Abstract
Synaptic vesicle loading of glutamate is a pivotal step in glutamate synaptic transmission. The molecular machinery responsible for this step is comprised of v-type proton-pump ATPase and a vesicular glutamate transporter. Recent evidence indicates that synaptic vesicles are endowed with glycolytic ATP-synthesizing enzymes, providing energy for immediate use by vesicle-bound proton-pump ATPase. In this study, we provide evidence that synaptic vesicles are also capable of synthesizing the vesicular glutamate transporter substrate glutamate, from α-ketoglutarate and L-aspartate (as the amino group donor); glutamate thus produced is taken up into vesicles. We also report a finding thatα-ketoglutarate-derived glutamate uptake into synaptic vesicles and aspartate aminotransferase are inhibited by 2,3-pyrazinedicarboxylate. Evidence is given that this is a selective inhibitor for aspartate aminotransferase. These observations provide insight into understanding the nerve endings’ mechanism for high efficiency in glutamate transmission. Finding this inhibitor may have implications for further experimentation on the role of α-ketoglutarate-derived glutamate in glutamate transmission.
Keywords: biosynthesis, GOT, local synthesis, neurotransmitter, precursor
In glutamate transmission, glutamate uptake into synaptic vesicles is a pivotal initial step, enabling glutamate to enter the neurotransmitter pathway (Ueda1986; Maycox et al. 1990; Ozkan and Ueda 1998; Otsis 2001; Edwards 2007). This uptake system is comprised of v-type proton-pump ATPase (v-H+-ATPase) and a vesicular glutamate transporter known as VGLUT (Ueda 1986; Tabb and Ueda 1991; Lewis and Ueda 1998; Takamori et al. 2000; Bellocchio et al. 2000; Juge et al. 2006). The electrochemical proton gradient generated by v-H+-ATPase is harnessed by VGLUT to concentrate glutamate in synaptic vesicles. The substrate for v-H+-ATPase is largely provided by the synaptic vesicle-bound glycolytic ATP-generating enzyme systems, the glyceraldehyde phosphate dehydrogenase/3-phosphoglycerate kinase complex and pyruvate kinase (Ikemoto et al. 2003; Ishida et al. 2009). Recent evidence suggests that ATP generated by vesicle-bound pyruvate kinase is more readily utilized by v-H+-ATPase than is ATP added to the medium (Ishida et al. 2009). This supports the notion that locally produced ATP plays an important role in providing energy required for vesicular neurotransmitter loading (Ueda and Ikemoto 2007). In this study, we provide evidence suggesting that local synthesis is also extended to the VGLUT substrate glutamate, enhancing efficiency in glutamate loading into synaptic vesicles.
Biosynthesis of the VGLUT substrate remains to be established. It is widely thought that glutamine made in astrocytes serves as the principal precursor of the neurotransmitter glutamate, which is produced by neuronal glutaminase. However, growing evidence indicates that the role of the glutamate-glutamine cycle in basal glutamate synaptic transmission is less clear (for reviews, see Edwards 2007). Biochemically, metabolic glutamate is produced from α-ketoglutarate (α-KGA) by aspartate aminotransferase (AAT) and glutamate dehydrogenase as well as from glutamine by glutaminase. We have assessed the possibility that α-KGA could serve as an immediate precursor for synthesis of the synaptic vesicular pool of glutamate.
Materials and methods
Tissues
All the tissues used in this study were purchased: frozen rat (male) brains from Pel-Freez Biologicals and fresh bovine brains (adult male) from a local slaughter house. Thus, this research did not involve handling of live animals.
Reagents and antibodies
[1-14C]α-Ketoglutaric acid (57 mCi/mmol), L-[3,4-3H(N)]glutamine (50 Ci/mmol), and L-[3,4-3H]glutamic acid (50 Ci/mmol) were purchased from PerkinElmer. Monoclonal anti-synaptophysin1 antibody was prepared, at the University of Michigan Hybridoma Facility, from the hybridoma clone 3G12 (Berwin et al. 1998), which was generously provided by Dr. Eric Floor, Department of Physiology and Cell Biology, University of Kansas. The culture medium supernatant of hybridoma 3G12 was used. Polyclonal antibodies to cytosolic and mitochondrial aspartate aminotransferase (AAT) (EC 2.6.1.1), referred to as GOT1 and GOT2 antibodies, respectively, were obtained from Aviva Systems Biology and Abnova, respectively. These antibodies were raised against the N-terminal region (1–50) of human GOT1 and the C-terminal region (331–430) of human GOT2; these regions show no significant sequence homology. Monoclonal anti-VGLUT1 antibody was purchased from Synaptic Systems; biotin-conjugated rabbit anti-mouse IgG from ZYMED; Monoclonal anti-cytochrome oxidase subunit IV (20E8) from Molecular Probes; anti-rabbit Ig, biotinylated species-specific antibody and streptavidin-conjugated horseradish peroxidase from GE Healthcare; and an enhanced chemiluminescence detection agent (SuperSignal® West Femto Maximum Sensitivity Substrate) from Thermo Scientific. All other agents were purchased from Sigma-Aldrich.
Preparation of subcellular fractions
Synaptic vesicles were prepared from frozen rat brain (Pel-Freez Biologicals) by differential and sucrose gradient centrifugation as described previously (Ishida et al. 2009). Rat crude synaptic vesicles were prepared as described (Kish and Ueda 1989) and suspended in 4 mM HEPES-KOH (pH 7.4) containing 0.1 mM dithiothreitol. Crude synaptic vesicle fractions (1.0 ml × 6) obtained from 100 rat brains were layered on top of the sucrose gradients in the above buffer (10.5 ml × 6), each consisting of 3.5 ml 0.2, 0.3, and 0.4 M sucrose, and centrifuged at 4° C at 24,800 rpm (109,400 gmax) in a Beckman SW 40Ti rotor for 2 h. The sample layer was discarded. The interface between the sample layer and the 0.2 M sucrose layer (SV-A), the 0.2 M sucrose layer (SV-A′), the interface between 0.2 M and 0.3 M sucrose layers (SV-B), the 0.3 M sucrose layer (SV-B′), the interface between 0.3 M and 0.4 M sucrose layers (SV-C), the 0.4 M sucrose layer (SV-C′), and the pellet (D) were collected. These fractions, except D, were diluted 2–3 fold with 4 mM HEPES-KOH (pH 7.4) containing 0.1 mM dithiothreitol, and centrifuged at 4° C at 55,000 rpm (278,000 gmax) in a Beckman 70.1Ti rotor for 60 min. All the pellets were suspended in a solution containing 0.32 M sucrose, 4 mM HEPES-KOH (pH 7.4) and 0.1 mM dithiothreitol, and frozen in liquid nitrogen. Purified synaptic vesicles (0.4 M sucrose layer) were also prepared from fresh bovine brain by a conventional method using sucrose density gradient centrifugation at 28,000 rpm (141,000 gmax) for 2 h in a Beckman SW 28 rotor, as described previously (Kish and Ueda, 1989). The synaptosomal cytosol (synsol) fraction and the plasma membrane plus mitochondria fraction were prepared from frozen rat brain, as previously described (Ueda et al. 1979).
Crude synaptosomes (P2 fraction) were prepared from fresh calf frontal cortex (Ueda et al. 1979), and purified by Percoll gradient centrifugation (Dunkley et al. 2008). Fraction 4, the richest in synaptosomes, was used as purified synaptosomes in this study. Protein was determined by the method of Bradford (1976), with bovine serum albumin as standard. The nonsynaptic mitochondria fraction was prepared from frozen rat brain as described by Lai and Clark (1979).
Uptake of α-KGA-derived products into isolated synaptic vesicles
Vesicular α-KGA-derived uptake was measured by the filtration-based assay using Whatman GF/C filters, as described previously (Kish and Ueda 1989), with minor modifications. In the standard assay, aliquots (50–100 μg protein) of rat brain synaptic vesicle fraction SV-A′ were incubated at 30° C for 15 min with 30 or 60 μM [14C]α-KGA (57 mCi/mmol) in a mixture (final volume, 0.1 ml) containing 20 mM HEPES-Tris (pH 7.4), 100 or 250 mM sucrose, 4 mM MgSO4, 4 mM KCl, and 2 mM L-aspartate, with or without 2 mM ATP. ATP-dependent uptake of α-KGA-derived glutamate into vesicles in the presence of 100 mM sucrose was found to be virtually the same as uptake in the presence of 250 mM sucrose, using a set of three independent rat SV-A′ preparations: 326 ± 8 vs. 328 ± 8 pmol/mg/15 min.
Analysis of α-KGA-derived products accumulated in isolated synaptic vesicles
Synaptic vesicle fraction SV-A′ was incubated with 30 μM [14C]α-KGA in the presence of ATP and aspartate, and filtered as described above; radioactive material on the filter was extracted with 80% ethanol. The extract from multiple filters was concentrated and dissolved in 0.5 ml of 13 mM trifluoroacetic acid containing 1 mM 1-octanesulfonate, and an aliquot subjected to HPLC, as described previously (Ishida et al. 2009).
Aspartate aminotransferase enzyme activity assay
Enzymatic activity of synaptic vesicle-associated AAT was measured by the method of Rej and Hφrder (1983) with the following modifications: in order to minimize the effect of endogenous malate dehydrogenase-like activity associated with synaptic vesicles, NADPH was used instead of NADH. Aspartate aminotransferase enzyme activity was monitored by changes in fluorescence of NADPH at 460 nm (excitation wave length, 390 nm) rather than by changes in absorption at 340 nm. Purified synaptic vesicle fraction SV-A′ (40–50 μg) was incubated at 30° C for 2 min in 100 mM Tris-HCl (pH 7.5) containing 193 μM NADPH, malate dehydrogenase (107 units/ml), and a test amino acid as amino donor (50 mM). The reaction was initiated by addition of 10 mM α-KGA to the mixture (final volume, 0.15 ml).
Glutaminase activity assay
Phosphate-dependent glutaminase activity of nonsynaptic mitochondria was determined at 30° C by the method of Curthoys and Lowry (1973). Fluorescence of NADPH formed was detected at 460 nm (excitation wave length, 340 nm).
Western blot analysis
Protein samples were electrophoresed and transferred onto Immobilon-P polyvinylidene difluoride membrane (Millipore) as described (Harlow and Lane 1988). After blocking, the membrane was incubated overnight at 4° C with respective first antibodies, and then with biotinylated rabbit anti-mouse IgG (1:2000 dilution) or anti-rabbit Ig, biotinylated species-specific antibody (1:2000 dilution) for 2 h at room temperature. This was followed by incubation with streptavidin-horseradish peroxidase conjugate (1:500 dilution) for 40 min at room temperature. Western blots were visualized by an enhanced chemiluminescence detection procedure, using FAS-1000 (Toyobo) or LAS-1000 (GE Healthcare) image analyzers.
Solubilization of vesicle-bound AAT
The crude synaptic vesicle fraction (1 mg/ml) was treated on ice for 60 min with various concentrations of KCl, and centrifuged at 438,000 gmax for 60 min; the pellets were suspended in the original volume of 2 mM Tris-maleate (pH 8.0) containing 80 mM sucrose and 0.1 mM dithiothreitol. The supernatant and pellet proteins were precipitated with trichloroacetic acid, and dissolved in 60 μl of SDS-PAGE sample buffer. Aliquots (20 μl) were subjected to SDS/western blotting and probed with GOT2 antibodies.
Synaptosomal uptake of α-KGA and glutamine
Purified synaptosomes (Percoll gradient fraction 4, 155 or 164 μg protein) were suspended in 0.2 ml oxygenated (95% O2-5% CO2) Krebs-Ringer buffer containing 140 mM NaCl, 1.5 mM K2HPO4, 2 mM MgCl2, 1.3 mM CaCl2, 5 mM glucose and 10 mM HEPES-NaOH (pH 7.4), and preincubated at 37° C for 10 min. After addition of 50 μM [14C]α-KGA (57 mCi/mmol) and 500 μM [3H]glutamine (37 mCi/mmol), incubation was continued for an additional 30 min. Aliquots (40 μl) were removed and filtered on Whatman GF/C filters, and radioactivity (14C and 3H) retained on the filter determined in a Beckman LS 6500 scintillation spectrophotometer.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analysis was performed with student t-test for difference between two values. Significance was set at p < 0.05.
Results
Synaptic vesicles are endowed with the capacity to synthesize the VGLUT substrate for immediate use
The concept that local synthesis of ATP is an efficient mechanism for fueling vesicular glutamate uptake is supported by the observations (Ikemoto et al. 2003; Ishida et al. 2009) that synaptic vesicles bear two glycolytic ATP-generating enzyme systems, and that they are able to provide sufficient energy to activate VGLUT. In further support for this concept, we have observed that endogenous ATP generated from phosphoenol pyruvate and ADP by vesicle-bound pyruvate kinase is more effective than added exogenous ATP (Ishida et al. 2009; also Takeda and Ueda, unpublished observation).
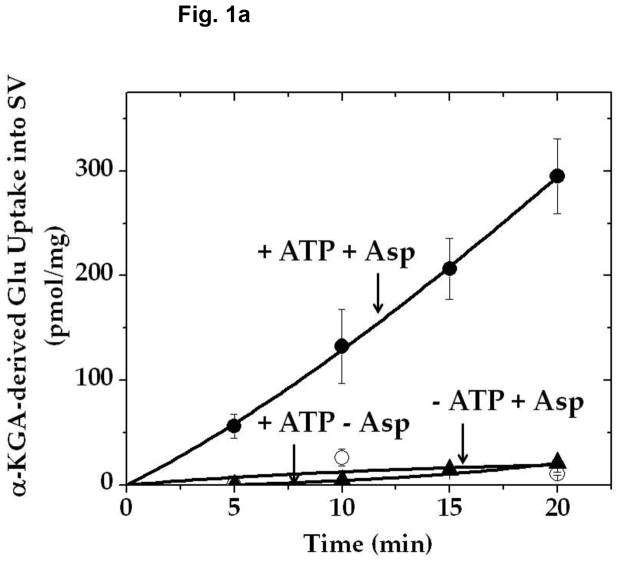
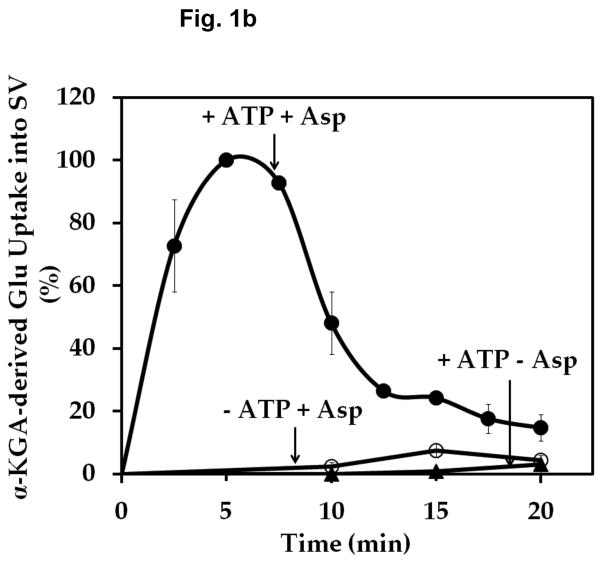
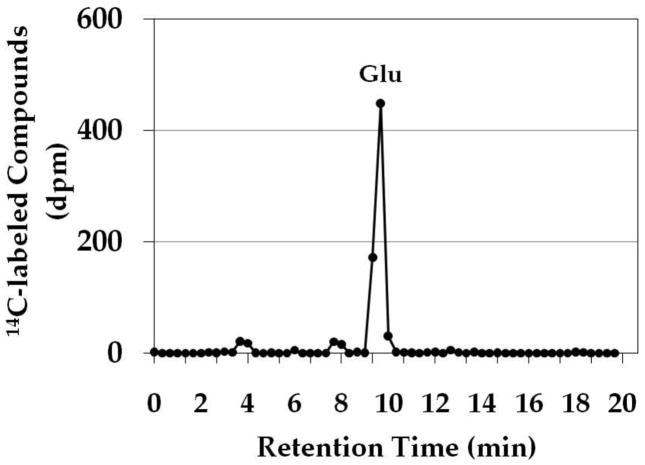
In light of these observations, it was considered reasonable that the VGLUT substrate glutamate would also be supplied locally by a biosynthetic enzyme bound to synaptic vesicles. We explored the possibility that AAT might serve this function. Aspartate aminotransferase catalyzes the reversible formation of glutamate from α-KGA at the expense of the aspartate (Hayashi et al. 1990). We incubated isolated synaptic vesicles with [14C]α-KGA in the presence of L-aspartate, and determined the ability of synaptic vesicles to accumulate endogenously generated [14C]glutamate. As shown in Fig. 1, synaptic vesicles incorporated radioactive material in a time-dependent manner. This required the presence of L-aspartate as well as ATP. Aspartate-, ATP-, and time-dependent uptake of radioactive material into vesicles was observed with synaptic vesicles purified from frozen rat brain (Ishida et al 2009) as well as from fresh bovine brain (Kish and Ueda, 1989). An efflux system for accumulated radioactive material appears to be quite active in bovine vesicles, however. The time course difference between rat and bovine synaptic vesicles remains to be understood. This might be due to the difference in developmental stage or represent species difference. The physiological significance of the efflux is not clear at present. The radioactive material was extracted from rat brain vesicles, and identified largely as glutamate (Fig. 2). These observations suggest that synaptic vesicles are endowed with AAT converting [14C]α-KGA to [14C]glutamate, which is readily transported into vesicles.
Fig. 1.
Time course of α-KGA-derived glutamate uptake into isolated synaptic vesicles. Rat synaptic vesicles (a) or bovine synaptic vesicles (b), 100 μg protein each, were preincubated at 30° C for 2 min in the medium containing 250 mM sucrose described in Materials and Methods, in the absence (○) or presence (●) of 2 mM ATP each with 2 mM L-aspartate, or in the absence of aspartate but in the presence of 2 mM ATP (▲). Following addition of 60 μM [14C]α-KGA, incubation was continued at 30° C for the various periods of time indicated. Vesicular accumulation of radioactive material was determined, as described in Materials and Methods. The value (72 ± 10 pmol/mg) obtained in the absence of ATP with 0 min incubation was subtracted from all values in each experiment shown in (a), and the value (40 ± 5 pmol/mg) from all values in each experiment shown in (b). The results in (a) show the mean ± SEM from three separate rat brain SV-A′ preparations, and those in (b) the average in percentage of two separate bovine synaptic vesicle preparations; 100% represent 215 ± 65 pmol/mg. SV, synaptic vesicle; Glu, glutamate; Asp, aspartate.
Fig. 2.
HPLC chromatogram of α-KGA-derived products in synaptic vesicles. Synaptic vesicle fraction SV-A′ was incubated with 30 μM [14C]α-KGA for 15 min in the presence of 2 mM L-aspartate and 2 mM ATP, and filtered. Radioactive compounds on the filter were analyzed by HPLC, as described in Materials and Methods. Authentic L-[3H]glutamate was used to identify the major radioactive peak as glutamate. Glu, glutamate.
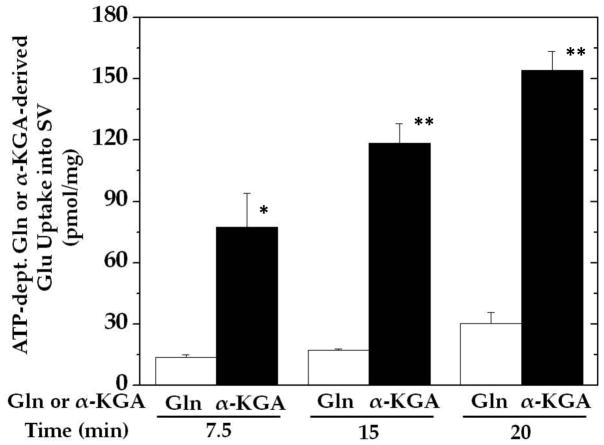
In contrast, synaptic vesicles have a substantially reduced ability to convert glutamine to glutamate for vesicular accumulation, even in the presence of the glutaminase activator inorganic phosphate at a concentration higher than is physiologically relevant (Fig. 3). The rate of α-KGA-derived glutamate uptake is reduced in the presence of 3 mM phosphate compared with the uptake in its absence (cf. Fig. 3 and Fig. 1: 118 ± 9 vs. 207 ± 21 pmol/mg/15 min, n= 3). This inhibition by phospahe could be largely due to competition of phosphate (3 mM) with α-KGA (50 μM) for the α-KGA binding site of AAT; phosphate (3 mM) does not cause significant inhibition of vesicular uptake of glutamate at 50 μM. Glutamine-derived glutamate uptake (17 pmol/mg) was only 8% of unsuppressed α-KGA-derived glutamate uptake (207 pmol/mg) at 15 min.
Fig. 3.
The ability of isolated synaptic vesicles to convert glutamine to glutamate and accumulate is minimal compared with that to cause α-KGA-derived glutamate uptake. Rat synaptic vesicle fraction SV-A′ (50μg) was incubated in the presence of 250 mM sucrose at 30° C for various periods of time with 50 μM [3H]glutamine (0.4 mCi/mmol) or 50 μM [14C]α-KGA (57 mCi/mmol), 2 mM aspartate, and 3 mM inorganic phosphate, each with or without 2 mM ATP. The mixture without [3H]glutamine or [14C]α-KGA was preincubated at 30° C for 2 min. After addition of 50 μM [3H]glutamine or 50 μM [14C]α-KGA, the mixture was further incubated for various periods indicated. Values obtained in the absence of ATP were subtracted from all values. The data represent the mean ± SEM from three separated preparations. *, p<0.05; **, p<0.005. Glu, glutamate; Gln, glutamine; SV, synaptic vesicle.
Vesicle-bound transaminase is AAT specific to L-aspartate as amino donor
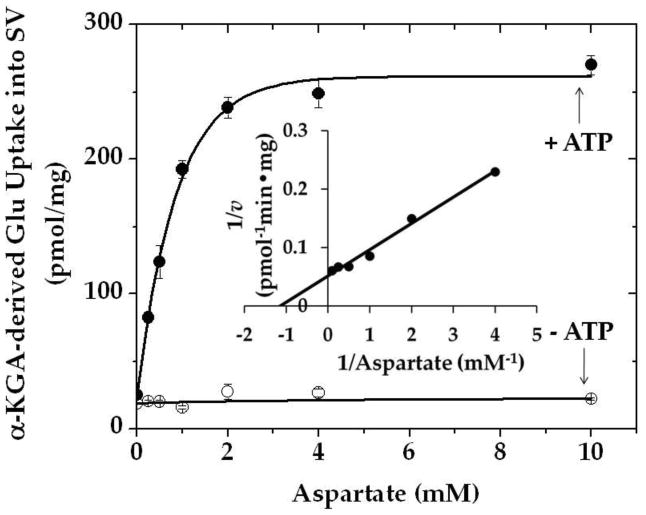
Figure 4 shows that vesicular uptake of α-KGA-derived glutamate is dependent on aspartate concentration; the concentration required for half-maximal uptake is about 0.9 mM. The ATP-dependent uptake obeyed Michaelis-Menten kinetics, and Km for L-aspartate was determined to be 0.9 mM. A similar Km value (1.5 mM) was obtained with bovine brain synaptic vesicles incubated in the presence of 250 mM sucrose (data not shown). Direct measurement of synaptic vesicle-bound AAT enzyme activity yielded a similar Km value (data not shown). These values are in agreement with the Km values (0.87 mM) for L-aspartate of brain synaptic mitochondrial AAT (Dennis et al. 1977), but are different from that (6.7 mM) of the cytosolic enzyme (Magee and Phillips 1971).
Fig. 4.
Effect of various concentrations of aspartate on α-KGA-derived glutamate uptake into isolated synaptic vesicles. Two preparations of rat synaptic vesicle fraction SV-A′ (50 μg protein) were combined and incubated at 30° C for 15 min with 30 μM [14C]α-KGA in the medium containing 100 mM sucrose described in Materials and Methods, in the absence (○) or presence (●) of 2 mM ATP with various concentrations of L-aspartate. Values are the mean ± SEM of triplicate determinations. Glu, glutamate; v, the initial velocity of vesicular uptake of α-KGA-derived Glu uptake expressed in pmol·min−1·mg−1.
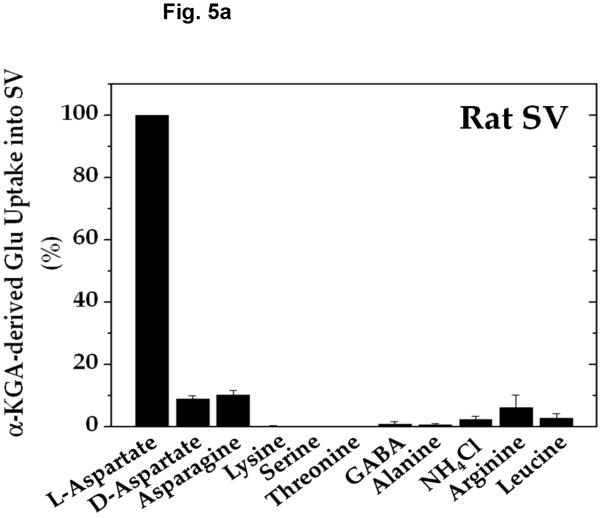
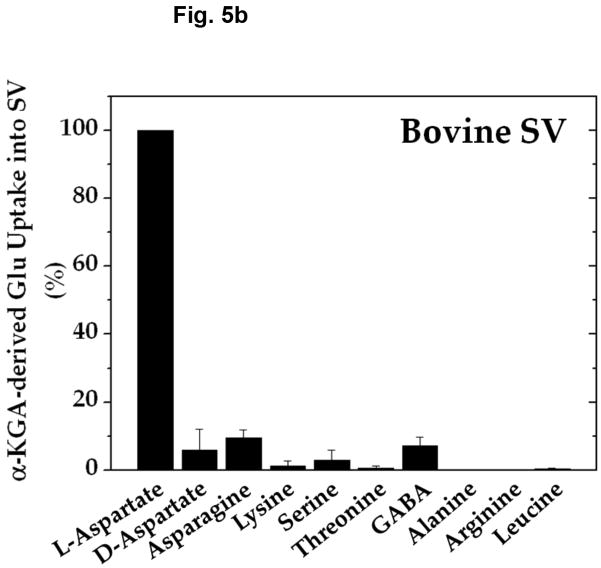
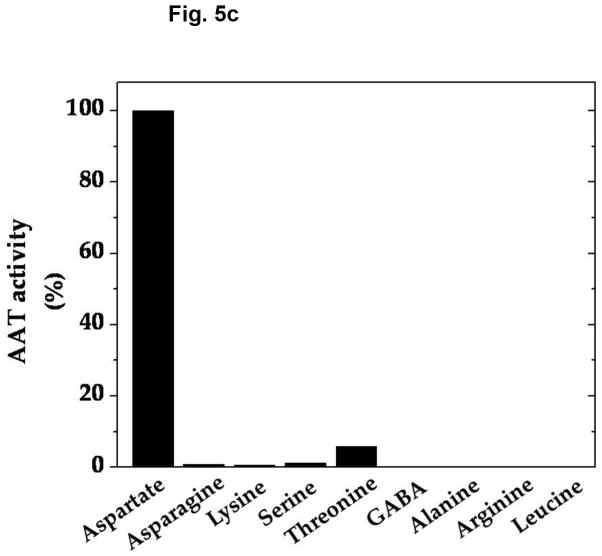
Amino group donor specificity for rat brain vesicular loading of glutamate derived from α-KGA is shown in Fig. 5a. We have tested various amino group donors, including D-aspartate, L-asparagine, L-lysine, L-serine, L-threonine, GABA, L-alanine, ammonium chloride, L-arginine, and L-leucine for the ability to serve as an amino group donor in the presence of 100 mM sucrose, and compared their ability with that of L-aspartate. L-Aspartate was found to be the most effective of all the amino group donors tested. Figure 5b shows that nearly identical results were obtained with synaptic vesicles purified from fresh bovine brain by a conventional method (Kish and Ueda, 1989, Ueda et al, 1979), even when they were incubated in the presence of 250 mM sucrose. These data indicate that vesicular filling of α-KGA-derived glutamate almost exclusively depends upon L-aspartate with respect to amino group donor. This suggests that synaptic vesicle-bound transaminase is highly specific to L-aspartate, in support of the presence of AAT in synaptic vesicles. We have directly measured the enzyme activity of AAT in the presence of the various amino group donors. As shown in Fig. 5c, the amino group donor specificity of vesicle-bound AAT is essentially identical to that of α-KGA-derived glutamate vesicular uptake. In contrast, alanine has been shown to serve as an amino group donor to form releasable glutamate from α-KGA in cerebellar granule cells in culture (Peng et al. 1991).
Fig. 5.
Amino-group donor specificity of vesicular α-KGA-derived glutamate uptake and vesicle-bound AAT enzyme activity. (a) Rat vesicular α-KGA-derived glutamate uptake. Rat synaptic vesicle fraction SV-A′ (50 μg protein) was incubated at 30° C for 15 min with 30 μM [14C]α-KGA in the presence of the indicated amino group donors, in the vesicular uptake medium containing 100 mM sucrose, as described in Materials and Methods. All amino donor agents were tested at 2 mM. The results are expressed as percentage of activity in the presence of 2 mM ATP with 2 mM L-aspartate (238 ± 59 pmol/mg, n=3). Values obtained in the presence of 2 mM ATP without aspartate were subtracted from all values. Each value represents the mean ± SEM, obtained using three separate preparations, except for D-aspartate (triplicates with one preparation). Glu, glutamate. (b) Bovine vesicular α-KGA-derived glutamate uptake. Bovine synaptic vesicles (100 μg protein) were preincubated at 30° C for 2 min, without [14C]α-KGA, in the vesicular uptake medium containing 250 mM sucrose. After addition of 30 μM [14C]α-KGA, the mixture was incubated for additional 3.5 min. The results are expressed as percentage of activity in the presence of 2 mM ATP with 2 mM L-aspartate (112 ± 49 pmol/mg, n=2). Values obtained in the presence of 2 mM ATP without aspartate were subtracted from all values. (c) AAT activity. Rat synaptic vesicle fraction SV-A′ (46 μg) was incubated at 30° C for 2 min to determine AAT activity in the presence of various amino donors (50 mM) in the medium described in Materials and Methods. The results are expressed as percentage of activity with 50 mM L-aspartate (122 nmol/min/mg).
Vesicle-bound AAT is of the mitochondrial AAT type, but associated with synaptic vesicles via charge-charge interaction
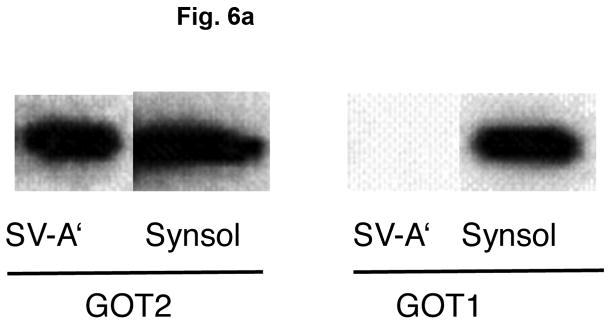
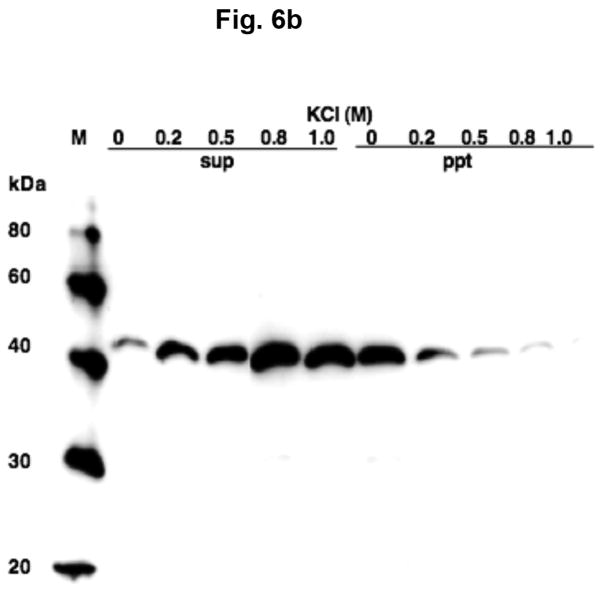
Aspartate aminotransferase is comprised of two identical subunits, whose molecular weight is approximately 45,000 (Hayashi et al. 1990). Two types of AAT are well known, one present in the cytosol and another in mitochondria, referred to as GOT1 and GOT2, respectively, which are immunologically and genetically distinct (Panteghin 1990). Figure 6a shows that AAT in synaptic vesicle fraction SV-A′ strongly interact with GOT2 antibodies, but are hardly recognized by GOT1 antibodies. This indicates that synaptic vesicle-bound AAT responsible for synthesis of the VGLUT substrate is distinct from cytosolic AAT (GOT1); it is highly similar if not identical to mitochondrial AAT (GOT2). This is consistent with the observation mentioned above that the affinity for aspartate of vesicle-bound AAT resembles that of the mitochondrial enzyme, but differs from the cytosolic enzyme. It was noted that AAT in the synsol fraction was recognized by both GOT1 and GOT2 antibodies (Fig. 6a). This could be due in part to the possibility that a fraction of originally vesicle-bound AAT could be dissociated from vesicles during the vesicle preparation. Vesicle-bound AAT was dissociated from vesicle membranes by increasing salt concentration; it was partially extracted with 0.2 M KCl, and almost entirely with 0.8 M KCl (Fig. 6b), indicating it is a peripheral protein associated with synaptic vesicles via ionic bonding.
Fig. 6.
Vesicle-bound AAT belongs to the mitochondrial-bound AAT type and is associated with vesicles via ionic interaction. (a) Reactivity of vesicle-bound AAT and synsol AAT with antibodies against mitochondrial AAT and cytosolic AAT. Synaptic vesicle fraction SV-A′ (4 μg) and the synsol fraction (4 μg) were subjected to SDS/western blotting, and each probed with antibodies against the cytosolic AAT (GOT1, 1:500 dilution) and the mitochondrial AAT (GOT2, 1:1000 dilution). (b) Vesicle-bound AAT is extracted with high salt. Synaptic vesicle fraction SV-A′ was treated with various concentrations of KCl and centrifuged, as described in Materials and Methods, and supernatants (sup) and pellets (ppt) subjected to SDS/western blotting and probed with GOT2 antibody.
Vesicular uptake of glutamate made from α-KGA by vesicle-bound AAT is mediated by v-H+-ATPase and VGLUT
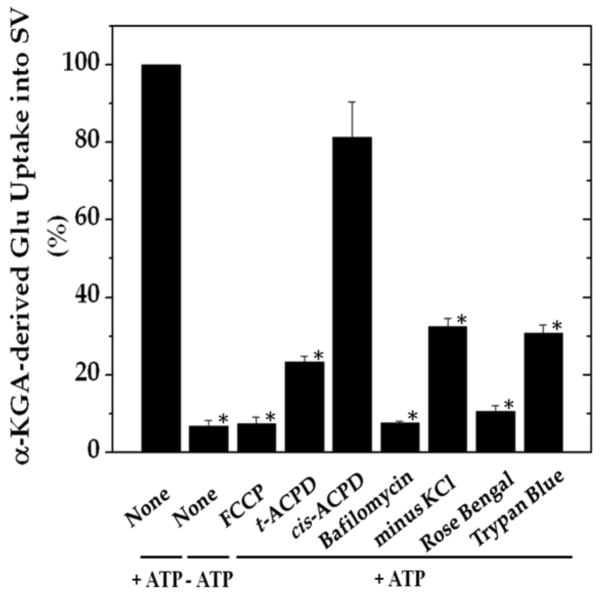
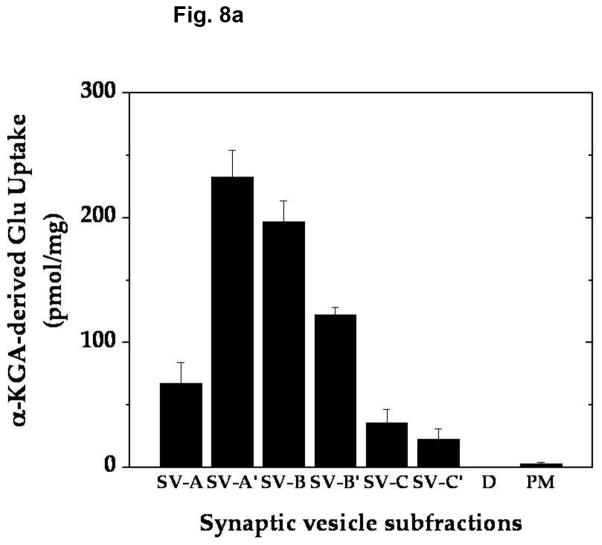
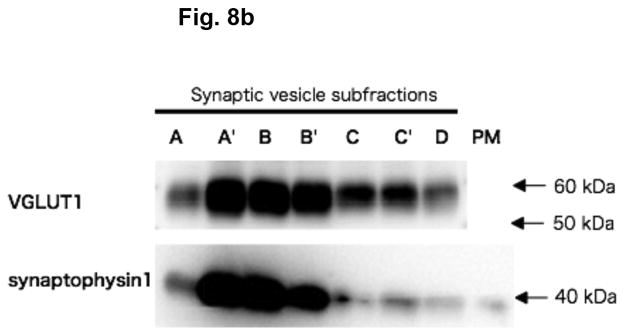
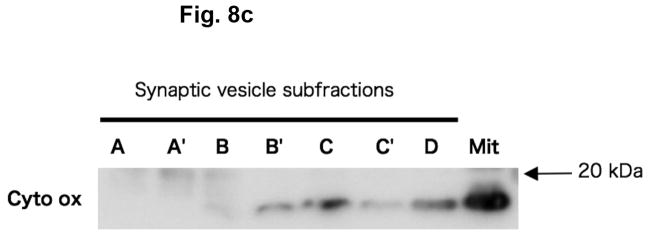
In order to determine if α-KGA-derived glutamate uptake into synaptic vesicles is mediated by v-H+-ATPase and VGLUT, we have examined the effect of the v-H+-ATPase inhibitor bafilomycin (Tabb and Ueda 1991), the electrochemical gradient dissipator carbonyl cyanide p-(trifluoromethoxy)-phenylhydrazone (FCCP) (Naito and Ueda 1983; Maycox et al. 1988; Tabb et al. 1992), the VGLUT substrate/inhibitor trans-1-aminocyclopentane-1,3-dicarboxylate (ACPD) (Winter and Ueda 1993; 2008; Moriyama and Yamamoto 1995), and the VGLUT inhibitors Trypan Blue (Roseth et al. 1998) and Rose Bengal (Ogita et al. 2001). As shown in Fig. 7, all these agents inhibited vesicular uptake of α-KGA-derived glutamate. Omission of a low millimolar concentration of chloride (e.g., 4 mM), a well-known stimulator of vesicular glutamate uptake (for review, see Ozkan and Ueda 1985; Schenck et al. 2009), from the incubation medium also resulted in a reduction of α-KGA-derived glutamate uptake. In contrast to trans-ACPD, cis-ACPD, which does not interact with VGLUT (Winter and Ueda, 1993; 2008; Moriyama and Yamamoto, 1995), hardly affected α-KGA-derived glutamate uptake into synaptic vesicles. These results indicate that glutamate produced by vesicle-bound AAT is taken up by VGLUT at the expense of an electrochemical gradient generated by v-H+-ATPase. All these observations together support the notion that synaptic vesicles have the capacity to biosynthesize the VGLUT substrate from α-KGA, using L-aspartate as the specific amino group donor, and that glutamate thus locally produced is readily transported into vesicles by VGLUT. The vesicular localization of the glutamate synthesis-uptake system is supported by the observation that α-KGA-derived glutamate uptake is the highest in synaptic vesicle fraction SV-A′, the fraction richest in synaptic vesicles of the various subcellular fractions tested (Figs. 8a and 8b). This figure shows that the subcellular distribution of α-KGA-derived glutamate uptake activity parallels that of the synaptic vesicle-specific proteins VGLUT1 and synaptophysin1. As expected, the plasma membrane plus mitochondria fraction was shown to be essentially devoid of such a process and of synaptophysin1, a vesicular marker protein. Fraction SV-A′ is free of mitochondrial contamination as indicated in Fig. 8c, which shows that SV-A′ is devoid of the mitochondrial marker cytochrome oxdase subunit VI.
Fig. 7.
Vesicular uptake of α-KGA-derived glutamate uptake is affected by agents known to act on v-H+-ATPase and VGLUT. Synaptic vesicle fraction SV-A′ (50 μg protein) was incubated at 30° C for 15 min with 30 μM [14C]α-KGA in 0.1 ml of the medium described in Materials and Methods, with addition or omission of the indicated compounds. The concentrations used were: 12.5 μM (FCCP); 2 mM (trans-ACPD); 2 mM (cis-ACPD); 1 μM (bafilomycin); 1 μM (Trypan Blue); 1 μM (Rose Bengal). The value obtained in the absence of ATP with 0 min incubation was subtracted from all values. The results are expressed as percentage of control values (196 ± 22 pmol/mg, activity in the presence of ATP with no addition of test agents and no omission of chloride). Each value represents the mean ± SEM, obtained using three separate preparations. *, p<0.001. Glu, glutamate.
Fig. 8.
The α-KGA-derived glutamate uptake system is the most abundant in the subcellular fraction, the richest in synaptic vesicles and devoid of mitochondrial contamination. (a) α-KGA-derived glutamate uptake. Various subcellular fractions were analyzed for ATP-dependent α-KGA-derived glutamate uptake in the presence of 60 μM [14C]α-KGA, using 75 μg protein. SV-A, SV-A′, SV-B, SV-B′, SV-C, SV-C′, and D: synaptic vesicle (SV) subfractions obtained upon sucrose density-gradient centrifugation of the crude synaptic fraction, as described in Materials and Methods. Values are the mean ± SEM of 4 or 5 separate preparations. (b) Relative contents of VGLUT1 and synaptophysin1 in various synaptic vesicle fractions and plasma membranes. Various synaptic vesicle fractions and the plasma membrane fraction, 4 μg each, were subjected to SDS/western blotting, and probed with antibodies against VGLUT1 (1:1000 dilution) and synaptophysin1 (1:5000 dilution). Glu, glutamate; PM, plasma membrane plus mitochondrial fraction. (c) Relative contents of cytochrome oxidase subunit IV in various synaptic vesicle fractions and the mitochondria fraction. Various synaptic vesicle fractions and the mitochondria fraction, 10μg each, were subjected to SDS/western blotting, and probed with antibodies against cytochrome oxidase subunit IV (1:5000 dilution). Mit, mitochondria; A, SV-A; A′, SV-A′; B, SV-B; B′, SV-B′; C, SV-C; C′, SV-C′; D, pellet.
AAT-selective inhibitor
In order to determine whether α-KGA-derived glutamate is used as a neurotransmitter in electrophysiological experiments, it would be essential to have a specific or selective inhibitor of AAT at hand. However, to our knowledge, no such a readily available agent has been reported. Hence, we have sought such an agent. The following aspartate-related compounds were tested for the ability to inhibit uptake of α-KGA-derived glutamate into isolated synaptic vesicles: 2,3-pyrazinedicarboxylate (2,3-PDC); dimethyl 4, 5-imidazoledicarboxylate; 2,3-pyridinedicarboxylate; 4,5-imidazoledicarboxylate; L-trans-pyrrolidine-2,4-dicarboxylate; pyridine-2,3-dicarboxylic acid dimethyl ester; 2-isopropyl-1H-imidazole-4,5-dicarboxylate; and 4-methyl-quinoline-2,3-dicarboxylic acid dimethyl ester. Of these agents tested, 2,3-PDC exhibited the most potent inhibitory effect, whereas 2,3-pyridinedicarboxylate and 4,5-imidazoledicarboxylate were less potent, and L-trans-pyrrolidine-2,4-dicarboxylate, a glutamate analog, had no effect (data not shown).
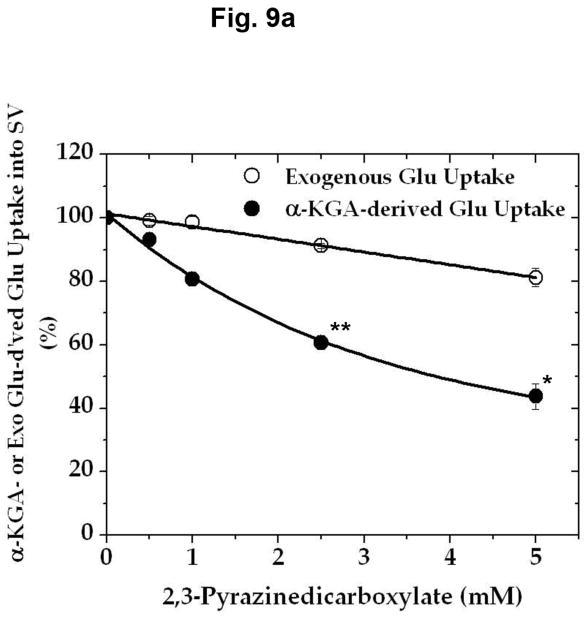
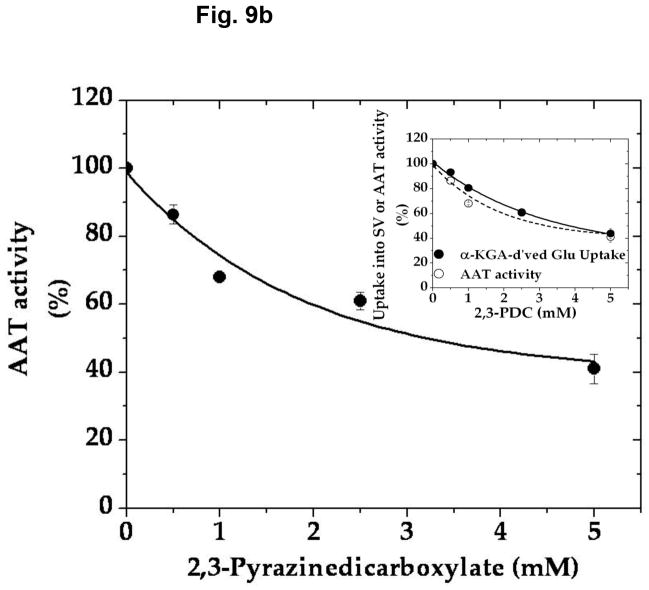
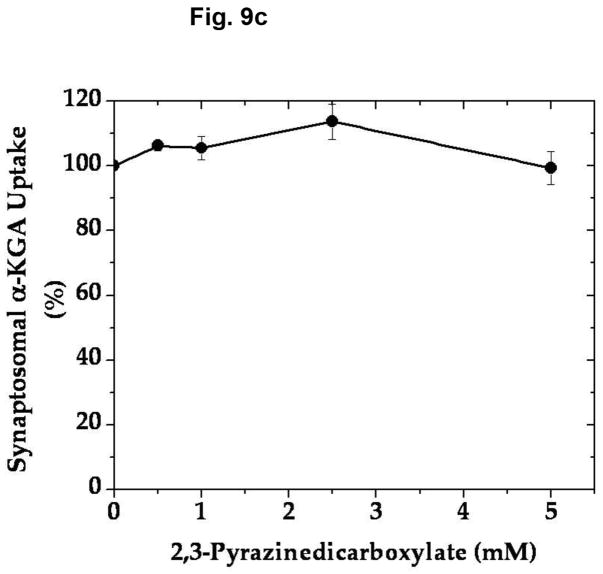
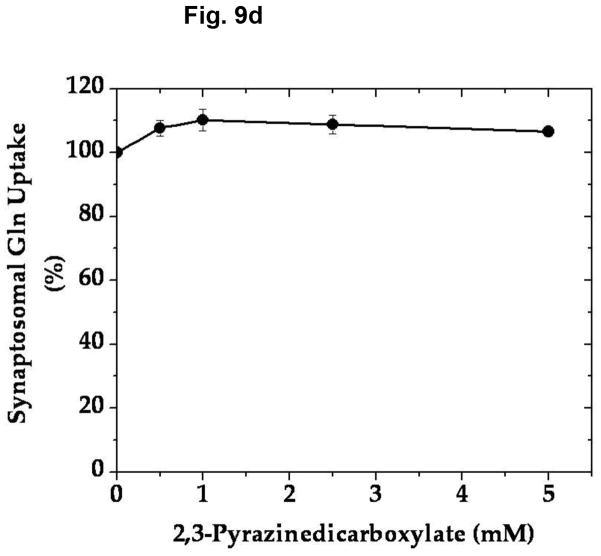
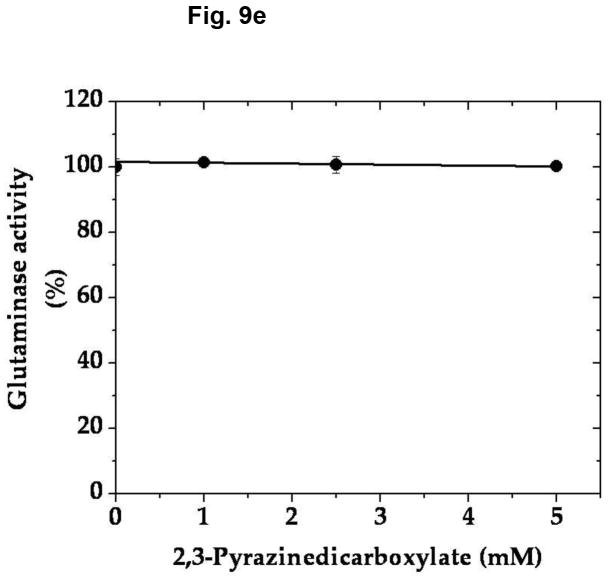
In an effort to determine the specific/selective action of this compound on AAT, we have compared the effect of various concentrations of 2,3-PDC on the vesicular uptake of α-KGA-derived glutamate with that on the vesicular uptake of exogenous (directly added) glutamate. As shown in Fig. 9a, 2,3-PDC inhibited α-KGA-derived glutamate in a concentration-dependent manner, whereas it had a minimal effect on exogenous glutamate uptake in the same concentration range tested, indicating that it has little if any effect on v-H+-ATPase or VGLUT. As expected, it exhibited a dose-dependent curve indistinguishable from that of AAT enzymatic activity (Fig. 9b). These observations indicate that the inhibitory effect of 2,3- PDC on α-KGA-derived vesicular glutamate uptake is largely due to its action on vesicle-bound AAT. However, this agent exhibited no effect on Na+-dependent α-KGA or glutamine uptake into synaptosomes (Fig. 9c, 9d) or on phosphate-dependent glutaminase activity in mitochondria (Fig. 9e). Thus, the inhibitory action of 2,3-PDC is quite selective to vesicle-bound AAT among vesicle-bound AAT, v-H+-ATPase, VGLUT, synaptosomal α-KGA uptake, synaptosomal glutamine uptake, and mitochondrial glutaminase. These data further support the notion that α-KGA-derived glutamate uptake into synaptic vesicles is mediated by vesicle-bound AAT.
Fig. 9.
2,3-Pyrazinedicarboxylate is an AAT-selective inhibitor among AAT, v-H+-ATPase, VGLUT, plasma membrane α-KGA and glutamine transporters, and glutaminase. (a) Vesicular uptake of α-KGA-derived glutamate vs. exogenous glutamate. Synaptic vesicle fraction SV-A′ (50 μg) was incubated at 30° C for 10 min in the presence of 2 mM ATP, 2 mM L-aspartate, and 100 mM sucrose, with 50 μM [14C]α-KGA or 50 μM [3H]glutamate (180 mCi/mmol), in the presence of various concentrations of 2,3-PDC, as described in Materials and Methods. Values obtained in the absence of ATP and test agents were subtracted from all values. Control values (100%): 204 ± 35 pmol/mg for α-KGA-derived glutamate uptake; 787 ± 94 pmol/mg for exogenous glutamate uptake. Each value represents the mean ± SEM, obtained using five separate preparations. *, p<0.005; **, p<0.001. (b) AAT enzymatic activity. Synaptic vesicle-associated AAT activity was measured as described in Materials and Methods, except that the reaction was carried out in 50 mM Tris-HCl (pH 7.5) containing 2 mM L-aspartate, 200 μM NADPH, 140 units/ml malate dehydrogenase, and the indicated concentrations of 2,3-PDC. The reaction was initiated by addition of 50 μM α-KGA to the mixture. Control values (100%): 11.6 ± 3.1 nmol/min/mg. Each value represents the mean ± SEM, obtained using 3 separate preparations. The insert shows the direct comparison of (b) with (a). (c and d) Synaptosomal α-KGA uptake and glutamine uptake, respectively. Purified synaptosomes were incubated 0.2 ml oxygenated (95% O2-5% CO2) Krebs-Ringer buffer with both 50 μM [14C]α-KGA and 0.5 mM [3H]glutamine (double labeling), in the presence of various concentrations of 2,3-PDC, and the amount of the radioactive materials determined, as described in Materials and Methods. Control values (100%): 3.3 ± 0.1 nmol/mg and 24.9 ± 2.4 nmol/mg for α-KGA uptake and glutamine uptake, respectively. Each value represents the average of two separate preparations with the variations indicated. (e) Glutaminase activity. Phosphate-dependent glutaminase activity in the mitochondria fraction (1.7 μg) was determined as described in Materials and Methods, in the presence or absence of the indicated concentrations of 2,3-PDC. Phosphate–independent glutaminase activity, which was less than 10% of phosphate–dependent glutaminase activity, was subtracted from all values. Control values (100%): 0.983 ± 0.025 μmol/min/mg. Each value represents the average ± variations of duplicate determinations. Shown is the representative of two independent experiments with similar results. Glu, glutamate; Gln, glutamine.
Discussion
We have provided evidence that synaptic vesicles are endowed with AAT, which synthesizes the VGLUT substrate glutamate from α-KGA and L-aspartate. This vesicle-bound transaminase is highly specific to L-aspartate with respect to the amino donor. It belongs to the mitochondrial AAT type, as judged by its kinetic properties and cross-reactivity with GOT2 antibodies, which are in agreement with proteomics analysis of highly purified synaptic vesicles, reporting detection of GOT2 peptides (Takamori et al. 2006, Supplemental data; Burré et al. 2006). However, the manner in which it is solubilized suggests that it is bound to the cytosolic side of the vesicle membrane, whereas mitochondrial AAT is localized to the mitochondrial interior (McKenna et al. 2000). The L-aspartate affinity of vesicle-bound AAT is higher than that of the cytosolic enzyme. The vesicle-bound enzyme’s Km value for L-aspartate is below the cytosolic concentration of L-aspartate (Benjamin and Quastel 1972; Taguchi et al. 1993), whereas that of the cytosolic enzyme is higher. At physiologically relevant L-aspartate concentrations, vesicle-bound AAT should function close to maximal velocity, the cytosolic enzyme far below it. This distinct difference in the L-aspartate affinity would endow vesicle-bound AAT with a kinetic advantage, i.e., more rapid cytosolic α-KGA-glutamate conversion than in the case of cytosolic AAT. Glutamate thus produced from α-KGA by vesicle-bound AAT would be immediately taken up by VGLUT energized by a v-H+-ATPase-generated-electrochemical gradient. In contrast to α-KGA, glutamine is not converted to glutamate by synaptic vesicles (Fig. 3); this conversion occurs largely in mitochondria, which are situated away from synaptic vesicles. Hence, vesicle-bound AAT could play a role in prompt, efficient supply of the neurotransmitter pool of glutamate from its precursor α-KGA.
Nerve terminals have the capacity to take up α-KGA (Shank and Campbell 1984; also Fig. 9c). Astrocytes has been shown to release α-KGA (Westergaard et al. 1994). Astrocytes are rich in the CO2-fixing enzyme pyruvate carboxylate (Cesar 1995), which form oxaloacetate, resulting in de novo synthesis of α-KGA via the TCA cycle. Hassel and Brathe (2000) have provided evidence that neurons are also capable of incorporating CO2 into pyruvate in mitochondria by malic enzyme, abundant in neurons (Vogel et al. 1998); this leads to de novo synthesis of releasable glutamate through α-KGA formation. α-Ketoglutarate is also produced from glutamine-derived glutamate by glutamate dehydrogenase rich in nerve terminal mitochondria (McKenna 2007), as well as from pyruvate via the TCA cycle. α-Ketoglutarate supplied from either astrocytes (Westergaard et al. 1994) or mitochondria (Palaiologos et al. 1988; Bolli et al. 1989; Hassel and Brathe 2000; McKenna et al. 2000; McKenna 2007) in nerve terminals, or from both sources, could be rendered available for preferential use by vesicle-bound AAT in order to generate the VGLUT substrate glutamate. The other AAT substrate L-aspartate would be provided from mitochondria via the malate-aspartale shuttle (Palaiologos et al. 1988; Yudkoff et al. 1994; McKenna et al. 2000; McKenna, 2007). Thus, we propose here that vesicle-bound AAT could play a role in glutamate transmission by supplying the transmitter glutamate via local synthesis at the site of utilization (Fig. 10). Local synthesis of the VGLUT substrate at the synaptic vesicle site, coupled to the mitochondrial aspartate-malate shuttle and α-KGA transport, as well as to the nerve terminal uptake of α-KGA, could represent an efficient mechanism for vesicular glutamate loading, hence for synaptic glutamate transmission.
Fig. 10.
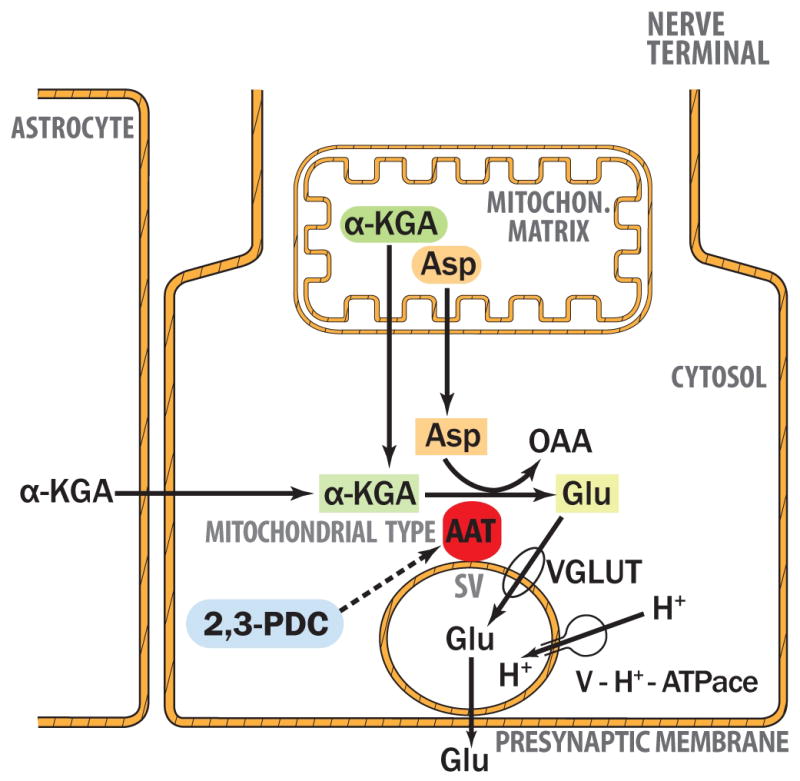
VGLUT substrate glutamate is synthesized from α-KGA by vesicle-bound AAT at the expense of Asp. AAT, aspartate aminotransferase; α-KGA, α-ketoglutarate; Asp, aspartate; Glu, glutamate; OAA, oxaloacetate; 2,3-PDC, 2,3-pirazinedicarboxylate; SV, synaptic vesicle; VGLUT, vesicular glutamate transporter; v-H+-ATPase, v-type proton-pump ATPase.
The principal precursor of the neurotransmitter glutamate is generally thought to be glutamine (Bradford et al.; Hamberger et al. 1979; Laake et al. 1995; Sibson et al. 1997; Hertz and Zielke 2004), which is provided by astrocyes (Shank and Aprison 1988; Schousboe et al. 1997). Glutamate released from neurons is taken up by astrocytes (Danbolt 2001) and transformed to glutamine by glutamine synthetase, which is highly enriched in astrocytes (Martinez-Hernandez et al. 1977). Glutamine is then transferred to neurons via neuronal glutamine transporters (Varoqui et al. 2000) and converted back to glutamate by mitochondrial glutaminase (Bradford and Ward 1976; Roberg et al. 1995). Glutamate thus produced is utilized as a neurotransmitter.
However, several recent lines of evidence suggest that the role of the glutamate-glutamine cycle is not unequivocally established in providing the immediate precursor for the transmitter glutamate, which is responsible, in particular, for spontaneous synaptic transmission (for reviews, see Edwards 2007). Kam and Nicoll (2007) provided evidence that spontaneous excitatory synaptic transmission was not affected by pharmacological interruption of the glutamate-glutamine cycle. Masson et al. (2006) showed that the knocking out of glutaminase1 failed to disrupt spontaneous glutamate transmission. McKenna et al. (2000) have reported a high level of glutamate dehydrogenase in synaptic mitochondria, suggesting an important role for this enzyme in converting glutamine-derived glutamate to α-KGA in mitochondria (McKenna 2007). Moreover, Hassel and Brathe (2000) have demonstrated that de novo synthesis of exocytotically releasable glutamate by CO2 fixation occurs in neurons, and suggested re-evaluating the importance of the glutamate-glutamine cycle in glutamate synaptic transmission.
Evidence presented here supports the notion that α-KGA could serve as an immediate precursor for a neurotransmitter pool of glutamate. A specific/selective inhibitor of AAT would be instrumental in testing this hypothesis, using electrophysiological experimental paradigms. The well known AAT inhibitors aminooxyacetate and hydroxylamine are not specific to AAT; they inhibit a number of pyridoxal phosphate-conjugated enzymes, including transaminases, DOPA carboxylase (John et al. 1978), glutamate carboxylase (Roberts and Simonsen 1963), histidine carboxylase (Leinweber 1968), and cystathionase (Beeler and Churchich 1976). Moreover, we have found that they also exhibit substantial inhibition of Na+-dependent α-KGA and glutamine uptake into synaptosomes (data not shown), most likely due to breaking the acyl (aspartic acid residue)-phosphate bond of the activated intermediate of Na+/K+ ATPase, the enzyme responsible for maintaining the Na+ gradient.
In contrast to hydroxylamine and the hydroxylamine analog aminooxyacetate, 2,3-PDC (an alternative AAT inhibitor which we identified) caused no inhibition of Na+-dependent uptake of α-KGA or glutamine into synaptosomes, or of mitochondrial glutaminase activity. This indicates that 2,3-PDC is distinct from hydroxylamine analogs, which are known to react not only with the pyridoxal group, but also with acid anhydrides and thioesters; hence 2,3-PDC fails to disrupt the acyl phosphate bond of Na+/K+-ATPase. Thus, this compound may inhibit AAT without interacting with its pyridoxal moiety. Notably, 2,3-PDC had minimal effect on v-H+-ATPase/VGLUT, yet it displayed differential inhibitory effects on vesicle-bound AAT and v-H+-ATPase/VGLUT (reflected in the effects on α-KGA-derived glutamate uptake and exogenous glutamate uptake, respectively). However, improvement for higher potency and stringency is awaited. That 2,3-PDC has no effect on glutaminase is of particular interest, since this suggests this agent is expected not to affect the neurotransmitter pool of glutamate directly derived from glutamine. Thus 2,3-PDC or, better yet, a more potent and specific inhibitor derivative of this compound, could be of use in testing the hypothesis that α–KGA serves as an immediate precursor for synthesizing the vesicular pool of glutamate, which functions as an excitatory neurotransmitter.
Acknowledgments
This work was supported by NIH/NIMH grant MH 071384 (TU). We thank Dr. Stephen K. Fisher for critical reading of the manuscript, Dr. Takeshi Yamazaki for helpful discussions and continuous interest in this work, and Computer Consultant Douglas J. Smith for excellent illustration of the model figure.
Abbreviations
- AAT
aspartate aminotransferase
- ACPD
1-aminocyclopentane-1,3-dicarboxylate
- α-KGA
α-ketoglutarate
- FCCP
carbonyl cyanide p-(trifluoromethoxy)-phenylhydrazone
- 2,3-PDC
2,3-pirazinedicarboxylate
- synsol
synaptosomal cytosol
- VGLUT
vesicular glutamate transporter
- v-H+-ATPase
v-type proton-pump ATPase
Footnotes
The authors declare no conflict of interest regarding the work reported here.
References
- Beeler T, Churchich JF. Reactivity of the phosphopyridoxal groups of cystathionase. J Biol Chem. 1976;251:5267–5271. [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Benjamin AM, Quastel JH. Locations of amino Acids in brain slices from the rat. Biochem J. 1972;128:631–646. doi: 10.1042/bj1280631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwin B, Floor E, Martin TFJ. CAPS (Mammalian UNC-31) Protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron. 1998;21:137–145. doi: 10.1016/s0896-6273(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Bolli R, Narecz KA, Azzi A. Monocarboxylate and α-ketoglutarate carriers from bovine heart mitochondria. Purification by affinity chromatography on immobilized 2-cyano-4-hydroxycinnamate. J Biol Chem. 1989;264:18024–8030. [PubMed] [Google Scholar]
- Bradford HF, Ward HK. On glutaminase activity in mammalian synaptosomes. Brain Res. 1976;110:115–125. doi: 10.1016/0006-8993(76)90212-2. [DOI] [PubMed] [Google Scholar]
- Bradford HF, Ward HK, Thomas AJ. Glutamine--a major substrate for nerve endings. J Neurochem. 1978;30:1453–1459. doi: 10.1111/j.1471-4159.1978.tb10477.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burré J, et al. Analysis of the synaptic vesicle proteome using three gel-based protein separation techniques. Proteomics. 2006;6:6250–6262. doi: 10.1002/pmic.200600357. [DOI] [PubMed] [Google Scholar]
- Cesar M, Hamprecht B. Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J Neurochem. 1995;64:2312–2318. doi: 10.1046/j.1471-4159.1995.64052312.x. [DOI] [PubMed] [Google Scholar]
- Curthoys NP, Lowry OH. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973;248:162–168. [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dennis SC, Lai JC, Clark JB. Comparative studies on glutamate metabolism in synpatic and non-synaptic rat brain mitochondria. Biochem J. 1977;164:727–736. doi: 10.1042/bj1640727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nature Protocols. 2008;3:1718–1728. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hamberger AC, Chiang GH, Nylén ES, Scheff SW, Cotman CW. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979;168:513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory; New York: 1988. [Google Scholar]
- Hassel B, Brathe A. Neuronal pyruvate carboxylation supports formation of transmitter glutamate. J Neurosci. 2000;20:1342–1347. doi: 10.1523/JNEUROSCI.20-04-01342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Wada H, Yoshimura T, Esaki N, Soda K. Recent topics in pyridoxal 5′-phosphate enzyme studies. Annu Rev Biochem. 1990;59:87–110. doi: 10.1146/annurev.bi.59.070190.000511. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles: Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- Ishida A, Noda Y, Ueda T. Synaptic vesicle-bound pyruvate kinase can support vesicular glutamate uptake. Neurochem Res. 2009;34:807–818. doi: 10.1007/s11064-008-9833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John RA, Charteris A, Fowler LJ. The reaction of amino-oxyacetate with pyridoxal phosphate-dependent enzymes. Biochem J. 1978;171:771–779. doi: 10.1042/bj1710771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Yoshida Y, Yatsushiro S, Omote H, Moriyama Y. Vesicular glutamate transporter contains two independent transport machineries. J Biol Chem. 2006;281:39499–39506. doi: 10.1074/jbc.M607670200. [DOI] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27:9192–9200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish PE, Ueda T. Glutamate accumulation into synaptic vesicles. Meth Enzymol. 1989;174:9–25. doi: 10.1016/0076-6879(89)74005-2. [DOI] [PubMed] [Google Scholar]
- Laake JH, Slyngstad TA, Haug FM, Ottersen OP. Glutamine from glial cells Is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J Neurochem. 1995;65:871–881. doi: 10.1046/j.1471-4159.1995.65020871.x. [DOI] [PubMed] [Google Scholar]
- Lai JC, Clark JB. Preparation of synaptic and nonsynaptic mitochondria from mammalian brain. Meth Enzymol. 1979;55:51–60. doi: 10.1016/0076-6879(79)55008-3. [DOI] [PubMed] [Google Scholar]
- Leinweber FJ. Mechanism of histidine decarboxylase inhibition by NSD-1055 and related hydroxylamines. Mol Pharmacol. 1968;4:337–348. [PubMed] [Google Scholar]
- Lewis SM, Ueda T. Solubilization and reconstitution of synaptic vesicle glutamate transport system. Meth Enzymol. 1998;296:125–144. doi: 10.1016/s0076-6879(98)96011-6. [DOI] [PubMed] [Google Scholar]
- Magee SC, Phillips AT. Molecular properties of the multiple aspartate aminotransferases purified from rat brain. Biochemistry. 1971;31:3397–3405. doi: 10.1021/bi00794a013. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Masson J, Darmon M, Conjard A, Chuhma N, Ropert N, Thoby-Brisson M, Foutz AS, Parrot S, Miller GM, Jorisch R, Polan J, Hamon M, Hen R, Rayport S. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26:4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycox PR, Deckwert T, Hell JW, Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988;263:15423–15428. [PubMed] [Google Scholar]
- Maycox PR, Hell JW, Jahn R. Amino Acid Neurotransmission: spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. doi: 10.1016/0166-2236(90)90178-d. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Stevenson JH, Huan X, Hopkins IB. Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int. 2000;37:229–241. doi: 10.1016/s0197-0186(00)00042-5. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle Is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Yamamoto A. Vesicular L-glutamate transporter in microvesicles from bovine pineal glands. J Biol Chem. 1995;270:22314–22320. doi: 10.1074/jbc.270.38.22314. [DOI] [PubMed] [Google Scholar]
- Naito S, Ueda T. Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J Biol Chem. 1983;258:696–699. [PubMed] [Google Scholar]
- Naito S, Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985;44:99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Ogita K, Hirata K, Bole DG, Yoshida S, Tamura Y, Leckenby AM, Ueda T. Inhibition of vesicular glutamate storage and exocytoticrelease by Rose Bengal. J Neurochem. 2001;77:34–42. doi: 10.1046/j.1471-4159.2001.t01-1-00200.x. [DOI] [PubMed] [Google Scholar]
- Otis TS. Vesicular glutamate transporters in cognito. Neuron. 2001;29:11–14. doi: 10.1016/s0896-6273(01)00176-3. [DOI] [PubMed] [Google Scholar]
- Ozkan ED, Ueda T. Glutamate transport and storage in synaptic vesicles. Jpn J Pharmacol. 1998;77:1–10. doi: 10.1254/jjp.77.1. [DOI] [PubMed] [Google Scholar]
- Palaiologos G, Hertz L, Schousboe A. Evidence that aspartate aminotransferase activity and ketodicarboxylate carrier function are essential for biosynthesis of transmitter glutamate. J Neurochem. 1988;51:317–320. doi: 10.1111/j.1471-4159.1988.tb04872.x. [DOI] [PubMed] [Google Scholar]
- Panteghin M. Aspartate aminotransferase isoezymes. Clin Biochem. 1990;23:311–319. doi: 10.1016/0009-9120(90)80062-n. [DOI] [PubMed] [Google Scholar]
- Peng L, Schousboe A, Hertz L. Utilization of alpha-ketoglutarate as a precursor for transmitter glutamate in cultured cerebellar granule cells. Neurochem Res. 1991;16:29–34. doi: 10.1007/BF00965824. [DOI] [PubMed] [Google Scholar]
- Rej R, Hφrder M. Aspartate aminotransferase. In: Bermeyer J, Grasßl M, editors. Methods of Enzymatic Analysis. 3. III. Verlag Chemie GmbH; Weinheim: 1983. pp. 416–427. [Google Scholar]
- Roberg B, Torgner IA, Kvamme E. The orientation of phosphate activated glutaminase in the inner mitochondrial membrane of synaptic and non-synaptic rat brain mitochondria. Neurochem Int. 1995;27:367–376. doi: 10.1016/0197-0186(95)00018-4. [DOI] [PubMed] [Google Scholar]
- Roberts E, Simonsen DG. Some properties of L-glutamic decarboxylase in mouse brain. Biochem Pharmacol. 1963;12:113–134. doi: 10.1016/0006-2952(63)90177-1. [DOI] [PubMed] [Google Scholar]
- Roseth S, Fykse EM, Fonnum F. Uptake of L-glutamate into synaptic vesicles: Competitive inhibition by dyes with biphenyl and amino-and sulphonic acid-substituted naphthyl roups. Biochem Pharmacol. 1998;56:1243–1249. doi: 10.1016/s0006-2952(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Schenck S, Wojcik SM, Brose N, Takamori S. A chloride conductance in VGLUT1 underlies maximal glutamate loading into synaptic vesicles. Nat Neurosci. 2009;12:156–162. doi: 10.1038/nn.2248. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Waagepetersen H, Larsson OM, Bakken IJ, Sonnewald U. Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia. 1997;21:99–105. [PubMed] [Google Scholar]
- Shank RP, Aprison MH. Glutamate as a neurotransmitter. In: Kvamme E, editor. Glutamine and Glutamate in Mammals. II. CRC Press; Boca Raton: 1988. pp. 3–20. [Google Scholar]
- Shank RP, Campbell GL. Alpha-ketoglutarate and malate uptake and metabolism by synaptosomes: further evidence for an astrocyte-to-neuron metabolic shuttle. J Neurochem. 1984;42:1153–1161. doi: 10.1111/j.1471-4159.1984.tb12724.x. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG. In vivo13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate–glutamine cycling. Proc Natl Acad Sci U S A. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb JS, Ueda T. Phylogenetic studies on the synaptic vesicle glutamate transport system. J Neurosci. 1991;11:1822–1828. doi: 10.1523/JNEUROSCI.11-06-01822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb JS, Kish PE, Van Dyke R, Ueda T. Glutamate transport into synaptic vesicles. Roles of membrane potential, pH gradient and intravesicular pH. J Biol Chem. 1992;267:15412–15418. [PubMed] [Google Scholar]
- Taguchi T, Miyake K, Tanonaka K, Okada M, Takagi N, Fujimori K, Takeo S. Sustained changes in acetylcholine and amino acid contents of brain regions following microsphere embolism in rats. Jpn J Pharmacol. 1993;62:269–78. doi: 10.1254/jjp.62.269. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, et al. Molecular Anatomy of a Trafficking Organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Ueda T. Glutamate transport in the synaptic vesicle. In: Roberts PJ, Storm-Mathisen J, Bradford HF, editors. Excitatory Amino Acids. Macmillan; London: 1986. pp. 173–195. [Google Scholar]
- Ueda T, Greengard P, Berzins K, Cohen RS, Blomberg F, Grab DJ, Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979;83:308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Ikemoto A. Cytoplasmic glycolytic enzymes. Synaptic vesicle-associated glycolytic ATP-generating enzymes: Coupling to neurotransmitter accumulation. In: Gibson G, Dienel G, editors. Handbook of Neurochemistry and Molecular Neurobiology, Brain Energetics, Cellular and Molecular Integration. 3. Springer; Heidelberg: 2007. pp. 241–259. [Google Scholar]
- Varoqui H, Zhu H, Yao D, Ming H, Erickson JD. Cloning andfunctional identification of a neuronal glutamine transporter. J Biol Chem. 2000;275:4049–4054. doi: 10.1074/jbc.275.6.4049. [DOI] [PubMed] [Google Scholar]
- Vogel R, Jennemann G, Seitz J, Wiesinger H, Hamprecht B. Mitochondrial malic enzyme: purification from bovine brain, generation of an antiserum, and immunocytochemical localization in neurons of rat brain. J Neurochem. 1998;71:844–852. doi: 10.1046/j.1471-4159.1998.71020844.x. [DOI] [PubMed] [Google Scholar]
- Wada H, Snell EE. Enzymatic transamination of pyridoxamine. I. With oxaloacetate and α-ketoglutarate. J Biol Chem. 1962;237:127–132. [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Release of α-ketoglutarate, malate and succinate from cultured astrocytes: possible role in amino acid neurotransmitter homeostasis. Neurosci Lett. 1994;176:105–09. doi: 10.1016/0304-3940(94)90882-6. [DOI] [PubMed] [Google Scholar]
- Winter HC, Ueda T. Glutamate uptake system in the presynaptic vesicle: glutamic acid analogs as inhibitors and alternate substrates. Neurochem Res. 1993;18:79–85. doi: 10.1007/BF00966925. [DOI] [PubMed] [Google Scholar]
- Winter HC, Ueda T. The glutamate uptake system in synaptic vesicles: further characterization of structural requirements for inhibitors and substrates. Neurochem Res. 2008;33:223–231. doi: 10.1007/s11064-007-9493-8. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J Biol Chem. 1994;269:27414–27420. [PubMed] [Google Scholar]