Abstract
Intravenous infusion studies in humans suggest that both VWF and FVIII remain intravascular in contrast to other coagulation proteins. We explored whether infusion of VWF and FVIII by either intraperitoneal (IP) or subcutaneous (SC) injection would result in efficient absorption of these large proteins into the vascular circulation. FVIIInull or VWFnull mice were infused with plasma-derived or recombinant VWF and/or FVIII by IP, SC, or intravenous (IV) injection. Both VWF and FVIII were absorbed into the blood circulation after IP injection with a peak between 2 to 4 hours at levels similar to those observed in mice infused intravenously. In contrast, neither VWF nor FVIII was detected in the plasma following SC injection. Although IV injection achieved peak plasma levels quickly, both human VWF and FVIII rapidly decreased during the first 2 hours following IV injection. Following both IV and IP infusion of VWF, the multimeric structure of circulating VWF was similar to that observed in the infusate. These results demonstrate that both VWF and FVIII can be efficiently absorbed into the blood circulation following IP but not SC injection, indicating that IP administration could be an alternative route for VWF or FVIII infusion.
Keywords: FVIII, VWF, Infusion, Intraperitoneal
Introduction
von Willebrand factor (VWF) is a large multivalent adhesive protein that serves as a carrier protein for factor VIII (FVIII) and an adhesive link between platelets and the injured blood vessel wall.1, 2 The basic VWF monomer is a 2050 amino acid protein. Multimers of VWF can be extremely large, ranging in size from about 450 kDa dimers to greater than 20,000 kDa.3-5 FVIII is synthesized as a 2351 amino acid single-chain glycoprotein with a molecular weight of 280 kDa, which is proteolytically processed to its circulating heterodimeric form, composed of a heavy chain and a light chain.6 FVIII binds VWF non-covalently, forming a VWF/FVIII complex in plasma.7, 8 The deficiency of VWF results in von Willebrand disease (VWD), which is a genetic bleeding disorder.9 The deficiency of FVIII causes hemophilia A, which is an X-chromosome linked genetic bleeding disorder.10
Both severe VWD and hemophilia A are normally treated by intravenous infusion of either plasma-derived or recombinant proteins.11-13 Both VWF and FVIII are large adhesive proteins and intravenous infusion studies in humans suggest that both proteins remain intravascular in contrast to other coagulation proteins, such as factor IX (FIX).14-16 When FIX is injected intraperitoneally (IP) or subcutaneously (SC) it does achieve access to the vasculature.17 Large proteins, like VWF and FVIII, have been presumed to be inefficiently transferred into the vascular space after SC or IP administration. There are at least two reasons driving us to investigate whether IP or SC administration of these two large proteins can transfer into the vascular space. One is to explore an effective alternative route for VWF and FVIII infusion without venous access, which at times might be difficult particularly in the pediatric population. The other is for gene therapy of von Willebrand disease (VWD) or hemophilia A. It is well known that the bulk of plasma VWF is derived from endothelial synthesis.18 In contrast, the precise site of FVIII biosynthesis remains unclear,19-22 although it has been proposed that synthesis of FVIII occurs in a subpopulation of endothelial cells.2, 23-27 While the cell that naturally produces VWF might be the ideal target for VWF expression for severe VWD gene therapy, it is difficult to genetically modify endothelial cells and implant them into the vasculature. Instead, if VWF can be transferred into the vascular circulation from extra-vascular space such as the peritoneal cavity, implanting genetically manipulated cells that express VWF may serve as a mechanism for VWD gene therapy. The similar approach may also serve as a mechanism for hemophilia A gene therapy. The aim of this study is to investigate whether VWF and FVIII can be transferred from extra-vascular space into the vascular space. To address this issue, we infused VWF and / or FVIII from various sources into mice using various routes to investigate whether infusion of VWF and FVIII by either IP or SC injection would result in efficient absorption of these large proteins into the vascular circulation.
Materials and Methods
Materials
Alphanate, a human plasma-derived VWF/FVIII concentrate, was obtained from Grifols Biological Inc (Los Angeles, CA). Humate P, another human plasma-derived VWF/FVIII concentrate containing high molecular multimers,28 was obtained from CSL Behring (King of Prussia, PA). Refacto, a recombinant human B-domain deleted FVIII (rhFVIII), was obtained from Wyeth Pharmaceuticals (Collegeville, PA). Human recombinant VWF was kindly provided by Baxter International Inc. (Vienna, Austria). The Coatest SP4 FVIII Kit was purchased from DiaPharma (Franklin, OH). Biotin-conjugated and non-conjugated rabbit anti-human VWF antibody, which cross-reacts with murine VWF, was purchased from Dako (Carpinteria, CA). Both mouse anti-human VWF monoclonal antibody, AVW1, and mouse anti-mouse VWF monoclonal antibody, 344.3, were produced by our laboratory. PNPP (p-Nitrophenyl Phosphate, Disodium Salt) substrate was purchased from Thermo Fisher Scientific (Rockford, IL). PPACK (D-Phe-Pro-Arg-chloromethylketone) was purchased from Enzo Life Sciences International, Inc. (Plymouth Meeting, PA). Horseradish Peroxidase (HRP) conjugated Goat anti-rabbit antibody was purchased from Pierce (Rockford, IL).
Animal procedures
Animal studies were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Infusates were diluted in Blood Bank saline (0.85% NaCl, pH 6.7) (OpticsPlanet, Inc., Northbrook, IL) to 5 U/ml. FVIII knockout (FVIIInull) mice were infused with human plasma-derived VWF and FVIII (Alphanate or Human P) or recombinant FVIII (ReFacto) at a dose of 50 U / kg by intraperitoneal (IP), sub-cutaneous (SC), or intravenous (IV) administration. VWF knockout (VWFnull) mice were used for recombinant VWF (Baxter) infusion studies. All infusions were the first exposure for the animals.
As a comparison, normal mouse plasma, as a source of plasma derived VWF, was infused into VWFnull mice at a dose of 12.5 U / kg by IP or IV administration. For these experiments, plasma containing 1 U/ml of murine VWF was collected from C57BL/6 mice using PPACK anticoagulant at a final concentration of 80 μM.
For blood sampling, 100 μl of blood was collected by tail bleed into microcentrifuge tubes containing 0.1 vol of 0.1 M sodium citrate at various time points after infusion. Blood cells were removed by centrifugation at 5000 rpm for 10 min, platelet poor plasma was centrifuged once more at 10,000 rpm for 10 min and plasma was used for assays.
VWF antigen (VWF:Ag) assay
The levels of VWF:Ag were determined by means of a solid phase capture enzyme-linked immunosorbent assay (ELISA). For human VWF ELISA, AVW1 was used as a capture antibody. For mouse VWF ELISA, 344.3, was used as a capture antibody. Bound VWF was detected with biotin-conjugated rabbit anti-human VWF antibody (Dako) that cross reacts with murine VWF and Alkaline Phosphatase-conjugated Steptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA). After the final washing step, plates were developed with PNPP substrate and the change in optical density read at 405 nm. A standard curve was constructed by plotting known amounts of infusate against Vmax (mOD/min) at 405 nm.
FVIII:C assay
The levels of FVIII activity (FVIII:C) in the infused animals were quantitated by a modified FVIII chromogenic assay that we have developed using the Coatest SP4 FVIII Kit as previously reported.29 Briefly, plasma was diluted in 1x Coatest buffer, and 25 μl of diluted plasma was added to 96-well microtiter plates in duplicate. Assay components, including FIXa, FX, CaCl2, and phospholipid, were added to each well, and the plate was incubated at 37°C for 10 minutes. The chromogenic FXa substrate S-2765 was added and the plate was transferred immediately to a ThermoMax microplate reader (Molecular Devices, Sunnyvale, CA) preset at 37°C. A standard curve was constructed by plotting known amounts of rhFVIII (Refacto) in 1x Coatest buffer against Vmax (mOD/min) at 405 nm minus 490 nm.
VWF multimer analysis
VWF multimer structure was analyzed using non-reducing LiDS-agarose electrophoresis. VWF Multimers from plasma samples or infusates were separated through a 0.65% (w/v) HGT(P) agarose gel (Lonza, Rockland, ME) containing 0.1% lithium dodecyl sulfate (LiDS) (Fisher Scientific, Fair Lawn, NJ) for 4 hours at 120 V using a horizontal gel unit with running buffer containing 100 mmol/L Tris, 133 mmol/L glycine, and 0.1% LiDS. Proteins were then transblotted onto Immobilon-P PVDF membrane (Millipore, Medford, MA) for 2 hours at 12 V in 11 mmol/L NaH2PO4 and 40 mmol/L Na2HPO4. Membranes were then blocked with 5% nonfat dry milk for 1 hour and incubated overnight with rabbit anti-VWF antibody (Dako) at a concentration of 1 μg/ml. After washing with PBS containing 0.05% (v/v) Tween 20, membranes were incubated with Horseradish Peroxidase (HRP) conjugated Goat anti-rabbit antibody at 1:20,000 for 1 hour, developed with Pierce SuperSignal Chemiluminescent substrate, and bands were visualized by exposure to x-ray film (BioMax film; Eastman Kodak, Rochester, NY).
Data analysis and statistical analysis
The half-lives of the VWF or FVIII were determined by calculation of the first-order rate constant for the elimination phase from the slope of the VWF:Ag or FVIII concentration against time.30, 31 The levels of VWF:Ag and FVIII:C from various time points were used to generate the factors in a formula for exponential decay [C (t) = C0 e−λt] using Excel software. C0 is the initial concentration of VWF or FVIII, and C (t) is the concentration remaining at a given time t. λ is the calculated decay constant. The half-life (t1/2) was calculated from exponential decay using a formula: t1/2 = ln2 / λ.
The data are presented as mean ± SD. The significance of differences between groups of mice was evaluated by 2-tailed Student’s t test. A value of P < 0.05 was considered statistically significant.
Results
Recovery of human VWF or FVIII in the vascular circulation in mice after protein administration using various routes
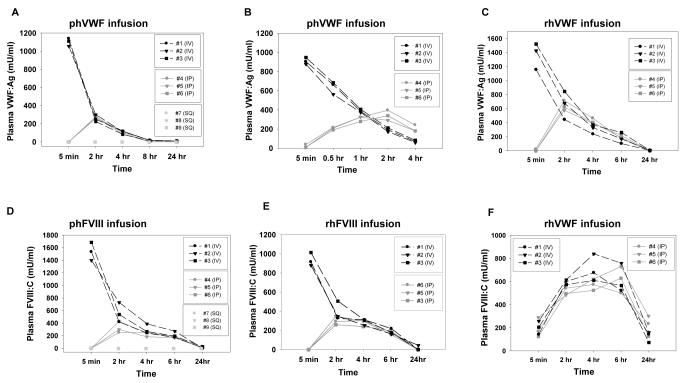
Both human VWF and FVIII, either plasma-derived or recombinant, were absorbed into the blood circulation after IP injection with a peak between 2 to 4 hours at levels similar to those observed at these time points in mice infused intravenously. In contrast, neither VWF nor FVIII was detected in the plasma following SC injection. Although IV injection achieved peak plasma levels quickly, both human VWF and FVIII rapidly decreased during the first 2 hours following IV injection (Figures 1A-E). As expected, endogenous murine FVIII was rescued in VWFnull mice after VWF infusion by both IV and IP administration (Figure 1F).
Figure 1. Circulating levels of VWF and FVIII in the infused animals.
Animals were infused with human VWF and /or FVIII from various sources at 50 IU/kg using varying routes. Plasma samples were collected at various time points for VWF:Ag ELISA or FVIII:C assay. Fig. 1A shows the levels of VWF in FVIIInull mice over a 24 hour period after infusion of Alphanate containing plasma derived human VWF (phVWF) and FVIII (phFVIII). A human-specific VWF:Ag ELISA assay was used. Fig. 1B shows the first 4 hours after infusion of Alphanate in another experiment. Fig. 1C shows the levels of VWF in VWFnull mouse plasma after infusion of recombinant human VWF (rhVWF). Fig. 1D shows the levels of FVIII in FVIIInull animals that were infused with Alphanate. Fig. 1E shows the levels of FVIII in FVIIInull mice that were infused with recombinant human FVIII (rhFVIII). Fig. 1 F. shows that endogenous murine FVIII was restored in VWFnull mice when rhVWF was infused whether by IP or IV injection.
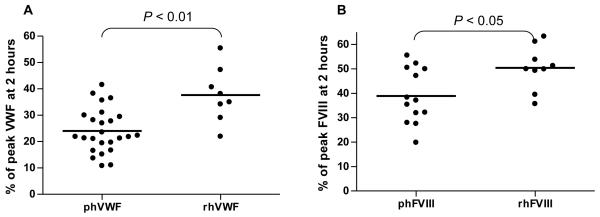
There was no difference in the levels of remaining VWF (P = 0.45) or FVIII (P = 0.97) in mouse plasma between Alphanate and Humate P infusion so those data were combined as plasma-derived human VWF (phVWF) or FVIII (phFVIII). Only 24.9 ± 8.3 % of peak plasma-derived human VWF levels remained in the plasma two hours after intravenous injection. In contrast, 37.7 ± 10.4% of peak recombinant human VWF (rhVWF) remained in the plasma two hours after IV injection, which is significantly higher than the residual plasma-derived VWF (P < 0.01) (Figure 2A). There was 50.4 ± 8.9% residual FVIII in plasma at two hours after IV infusion of recombinant FVIII (rhFVIII), which is significantly higher than the residual plasma-derived FVIII (38.9 ± 11.2%) (P < 0.05) (Figure 2B).
Figure 2. Residual VWF and FVIII in mouse plasma after intravenous infusion.
Animals were infused with VWF and / or FVIII by IV injection. Blood samples were collected at various time points. The levels of VWF:Ag were determined by ELISA. FVIII:C was evaluated by Chromogenic assay. Fig. 2A shows the residual VWF from either plasma-derived (Alphanate or Humante P) (phVWF) or recombinant VWF (rhVWF) at two hours after IV administration. Fig. 2B shows the residual FVIII from either plasma-derived (Alphanate or Humate P) (phFVIII) or recombinant FVIII (rhFVIII) at two hours after intravenous infusion.
The half-life (t1/2) of human VWF and FVIII in mouse plasma
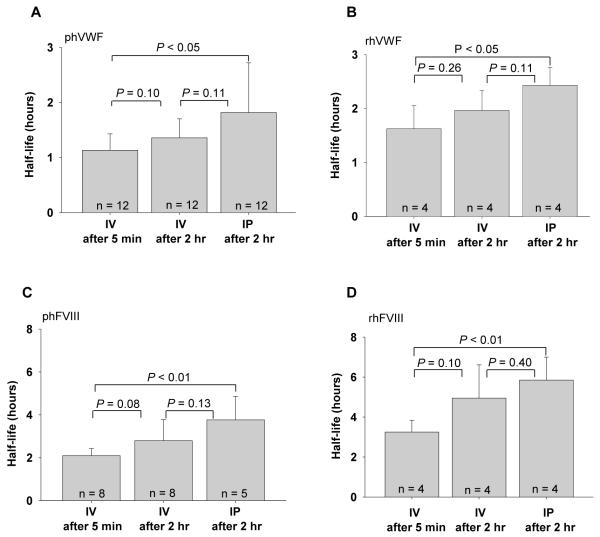
Following IV injection, the t1/2 for human plasma derived VWF and FVIII in mice were 1.13 ± 0.30 hours and 2.10 ± 0.34 hours, respectively, when all time points are included. The t1/2 for IV administration of recombinant human VWF and FVIII in mice were 1.63 ± 0.43 hours and 3.25 ± 0.58 hours, respectively. Half-life calculations following IP injection include only data following the peak at 2 hours because both proteins were still in the process of being absorbed into the blood circulation during the first two hours. Although not a strictly valid comparison, when the half-life calculations include data only following the peak after administration, the t1/2 of both human VWF and FVIII after IV administration is significantly shorter than for IP injection (Figures 3A-D).
Figure 3. The half-life of human VWF and FVIII in mouse plasma.
Animals were infused with VWF and / or FVIII by either IV or IP administration. The levels of VWF:Ag were determined by ELISA. FVIII:C was evaluated by Chromogenic assay. Half-life was calculated using exponential decay. Fig. 3A shows the half-life of plasma-derived human VWF (phVWF); Fig. 3B shows that the half-life of recombinant human VWF (rhVWF); Fig. 3C shows the half-life of plasma-derived human FVIII (phFVIII); Fig. 3D shows the half-life of recombinant FVIII (rhFVIII).
When we measured VWF and FVIII in the plasma of infused mice including data only after the 2 hour time point, the t1/2 of both proteins following IV injection was not significantly different from those obtained by IP administration, although IP half-life in general appears to be slightly longer for both plasma-derived and recombinant proteins. The 2 hour t1/2 of plasma-derived VWF was 1.36 ± 0.35 hours following IV injection and 1.82 ± 0.90 hours following IP administration (P = 0.11), and the t1/2 of plasma-derived FVIII was 2.79 ± 0.98 hours after delivery by IV injection and 3.76 ± 1.10 hours by IP injection (P = 0.13). The t1/2 of rhVWF was 1.97 ± 0.34 hours following IV injection and 2.43 ± 0.37 hours by IP injection (P = 0.11), and the t1/2 of rhFVIII was 4.97 ± 1.67 hours by IV injection and 5.85 ± 1.14 hours by IP injection (P = 0.40) (Figures 3A-D).
When we compared infused plasma-derived versus recombinant VWF and FVIII using combined IV and IP data after the two hour time point, the half-lives of human plasma-derived VWF and FVIII in mouse plasma were 1.56 ± 0.72 hours and 3.49 ± 1.25 hours, respectively, which was significantly shorter than those obtained following recombinant human VWF and FVIII infusions (2.17 ± 0.33 hours, and 5.03 ± 2.55 hours, respectively) (see Table 1). The difference between plasma-derived and recombinant VWF and FVIII was further compared by utilizing another pharmacokinetic parameter, the area under the curve (AUC). As seen in Table 2, AUC for recombinant VWF and FVIII were significantly higher (1.81 times and 1.38 times, respectively) than those obtained following plasma-derived VWF and FVIII infusion.
Table 1. The half-life of human VWF and FVIII in mouse plasma.
| VWF (hours) | FVIII (hours) | |
|---|---|---|
|
|
||
| Plasma-derived | 1.56 ± 0.72 (n = 34) † | 3.49 ± 1.25 (n = 21) ‡ |
| Recombinant | 2.17 ± 0.33 (n = 18) † | 5.03 ± 2.55 (n = 18) ‡ |
means P < 0.01.
means P < 0.05.
Table 2. The AUC (Area Under Curve) pharmacokinetic parameter of human VWF and FVIII estimated by analysis of residual protein profile.
| VWF (%. hr) | FVIII (%. hr) | |
|---|---|---|
|
|
||
| Plasma-derived | 188.73 ± 33.32 † | 375.4 ± 50.19 ‡ |
| Recombinant | 340.97 ± 44.89 † | 516.43 ± 109.52 ‡ |
Animals were infused with human VWF and /or FVIII from various sources using intravenous administration. Plasma samples were collected at various time points from 5 minutes to 24 hours for VWF:Ag ELISA or FVIII:C assay. Data were converted to the percent of peak VWF or FVIII at 5 minutes for analysis of the pharmacokinetic parameter AUC. The area under the time concentration curve was calculated based on the linear trapezoidal rule using the GraphPad Prism 4 software.
means P < 0.01.
means P < 0.05.
Recovery of mouse plasma-derived VWF in the vascular circulation in mice after administration by various routes
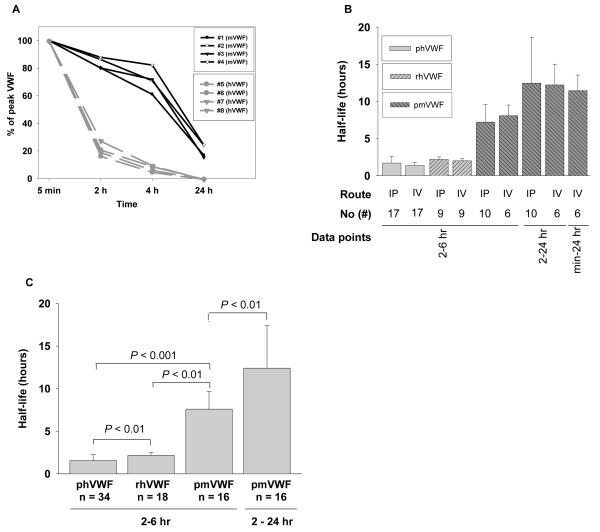
To study the recovery of mouse plasma-derived VWF in the vascular circulation after infusion using IP and IV routes, plasma from C57BL/6 mice was infused into VWFnull mice. Disappearance of infused mouse VWF is significantly slower than human VWF. Murine VWF was still detectable in mouse plasma 24 hours after infusion, while human plasma-derived VWF dropped to undetectable levels at 8 hours after infusion (Figure 1A). At two hours after IV infusion of mouse plasma-derived VWF, 83.6% of peak levels were still present, significantly higher survival compared to human plasma-derived VWF (24.9 ± 8.3 %) (P < 0.01). Twenty-four hours after infusion, 20.1 ± 4.7% of peak mouse VWF still remained (Fig 4A).
Figure 4. Comparison of human and murine plasma VWF clearance in mice.
Plasma from C57BL6 mice was used as a source of mouse plasma-derived VWF (mpVWF) for infusion using either IV or IP administration. The levels of VWF:Ag were determined by ELISA. Fig. 4A shows that the clearance of mouse VWF and human VWF in the infused animals. Fig. 4B demonstrates the half-life difference between human VWF and mouse VWF in mouse plasma after IP or IV administration. Fig. 4C shows the half-life of both plasma-derived and recombinant human VWF, including both IP and IV data, are significantly shorter than plasma-derived mouse VWF.
The t1/2 of mouse VWF in mouse plasma was 7.26 ± 2.38 hours and 8.13 ± 1.45 hours by IP administration and IV administration, respectively, if we calculated data obtained from 2 to 6 hours, for comparability with human VWF before it is cleared due to its much shorter survival. The t1/2 of mouse VWF in mouse plasma was 12.51 ± 6.20 hours and 12.27 ± 2.79 hours by IP administration and IV administration, respectively, if we used data points from 2 to 24 hours for half-life calculations. If we used all data points from the peak at 5 minutes to 24 hours for IV injection, the t1/2 of mouse VWF was 11.50 ± 2.10 hours, which is not significantly different from the t1/2 data obtained from 2 hours to 24 hours for both IP and IV injections (Fig 4B). The t1/2 of infused mouse plasma-derived VWF was significantly longer than those obtained from human VWF, including both plasma-derived human VWF and recombinant human VWF, regardless of the route of infusion (Figures 4B and 4C).
Multimeric structure of circulating VWF in the infused animals
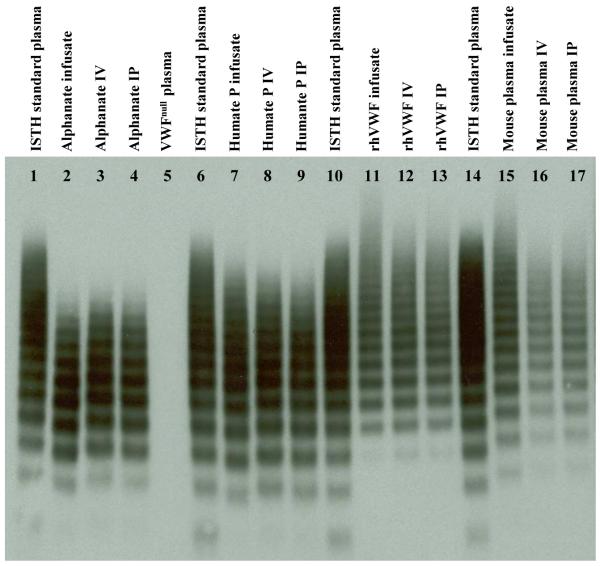
Since multimers of VWF can be extremely large, we wanted to know whether the whole range of multimers of VWF can be absorbed into the vascular circulation after IP administration. VWF multimer structure was analyzed using non-reducing LiDS-agarose electrophoresis. rhVWF contained higher molecular weight VWF multimers, but less intense low molecular multimers compared to plasma-derived VWF preparations including both Humate P and Alphanate. Following both IV and IP infusion, the multimeric structure of circulating VWF at 2 hours was similar to that observed in the infusate, whether plasma-derived or recombinant VWF (Figure 5), indicating that the entire spectrum of VWF multimers was similarly distributed into the vasculature using either the IV or IP route.
Figure 5. Multimeric structure of VWF in the infused mouse plasma.
Plasma samples were collected from mice two hours after infusion, analyzed by LiDS-agarose gel electrophoresis (0.65% agarose), and detected by Western blot using Dako polyclonal antibody. An ISTH standard plasma and the various infusates were used as controls. Multimer analysis of plasma from infused mice revealed vascular distribution of full-range multimers of similar density following either IP or IV administration.
Discussion
The standard method of treatment for both severe von Willebrand disease (VWD) and hemophilia A is intravenous infusion of either plasma-derived or recombinant replacement proteins (VWF for VWD and FVIII for hemophilia A). 11, 12 Since VWF and FVIII are large adhesive proteins, we wanted to know whether other routes, including IP and SC injection of these proteins, would result in efficient absorption into the vasculature. In the current studies, we infused VWF and FVIII into mice through various routes including IP, IV, and SC injections. We discovered that both proteins were efficiently absorbed into the blood circulation following IP injection with a peak between 2 to 4 hours after infusion at levels similar to those observed in mice infused intravenously, but were not detected in the circulation after SC injection.
There are two potential routes for the absorption of fluid and proteins from the peritoneal cavity into blood circulation. One is via the abdominal lymph vessels. The other is a transcapillary absorption processes. There is evidence demonstrating that fluids (with proteins) in the peritoneal cavity are absorbed into the vasculature through drainage into lymphatics lining the surface of the diaphragm under normal intraperitoneal pressure 32-35. Transient therapeutic levels of plasma FVIII were achieved in mice implanted intraperitoneally with encapsulated cells producing FVIII36 or injected intraperitoneally with lentiviral vector encoding human B-domain deleted FVIII,37 demonstrating that FVIII can reach the circulation from the peritoneal space. Our studies demonstrated that both VWF and FVIII were absorbed into the vasculature following IP injection, but not by SC injection, indicating that absorption of both proteins is most likely via the diaphragmatic lymph vessels. Transcapillary absorption may not be a major contributor because both subcutaneous tissues and the peritoneal cavity have abundant microvasculature. Previous studies of extravascular FIX injection have demonstrated that subcutaneous injection of FIX can result in significant levels of plasma FIX, indicating that FIX can be taken up via transcapillary absorption17. This may be due to differences of molecular size or protein structure between FIX and FVIII or VWF.
Conventional IV injection is the fastest way to raise plasma VWF and FVIII levels. Unlike intravenous injection, intraperitoneal administrations of both VWF and FVIII do not distribute immediately into the blood circulation. The protein concentrations in blood following intraperitoneal administration increased over the first two hours, then became similar to those after intravenous injection when equivalent doses of protein were injected. For the first two hours following IV injection, the levels of both VWF and FVIII in plasma decreased quickly, while plasma levels of proteins delivered by IP injection steadily increase to their peak over this same time period. After the 2 hour time point, clearance rates are similar for both IV and IP delivery. These results indicate that the IP route could be an effective route for VWF and FVIII infusion.
One important function of VWF is that it mediates the adhesion of platelets to sites of vascular injury 38. Since the largest multimers of VWF are most effective in promoting platelet adhesion 39, it is important to assure that the whole spectrum of VWF multimers is absorbed into the circulation to provide hemostatic activity after administration. We analyzed VWF multimer structure in plasma samples collected 2 hours after infusion and found that the structure of VWF multimers following IP or IV injection was similar to the infusates. The fact that the full range of VWF multimers present in infusates were absorbed into the vasculature with equivalent efficiency whether delivered by IP or IV infusion, indicates that VWF delivered by IP infusion should be fully functional in circulation.
Our studies have demonstrated that in mice the t1/2 of recombinant human VWF and FVIII are significantly longer than those of human plasma-derived VWF and FVIII. There are several possible explanations for these observations, including 1) the recombinant human VWF contains higher molecular weight VWF multimers that are not present in the plasma-derived VWF preparations used; 2) the structures of recombinant VWF or FVIII differ from the plasma-derived proteins in subtle or not so subtle ways (e.g. recombinant human FVIII used was a B-domain deleted product, while plasma-derived FVIII is the full-length protein. For human plasma-derived FVIII, the B-domain is much more divergent than the rest of the protein compared to mouse FVIII); 3) pooled plasma products contain a complex mixture of different polymorphic proteins, while recombinant proteins are a single type; and 4) glycosylation could be different between plasma-derived and recombinant proteins.
Previous reports have demonstrated that the half-life of human plasma-derived VWF in humans is about 11.2 hours 15. When human plasma-derived VWF (either Alphanate or Humate-P) was infused into mice, the half-life was only about 1.6 hours. When mouse plasma-derived VWF was infused into mice, the half-life was about 11.5 hours, which is very similar to the half-life in the human / human system. There is a nearly 20% difference in amino acid sequence between human and mouse VWF that is likely to result in recognition of the foreignness of a “xeno-transfusion” when human VWF is infused into mice. Other factors, such as altered cleavage by ADAMTS13 or other plasma proteases and differences in protein glycosylation, might also contribute to more rapid clearance of human VWF in the mouse model.
In conclusion, our studies demonstrate that intraperitoneal injection of both VWF and FVIII can efficiently transfer these proteins into the vascular space. This implies that intraperitoneal administration could be an effective alternative route for VWF and FVIII infusion when there is difficulty attaining venous access. Long-acting pharmaceuticals based on delayed-release biodegradable microspheres have been shown to have a long duration of action following extra-vascular injection.40, 41 Regardless of the need for an alternative route for infusion, administration of the encapsulated VWF or FVIII into peritoneal cavity may lead to a safe and effective long-acting sources of replacement proteins that would be beneficial for VWD or hemophilia A patients undergoing prophylaxis. Implanting cells that are genetically modified to produce recombinant proteins might provide a gene therapy strategy that could be beneficial in treatment of patients with VWD or hemophilia A.
Acknowledgements
This work was supported by American Heart Association National Center Scientist Development Award (0730183N) (QS), National Hemophilia Foundation Career Development Award (QS), Hemophilia Association of New York grant (QS), and National Institutes of Health grants HL-102035 (QS) and HL-44612 (RRM).
Footnotes
Authorship
Contribution: Q. S. designed and performed research, analyzed data, and wrote the manuscript; E. L. K. performed research and analyzed data; J. A. S. performed research; S. A. F. performed research, and made comments on manuscript; R. R. M. helped in the design of the project, provided research support, critiqued results, and made comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Reference List
- 1.Ruggeri ZM, Ware J. The structure and function of von Willebrand factor. Thromb Haemost. 1992;67(6):594–599. [PubMed] [Google Scholar]
- 2.Montgomery RR, Gill JC. Interactions between von Willebrand factor and Factor VIII: where did they first meet. J Pediatr Hematol Oncol. 2000;22(3):269–275. doi: 10.1097/00043426-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri ZM, Zimmerman TS. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981;57(6):1140–1143. [PubMed] [Google Scholar]
- 4.Mannucci PM, Abildgaard CF, Gralnick HR, et al. Multicenter comparison of von Willebrand factor multimer sizing techniques. Report of the Factor VIII and von Willebrand Factor Subcommittee. Thromb Haemost. 1985;54(4):873–876. [PubMed] [Google Scholar]
- 5.Ruggeri ZM, Ware J. von Willebrand factor. FASEB J. 1993;7(2):308–316. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman RJ, Wasley LC, Dorner AJ. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988;263(13):6352–6362. [PubMed] [Google Scholar]
- 7.Kaufman RJ, Pipe SW. Regulation of factor VIII expression and activity by von Willebrand factor. Thromb Haemost. 1999;82(2):201–208. [PubMed] [Google Scholar]
- 8.Kaufman RJ, Wasley LC, Davies MV, Wise RJ, Israel DI, Dorner AJ. Effect of von Willebrand factor coexpression on the synthesis and secretion of factor VIII in Chinese hamster ovary cells. Mol Cell Biol. 1989;9(3):1233–1242. doi: 10.1128/mcb.9.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyer LW. Von Willebrand’s disease. Prog Hemost Thromb. 1976;3:231–287. [PubMed] [Google Scholar]
- 10.Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361(9371):1801–1809. doi: 10.1016/S0140-6736(03)13405-8. [DOI] [PubMed] [Google Scholar]
- 11.Berntorp E, Boulyjenkov V, Brettler D, et al. Modern treatment of haemophilia. Bull World Health Organ. 1995;73(5):691–701. [PMC free article] [PubMed] [Google Scholar]
- 12.Rodeghiero F, Castaman G. Treatment of von Willebrand disease. Semin Hematol. 2005;42(1):29–35. doi: 10.1053/j.seminhematol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Bolton-Maggs PH, Lillicrap D, Goudemand J, Berntorp E. von Willebrand disease update: diagnostic and treatment dilemmas. Haemophilia. 2008;14(Suppl 3):56–61. doi: 10.1111/j.1365-2516.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 14.Favaloro EJ, Lloyd J, Rowell J, et al. Comparison of the pharmacokinetics of two von Willebrand factor concentrates [Biostate and AHF (High Purity)] in people with von Willebrand disorder. A randomised cross-over, multi-centre study. Thromb Haemost. 2007;97(6):922–930. [PubMed] [Google Scholar]
- 15.Dobrkovska A, Krzensk U, Chediak JR. Pharmacokinetics, efficacy and safety of Humate-P in von Willebrand disease. Haemophilia. 1998;4(Suppl 3):33–39. doi: 10.1046/j.1365-2516.1998.0040s3033.x. [DOI] [PubMed] [Google Scholar]
- 16.Hellstern P, Kiehl R, Miyashita C, et al. Factor VIII: C (FVIII: C) recovery and half-life after infusion of steam-treated high purity factor VIII concentrate in severe hemophilia A--comparison of one-stage assay, two-stage assay and a chromogenic substrate assay. Thromb Haemost. 1986;56(3):353–359. [PubMed] [Google Scholar]
- 17.Liles D, Landen CN, Monroe DM, et al. Extravascular administration of factor IX: potential for replacement therapy of canine and human hemophilia B. Thromb Haemost. 1997;77(5):944–948. [PubMed] [Google Scholar]
- 18.Bowie EJ, Solberg LA, Jr., Fass DN, et al. Transplantation of normal bone marrow into a pig with severe von Willebrand’s disease. J Clin Invest. 1986;78(1):26–30. doi: 10.1172/JCI112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274(28):19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- 20.Doering CB, Josephson CD, Craddock HN, Lollar P. Factor VIII expression in azoxymethane-induced murine fulminant hepatic failure. Blood. 2002;100(1):143–147. doi: 10.1182/blood.v100.1.143. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Nichols TC, Sarkar R, McCorquodale S, Bellinger DA, Ponder KP. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci U S A. 2005;102(17):6080–6085. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamont PA, Ragni MV. Lack of desmopressin (DDAVP) response in men with hemophilia A following liver transplantation. J Thromb Haemost. 2005;3(10):2259–2263. doi: 10.1111/j.1538-7836.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- 23.Kumaran V, Benten D, Follenzi A, Joseph B, Sarkar R, Gupta S. Transplantation of endothelial cells corrects the phenotype in hemophilia A mice. J Thromb Haemost. 2005;3(9):2022–2031. doi: 10.1111/j.1538-7836.2005.01508.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacquemin M, Neyrinck A, Hermanns MI, et al. FVIII production by human lung microvascular endothelial cells. Blood. 2006;108(2):515–517. doi: 10.1182/blood-2005-11-4571. [DOI] [PubMed] [Google Scholar]
- 25.Shovlin CL, Angus G, Manning RA, et al. Endothelial Cell Processing and Alternatively Spliced Transcripts of Factor VIII: Potential Implications for Coagulation Cascades and Pulmonary Hypertension. PLoS One. 2010;5(2):e9154. doi: 10.1371/journal.pone.0009154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Follenzi A, Benten D, Novikoff P, Faulkner L, Raut S, Gupta S. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest. 2008;118(3):935–945. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahani T, Lavend’homme R, Luttun A, Saint-Remy JM, Peerlinck K, Jacquemin M. Activation of human endothelial cells from specific vascular beds induces the release of a FVIII storage pool. Blood. 2010 doi: 10.1182/blood-2009-07-232546. [DOI] [PubMed] [Google Scholar]
- 28.Metzner HJ, Hermentin P, Cuesta-Linker T, Langner S, Muller HG, Friedebold J. Characterization of factor VIII/von Willebrand factor concentrates using a modified method of von Willebrand factor multimer analysis. Haemophilia. 1998;4(Suppl 3):25–32. doi: 10.1046/j.1365-2516.1998.0040s3025.x. [DOI] [PubMed] [Google Scholar]
- 29.Shi Q, Wilcox DA, Fahs SA, et al. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116(7):1974–1982. doi: 10.1172/JCI28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown SA, Eldridge A, Collins PW, Bowen DJ. Increased clearance of von Willebrand factor antigen post-DDAVP in Type 1 von Willebrand disease: is it a potential pathogenic process? J Thromb Haemost. 2003;1(8):1714–1717. doi: 10.1046/j.1538-7836.2003.00359.x. [DOI] [PubMed] [Google Scholar]
- 31.Haberichter SL, Balistreri M, Christopherson P, et al. Assay of the von Willebrand factor (VWF) propeptide to identify patients with type 1 von Willebrand disease with decreased VWF survival. Blood. 2006;108(10):3344–3351. doi: 10.1182/blood-2006-04-015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COURTICE FC, STEINBECK AW. The effects of lymphatic obstruction and of posture on the absorption of protein from the peritoneal cavity. Aust J Exp Biol Med Sci. 1951;29(6):451–458. doi: 10.1038/icb.1951.51. [DOI] [PubMed] [Google Scholar]
- 33.COURTICE FC, STEINBECK AW. Absorption of protein from the peritoneal cavity. J Physiol. 1951;114(3):336–355. doi: 10.1113/jphysiol.1951.sp004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MORRIS B. The effect of diaphragmatic movement on the absorption of protein and of red cells from the peritoneal cavity. Aust J Exp Biol Med Sci. 1953;31(3):239–246. doi: 10.1038/icb.1953.28. [DOI] [PubMed] [Google Scholar]
- 35.McKay T, Zink J, Greenway CV. Relative rates of absorption of fluid and protein from the peritoneal cavity in cats. Lymphology. 1978;11(3):106–110. [PubMed] [Google Scholar]
- 36.Garcia-Martin C, Chuah MK, Van DA, et al. Therapeutic levels of human factor VIII in mice implanted with encapsulated cells: potential for gene therapy of haemophilia A. J Gene Med. 2002;4(2):215–223. doi: 10.1002/jgm.248. [DOI] [PubMed] [Google Scholar]
- 37.Kootstra NA, Matsumura R, Verma IM. Efficient production of human FVIII in hemophilic mice using lentiviral vectors. Mol Ther. 2003;7(5 Pt 1):623–631. doi: 10.1016/s1525-0016(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 38.Scott JP, Montgomery RR. Therapy of von Willebrand disease. Semin Thromb Hemost. 1993;19(1):37–47. doi: 10.1055/s-2007-994004. [DOI] [PubMed] [Google Scholar]
- 39.Fischer BE, Kramer G, Mitterer A, et al. Effect of multimerization of human and recombinant von Willebrand factor on platelet aggregation, binding to collagen and binding of coagulation factor VIII. Thromb Res. 1996;84(1):55–66. doi: 10.1016/0049-3848(96)00161-2. [DOI] [PubMed] [Google Scholar]
- 40.Crotts G, Park TG. Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues. J Microencapsul. 1998;15(6):699–713. doi: 10.3109/02652049809008253. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]