Abstract
It is crucial to consider dynamics for understanding the biological function of proteins. We used a large number of molecular dynamics trajectories of non-homologous proteins as references and examined static structural features of proteins that are most relevant to fluctuations. We examined correlation of individual structural features with fluctuations and further investigated effective combinations of features for predicting the real-value of residue fluctuations using the support vector regression. It was found that some structural features have higher correlation than crystallographic B-factors with fluctuations observed in molecular dynamics trajectories. Moreover, support vector regression that uses combinations of static structural features showed accurate prediction of fluctuations with an average Pearson’s correlation coefficient of 0.669 and a root mean square error of 1.04 Å. This correlation coefficient is higher than the one observed for the prediction by the Gaussian network model. An advantage of the developed method over the Gaussian network models is that the former predicts the real-value of fluctuation. The results help improve our understanding of relationships between protein structure and fluctuation. Furthermore, the developed method provides a convienient practial way to predict fluctuations of proteins using easily computed static structural features of proteins.
Keywords: protein flexibility, protein dynamics, structure-dynamics relationship, support vector regression, molecular dynamics, fluctuation prediction
Introduction
Thanks to worldwide efforts in structural genomics 1–3 we now know over 75 thousand protein tertiary structures 4. This number is only a small fraction as compared with of the number of known protein sequences. Computational methods can predict structures for more than a half of newly sequenced proteins by means of template-based modeling with a sufficiently high accuracy 5–8. For some of the remaining proteins, it is possible to predict their structures in a de novo fashion if they are small and structurally simple 9–14. Thus, the problem of protein structure prediction is practically gradually being solved, and it may be completely solved in the near future. Obviously, for the most difficult (and “atypical”) cases of monomeric structures and to a much larger extent for the plethora of possible protein-protein (and protein-nucleic acid, protein-carbohydrate, etc.) complexes structure prediction will remain a challenging task for decades 9;15–17. The knowledge of protein tertiary structures facilitates fast developments in various branches of molecular medicine and biotechnology 18;19. It, however, becomes more and more obvious that in order to understand the underlying molecular mechanisms of life we need to see biomolecules “in action”.
Protein dynamics, resulting from a specific flexibility of their structures, has drawn much attention recently in both theoretical and experimental molecular biology. Studies of dynamics of protein structures and their assemblies are important for understanding the mechanisms of protein function in various cellular processes 20;21, in particular, ligand binding, enzymatic reactions 22, conformational diseases 23, and protein-protein interaction 24. The understanding of protein flexibility is also important for practical applications such as development of computer-aided methods of enzyme design 25;26 and drug development 27.
In X-ray protein crystallography, which determines the Cartesian coordinates of atoms in proteins, uncertainties/fluctuations of atomic positions are provided in the form of B-factors 28. The B-factor measures the mobility of atoms, but it also reflects some inherent aspects of crystallographic techniques. Moreover, fluctuations estimated by B-factors are influenced by the molecular environment of the crystal structure. Protein mobility in solution could differ qualitatively from that in a crystal. Eastman et al. showed that B-factors are an accurate measure of fluctuations for stable parts of proteins, but significantly underestimate motion in flexible regions 29. Somewhat more straightforward measures of structure fluctuations could be derived from NMR experiments, although resulting estimates can be flawed by various limitations of actual measurements and by the computational schemes of their interpretation 30–33. Therefore, these methods do not fully reflect actual fluctuations of proteins.
Molecular Dynamics (MD) is the most straightforward method for theoretical studies of dynamic aspects of molecular systems. Due to the progress in computing technology it is now practical to simulate protein systems in a timescale of tens of nanoseconds. Nevertheless, such simulations remain costly. With a significantly less computational requirement, the internal motion of a protein can be approximated by the normal mode analysis of a harmonic model of proteins 34. Another possibility is to employ simulations using coarse grained representations of protein structures. A simple approach is the Gaussian Network Model (GNM) and its derivatives 35–38. Long-time simulation at an intermediate resolution can be achieved using simplified protein models, such as UNRES 39 and CABS 40. These models enable a low resolution study of dynamics (or stochastic dynamics) in timescales by a few orders of magnitude longer than possible by all-atom MD 41–44. A weak point of studying dynamics using coarse grained models is a lack of straightforward scaling between the models’ time and the real time. Thus, all-atom MD simulations should be always used as a reference for coarse grained dynamics.
A number of computational methods for predicting protein fluctuations have been published; however, almost all of them evaluated their prediction results mainly in comparison with the crystallographic B-factor of proteins. As discussed above, the B-factor does not fully capture the mobility of proteins in solution. As we show in this work the fluctuations observed in MD and the B-factor correlate rather poorly, as was also concluded in a previous work 29.
There are a series of works that use GNM or its variants for predicting B-factors of proteins 35;38;45;46. Micheletti et al. extended GNM by adding Cβ atoms (βGM). The fluctuations of residues predicted by βGM were compared with the fluctuations from the MD simulation of HIV-1 protease 47. The self-consistent pair contact probability method, which is similar in its spirit to GNM, was used to predict fluctuations and compared with B-factors 48. The Zhou group developed an all-atom mean-field model to predict fluctuations 49.
Structural features of proteins were also investigated that can indicate fluctuations represented by B-factors. These features include solvent accessibility of residues50, distance from a residue to the center of mass of the protein 51, eigenvectors of the square distance matrix52, and predicted local fragment structures 53. An alternative direction pursued was to predict B-factors from protein sequences. Machine learning methods, such as Support Vector Machine 54;55, the random forest algorithm 56, or an artificial neural network 57, were used to predict fluctuations using sequence information and structural features that can be predicted from sequences, such as the secondary structure and the accessible surface area of residues.
In this work we used Support Vector Regression (SVR) to investigate the relationship between protein structure and dynamics. We employed various structural characteristics as well as structure fluctuation profiles predicted by GNM as input for SVR. The target reference is the dynamics observed in long MD simulations for a representative set of 592 globular proteins. To the best of our knowledge, this is the first time that protein fluctuations have been investigated on such a large dataset of MD simulations. In this context we also analyzed differences of protein dynamics deducted from the B-factors and the in-solvent dynamics computed by MD simulations. A more practical purpose of this work is to provide a fast (essentially instantaneous in comparison to MD) and reliable method that can be used for predicting fluctuations of protein structures. Unlike existing works mentioned above, we predict the real-value of residue fluctuations rather than simply showing correlation between predicted and actual fluctuations values. Remarkably, our method predicts fluctuation highly accurately with an average error of less than 1.1 Å. The correlation coefficient of our prediction with the actual fluctuations observed in MD simulations is higher than that of GNM. We also found that some of the static structural features, such as residue contact number, have higher correlation with the residue fluctuation in MD simulation than B-factors do. The developed software for predicting fluctuation, named flexPred, has been made freely available for the academic community.
Materials and Methods
Dataset of molecular dynamics trajectories
The MD trajectories of proteins were selected from MoDEL (Molecular Dynamics Extended Library) 58. Out of 1897 entries in the database, the following entries were discarded: trajectories for protein structures solved by nucleic magnetic resonance (NMR), those which include more than one protein chain in the simulation, and trajectories for proteins whose length differ from the corresponding entries in the Protein Data Bank (PDB) 4. These MD trajectories were computed using AMBER 59, GROMACS 60, or NAMD 61 force fields. If more than one simulation is available for a protein, we used the first one with an earlier entry date in the database. The MoDEL trajectory files were uncompressed with the PCASuite software 62. 837 trajectories remained after this filtering process. From this subset, we removed redundant proteins using the PISCES server 63 with a sequence identity cutoff of 35%. The final number of trajectories is 592. This dataset contains proteins from all main classes in the CATH database 64: 111 proteins in the α class (18.75%), 149 proteins in the β class (25.17%), 256 in the αβ class (43.24%), and 76 in the few secondary structure class (12.84%). The length of the proteins ranges from 21 to 994 residues (Figure 1). The simulation time was 10 ns for most of the proteins (96.11%), while the rest of the proteins had shorter trajectories: 5 nanoseconds (ns) (0.33%), 2 ns (2.36%), 1 ns (0.5%), and one protein each with 6.5 ns, 6.0 ns, 5.5 ns, and 4.5 ns.
Figure 1.
Histogram of the length of proteins in the dataset. There are in total 592 proteins.
Definition of fluctuation
The fluctuation of amino acid residue i is defined in two ways. It can be defined as a root mean square deviation (RMSD) of the mean position of an atom in an MD trajectory:
| (Eq. 1) |
xi(tj) is the Cartesian coordinates of the Cα atom of residue i at time tj in the trajectory, T is the number of time frames in the trajectory, and <xi> is the average position of the Cα atom of residue i in the trajectory. We also used the coordinates in the PDB file as the reference:
| (Eq. 2) |
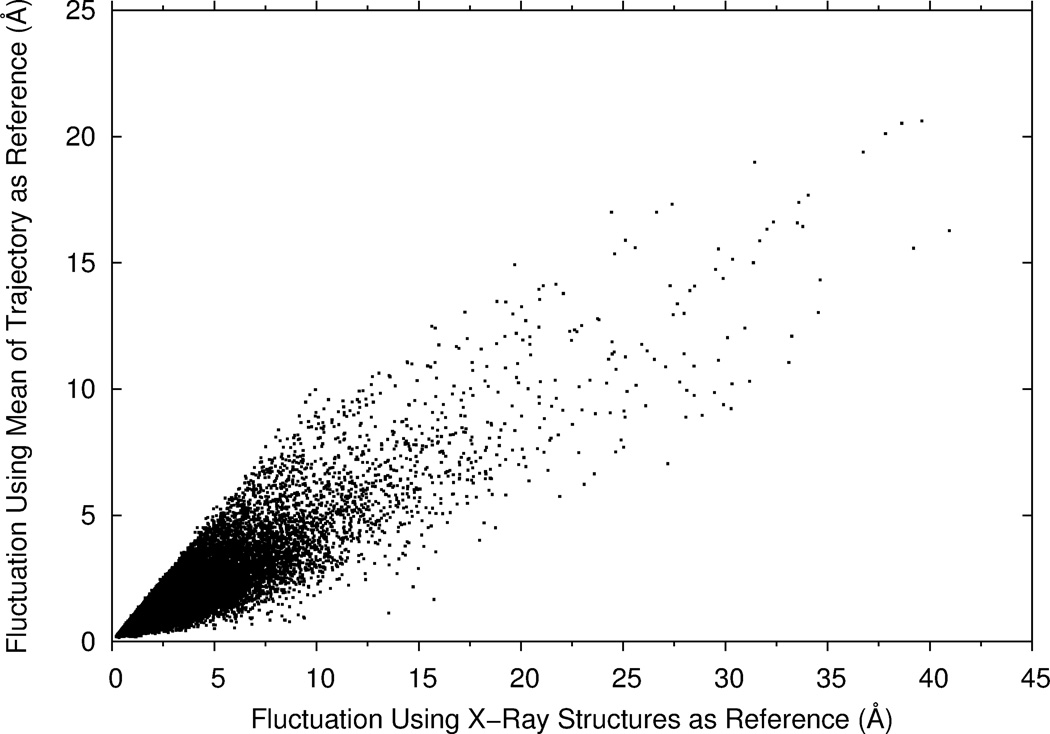
where xiref is the coordinates of the Cα atom of residue i in the PDB file. The distance of residue positions is computed after superimposing the PDB structure on each frame. If alternative positions of the atom are recorded in the PDB files, the first position of the atom was used. As shown in Figure 2, these two definitions give similar fluctuations of residues, but not identical. The correlation coefficient of the two fluctuation values is 0.86. The fluctuation value is smaller when the mean of a trajectory is used as the reference (Eq. 1) in almost all the cases (99.9 %). Unless noted, we use the second definition of fluctuation (Eq. 2) in the results that will be shown below, because we compare the fluctuations from MD with B-factors and GNM, both of which are attributed to PDB structures.
Figure 2.
Average fluctuations of proteins in MD trajectories using two definitions. X values show fluctuations of residues relative to the crystal structures of proteins in the PDB (Eq. 2) while y values are fluctuations relative to the mean structure of each MD trajectory (Eq. 1).
Structural features of proteins
We considered the following static protein structural features.
B-factor (temperature factor) 28. The B-factor reflects dynamic motion, the static disorder of the atom in the crystal structure, and also errors in model building. The B-factor values are taken from the PDB file.
Residue contact number, which is defined as the number of surrounding residues whose Cα atom is closer than a cutoff distance. The contact number was also shown to correlates well with the B-factor 65;66.
Number of hydrophobic/hydrophilic residue contacts, where the number of residue contacts is separately counted for hydrophobic and hydrophilic residues. Hydrophobic/hydrophilic residues are those which have a positive/negative value on the Kyte-Doolittle hydrophobicity scale 67.
Solvent accessibility surface area (Å2). This parameter is defined as water exposed surface of a residue. We used the DSSP program 68 to compute the accessibility surface area of amino acids, which are then normalized with the value in the tripeptide with glycines on both sides of the target amino acid residue 69.
Residue depth, which is defined as the distance of the Cα atom or the average distance of all the atoms in a residue to the closest water molecule 70. Protein surface was computed with the MSMS program 71. The hsexpo program was used to compute residue depth 72.
Lower/upper half-sphere exposure of a residue 72, which is defined as the number of contacts within a half-sphere of a radius of 13 Å centering at either the Cα or the Cβ atom of the residue. The sphere is divided into half by a plane perpendicular to the Cα-Cβ vector.
Secondary structure. Each residue is classified into eight classes, i.e. seven secondary structure types defined by DSSP 68 or other.
- Fluctuations predicted by the Gaussian Network Model (GNM) 35;36. GNM is a coarse-grained model where Cα atoms are connected by springs. GNM has been used for investigating protein dynamics including the prediction of B-factor values of proteins 38. We downloaded GNM codes from the Jernigan laboratory (http://ribosome.bb.iastate.edu/). Fluctuations were computed with a residue contact distance cutoff of 16 Å 73 and without using cutoff 38. Residue contacts in a protein are represented as the Kirchhoff matrix in GNM:
where rij is the distance between two atoms, i and j, and rc (= 16 Å) is the cutoff value. GNM without cutoff uses the following modified Kirchhoff matrix:(Eq. 4)
In both methods, the average fluctuation of residue i over time is defined by:(Eq. 5)
where C is constant.(Eq. 6)
Support Vector Regression
We combined the structural features listed above to predict fluctuations using Support Vector Regression (SVR). The LIBSVM package 74 with Gaussian kernels was used. Since it was not feasible to test all the possible combinations of features, features were added or changed one at a time starting from the one which has the largest correlation coefficient with residue fluctuation. We performed five-fold cross validation using the dataset of trajectories. The default set of parameters in libsvm, C = 64.0, γ = 1, and ε = 0.5, was used, which was shown to perform best among others tested in the first few feature combinations in the five-fold cross validation (data not shown).
Evaluation of fluctuations prediction
Pearson’s correlation coefficient was used to examine how well individual features or predicted fluctuations correlated with actual fluctuations in the MD trajectories. Average correlation coefficients were computed using all the trajectories in the dataset.
In addition, the error of predicted fluctuations was quantified as the RMSD to the reference trajectory fluctuation:
| (Eq. 7) |
where N is the length of the protein, ΔRipred is predicted, and is actual fluctuation (Eq. 2) of residue i.
Availability of the developed program
The program for predicting the fluctuation of residues in a protein structure is made freely available for the academic community at http://kiharalab.org/flexPred/. Both the web server and the source code written in Python are available. It takes a PDB file of a query protein for input data and outputs a predicted fluctuation value for each residue. The computational time for a protein is typically within a couple of seconds to twenty seconds depending on the length of the protein.
Results and Discussion
The relationships between structural features and residue fluctuations are examined in several aspects. First, we compare the correlation coefficient of individual static structural features with actual fluctuations. Then, we explore different combinations of features to make accurate prediction of fluctuations using SVR. Then, the accuracy of the fluctuation prediction by SVR and by GNM is further examined. Finally, we also consider the structural variation of models by NMR in comparison with prediction as well as the fluctuations observed in MD trajectories.
Correlation of static structural features of proteins with fluctuations
In Table 1, we compared the correlation coefficient of individual structural features with the fluctuation of residues observed in the MD trajectories. Eight different distance cutoff values, 6, 8, to 16 Å, were used for the residue contact number. The top of the table shows the correlation of the B-factor (0.484). Interestingly, several static structural features, namely, the distance to the center of mass, the contact number computed with the cutoff of 12–22 Å, have more significant correlation with the fluctuations than the B-factor. Among the static features, the largest correlation coefficients were observed for the residue contact number (15 and 16 Å). These results indicate that the motion of chains in the MD trajectories is better captured by the coarse-grained topological structures of proteins rather than the B-factor.
Table 1.
Correlation coefficients between structural features and fluctuations.
| Structural Features | Number of proteins with p- value < 0.05 (%) a) |
Avg. corr. coeff. b) |
|---|---|---|
| B-Factor | 565 (95.4) | 0.484 (0.504) |
| Distance to center of mass | 584 (98.6) | 0.509 (0.514) |
| Square of distance to center of mass | 586 (99.0) | 0.545 (0.549) |
| Contact number (cutoff 6 Å) | 571 (96.5) | −0.374 (−0.384) |
| Contact number (8 Å) | 591 (99.8) | −0.480 (−0.481) |
| Contact number (12 Å) | 590 (99.7) | −0.554 (−0.556) |
| Contact number (15 Å) | 587 (99.2) | −0.568 (−0.571) |
| Contact number (16 Å) | 571 (96.5) | −0.567 (−0.571) |
| Contact number (18 Å) | 587 (99.2) | −0.562 (−0.565) |
| Contact number (20 Å) | 585 (98.8) | −0.555 (−0.559) |
| Contact number (22 Å) | 584 (98.6) | −0.545 (−0.551) |
| Accessible Surface Area (ASA) c) | 580 (98.0) | 0.404 (0.407) |
| ASA normalized | 590 (99.7) | 0.476 (0.477) |
| Residue depth (residue mean) d) | 559 (94.4) | −0.352 (−0.371) |
| Residue depth (Cα) | 553 (93.4) | −0.339 (−0.359) |
| Half upper sphere exposure (Cα) e) | 568 (95.9) | −0.385 (−0.398) |
| Half lower sphere exposure (Cα) | 567 (95.8) | −0.389 (−0.402) |
| Half upper sphere exposure (Cβ) | 537 (90.7) | −0.339 (−0.363) |
| Half lower sphere exposure (Cβ) | 561 (94.8) | −0.383 (−0.399) |
| Prediction by GNM (cutoff 16 Å) f) | 586 (99.0) | 0.643 (0.648) |
| Prediction by GNM (no cutoff) | 591 (99.8) | 0.646 (0.646) |
The largest correlation coefficients among the static structural features are highlighted in bold.
The number of proteins that have significant correlation coefficient to the fluctuations (with p-value < 0.05) are counted. The total number of trajectories (proteins) is 592.
The average value calculated only for the subset of proteins with p-value<0.05 is shown in the parentheses.
Accessible surface area (Å2) of amino residues without normalization. The next row is the correlation with the normalized Accessible surface area.
The residue depth computed as the average distance for each atom in the residue and the distance for the Cα atom (next row).
The lower/upper half-sphere exposure of a residue using the Cα or the Cβ atom to determine the position of the plane which cut the sphere to half.
Fluctuations predicted by GNM (Eq. 6).
As a reference, we also show the correlation of the fluctuations predicted by GNM (bottom rows of Table 1). GNM showed higher correlation than the other structural features. Note that GNM actually simulates dynamic motion of protein structures; thus it has a different nature from the other static features compared in the table. Consistently with the previous work by Yang et al. 38, GNM without using a distance cutoff showed higher correlation than GNM with a distance cutoff.
Since the residue contact number (with a 16 Å cutoff) and the square of distance to the center of mass showed two largest correlation coefficients among the static structure features examined, we used these two features as the basis for combinations of input features for training SVR in the next section.
SVR models for predicting residue fluctuation using static structure features
Next, we employed SVR to predict the fluctuation of residue positions in the MD trajectories using various combinations of static structural features. Fluctuation predictions by GNM (at the bottom of Table 1) were not included as features. Five-fold cross validation was performed, in which SVR parameters were trained on four-fifths of the dataset, while prediction was made for the rest of the one-fifth of the dataset. This procedure was repeated five times to make prediction for all data in the dataset. Starting from the combination of the residue contact number (with 16 Å cutoff) and the square of distance to the center of mass, which are the two features that showed the highest correlation with fluctuations (Table 1), seventeen different feature combinations were tested by adding one feature at a time (Table 2).
Table 2.
Summary of fluctuation prediction using SVR models with different feature combinations.
| Feature Set |
Features used a) | Number of proteins with p-value < 0.05 (%) |
Average corr. coeff. b) |
RMS (Å)c) |
|---|---|---|---|---|
| 1 | C(16), D2 | 584 (98.6) | 0.638 (0.644) | 1.075 |
| 2 | C(16), D2, B | 587 (99.2) | 0.654 (0.658) | 1.067 |
| 3 | C(16), D2, B, C(18) | 587 (99.2) | 0.655 (0.659) | 1.060 |
| 4 | C(16), D2, B, C(18), Sec | 589 (99.5) | 0.661 (0.664) | 1.048 |
| 5 | C(16), D2, B, C(18), Res-type | 586 (99.0) | 0.652 (0.657) | 1.063 |
| 6 | C(16), D2, B, C(18), Sec, C(12) | 589 (99.5) | 0.665 (0.668) | 1.042 |
| 7 | C(16), D2, B, C(18), Sec, C(12), C(8) | 588 (99.3) | 0.667 (0.668) | 1.042 |
| 8 | C(16), D2,C(18), C(12), C(8), C(6) | 588 (99.3) | 0.656 (0.660) | 1.053 |
| 9 | C(16), D2, B, C(18), C(12), C(8), C(6) | 588 (99.3) | 0.666 (0.669) | 1.045 |
| 10 | C(16), D2, B, C(18), C(12), C(8), C(6), Sec | 589 (99.5) | 0.665 (0.667) | 1.043 |
| 11 | C(16), D2, B, C(18), C(12), C(8), C(6), Acc | 587 (99.2) | 0.665 (0.669) | 1.045 |
| 12 | C(16), D2, B, C(18), C(12), C(8), C(6), C(20) | 588 (99.3) | 0.666 (0.670) | 1.042 |
| 13 | C(16), D2, B, C(18), C(12), C(8), C(6), C(20), C(22) | 588 (99.3) | 0.667 (0.670) | 1.042 |
| 14 | C(16), D2, B, C(18), C(12), C(8), C(6), C(15), C(20), C(22) | 588 (99.3) | 0.666 (0.670) | 1.042 |
| 15 | C(16),B, C(18), C(12), C(8), C(6), C(20), C(22) | 588 (99.3) | 0.669 (0.673) | 1.073 |
| 16 | C(16), C(18), C(12), C(8), C(6), C(15), C(20), C(22) | 587 (99.2) | 0.660 (0.665) | 1.092 |
| 17 | C(16), B, C(18), C(12), C(8), C(6), C(20), C(22), HP | 587 (99.2) | 0.647 (0.651) | 1.092 |
C(x), the residue contact number with x Å distance cutoff; B, B-factor; D2 - square of the distance between the Cα atom to the protein center of mass; Sec, the secondary structure; Acc, normalized accessible surface area; HP, the number of hydrophilic/hydrophobic contacts, Res-Type, amino acid type of residues.
The average correlation coefficients between predicted and actual fluctuations. Values calculated only for the subset of proteins that have significant correlation with p-value < 0.05 is shown in the parentheses.
The RMS (Eq. 7) was averaged over all the proteins in the dataset.
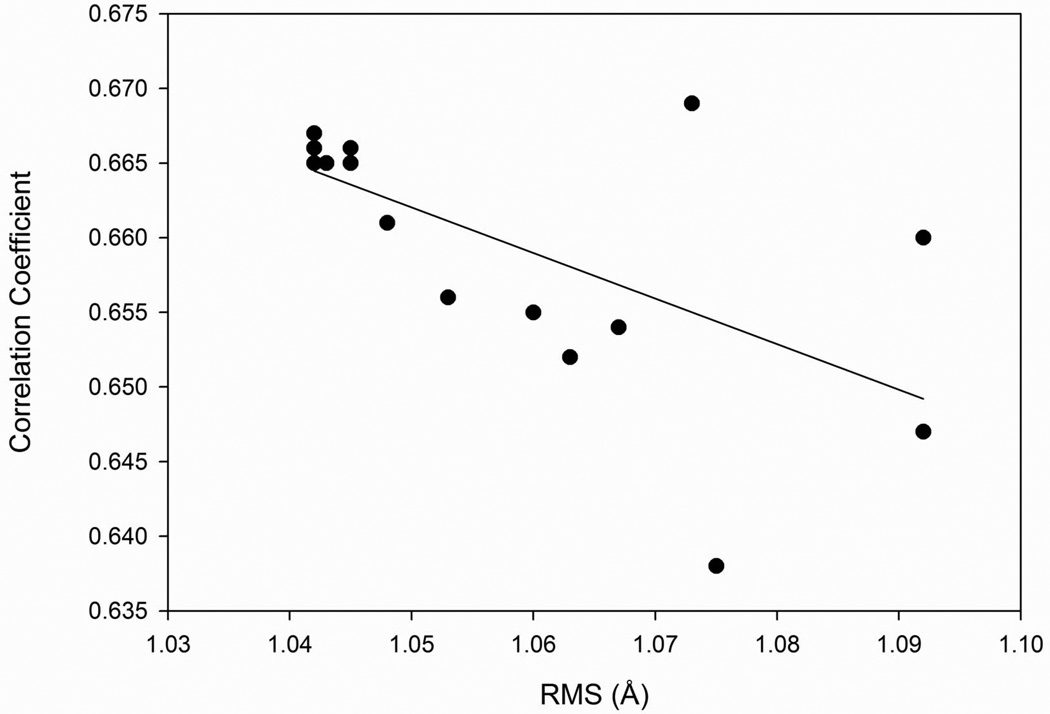
Among the seventeen feature combinations examined, all except for two (the feature set 1 and set 17) showed higher correlation with actual fluctuations than GNM (Table 1). The largest correlation coefficient, 0.669, was achieved for the feature set 15, which uses the residue contact numbers with different distance cutoffs. In terms of average RMS, all the feature combinations predicted residue fluctuations within an RMS of 1.1 Å, ranging from 1.042 Å to 1.092 Å. The smallest RMS was achieved for feature sets 6, 7, 12, 13, and 14, which combine the residue contact numbers, the square distance from the center of mass, and the B-factor. Sets 6 and 7 additionally used information about the secondary structure. The RMS and the average correlation coefficients (Table 2) correlate moderately with a correlation coefficient of 0.627 (Fig. 3). Figure 4 shows the distribution of the average correlation coefficients between predicted and actual fluctuations (Fig. 4A) and the average RMS (Fig. 4B) for each protein, which were predicted using feature set 12. Remarkably, the majority (70%) of proteins fluctuations were predicted within an RMS of 1.0 Å. The strong advantage of the developed SVR models is that they predict the real-value of fluctuation, unlike GNM which predicts only the relative magnitude of residue fluctuations that need to be rescaled to obtain actual fluctuation values.
Figure 3.
The average correlation coefficient and RMS of predicted and actual fluctuations. Predictions were made with SVR using seventeen different feature combinations (Table 2).
Figure 4.
Distribution of A, corelation coefficients; B, RMS (Å); of predicted and actual fluctuations computed for 592 proteins in the dataset.
Incorporating dynamic (GNM) features to SVR models
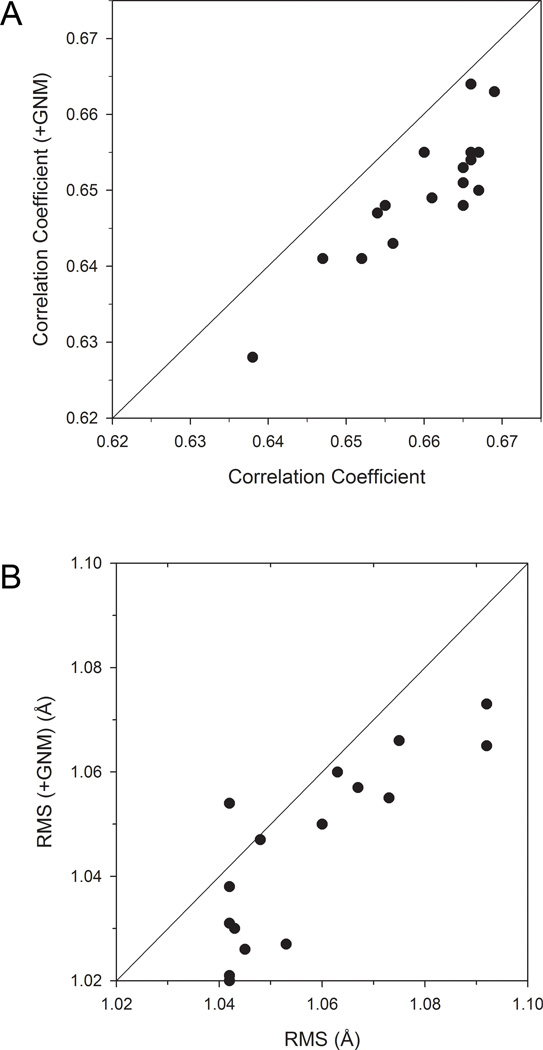
We further investigated whether adding GNM as an input feature can improve fluctuations prediction with SVR. We used 〈(ΔRi)2〉 for the fluctuations from GNM (Eq. 6) without a distance cutoff because it has higher correlation with the actual fluctuations than does. To each of the feature sets examined in Table 2 we added 〈(ΔRi)2〉 predicted by GNM and performed five-fold cross validation. The resulting fluctuation prediction with and without GNM was compared in terms of the correlation coefficient (Fig. 5A) and the RMS (Fig. 5B) with the actual fluctuations.
Figure 5.
Comparison of the prediction performance with and without using GNM as a feature. 〈(ΔRi)2〉 predicted by GNM was added to each SVR feature set listed in Table 2. A, Average correlation coefficient; B, average RMS predicted by SVR with and without 〈(ΔRi)2〉 from GNM are plotted.
Adding GNM in the feature set made slight improvement in the RMS of the predicted fluctuations (Fig. 5B) except for one case (feature set 12), lowering RMS on average by 0.010. However, small consistent deterioration of the correlation coefficient was observed (Fig. 5A) when GNM was added. The average decrease in the correlation coefficient is 0.013. Thus, GNM did not make significant contribution to improving fluctuation prediction.
Comparison of SVR model prediction results with B-factor fluctuation values
In Figure 6, we show four examples of actual and predicted fluctuations as well as fluctuations derived from the B-factors. For residue i with a B-factor of Bi, the fluctuation is defined as
| (Eq. 8) |
The fluctuations from the B-factor was also rescaled to achieve a smaller RMS with the actual fluctuations (i.e. fluctuations from MD trajectories) as follows
| (Eq. 9) |
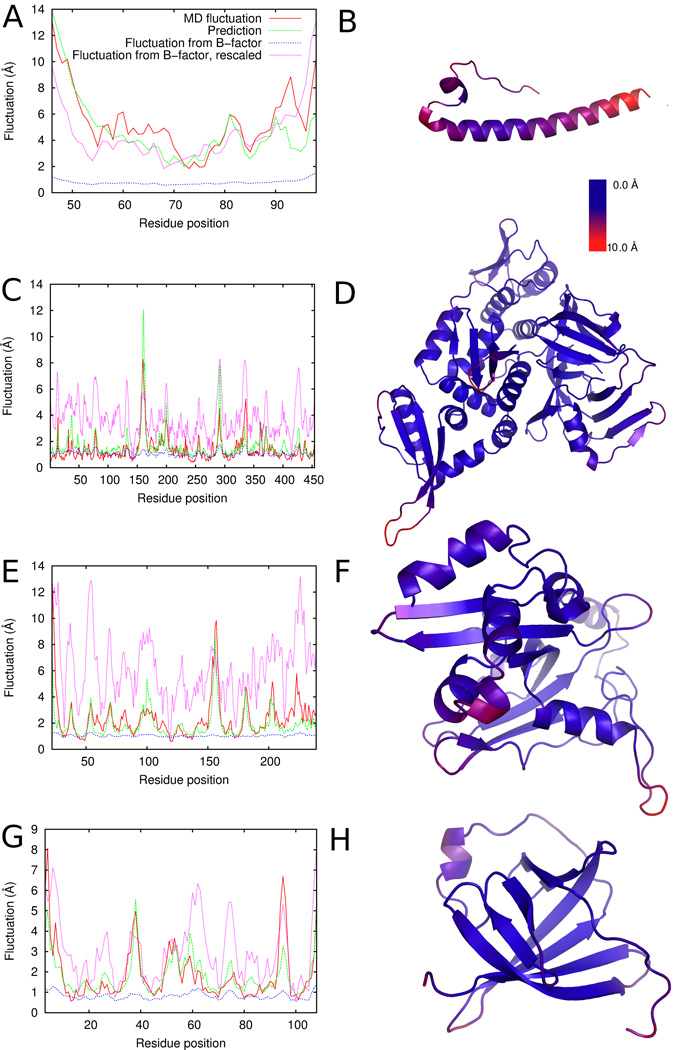
where are the maximum and the minimum values of actual fluctuations, and are the maximum and the minimum fluctuation values computed from B-factor values (Eq. 8) in the protein. α is a weighting factor explored from 0.1 to 1.0 with an interval of 0.1 to seek smaller RMS for the actual fluctuations (Table 3). In Figure 6, α is set to 1.0 for the plots of “Fluctuation from B-factor, rescaled”. Note that this rescaling obviously changes the RMS but does not change the correlation coefficient to the actual fluctuation. The acutal fluctuations in the MD trajectories are defined by Equation 2 and predictions were made using feature set 15 in Table 2. The right panel of each protein visualizes the magnitude of actual fluctuations in a color scale from blue to red with blue showing small while red for large fluctuation.
Figure 6.
Examples of predicted fluctuations in comparison with B-factor derived fluctuations and MD simulation fluctuations. Left panels show the values of fluctuations: red, fluctuations observed in the MD trajectories; green, predicted fluctuations; dotted blue line, fluctuations computed from B-factors; dotted magenta line, rescaled fluctuations from B-factors (α = 1.0). The correlation coefficients and RMS are summarized in Table 3. Right-hand panels show the magnitude of fluctuations in a color scale with blue indicating lower fluctuations and red for higher fluctuations. A, B, 1mof; C, D, 1dq3; E, F, 1gpc; G, H, 1a1x.
Table 3.
Correlation coefficients and RMS of the four example predictions.
| PDB ID | Correlation Coefficient | RMS (Å) | ||||
|---|---|---|---|---|---|---|
| B-factor | Prediction | B-factor | B-factor, rescaled α =1.0 a) |
B-factor, rescaled (α) b) |
Prediction | |
| 1mof | 0.69 | 0.80 | 4.92 | 1.91 | 1.91 (1.0) | 1.55 |
| 1dq3 | 0.50 | 0.81 | 0.94 | 2.64 | 0.85 (0.4) | 0.71 |
| 1gpc | 0.55 | 0.78 | 1.93 | 4.32 | 1.42 (0.4) | 1.04 |
| 1alx | 0.61 | 0.82 | 1.60 | 1.72 | 1.09 (0.6) | 0.79 |
The data correspond to plots at the left panels in Figure 6.
Fluctuations computed from B-factor were rescaled with α = 1.0 in Equation 9. This value corresponds to the curve “Fluctuation from B-factor, rescaled” in Figure 6.
Fluctuations computed from B-factor were rescaled with the weight factor α (Eq. 9) ranging from 0.1 to 1.0 with an interval of 0.1. Then, the smallest RMS obtained is shown together with the used α value in the parentheses.
The first example, retrovirus coat protein (PDB ID: 1mof) (Figs. 6A, B), exhibits a large fluctuation at two termini and at the end of the long helix. Prediction by SVR captured fluctuating residues and the magnitude fairly well with a correlation coefficient of 0.80 and an RMS of 1.55 Å. The fluctuations derived from B-factor have lower correlation with the actual fluctuations (correlation coefficient of 0.69) with a larger RMS of 1.91 Å even after rescaling. In the second example (Figs. 6C, D) of homing endonuclease PI-PfuI (PDB ID: 1dq3), overall fluctuation is not large but shows high peaks of fluctuation at loop regions. The predicted fluctuations have a correlation coefficient of 0.81 while the fluctuations from B-factor have a moderate correlation of 0.50. The third example, DNA-binding protein gp32 (PDB ID: 1gpc) (Figs. 6E, F), has the largest fluctuation at the loop of residues 150–160 and over 3 Å fluctuation at the other loop regions, which are captured well by the prediction. Predicted fluctuations have a correlation coefficient of 0.78 and a small RMS of 1.04 Å. In contrast, the correlation of fluctuations from B-factor is 0.55 with a larger RMS of 1.93 Å. The last example, MTCP-1 (PDB ID: 1a1x) (Figs. 6G, H) is a β-barrel protein with a long loop at residues 50 to 60. Relatively large fluctuation was observed at the N-terminus and at the loop regions that connect β-strands (e.g. residues 35–40), which are well predicted. The overall RMS of the prediction is 0.79 Å and the correlation coefficient with the actual fluctuations is 0.82, better than the fluctuations derived from B-factors.
Consistent with Table 1, the fluctuations from B-factors correlate only moderately with the actual fluctuations. Fluctuations computed from B-factors using Eq. 8 have always a larger RMS than the SVR prediction. The agreement of the fluctuations from B-factors can be improved if it is rescaled individually for each protein as shown in the second column from the right in Table 3; however, the value of the optimal scaling factor α differs from protein to protein and thus cannot be known beforehand. In contrast, our prediction by SVR has a significantly higher correlation with the actual prediction and it predicts the real-value of the fluctuations satisfactorily without any rescaling.
MD fluctuations and fluctuations from NMR models
The MoDEL database also contains simulations of protein structures determined by NMR. We selected 140 non-redundant protein structures determined by NMR that contain more than ten models in their PDB files. Redundant proteins were removed by considering sequence identity according to the PISCES database 63. Using the 140 proteins, we compared fluctuations observed in the NMR models, MD trajectories, and the predicted fluctuations. The results are summarized in Table 4. The fluctuation prediction was carried out using feature set 16, which does not contain the B-factor term (NMR structures do not have B-factors).
Table 4.
Comparison of fluctuations of NMR models, MD, and our prediction.
| Compared data |
Number of proteins with p- value < 0.05 (%) |
Corr. coeff. |
RMS(Å) |
|---|---|---|---|
| NMR vs. MD | 136 (97.1) | 0.651 (0.667) | 2.425 |
| NMR vs. prediction | 138 (98.6) | 0.739 (0.747) | 1.808 |
| MD vs. prediction | 138 (98.6) | 0.686 (0.693) | 2.165 |
140 non-redundant proteins in the MoDEL database were used whose structures were determined by NMR.
It is shown that the prediction has a significant correlation (0.739) with the structural variation of the models derived from NMR. Interestingly, the correlation coefficient between the prediction and NMR is highest among the other two pairs, prediction vs. MD and NMR vs. MD.
Conclusion
We used a large number of MD trajectories of non-homologous proteins as references and examined static structural features of the proteins that are most relevant to fluctuations. We examined the correlation of individual structural features with fluctuations and then investigated effective combinations of features for SVR to predict the real-value of fluctuation of residues. The main findings of this work are summarized as follows. First of all, two types of structural features, the distance to the center of mass of the protein and the residue contact number, showed a higher correlation coefficient with fluctuations than B-factor does. Combinations of static features used as input for SVR achieved accurate prediction of fluctuations with a correlation coefficient of 0.67 and RMS of 1.042 Å. This correlation coefficient is higher than GNM has to the actual fluctuation. Our method predicts the structural variation of NMR models also well. The current study demonstrates that flexibility of proteins is inherently coded in coarse-grained static protein structural features, even more than in the crystallographic B-factors. Thus, protein motion is determined by its static structure that is coded by its sequence, which could be considered as an extension of the Anfinsen’s dogma 75. Indeed series of studies on GNM has also demonstrated that motion of a protein is determined by its structure. However, the current work further shows that static structural features can predict the real-value of fluctuations, which GNM has not been shown to be able to do. As the importance of protein dynamics has been more recognized for biological function, the prediction method we developed has also a practical value in the wide areas of biology and biotechnology.
Acknowledgments
We thank Dr. Jordi Camps (Centre Nacional d'Anàlisis Genòmica, Spain) and Dr. Tim Meyer (Institute for Research in Biomedicine, Spain) for help with the PCAsuite software and the MoDEL database. MJ acknowledges the support from a Project operated within the Foundation for Polish Science MPD Programme co-financed by the EU European Regional Development Fund. This work was supported by a grant from the National Science Foundation (IIS0915801). DK also acknowledges grants from the National Institutes of Health (R01GM075004 and R01GM097528) and the National Science Foundation (DMS0800568, EF0850009).
Footnotes
The authors declare no conflict of interest.
Reference List
- 1.Chandonia JM, Brenner SE. The impact of structural genomics: expectations and outcomes. Science. 2006;311:347–351. doi: 10.1126/science.1121018. [DOI] [PubMed] [Google Scholar]
- 2.Todd AE, Marsden RL, Thornton JM, Orengo CA. Progress of structural genomics initiatives: an analysis of solved target structures. J Mol Biol. 2005;348:1235–1260. doi: 10.1016/j.jmb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 3.Westbrook J, Feng Z, Chen L, Yang H, Berman HM. The Protein Data Bank and structural genomics. Nucleic Acids Res. 2003;31:489–491. doi: 10.1093/nar/gkg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pieper U, Eswar N, Davis FP, Braberg H, Madhusudhan MS, Rossi A, Marti-Renom M, Karchin R, Webb BM, Eramian D, Shen MY, Kelly L, Melo F, Sali A. MODBASE: a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res. 2006;34:D291–D295. doi: 10.1093/nar/gkj059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kihara D, Skolnick J. Microbial Genomes have over 72% structure assignment by the threading algorithm PROSPECTOR_Q. Proteins. 2004;55:464–473. doi: 10.1002/prot.20044. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y. Progress and challenges in protein structure prediction. Curr Opin Struct Biol. 2008;18:342–348. doi: 10.1016/j.sbi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Kihara D. Effect of using suboptimal alignments in template-based protein structure prediction. Proteins. 2011;79:315–334. doi: 10.1002/prot.22885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das R, Baker D. Macromolecular modeling with rosetta. Annu Rev Biochem. 2008;77:363–382. doi: 10.1146/annurev.biochem.77.062906.171838. [DOI] [PubMed] [Google Scholar]
- 10.Bradley P, Misura KM, Baker D. Toward high-resolution de novo structure prediction for small proteins. Science. 2005;309:1868–1871. doi: 10.1126/science.1113801. [DOI] [PubMed] [Google Scholar]
- 11.Kihara D, Lu H, Kolinski A, Skolnick J. TOUCHSTONE: an ab initio protein structure prediction method that uses threading-based tertiary restraints. Proc Natl Acad Sci U S A. 2001;98:10125–10130. doi: 10.1073/pnas.181328398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kihara D, Zhang Y, Lu H, Kolinski A, Skolnick J. Ab initio protein structure prediction on a genomic scale: application to the Mycoplasma genitalium genome. Proc Natl Acad Sci U S A. 2002;99:5993–5998. doi: 10.1073/pnas.092135699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borreguero JM, Skolnick J. Benchmarking of TASSER in the ab initio limit. Proteins. 2007;68:48–56. doi: 10.1002/prot.21392. [DOI] [PubMed] [Google Scholar]
- 14.Trojanowski S, Rutkowska A, Kolinski A. TRACER. A new approach to comparative modeling that combines threading with free-space conformational sampling. Acta Biochim Pol. 2010;57:125–133. [PubMed] [Google Scholar]
- 15.Venkatraman V, Sael L, Kihara D. Potential for protein surface shape analysis using spherical harmonics and 3D Zernike descriptors. Cell Biochem Biophys. 2009;54:23–32. doi: 10.1007/s12013-009-9051-x. [DOI] [PubMed] [Google Scholar]
- 16.Puton T, Kozlowski L, Tuszynska I, Rother K, Bujnicki JM. Computational methods for prediction of protein-RNA interactions. J Struct Biol. 2011 doi: 10.1016/j.jsb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie DW. Recent progress and future directions in protein-protein docking. Curr Protein Pept Sci. 2008;9:1–15. doi: 10.2174/138920308783565741. [DOI] [PubMed] [Google Scholar]
- 18.Hillisch A, Pineda LF, Hilgenfeld R. Utility of homology models in the drug discovery process. Drug Discov Today. 2004;9:659–669. doi: 10.1016/S1359-6446(04)03196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda-Shitaka M, Takaya D, Chiba C, Tanaka H, Umeyama H. Protein structure prediction in structure based drug design. Curr Med Chem. 2004;11:551–558. doi: 10.2174/0929867043455837. [DOI] [PubMed] [Google Scholar]
- 20.Teilum K, Olsen JG, Kragelund BB. Functional aspects of protein flexibility. Cell Mol Life Sci. 2009;66:2231–2247. doi: 10.1007/s00018-009-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z. Intrinsically disordered protein. J Mol Graph Model. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 22.Hammes GG, Benkovic SJ, Hammes-Schiffer S. Flexibility, Diversity, and Cooperativity: Pillars of Enzyme Catalysis. Biochemistry. 2011 doi: 10.1021/bi201486f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiti F, Dobson CM. Amyloid formation by globular proteins under native conditions. Nat Chem Biol. 2009;5:15–22. doi: 10.1038/nchembio.131. [DOI] [PubMed] [Google Scholar]
- 24.Zacharias M. Accounting for conformational changes during protein-protein docking. Curr Opin Struct Biol. 2010;20:180–186. doi: 10.1016/j.sbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Mandell DJ, Kortemme T. Backbone flexibility in computational protein design. Curr Opin Biotechnol. 2009;20:420–428. doi: 10.1016/j.copbio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Lassila JK. Conformational diversity and computational enzyme design. Curr Opin Chem Biol. 2010;14:676–682. doi: 10.1016/j.cbpa.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lill MA. Efficient incorporation of protein flexibility and dynamics into molecular docking simulations. Biochemistry. 2011;50:6157–6169. doi: 10.1021/bi2004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Debye P. Interferenz von Röntgenstrahlen und Wärmebewegung. Annalen der Physik. 1913;348:49–92. [Google Scholar]
- 29.Eastman P, Pellegrini M, Doniach S. Protein flexibility in solution and in crystals. Journal of Chemical Physics. 1999;110:10141–10152. [Google Scholar]
- 30.Ishima R, Torchia DA. Protein dynamics from NMR. Nat Struct Biol. 2000;7:740–743. doi: 10.1038/78963. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin AJ, Kay LE. NMR spectroscopy brings invisible protein states into focus. Nat Chem Biol. 2009;5:808–814. doi: 10.1038/nchembio.238. [DOI] [PubMed] [Google Scholar]
- 32.Nilges M, Habeck M, O'Donoghue SI, Rieping W. Error distribution derived NOE distance restraints. Proteins. 2006;64:652–664. doi: 10.1002/prot.20985. [DOI] [PubMed] [Google Scholar]
- 33.Chalaoux FR, O'Donoghue SI, Nilges M. Molecular dynamics and accuracy of NMR structures: effects of error bounds and data removal. Proteins. 1999;34:453–463. [PubMed] [Google Scholar]
- 34.Brooks B, Karplus M. Harmonic dynamics of proteins: normal modes and fluctuations in bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci U S A. 1983;80:6571–6575. doi: 10.1073/pnas.80.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haliloglu T, Bahar I, Erman B. Gaussian dynamics of folded proteins. Physical Review Letters. 1997;79:3090–3093. [Google Scholar]
- 36.Tirion MM. Large Amplitude Elastic Motions in Proteins from a Single-Parameter, Atomic Analysis. Phys Rev Lett. 1996;77:1905–1908. doi: 10.1103/PhysRevLett.77.1905. [DOI] [PubMed] [Google Scholar]
- 37.Bahar I, Erman B, Haliloglu T, Jernigan RL. Efficient characterization of collective motions and interresidue correlations in proteins by low-resolution simulations. Biochemistry. 1997;36:13512–13523. doi: 10.1021/bi971611f. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Song G, Jernigan RL. Protein elastic network models and the ranges of cooperativity. Proc Natl Acad Sci U S A. 2009;106:12347–12352. doi: 10.1073/pnas.0902159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liwo A, Oldziej S, Pincus MR, Wawak RJ, Rackovsky S, Scheraga HA. A united-residue force field for off-lattice protein-structure simulations. I. Functional forms and parameters of long-range side-chain interaction potentials from protein crystal data. J Comp Chem. 1997;18:849–873. [Google Scholar]
- 40.Kolinski A. Protein modeling and structure prediction with a reduced representation. Acta Biochim Pol. 2004;51:349–371. [PubMed] [Google Scholar]
- 41.He Y, Liwo A, Weinstein H, Scheraga HA. PDZ binding to the BAR domain of PICK1 is elucidated by coarse-grained molecular dynamics. J Mol Biol. 2011;405:298–314. doi: 10.1016/j.jmb.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kmiecik S, Kolinski A. Characterization of protein-folding pathways by reduced-space modeling. Proc Natl Acad Sci U S A. 2007;104:12330–12335. doi: 10.1073/pnas.0702265104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kmiecik S, Kolinski A. Folding pathway of the b1 domain of protein G explored by multiscale modeling. Biophys J. 2008;94:726–736. doi: 10.1529/biophysj.107.116095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kmiecik S, Kolinski A. Simulation of chaperonin effect on protein folding: a shift from nucleation-condensation to framework mechanism. J Am Chem Soc. 2011;133:10283–10289. doi: 10.1021/ja203275f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondrashov DA, Cui Q, Phillips GN., Jr Optimization and evaluation of a coarse-grained model of protein motion using x-ray crystal data. Biophys J. 2006;91:2760–2767. doi: 10.1529/biophysj.106.085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin TL, Song G. Generalized spring tensor models for protein fluctuation dynamics and conformation changes. BMC Struct Biol. 2010;10 Suppl 1:S3. doi: 10.1186/1472-6807-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micheletti C, Carloni P, Maritan A. Accurate and efficient description of protein vibrational dynamics: comparing molecular dynamics and Gaussian models. Proteins. 2004;55:635–645. doi: 10.1002/prot.20049. [DOI] [PubMed] [Google Scholar]
- 48.Canino LS, Shen T, McCammon JA. Changes in flexibility upon binding: Application of the self-consistent pair contact probability method to protein-protein interactions. J Chem Phys. 2002;117:9927–9933. [Google Scholar]
- 49.Pandey BP, Zhang C, Yuan X, Zi J, Zhou Y. Protein flexibility prediction by an all-atom mean-field statistical theory. Protein Sci. 2005;14:1772–1777. doi: 10.1110/ps.041311005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Zhang T, Chen K, Shen S, Ruan J, Kurgan L. On the relation between residue flexibility and local solvent accessibility in proteins. Proteins. 2009;76:617–636. doi: 10.1002/prot.22375. [DOI] [PubMed] [Google Scholar]
- 51.Shih CH, Huang SW, Yen SC, Lai YL, Yu SH, Hwang JK. A simple way to compute protein dynamics without a mechanical model. Proteins. 2007;68:34–38. doi: 10.1002/prot.21430. [DOI] [PubMed] [Google Scholar]
- 52.Kloczkowski A, Jernigan RL, Wu Z, Song G, Yang L, Kolinski A, Pokarowski P. Distance matrix-based approach to protein structure prediction. J Struct Funct Genomics. 2009;10:67–81. doi: 10.1007/s10969-009-9062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bornot A, Etchebest C, De Brevern AG. Predicting protein flexibility through the prediction of local structures. Proteins. 2011;79:839–852. doi: 10.1002/prot.22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu J, Gribskov M, Bourne PE. Wiggle-predicting functionally flexible regions from primary sequence. PLoS Comput Biol. 2006;2:e90. doi: 10.1371/journal.pcbi.0020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen P, Wang B, Wong HS, Huang DS. Prediction of protein B-factors using multi-class bounded SVM. Protein Pept Lett. 2007;14:185–190. doi: 10.2174/092986607779816078. [DOI] [PubMed] [Google Scholar]
- 56.Hirose S, Yokota K, Kuroda Y, Wako H, Endo S, Kanai S, Noguchi T. Prediction of protein motions from amino acid sequence and its application to protein-protein interaction. BMC Struct Biol. 2010;10:20. doi: 10.1186/1472-6807-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlessinger A, Rost B. Protein flexibility and rigidity predicted from sequence. Proteins. 2005;61:115–126. doi: 10.1002/prot.20587. [DOI] [PubMed] [Google Scholar]
- 58.Meyer T, D'Abramo M, Hospital A, Rueda M, Ferrer-Costa C, Perez A, Carrillo O, Camps J, Fenollosa C, Repchevsky D, Gelpi JL, Orozco M. MoDEL (Molecular Dynamics Extended Library): a database of atomistic molecular dynamics trajectories. Structure. 2010;18:1399–1409. doi: 10.1016/j.str.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 59.Case DA, Cheatham TE, III, Darden T, Gohlke H, Luo R, Merz KM, Jr, Onufriev A, Simmerling C, Wang B, Woods RJ. The Amber biomolecular simulation programs. J Comput Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly Efficient, load-aalanced, and scalable molecular simulation. J Chem Theory and Computation. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 61.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer T, Ferrer-Costa C, Perez A, Rueda M, Bidon-Chanal A, Luque FJ, Laughton CA, Orozco M. Essential dynamics: A tool for efficient trajectory compression and management. J Chem Theory and Computation. 2006;2:251–258. doi: 10.1021/ct050285b. [DOI] [PubMed] [Google Scholar]
- 63.Wang G, Dunbrack RL., Jr PISCES: a protein sequence culling server. Bioinformatics. 2003;19:1589–1591. doi: 10.1093/bioinformatics/btg224. [DOI] [PubMed] [Google Scholar]
- 64.Orengo CA, Michie AD, Jones S, Jones DT, Swindells MB, Thornton JM. CATH--a hierarchic classification of protein domain structures. Structure. 1997;5:1093–1108. doi: 10.1016/s0969-2126(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 65.Lin CP, Huang SW, Lai YL, Yen SC, Shih CH, Lu CH, Huang CC, Hwang JK. Deriving protein dynamical properties from weighted protein contact number. Proteins. 2008;72:929–935. doi: 10.1002/prot.21983. [DOI] [PubMed] [Google Scholar]
- 66.Halle B. Flexibility and packing in proteins. Proc Natl Acad Sci U S A. 2002;99:1274–1279. doi: 10.1073/pnas.032522499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 68.Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 69.Miller S, Janin J, Lesk AM, Chothia C. Interior and surface of monomeric proteins. J Mol Biol. 1987;196:641–656. doi: 10.1016/0022-2836(87)90038-6. [DOI] [PubMed] [Google Scholar]
- 70.Chakravarty S, Varadarajan R. Residue depth: a novel parameter for the analysis of protein structure and stability. Structure. 1999;7:723–732. doi: 10.1016/s0969-2126(99)80097-5. [DOI] [PubMed] [Google Scholar]
- 71.Sanner M, Olson AJ, Spehner JC. Fast and robust computation of molecular surfaces. Proceedings of 11th ACM Symposium on Computational Geometry; 1995. pp. C6–C7. [Google Scholar]
- 72.Hamelryck T. An amino acid has two sides: a new 2D measure provides a different view of solvent exposure. Proteins. 2005;59:38–48. doi: 10.1002/prot.20379. [DOI] [PubMed] [Google Scholar]
- 73.Kundu S, Melton JS, Sorensen DC, Phillips GN., Jr Dynamics of proteins in crystals: comparison of experiment with simple models. Biophys J. 2002;83:723–732. doi: 10.1016/S0006-3495(02)75203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang C-C, Jin C-J. LIBSVM : a library for support vector machines. ACM Transactions on Intelligent Systems and Technology. 2001;2:27:1–27:27. [Google Scholar]
- 75.Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]