 μl protease inhibitor cocktail solution from RIPA Lysis buffer System (Santacruz 24948) per ml of CelLytic (Sigma C3228)]. The tissue was homogenized by sonication and then samples were centrifuged at 13,000 rpm for 10 minutes at 4°C. Protein concentration was estimated using Nanodrop. The rest of the supernatant was transferred to a pre-chilled tube into which was added 1X Pierce lane dye marker (Thermo Scientific, 39001). Samples were boiled for 10 minutes at 95°C. Thirty ug of protein per sample were resolved on 12% gels (BioRad 456-1043) and then transferred to nitrocellulose or PVDF membranes. The membranes were incubated in a blocking solution of 5% non-fat milk in 0.05% Tween-20 in PBS (PBST) for 30 minutes and then incubated overnight at 4°C with primary antibodies (rabbit anti-pJUN, Cell Signaling, 1:500; mouse anti-β-tubulin, Covance, 1:1000) diluted in PBST. The following day, membranes were washed in PBST three times for a total of 30 minutes and then incubated with secondary antibodies (HRP-conjugated anti- rabbit IgG, Biorad Laboratories, 1:5000-1:10,000; HRP-conjugated anti- mouse IgG, Jackson Laboratories, 1:10,000) diluted in PBST for 1 hour at room temperature. Membranes were washed three times with PBS-T for 10 minutes each at room temperature. Immunoreactive bands were detected using an enhanced chemiluminescence reagent kit (Supersignal West Dura Extended Substrate, Pierce, 34075 or Immun-star, BioRad 170-5070). The relative abundance of proteins was determined by densitometric analyses (using Quantity One software: BioRad), expressed relative to control groups and then normalized to a β-tubulin loading control.
Statistical Analysis
At least 4 retinas were assessed for each genotype for all experimental conditions. For experiments involving quantification of results, the experimenter was masked to genotype and/or experimental group. Experiments with two groups were analyzed for differences using the unpaired Student's t-test. Experiments with three or more groups were subjected to statistical analyses using one-way ANOVA with significance determined at P values < 0.05, followed by the Tukey-Kramer post hoc test for group comparisons.
Results
JNK signaling is activated in RGCs in vivo following axonal injury
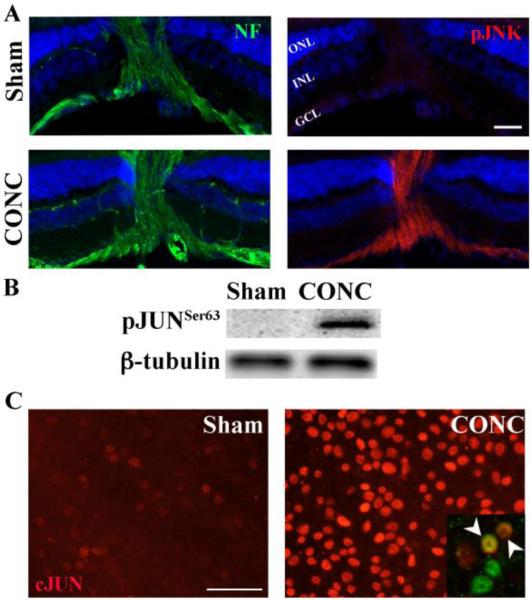
Axonal injury is an early critical insult to RGCs in glaucoma (Anderson and Hendrickson, 1974; Howell et al., 2007; Quigley et al., 1983; Schlamp et al., 2006). Recent reports have also demonstrated distortion of the RGC axonal cytoskeleton in rodent models of glaucoma (Huang et al., 2011). Cytoskeleton disruption is known to activate JNK (Muller et al., 2006). Therefore, it is possible that JNK is activated at/near the site of axon injury in RGCs. To determine whether JNK signaling is activated in injured RGCs in vivo following exposure to a glaucoma relevant insult, the expression of phosphorylated JNK (pJNK) in RGC axons at the optic nerve head was characterized following controlled optic nerve crush (CONC) injury. Robust activation of JNK was detectable in RGC axons labeled with neurofilament one hour after CONC but not in sham surgery eyes (Fig. 1A). Given that JNK can signal axonal injury back to the soma (Cavalli et al., 2005; Lindwall and Kanje, 2005), its early expression after injury suggests that JNK signaling is a critical early signaling pathway of axonal injury in RGCs. JNK's canonical role is to phosphorylate (activate) JUN and much of JNK's control of cell physiology goes through JUN activation (Raivich and Behrens, 2006). JNK phosphorylates the Ser-63 and Ser-73 residues of JUN and increases the transcriptional activity of the JUN/AP-1 complex (Pulverer et al., 1991). To determine whether this pathway, once initiated, remains active until cell death, phosphorylation of JUN was characterized 3 days following CONC, the time point when significant RGCs death begins (Harder and Libby, 2011). Phosphorylated JUN was only observed following CONC (Fig. 1B). Unfortunately, while several JUN antibodies were confirmed to be specific for pJUN in Western blotting, they were not specific for pJUN in immunohistochemistry (Suppl Fig 1). However, since JUN is known to induce its own expression following its phosphorylation by JNK, (Angel et al., 1988), an antibody against JUN was used to determine if JUN expression changes in axonally injured RGCs. While expression of JUN was weak in RGCs from sham-treated eyes, expression of JUN appeared to be increased in RGCs following CONC (Fig. 1C, inset). Axonal injury-induced activation of JNK at the site of insult and sustained activation of JNK signaling till the commencement of RGC death suggests that JNK signaling might regulate RGC death.
Figure 1. JNK signaling is activated in RGCs in vivo following axonal injury.
A, Phosphorylated JNK (pJNK; Red) is not present in the retina or optic nerve head (area where retinal axons, labeled with neurofilament (NF, green) transverse the retina) in the uninjured sham animals. However, 1hr after controlled optic nerve crush injury (CONC), pJNK is in RGC axons (neurofilament positive). B, Western blot analysis shows that phosphorylated JUN (pJUN) is not present in uninjured retinas but is present after CONC (3 days after injury, representative blot, n = 3). C, Immunostaining of retina flatmounts for JUN (red) confirms the upregulation seen by western blot analysis and shows JUN staining in RGCs (Right inset, BRN3B+ cells, green; arrow heads indicate colocalization of BRN3B and JUN). Blue staining in A is DAPI; Scale bar, 50um. ONL, Outer Nuclear Layer; INL, Inner Nuclear Layer; GCL, Ganglion Cell Layer.
Jnk2 and Jnk3 are the major isoforms activated in RGC axons after axonal-injury
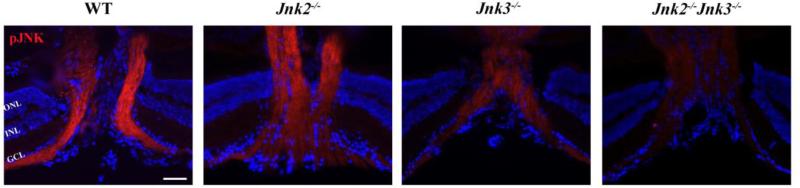
Each of the three JNK isoforms is thought to have independent functions (Brecht et al., 2005), thus it is important to understand which JNK(s) is involved in the RGC axonal injury response. Jnk3 is the major Jnk isoform expressed in neural tissue (Martin et al., 1996). Deficiency in Jnk3 protects neurons from insults such as excitotoxicity or ischemia (Kuan et al., 2003; Yang et al., 1997). However, in some neurodegenerative diseases, deficiency of both Jnk2 and Jnk3 are required to prevent apoptosis (Ries et al., 2008). Therefore, we used mice null for Jnk2, Jnk3 or both Jnk2 and Jnk3 to determine which Jnk isoforms contribute to axonal injury-induced JNK activation (Figure 2). Compared to the axonal pJNK expression in wildtype mice at one hour after CONC (Figure 1A and Figure 2), the activation of JNK appeared similar or slightly less in RGC axons of Jnk2-/- mice. In Jnk3-/- mice, there appears to be a substantial reduction of pJNK in RGC axons. In Jnk2-/- Jnk3-/- mice, pJNK was undetectable in RGC axons at the optic nerve head. Thus it appears that Jnk2 and Jnk3 contribute to the pool of activated JNK in RGCs after axonal injury, with Jnk3 perhaps providing a larger contribution.
Figure 2. Activation of JNK in RGC axons following CONC is attenuated in Jnk3-/- and Jnk2-/-Jnk3-/- retinas.
Longitudinal retinal sections through the optic nerve head 1hr following controlled optic nerve crush injury (CONC), immunostained for phosphorylated JNK and the nuclear marker DAPI (blue). Robust activation of JNK is observed in RGC axons of wild-type and Jnk2-/- mice. In contrast, deficiency of Jnk3 or Jnk2&3 dramatically attenuates activation of JNK in RGC axons. Representative images, n = 3. Scale bar, 50um. ONL, Outer Nuclear Layer; INL, Inner Nuclear Layer; GCL, Ganglion Cell Layer.
Both Jnk2 and Jnk3 are critical for RGC death after axonal injury
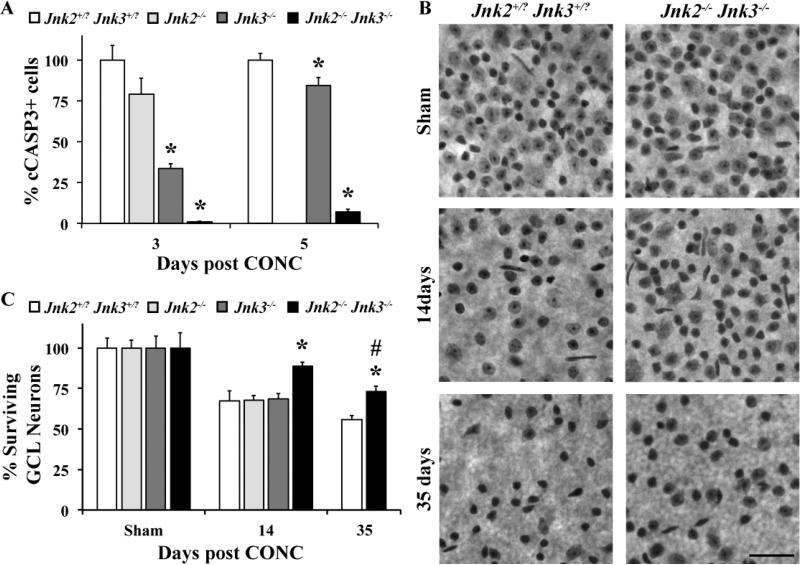
Since Jnk2 and Jnk3 are known to be important in injury induced neuronal death (Ries et al., 2008) and Jnk2/3 signaling appears to be active in RGC axons after injury, we hypothesized that a JNK dependent pathway was critical for RGC death after injury. Approximately three days after CONC, the first signs of RGC apoptosis, as judged by cleaved caspase 3 positive (cCASP3+) cells, can be detected in significant numbers (Harder and Libby, 2011). Since the number of cCASP3+ cells in the Jnk2+/-Jnk3+/- and the Jnk2+/+Jnk3+/+ mice following CONC were indistinguishable from each other (data not shown), mice of both these genotypes were used as wild-type controls (referred to as Jnk2+/?Jnk3+/?). Jnk3 deficiency (P < 0.001), but not Jnk2 deficiency (P = 0.16) significantly reduced the number of cCASP3+ cells three days after CONC (Fig. 3A). In Jnk2-/-Jnk3-/- mice cell death was almost completely prevented (<1% of the number of cCASP3+ cells observed in wildtype mice; Fig. 3A) at this early time point. Similarly, during the peak of RGC death at five days after CONC (Harder and Libby, 2011), Jnk2 deficiency did not protect RGCs while Jnk3 deficiency did provide minor yet significant (P = 0.03) protection. Jnk2/3 double deficient mice had significantly reduced cCASP3+ cells (7% of wildtype mice) at this time point.
Figure 3. Deficiency of Jnk2&3 protects RGCs from CONC-induced death.
A, At both 3 and 5 days following controlled optic nerve crush (CONC), the percent of dying cells (cleaved caspase 3 positive, cCASP3+) was significantly reduced in the Jnk3-/- and Jnk2-/-Jnk3-/- mice in comparison to the Jnk2+/?Jnk3+/? mice (n ≥ 4 for each genotype and time point; *, P < 0.05, comparing mutant to wildtype mice at same time point). B,C Counts of Nissl stained neurons in retinal flat mounts (RGC side up; representative images shown in B) shows that combined, but not single deficiency of Jnk2 and Jnk3 provides long term protection of RGCs out to at least 35 days (n ≥ 5 for all genotypes and time points). Though, by 35 days it is clear that RGCs are dying in the large numbers in Jnk2-/-Jnk3-/- mice (# P < 0.05, comparing 35 day double mutant mice to Sham of same genotype). Note, only RGCs die after CONC and 40-60% of RGC layer neurons are amacrine cells (Jeon et al., 1998; Li et al., 1999; Li et al., 2007; Quigley et al., 2011); thus, a loss of half of RGC layer neurons is approximately complete RGC loss. Scale bar, 50um.
After CONC, approximately 60% of RGCs are dead by 14 days (Harder and Libby, 2011; Li et al., 2007). To determine if combined deficiency of Jnk2/3 provides long-lasting protection to axonally injured RGCs, the number of Nissl stained RGC layer neurons surviving 14 days after CONC were counted. This method has been used to identify molecules that protect RGCs from cell death following exposure to various glaucoma-relevant insults (Howell et al., 2007; Howell et al., 2011; Li et al., 2000; Li et al., 2007; Libby et al., 2005). Note, approximately 40-60% of RGC layer neurons are amacrine cells in mice (Jeon et al., 1998; Li et al., 1999; Li et al., 2007; Quigley et al., 2011) and do not die after CONC injury (Kielczewski et al., 2005), therefore a loss of 50% of RGC layer neurons reflects near complete RGC loss. Developmentally, there is no difference in the number of RGC layer neurons in the Jnk2+/?Jnk3+/?and the Jnk2-/-Jnk3-/- mice (data not shown). Single deficiency in either Jnk2 or Jnk3 did not provide any protection by this time point (Fig. 3B, expressed as % of sham ± SEM: Jnk2+/?Jnk3+/?, 65 ± 6% ; Jnk2-/-, 68 ± 3%; Jnk3-/-, 69 ± 3%; P > 0.05). Remarkably, combined deficiency of both Jnk2 and Jnk3 significantly prevented RGC death at 14 days after CONC (Fig. 3C, expressed as % of sham ± SEM: Jnk2-/-Jnk3-/-, 89 ± 3%; P < 0.05). To further determine if Jnk2 and Jnk3 are required for axonal injury-induced death, RGC survival was assessed 35 days after CONC. At this time-point, while the number of surviving GCL neurons in Jnk2-/-Jnk3-/- mice was still significantly higher compared to Jnk2+/?Jnk3+/? mice (Fig. 3C, expressed as % of sham ± SEM: Jnk2+/?Jnk3+/?, 60 ± 3% ; Jnk2-/-Jnk3-/-, 73 ± 3%; P < 0.05 ), there was significant attrition of GCL neurons compared to sham-treated eyes of the same genotype (Fig. 3B, expressed as % of sham ± SEM: Sham Jnk2-/-Jnk3-/-, 100 ± 9% ; CONC 35 days Jnk2-/-Jnk3-/-, 73 ± 3%; P < 0.05 ) These data suggest that while Jnk2/3 provide long-lasting protection to axonally injured RGCs, a Jnk2/3 independent cell death pathway can ultimately kill at least some RGCs. The Nissl findings were confirmed by counting RGCs labeled with TUJ1, an RGC-marker whose expression is sustained following injury (Cui et al., 2003; Harder and Libby, 2011; Park et al., 2008). 89% of TUJ1 labeled cells survived in Jnk2-/-Jnk3-/- retinas (n = 3) compared to 48% in Jnk2+/?Jnk3+/? mice 14 days following CONC (n = 4). Notably, both of the methods, Nissl staining of GCL neurons and TUJ1 staining of RGCs, are consistent and show a similar significant RGC protection in Jnk2-/-Jnk3-/- mice following CONC.
Deficiency in Jnk2 and Jnk3 prevents BRN3B downregulation following axonal injury
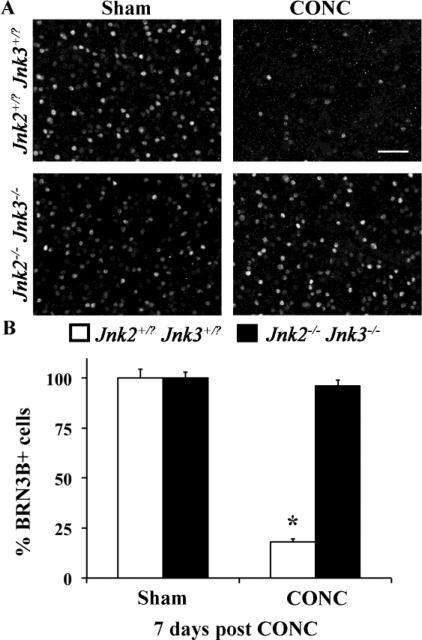
BRN3B (POU4F2) is a transcription factor that is specifically expressed in RGCs, labeling ~80% of RGCs in the retina (Badea et al., 2009; Moshiri et al., 2008). It is important for RGC survival during development (Gan et al., 1999) and is downregulated very early after RGC injury before RGC death, including, after optic nerve injury (Pelzel et al., 2010; Weishaupt et al., 2005). There was no difference in the number of BRN3B+ cells in sham treated eyes of either genotype, Jnk2+/?Jnk3+/? or Jnk2-/-Jnk3-/- eyes (cells per mm2 ± SEM: Jnk2+/?Jnk3+/?, 1799.3 ± 81.6; Jnk2-/-Jnk3-/-, 1771.4 ± 54.4; n = 3, P = 0.65). Confirming the downregulation of BRN3B after axonal injury, only 18% (Fig. 4; P < 0.001) of RGCs are BRN3B+ seven days after injury in the Jnk2+/?Jnk3+/? mice even though there are approximately half the RGCs still surviving 2 weeks after injury (see TUJ1 counts above). In the Jnk2-/-Jnk3-/- mutants, the number of BRN3B + cells seven days after CONC was similar to the number of BRN3B + cells in control eyes (96% of unmanipulated Jnk2-/-Jnk3-/- mutants; P = 0.41; Fig. 4). These data support that hypothesis that JNK signaling is an early event in the overall response after axonal injury in RGCs.
Figure 4. The RGC-specific marker, BRN3B is not downregulated in Jnk2-/-Jnk3-/- mice.
A, Representative images showing BRN3B immunolabeled cells in the GCL of Jnk2+/?Jnk3+/? or Jnk2-/-Jnk3-/- mice 7 days following sham treatment or controlled optic nerve crush (CONC). B,The number of BRN3B positive cells in the Jnk2+/?Jnk3+/? retinas was significantly reduced at 7 days post CONC, reflecting both RGC death and/or BRN3B downregulation. In contrast, in the Jnk2-/-Jnk3-/- mice, the number of BRN3B positive cells at 7 days post CONC was indistinguishable from the number of BRN3B positive cells in sham-treated eyes confirming the protection provided by Jnk2/3 deficiency. n = 3 for each genotype; *, P < 0.001; scale bar, 50um.
Phosphorylation of JUN parallels RGC death after axonal injury
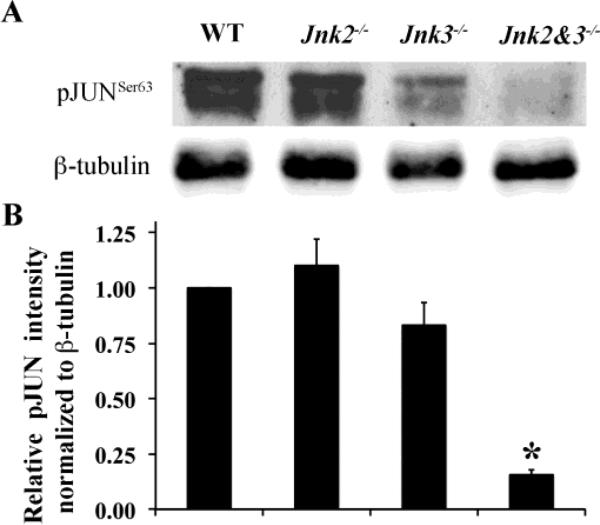
In other regions of the nervous system, mice deficient in specific Jnk isoforms show reduced apoptotic cell death following exposure to various insults (Pirianov et al., 2007; Yang et al., 1997). Protection in these studies has been attributed to a reduction in phosphorylated JUN (pJUN) leading to an attenuation of JUN/AP-1 activity. JUN is known to be upregulated after various axonal and/or glaucoma relevant insults in RGCs (Isenmann and Bahr, 1997; Levkovitch-Verbin et al., 2005; Munemasa et al., 2006). To determine if JNK could be mediating RGC death by a JUN dependent pathway after axonal injury, activation of JUN (as measured by phosphorylation of Ser-63) was assessed in wildtype and Jnk mutant mice after CONC. The expression of pJUN was detectable only after injury (Fig 1b). By three days after CONC, Jnk2-/- mice had similar levels of pJUN as control mice and Jnk3-/- mice consistently had less pJUN than control mice though this was not significantly different (Fig 5). Jnk2-/-Jnk3-/- mice had a significant reduction in pJUN (16% of Jnk2+/?Jnk3+/? levels; P < 0.001). The decreased levels of JUN activation in the Jnk3-/- and Jnk2-/-Jnk3-/- mutant mice parallel the decreased number dying cells (Fig 3a). These data suggest that JUN may be a critical pro-death target of pJNK in RGCs.
Figure 5. Phosphorylation of JUN is attenuated in the Jnk3-/- and Jnk2-/-Jnk3-/- nulls.
A, Representative western blot showing the expression of pJUNSer63 3 days post CONC (Ser63 is the residue that is phosphorylated by JNK, (Pulverer et al., 1991). B, Quantification by densitometric analyses of 4 different blots using retinal protein lysates from 4 different eyes shows that the relative amounts of pJUNSer63 (normalized to β-tubulin) is significantly reduced in the Jnk2-/-Jnk3-/- retinas compared to controls. *, P < 0.001.
JUN is critical for RGC death after CONC
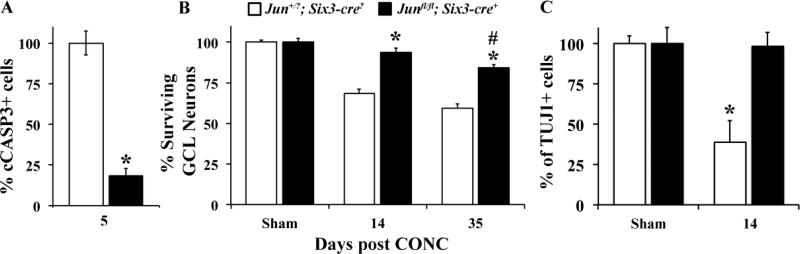
To determine if JUN is required for RGC death after axonal injury, CONC was performed on mice with retinal deletion of Jun. Since deletion of Jun is embryonically lethal (Hilberg et al., 1993; Johnson et al., 1993), JUN was conditionally deleted in the retina using a floxed allele of Jun (Junfl/fl, (Behrens et al., 2002)) and an early retinal deleter cre, Six3-cre (Furuta et al., 2000). In Junfl/fl; Six3cre+ mice JUN was deleted from almost all RGCs, as judged by the number of JUN+ RGCs compared to wildtype eyes 1 day after CONC (data not shown). Developmental deletion of JUN in the retina did not alter the number of RGCs (TUJ1+ cells per mm2 ± SEM: Sham Jun+/?; Six3cre?, 3142.18 ± 153.1; Sham Junfl/fl; Six3cre+, 2821.45 ± 284.6; n ≥ 3, P = 0.31). Jun deficiency significantly decreased the number of cCASP3+ cells during the peak of RGC cell death after CONC (5 days; Figure 6A). Nissl counts of surviving RGC layer neurons 14 days after CONC revealed robust survival of RGCs in the Junfl/fl; Six3cre+ retinas (Fig. 6B). Almost all GCL neurons survive in Junfl/fl; Six3cre+ mice 14 days after CONC. To confirm the presence of RGCs 14 days after CONC, immunohistochemistry was performed using a RGC marker, TUJ1 (Cui et al., 2003; Robinson and Madison, 2004). TUJ1 counts confirmed that 98% of RGCs survive 14 days after CONC in Junfl/fl; Six3cre+ mice (Fig. 6C; expressed as % of sham ± SEM: Jun+/?; Six3cre?, 39 ± 13%, Junfl/fl; Six3cre+, 98 ± 8.8% ; P < 0.05 ). At 35 days after CONC, nissl counts revealed that the number of surviving RGC layer neurons was still significantly higher in the Junfl/fl; Six3cre+ retinas compared to Jun+/?; Six3cre? (expressed as % of sham ± SEM: Junfl/fl; Six3cre+, 84 ± 3.7%, Jun+/?; Six3cre?, 59.2 ± 2.8% ; P < 0.05 ). Although at this time-point there was a significant reduction in GCL neuron number after CONC in Junfl/fl; Six3cre+ compared to sham treated Junfl/fl; Six3cre+ eyes (Fig. 6B; P < 0.01). Collectively these data demonstrate that JUN is critical for RGC death during the first several weeks following CONC; however, later on, JUN-independent pathways can ultimately kill an injured RGC.
Figure 6. Deficiency of JUN protects RGCs from CONC-induced death.
A, Deletion of Jun in the retina (Junfl/fl; Six3-cre+) significantly reduced (*, P < 0.001) the number of dying cells (cCASP3+) 5 days after CONC compared to Jun+/?; Six3-cre- mice. B, Nissl counts of the number of surviving GCL neurons showed that Jun deficiency protected from axonal-injury induced death at 14 days following CONC (n ≥ 5 per group; *, P < 0.001). Counts of surviving GCL neurons in Junfl/fl; Six3-cre+ mice 35 days following CONC indicate both a increased survival compared to Jun+/?; Six3-cre? mice (*, P = 0.001) and a significant loss compared to the uninjured Junfl/fl; Six3-cre+ mice (#, P = 0.008). C, Counts using the RGC marker TUJ1 at 14 days after injury confirmed both the significant loss of RGCs in the Jun+/?; Six3-cre- mice (*, P < 0.001) and the prevention of RGC death in Junfl/fl; Six3-cre+ retinas.
Discussion
Numerous studies have shown that axonal injury is a critical insult to RGCs in glaucoma and that axon damage precedes somal degeneration(Anderson and Hendrickson, 1974; Buckingham et al., 2008; Howell et al., 2007; Howell et al., 2011; Quigley et al., 1983; Schlamp et al., 2006). However, the molecular pathways triggered by the glaucomatous insult to the axon and the pathways that lead to RGC death are poorly defined. It is likely that among the first changes to occur following axonal injury are phosphorylation-dependent signal relay events that transfer the injury signal from the axon to the cell body (Abe and Cavalli, 2008; Lukas et al., 2009). A phospho-relay system can also alter gene expression by phosphorylating transcription factors and increasing their transactivation potential, thereby influencing a cell's response to injury. The c-Jun N-terminal kinase (JNK) family encoded by JNK1, JNK2 and JNK3, belongs to the MAPK phospho-relay system and is known to be involved in axon injury signaling (Abe and Cavalli, 2008). Retrograde transport of JNK signaling molecules, reported in peripheral nerve axons following injury, was found to influence the cell body's injury response (Lindwall and Kanje, 2005). Interestingly, the existence of a retrograde death signaling system in the axon has also been suggested in sympathetic neurons undergoing neurotrophic deprivation-induced death (Mok et al., 2009), a key insult in glaucoma (Johnson et al., 2009). Thus, JNK signaling could be a critical pathway linking axonal injury to prodeath signaling changes in injured retinal ganglion cells.
JNK signaling is known to occur in retinal ganglion cells after many glaucoma relevant injuries, and activated JNK is present in RGCs in human glaucoma patients (Tezel et al., 2003). Previous studies using JNK inhibitors have shown that JNK signaling does play a role in RGC death after injury (Liu et al., 2011; Tezel et al., 2004; Yang et al., 2008). However, no studies have determined whether JNK signaling is the predominant pro-apoptotic pathway regulating RGC death following axonal injury. Furthermore, it is unknown whether specific isoforms are involved and if so, which JNK isoforms are important. Different JNK isoforms have distinct localization within neurons, so it is important to identify which isoforms are involved in axonal injury-induced RGC death (Lee et al., 1999). Further, the specific JNK isoforms that get activated are stimulus dependent, thereby altering the requirement for specific isoforms (Brecht et al., 2005). Our data demonstrates that JNK signaling, mediated by activation of Jnk2 and Jnk3, is a major regulator of axonal injury-induced RGC death up to 35 days following axonal injury. In fact, Jnk2/3 deficiency almost completely prevents cell death during the peak of cell death after CONC (5 days post injury). Interestingly, Jnk3 but not Jnk2 deficiency reduced RGC death at early time points after injury, suggesting that JNK3 has a larger role in mediating RGC death. Quigley and colleagues (Quigley et al., 2011) recently reported that there was no protection from elevated intraocular pressure-induced RGC loss in Jnk3 deficient mice. However, this model may not be optimal for demonstrating a potential protective effect of Jnk3 deficiency since despite significant IOP elevation, there was only limited loss of RGCs (~3%) in both control and Jnk3 deficient retinas. Thus, it remains to be determined whether Jnk deficiency plays a significant role in glaucomatous RGC death.
Interestingly, JNK signaling has also been implicated in axon degeneration, one of the primary causes of glaucoma. Axon transport defects are known to trigger axon degeneration (Coleman, 2005) and have been implicated in the pathogenesis of several neurodegenerative diseases, including glaucoma (Chidlow et al., 2011). Jnk3 has been shown to inhibit fast axonal transport by phosphorylating the motor protein, kinesin-1, decreasing its ability to bind microtubules, in a mouse model of Huntington's disease (Morfini et al., 2009). In another study involving a dorsal root ganglion axotomy model, JNK activity was also found to be required for the commitment to degenerate as inhibiting JNK activity in the early phase following axotomy prevented axon fragmentation (Miller et al., 2009). Despite their potential importance in glaucoma, the signaling pathways activated in the RGC axon by axonal injury remain undefined. Interestingly, pJNK was found in the axons just after CONC injury (mainly JNK3 but some JNK2 as well). Thus, it is possible that JNK signaling is a common pathway linking axon degeneration to somal death. Importantly, activation of JNK signaling locally in RGC axons is one of the earliest molecular events to be directly correlated with axonal injury and RGC loss.
Identifying what pathways are activating JNK in the axon will be important in identifying key early insults that are responsible for triggering RGC death after axonal injury and potentially in glaucoma. Cell-intrinsic pathways activated by damage to the axonal cytoskeleton are likely to contribute to JNK activation in RGC axons following CONC. Mechanical injury to RGCs axons could cause microtubule disruption, a stress stimuli known to activate JNK (Huang et al., 2011).
Elevation of intra-axonal Ca2+ following optic nerve crush injury (Knoferle et al., 2010) can also result in microtubule disruption through Ca2+ mediated activation of calpain proteases (George et al., 1995). However, in glaucoma, RGC-extrinsic signals such as the inflammatory cytokines, IL-1 or TNFα could also activate JNK signaling in RGCs. Both of these are known to be potent activators of JNK signaling (Natoli et al., 1997) and are produced by glia (Lebrun-Julien et al., 2010; Tezel et al., 2001; Tezel et al., 2004).
Downstream of JNK, several of its substrates are known to have pro-apoptotic functions (Bogoyevitch and Kobe, 2006). Of these, the canonical target, JUN (Hibi et al., 1993), is a transcription factor known to be induced following axonal injury (Schenkel, 2004). However, JUN can exert either a pro-survival or pro-apoptotic role depending on the cellular context (Leppa and Bohmann, 1999). A pro-apoptotic role for JUN was established in RGCs following axotomy (Lingor et al., 2005; Yoshida et al., 2002). In both of these studies, only minor protection was reported at time points after the majority of RGC death had occurred, and earlier time points were not assessed. It is likely that the methods used by these studies failed to completely inhibit JUN. Lingor et al. (2005) used siRNA to inhibit JUN after axotomy, which may not have been able to provide stable, long-term knockdown in every cell. Yoshida et al. (2002) assessed RGC survival after axotomy in mice homozygous for a Jun allele that lacked two of the four JNK phosphorylation sites (SER63 and SER73). The fact that significant death still occurs in these mice 14 days after axotomy could be because JUN's transactivation potential is only attenuated, but not abolished (Behrens et al., 1999). To determine if JUN activation is an important primary pathway leading to RGC death, we directly investigated the importance of JUN after axonal injury. Consistent with previous studies (Levkovitch-Verbin et al., 2005; Yoshida et al., 2002), JUN was phosphorylated after axonal injury in RGCs. The differences in the amount of JUN phosphorylation in wild type and Jnk mutant mice correlated with the differences in the number of dying cells (see Fig 3A and Fig 5), suggesting that JUN was an important mediator of RGC death. Analysis of Jnk mutant mice showed that JNK2 and JNK3 appear to be the major isoforms that phosphorylate JUN in RGCs, which is consistent with the role these isoforms play in controlling RGC death after axonal injury. In the absence of JUN, RGC death was significantly delayed well past the peak of RGC death after CONC. In fact, at a time point in wildtype mice when more than half of RGCs are dead (14 days) almost 100% of RGCs survive in Jun deficient mice (Figure 6C). When RGC death is nearly complete in wildtype mice (35 days) there is still significant RGC survival in Jun deficient mice. Given JUN's primary function is as a transcription factor, these data suggest that transcription is critical for the pro-death response in axonally injured RGCs.
In summary, while upregulation of several pro-apoptotic molecules in RGC somas following axonal injury have been reported, the signaling pathways activated locally at/close to the site of insult remain undefined. Our results show that a JNK2/3 signaling pathway is a critical axonal injury response pathway in RGCs acting by predominately activating the transcription factor JUN. Interestingly, deficiency of Jnk2 and Jnk3 also prevented the downregulation of BRN3B, a RGC specific marker following axonal injury. During development, BRN3B is essential for RGC survival (Pan et al., 2005), and loss of BRN3B alters the expression of several genes associated with neuron integrity and function (Wang et al., 2002). The lack of BRN3B downregulation in these mice is consistent with JNK signaling being upstream of several injury response pathways in injured RGCs. Our data indicate that a JNK2/3 and JUN-dependent pathway is the major regulator of BAX activation responsible for nearly all RGC death within the first two weeks after injury. Going forward it will be important to determine the molecular pathway that activates JNK as well as the transcriptional targets of JUN that are responsible for killing RGCs. However, it is important to note that unlike in Bax deficient mice, RGCs ultimately die after axonal injury in Jun deficient animals. Therefore to fully understand BAX activation after axonal injury, the JUN independent pathways that ultimately trigger BAX activation will need to be identified. Since axonal injury is likely to be an early event in glaucoma and JNK signaling is active in glaucomatous eyes, understanding this pathway is likely to be key to understanding the pathophysiology of glaucoma.
Supplementary Material
Highlights.
JNK signaling is activated in retinal ganglion cell axons soon after axonal injury
Specific JNK isoforms, JNK2 and JNK3, are activated in the axon
Inhibition of JNK2 and 3 significantly delays death after axonal injury
JUN is the downstream effector of JNK activation
Acknowledgements
The authors would like to thank Thurma McDaniel and Donna Shannon for technical help and the members of Kiernan and Gan laboratories for helpful comments and discussion about the work. This work was supported by The Glaucoma Foundation (RTL), EY018606 (RTL), T32 EY007125 (JMH), David Bryant Trust (RTL), Research to Prevent Blindness Career Development Award (RTL), Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology at the University of Rochester. S.W.M. John is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Cavalli V. Nerve injury signaling. Current opinion in neurobiology. 2008;18:276–83. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–83. [PubMed] [Google Scholar]
- Angel P, et al. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–85. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Badea TC, et al. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–64. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, et al. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. The EMBO journal. 2002;21:1782–90. doi: 10.1093/emboj/21.7.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, et al. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nature genetics. 1999;21:326–9. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–95. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht S, et al. Specific pathophysiological functions of JNK isoforms in the brain. Eur J Neurosci. 2005;21:363–77. doi: 10.1111/j.1460-9568.2005.03857.x. [DOI] [PubMed] [Google Scholar]
- Buckingham BP, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. The Journal of neuroscience. 2008;28:2735–44. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, et al. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–87. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, et al. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta neuropathologica. 2011;121:737–51. doi: 10.1007/s00401-011-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nature reviews. Neuroscience. 2005;6:889–98. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Cui Q, et al. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Molecular and cellular neurosciences. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Eilers A, et al. Direct inhibition of c-Jun N-terminal kinase in sympathetic neurones prevents c-jun promoter activation and NGF withdrawal-induced death. J Neurochem. 2001;76:1439–54. doi: 10.1046/j.1471-4159.2001.00150.x. [DOI] [PubMed] [Google Scholar]
- Furuta Y, et al. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–2. [PubMed] [Google Scholar]
- Gan L, et al. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999;210:469–80. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- George EB, et al. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. The Journal of neuroscience. 1995;15:6445–52. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J, et al. c-Jun and the transcriptional control of neuronal apoptosis. Biochem Pharmacol. 2000;60:1015–21. doi: 10.1016/s0006-2952(00)00372-5. [DOI] [PubMed] [Google Scholar]
- Hanz S, Fainzilber M. Retrograde signaling in injured nerve--the axon reaction revisited. J Neurochem. 2006;99:13–9. doi: 10.1111/j.1471-4159.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- Harder JM, Libby RT. BBC3 (PUMA) regulates developmental apoptosis but not axonal injury induced death in the retina. Molecular neurodegeneration. 2011;6:50. doi: 10.1186/1750-1326-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M, et al. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–48. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hilberg F, et al. c-jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–81. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- Howell GR, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. The Journal of cell biology. 2007;179:1523–37. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. The Journal of clinical investigation. 2011;121:1429–44. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, et al. Distortion of axonal cytoskeleton: an early sign of glaucomatous damage. Investigative ophthalmology & visual science. 2011;52:2879–88. doi: 10.1167/iovs.10-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenmann S, Bahr M. Expression of c-Jun protein in degenerating retinal ganglion cells after optic nerve lesion in the rat. Exp Neurol. 1997;147:28–36. doi: 10.1006/exnr.1997.6585. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, et al. The major cell populations of the mouse retina. The Journal of neuroscience. 1998;18:8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, et al. Neurotrophin roles in retinal ganglion cell survival: lessons from rat glaucoma models. Exp Eye Res. 2009;88:808–15. doi: 10.1016/j.exer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RS, et al. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes & development. 1993;7:1309–17. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Kielczewski JL, et al. The effect of experimental glaucoma and optic nerve transection on amacrine cells in the rat retina. Investigative ophthalmology & visual science. 2005;46:3188–96. doi: 10.1167/iovs.05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoferle J, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6064–9. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CY, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15184–9. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JM, Caprioli J. Expression of phosphorylated c-Jun N-terminal protein kinase (JNK) in experimental glaucoma in rats. Experimental eye research. 2006;82:576–82. doi: 10.1016/j.exer.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, et al. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3817–22. doi: 10.1073/pnas.0909276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, et al. Distinct localization of SAPK isoforms in neurons of adult mouse brain implies multiple signaling modes of SAPK pathway. Brain research. Molecular brain research. 1999;70:116–24. doi: 10.1016/s0169-328x(99)00136-9. [DOI] [PubMed] [Google Scholar]
- Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–62. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, et al. The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma. Exp Eye Res. 2005;80:663–70. doi: 10.1016/j.exer.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Experimental induction of retinal ganglion cell death in adult mice. Investigative Ophthalmology & Visual Science. 1999;40:1004–8. [PubMed] [Google Scholar]
- Li Y, et al. Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Experimental eye research. 2000;71:209–13. doi: 10.1006/exer.2000.0873. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Dominant inheritance of retinal ganglion cell resistance to optic nerve crush in mice. BMC neuroscience. 2007;8:19. doi: 10.1186/1471-2202-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Kanje M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci. 2005;29:269–82. doi: 10.1016/j.mcn.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Lingor P, et al. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain : a journal of neurology. 2005;128:550–8. doi: 10.1093/brain/awh382. [DOI] [PubMed] [Google Scholar]
- Liu H, et al. Interference of the apoptotic signaling pathway in RGC stress response by SP600125 in moderate ocular hypertensive rats. The Chinese journal of physiology. 2011;54:124–32. [PubMed] [Google Scholar]
- Lukas TJ, et al. Early cellular signaling responses to axonal injury. Cell communication and signaling : CCS. 2009;7:5. doi: 10.1186/1478-811X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, et al. dp5/HRK is a c-Jun target gene and required for apoptosis induced by potassium deprivation in cerebellar granule neurons. The Journal of biological chemistry. 2007;282:30901–9. doi: 10.1074/jbc.M608694200. [DOI] [PubMed] [Google Scholar]
- Martin JH, et al. Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res Mol Brain Res. 1996;35:47–57. doi: 10.1016/0169-328x(95)00181-q. [DOI] [PubMed] [Google Scholar]
- Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nature neuroscience. 2009;12:387–9. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SA, et al. A retrograde apoptotic signal originating in NGF-deprived distal axons of rat sympathetic neurons in compartmented cultures. Cell research. 2009;19:546–60. doi: 10.1038/cr.2009.11. [DOI] [PubMed] [Google Scholar]
- Morfini GA, et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nature neuroscience. 2009;12:864–71. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, et al. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–60. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri A, et al. Near complete loss of retinal ganglion cells in the math5/brn3b double knockout elicits severe reductions of other cell types during retinal development. Developmental biology. 2008;316:214–27. doi: 10.1016/j.ydbio.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller GJ, et al. A role for mixed lineage kinases in granule cell apoptosis induced by cytoskeletal disruption. Journal of neurochemistry. 2006;96:1242–52. doi: 10.1111/j.1471-4159.2005.03590.x. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, et al. Pro-apoptotic role of c-Jun in NMDA-induced neurotoxicity in the rat retina. J Neurosci Res. 2006;83:907–18. doi: 10.1002/jnr.20786. [DOI] [PubMed] [Google Scholar]
- Natoli G, et al. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–3. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- Oliver G, et al. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–55. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Pan L, et al. Functional equivalence of Brn3 POU-domain transcription factors in mouse retinal neurogenesis. Development. 2005;132:703–12. doi: 10.1242/dev.01646. [DOI] [PubMed] [Google Scholar]
- Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–6. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzel HR, et al. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC neuroscience. 2010;11:62. doi: 10.1186/1471-2202-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin V, et al. Implication of the JNK pathway in a rat model of Huntington's disease. Exp Neurol. 2009;215:191–200. doi: 10.1016/j.expneurol.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Pirianov G, et al. Deletion of the c-Jun N-terminal kinase 3 gene protects neonatal mice against cerebral hypoxic-ischaemic injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1022–32. doi: 10.1038/sj.jcbfm.9600413. [DOI] [PubMed] [Google Scholar]
- Pulverer BJ, et al. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–4. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Quigley HA, et al. Lack of neuroprotection against experimental glaucoma in c-Jun N-terminal kinase 3 knockout mice. Experimental eye research. 2011;92:299–305. doi: 10.1016/j.exer.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, et al. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983;95:673–91. doi: 10.1016/0002-9394(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Raivich G, Behrens A. Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog Neurobiol. 2006;78:347–63. doi: 10.1016/j.pneurobio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ries V, et al. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. Journal of neurochemistry. 2008;107:1578–88. doi: 10.1111/j.1471-4159.2008.05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Axotomized mouse retinal ganglion cells containing melanopsin show enhanced survival, but not enhanced axon regrowth into a peripheral nerve graft. Vision research. 2004;44:2667–74. doi: 10.1016/j.visres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Roth S, et al. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003;44:5383–95. doi: 10.1167/iovs.03-0451. [DOI] [PubMed] [Google Scholar]
- Schenkel J. Activation of the c-Jun transcription factor following neurodegeneration in vivo. Neuroscience letters. 2004;361:36–9. doi: 10.1016/j.neulet.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, et al. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC neuroscience. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, et al. Protective effect of a JNK inhibitor against retinal ganglion cell loss induced by acute moderate ocular hypertension. Molecular vision. 2011;17:864–75. [PMC free article] [PubMed] [Google Scholar]
- Tezel G, et al. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–33. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- Tezel G, et al. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Investigative Ophthalmology & Visual Science. 2001;42:1787–94. [PubMed] [Google Scholar]
- Tezel G, et al. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996:202–12. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Wang SW, et al. Retinal ganglion cell differentiation in cultured mouse retinal explants. Methods. 2002;28:448–56. doi: 10.1016/s1046-2023(02)00264-5. [DOI] [PubMed] [Google Scholar]
- Weishaupt JH, et al. Axotomy-induced early down-regulation of POU-IV class transcription factors Brn-3a and Brn-3b in retinal ganglion cells. Journal of molecular neuroscience : MN. 2005;26:17–25. doi: 10.1385/JMN:26:1:017. [DOI] [PubMed] [Google Scholar]
- Whitfield J, et al. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–43. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Xia XG, et al. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:10433–8. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DD, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–70. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Yang X, et al. Phosphorylation-dependent interaction with 14-3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008;49:2483–94. doi: 10.1167/iovs.07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, et al. Amino-terminal phosphorylation of c-Jun regulates apoptosis in the retinal ganglion cells by optic nerve transection. Invest Ophthalmol Vis Sci. 2002;43:1631–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.