SUMMARY
Mechanisms by which Salmonella establish chronic infections are not well understood. Microbes respond to stress by importing or producing compatible solutes, small molecules that stabilize proteins and lipids. The Salmonella locus opuABCD (also called OpuC) encodes a predicted importer of the compatible solute glycine betaine. Under stress conditions, if glycine betaine cannot be imported, S. enterica produce the disaccharide trehalose, a highly effective compatible solute. We demonstrate that strains lacking opuABCD accumulate more trehalose under stress conditions than wild-type strains. ΔopuABCD mutant strains are more resistant to high salt, low pH and hydrogen peroxide, conditions that mimic aspects of innate immunity, in a trehalose-dependent manner. In addition, ΔopuABCD mutant strains require the trehalose production genes to out-compete wild-type strains in mice and macrophages. These data suggest that in the absence of opuABCD, trehalose accumulation increases bacterial resistance to stress in broth and mice. Thus, opuABCD reduces bacterial colonization via a mechanism that limits trehalose production. Mechanisms by which microbes limit disease may reveal novel pathways as therapeutic targets.
Keywords: Salmonella enterica serovar Typhimurium, antivirulence, innate immunity, otsA, otsB
INTRODUCTION
The Gram-negative bacterium Salmonella enterica can be transmitted to naive hosts in contaminated water or food. To cause systemic infection, the bacteria cross the intestinal epithelium and reside in the liver, spleen and lymph nodes inside of professional phagocytes, particularly macrophages. S. enterica serovars Typhi and Paratyphi A–C cause human typhoid fever, a systemic acute infection that resolves into subacute chronic infection in approximately 4% of patients (Parry et al., 2002). Prior to the use of antibiotics, approximately 5–20% of typhoid patients died within two to three weeks of infection, but most people began to convalesce within four weeks (Osler, 1892). Today, the increasing prevalence of multi-drug resistant Salmonellae is significant cause for concern (Cooke and Wain, 2004).
In the wild and in the laboratory, mice infected with S. enterica serovar Typhimurium develop a natural, systemic typhoid-like disease, in which acute infections become persistent and generally asymptomatic (Tsolis et al., 1999). S. Typhimurium reside within macrophages in mice, which contain the bacteria in vesicles and restrict S. Typhimurium replication (Fortier et al., 2005). Macrophage vesicles are a challenging microenvironment for S. Typhimurium because they are at low pH and contain oxidative radicals, antimicrobial peptides, and limiting levels of nutrients (Knodler and Steele-Mortimer, 2003). For example, S. Typhimurium access to iron is restricted by Nramp1 (Slc11a1), which transports iron from pathogen-containing vesicles to the cytosol (Nairz et al., 2009; Blackwell et al., 2003; Fritsche et al., 2008).
S. Typhimurium adapts to stresses encountered within macrophage vesicles using a variety of strategies. Resistance to antimicrobial peptides is mediated, for instance, by chemical modifications to lipopolysaccharide (Gunn, 2008). Saccharide deprivation stimulates utilization of host fatty acids as a carbon source, as inferred from the requirement for the glyoxylate bypass pathway during persistent infection (Fang et al., 2005). More general responses to stress include the import or production of compatible solutes, low molecular weight, highly soluble organic molecules that maintain fluid balance in the cell and protect proteins and lipids form denaturation (Luzardo et al., 2000; Hincha and Hagemann, 2004). Compatible solutes help cells acclimate to changes in osmolarity, pH and temperature, stressors encountered by S. Typhimurium during infection. Two major compatible solutes imported by S. Typhimurium are glycine betaine and proline, but only glycine betaine, an N-trimethylated amino acid, is present at physiologic concentrations in host tissues (Lever et al., 1994). A third major compatible solute is trehalose, a disaccharide consisting of two glucose monomers produced by microbes, plants and invertebrates, but not mammals (Richards et al., 2002). Trehalose is a particularly effective stress protectant but is not needed for acute S. Typhimurium infection in mice lacking the Nramp1 cation transporter (Fang et al., 1996; Howells et al., 2002).
Systemic S. enterica has been described as a “stealth pathogen”, which remains under the radar of the vertebrate immune system (Tsolis et al., 2008). Only four to five bacterial rods are typically found within each macrophage in mouse tissues, suggesting the bacteria replicate only two to three times within a given macrophage (Sheppard et al., 2003; Nix et al., 2007). Thus, S. enterica may have evolved to limit replication within tissues to minimize host damage and/or the intensity of the inflammatory response. Consistent with this hypothesis, S. Typhimurium genes that limit bacterial pathogenicity have been discovered based on hypervirulence phenotypes of strains with loss-of-function mutations (Ho and Slauch, 2001). Phenotypes of hypervirulence include reduced lethal or infectious dose, colonization advantages, and decreased survival time of the host (Foreman-Wykert and Miller, 2003; Gal-Mor et al., 2008; Baek et al., 2009). Multiple examples of microbial loci that limit virulence, also called “antivirulence” loci, are known in S. Typhimurium and other persistent pathogens. However, direct mechanisms by which they curtail infection have not yet been described. How microbial genes limit disease is important to establish because they have the potential to suggest novel avenues for treatment. In this study, we demonstrate that the S. Typhimurium OpuABCD glycine betaine transport system limits virulence via a mechanism that reduces trehalose production.
RESULTS
opuABCD is a putative Salmonella enterica glycine-betaine importer
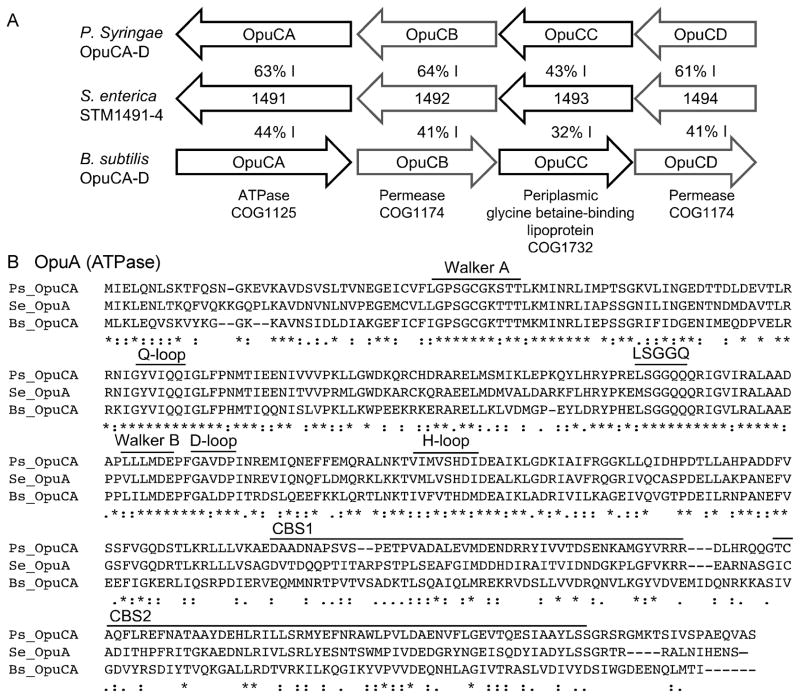
The RcsBCD (Regulation of capsule synthesis) sensor-kinase signaling system is important for virulence in murine models of acute and chronic infection (Detweiler et al., 2003; Domínguez-Bernal et al., 2004). A previous study used DNA microarrays to identify genes potentially regulated by RcsBCD, including STM1491 and STM1492 (Erickson and Detweiler, 2006). STM1491 and STM1492 are part of a four-gene putative operon that has significant homology to Osmoprotectant uptake locus C (OpuC), within Gram-negative and -positive bacteria. The four genes, STM1491-4, encode a putative ATPase, two permeases, and a periplasmic binding protein, each with 42–63% amino acid identity over the entire length of the corresponding protein to Pseudomonas syringae OpuABCD (Figure 1A). The P. syringae and Bacillus subtilis OpuABCD loci are demonstrated high affinity glycine betaine importers (Kappes et al., 1996, 1999; Chen and Beattie, 2007). In P. syringae, OpuABCD is the primary transporter for glycine betaine under high osmolarity (Chen and Beattie, 2007). In addition, the OpuA ATPases, including STM1491, are characterized by tandem cystathionine-β-synthase (CBS) domains, which are required for and predictive of functional osmoregulatory transport (Figure 1B) (Chen and Beattie, 2007; Biemans-Oldehinkel et al., 2006). These observations support designation of STM1491-4 as opuA-D.
Figure 1. STM1491-4 is a putative glycine betaine importer.
A) The opu loci from Pseudomonas syringae (P.s. pathovar tomato strain DC3000), S. enterica STM1491-4 locus (S.e. serotype Typhimurium), and Bacillus subtilis (B.s. subspecies subtilis strain 168). Percent values between arrows indicates shared amino acid identity. Predicted encoded proteins and COG families are indicated at the bottom. B) Alignment of OpuA predicted ATPases from S. Typhimurium (SeT; STM1491, NP_460451.1), P. syringae (Ps; PSTPO_4575, AAO58021.1), and B. subtilis (Bs NP_391263.1). Conserved nucleotide-binding protein motifs are labeled, including the Walker A (P-loop), Q-loop, LSGGQ motif, Walker B, D-loop, and H-loop. CLUSTAL W (1.81) multiple sequence alignment and consensus key: single, fully conserved residue (*); conservation of strong groups (:); conservation of weak groups (.).
ΔopuA-D mutants are resistant to salt stress
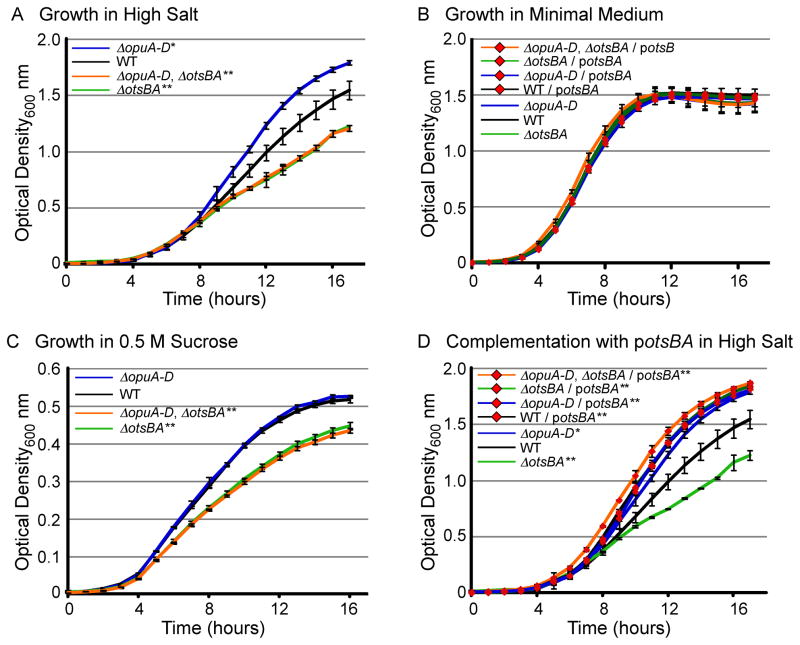
In E. coli and S. enterica, the compatible solute trehalose is produced under conditions of stress if glycine betaine is unavailable or cannot be imported (Cayley et al., 1992; Larsen et al., 1987). Since trehalose is a more effective compatible solute than glycine betaine (Hincha and Hagemann, 2004), strains lacking the opuA-D locus may have increased resistance to high salt. In M9 with 0.4 M sodium chloride, the ΔopuA-D mutant strain grew more rapidly than wild-type and by 17 hours, had attained an OD600 of 1.8 compared to only 1.5 (P < 0.01), respectively (Figure 2A). Plating for colony forming units (CFU) confirmed the observed differences in optical density, and examination of cell shape and size by microscopy revealed no distinctions (data not shown). In control experiments, there were no apparent differences in growth between wild-type and ΔopuA-D mutants in nutrient rich (LB) (data not shown) and poor (M9 minimal) media at 37°C (Figure 2B). To establish whether ΔopuA-D mutants are resistant to sucrose, another distinct osmotic stress, bacteria were grown in nutrient poor media with 0.5 M sucrose, an osmolarity equivalent to that of 0.4 M sodium chloride. No differences in growth between mutant and wild-type strains were observed (Figure 2C). This observation indicates that salt and sucrose elicit distinct responses in not only wild-type (Botsford et al., 1994) but also in ΔopuA-D mutant strains. S. Typhimurium responds differently to osmotic stress caused by sucrose as compared to sodium chloride in that sucrose exposure elicits glutamate accumulation at a lower osmolarity than salt (Botsford et al., 1994). Collectively the data suggest that S. enterica strains lacking opuA-D respond to salt stress in a specific manner that potentially reflects differential gene regulation.
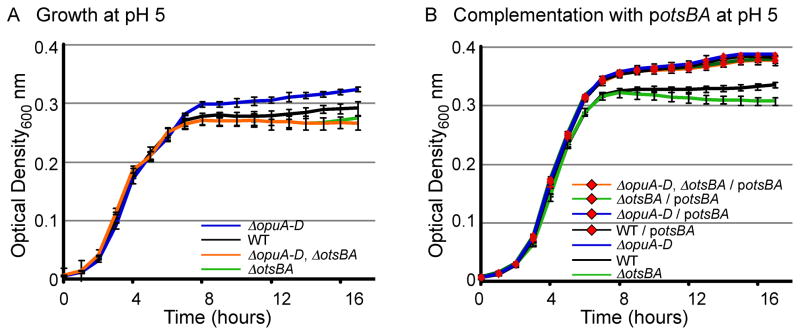
Figure 2. Growth advantage of ΔopuA-D strains in high salt requires otsBA.
Strains (as noted) were grown in M9 with 0.4 M NaCl (A, D), 0.01 M NaCl (B), or 0.5 M sucrose (C). Optical density at 600 nm (OD600) was monitored for 17 hours. Error bars are SD, N > 5 experiments. Asterisks indicate P < 0.01 (*) and 0.001 (**) compared to WT, as determined using an one-way ANOVA with a Tukey post test on the time required to grow from an OD600 of 0.1 to the OD600 reached by the strain with the lowest saturation level in the experiment (Smith and Bidochka, 1998).
Resistance of ΔopuA-D mutants to salt requires trehalose-production genes
Trehalose generation in S. Typhimurium and E. coli requires the osmoregulated trehalose synthesis (ots) operon encoding otsB and otsA, and involves the transfer of glucose from UDP-glucose to glucose-6-phosphate to yield trehalose-6-phosphate (Cánovas et al., 2001; Giaever et al., 1988). To establish whether the salt-resistance phenotype of ΔopuA-D mutants requires the otsBA trehalose production operon, ΔotsBA mutant strains were constructed. As expected, the trehalose production operon is required for normal growth in 0.4 M NaCl (Figure 2A). In addition, deletion of otsBA eliminated the growth advantage of ΔopuA-D mutant strains and reduced growth rates to below that of wild-type. Complementation of the ΔopuA-D, ΔotsBA double mutant with otsBA under the endogenous promoter on a mid-copy plasmid (potsBA) restored salt-resistance (Figure 2D). In addition, the plasmid containing otsBA increased the growth rates of all strains, including wild-type. These observations demonstrate that otsBA is necessary and sufficient for the phenotype of increased salt-resistance in ΔopuA-D mutant strains.
Under stress, ΔopuA-D mutants accumulate more trehalose than wild-type strains
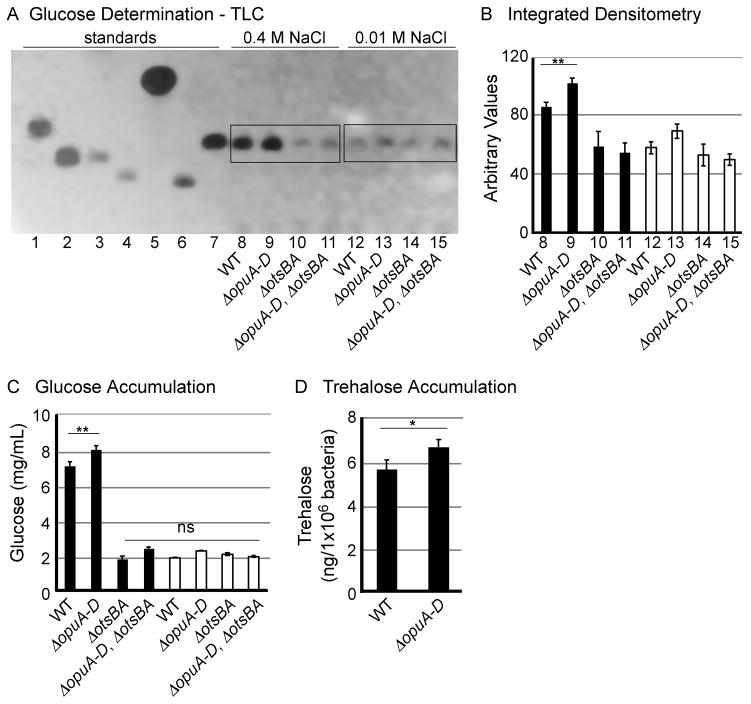
Under stress, improved E. coli growth results from small increases, as little as 3%, in trehalose accumulation (Mahmud et al., 2009; McDougall et al., 1993). To establish whether trehalose accumulates in strains lacking the opu locus, bacteria were grown with 0.01 M or 0.4 M sodium chloride without glycine betaine. Standard methods for measuring trehalose accumulation involve quantifying glucose as a proxy for trehalose (Nigam, 2007; Cánovas et al., 2001). Saccharides were extracted under conditions in which trehalose is hydrolyzed to glucose (Whistler and Wolfrom, 1980), resolved by thin layer chromatography (TLC) and stained with potassium permanganate (Figure 3A and 4A). Glucose levels were also analyzed by densitometry (Figure 3B and 4B). Background levels of glucose were observed in all strains grown in 0.01 M sodium chloride (Figure 3A, B, lanes 12–15) (Record et al., 1998). Wild-type strains had significant levels of glucose, as expected (Figure 3A and 4A, lane 8), but the ΔopuA-D mutant had 12% more glucose than the wild-type strain (Figure 3B). Glucose accumulation in all strain backgrounds required an intact otsBA locus. Thus, ΔopuA-D mutant strains exposed to salt stress produce more trehalose than their wild-type counterparts.
Figure 3. ΔopuA-D mutant strain produces excess trehalose.
Bacteria were grown in M9 with 0.01 M NaCl or 0.4 M NaCl. A) Sugars were extracted from equal numbers of bacteria and analyzed by thin-layer chromatography (TLC). Sugar standards, lanes 1–7, arabinose (10μg), galactose (10 μg), sucrose (10 μg), maltose (10 μg), glycerol (80μg), trehalose (20 μg), glucose (10 μg), respectively. Lanes 8–15 as noted. B) Integrated densitometric analysis of four TLCs was performed using Image J software. Arbitrary values correspond to the product of the area and mean grey values, 0.4 M NaCl (black) or 0.01 M NaCl (white). Error bars are SEM. C) Total glucose accumulation (mg/mL) quantified by the glucose-hexokinase conversion assay. Strains as noted were grown in M9 in 0.4 M NaCl (black) or 0.01 M NaCl (white). Error bars are SEM, N > 3 experiments. D) Trehalose accumulated (ng per 1x106 bacteria) quantified as described in the Experimental Procedure from bacteria grown in M9 with 0.4 M NaCl. Error bars are SEM, N > 3 experiments. ns, not significant. Asterisks indicate P < 0.05 (*) and 0.01 (**).
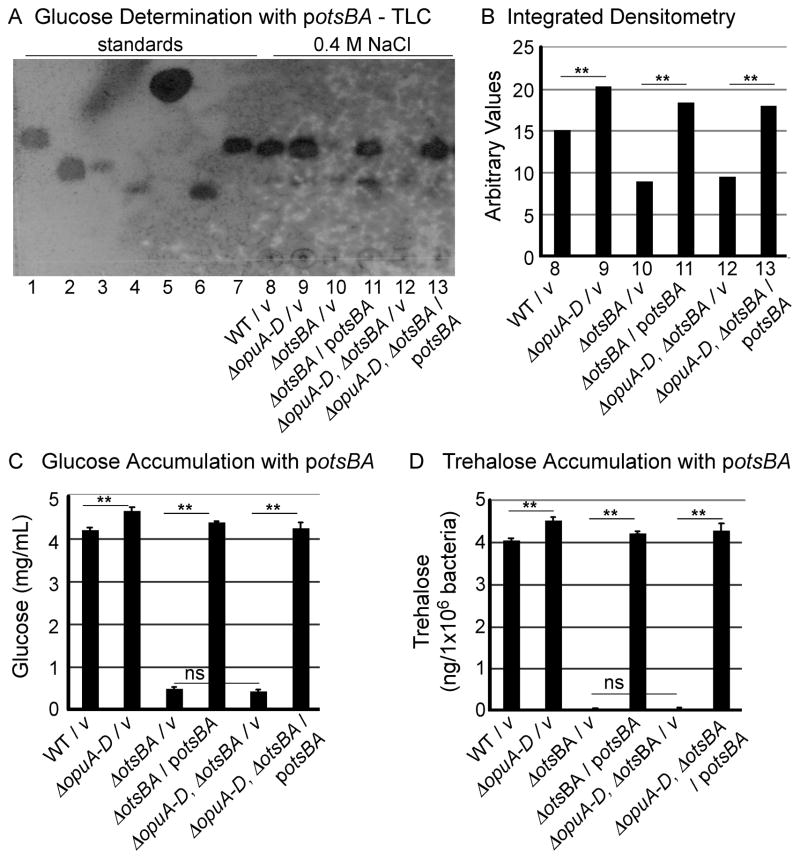
Figure 4. Complementation with potsBA restores trehalose accumulation.
Bacteria were grown in M9 with 0.4 M NaCl. A) Sugars were extracted from equal numbers of bacteria and analyzed by thin-layer chromatography (TLC). Sugar standards, lanes 1–7, arabinose (10 μg), galactose (10 μg), sucrose (10 μg), maltose (10 μg), glycerol (80 μg), trehalose (20 μg), glucose (10 μg), respectively. Lanes 8–15 as noted. B) Integrated densitometric analysis of the TLC presented in panel B was performed using Image J software. Arbitrary values correspond to the product of the area and mean grey values. C) Total glucose accumulated (mg/mL) was measured from bacteria grown in M9 with 0.4 M NaCl and complemented with potsBA. Error bars are SEM, N > 3 experiments. D) Trehalose accumulated in the presence of potsBA. Error bars are SEM, N > 3 experiments. v: vector strains have pRB3-273c, ns, not significant. Asterisks indicate P < 0.01 (*) and 0.001 (**).
Quantification of glucose using a different method yielded similar results (Figure 3C and 4C). Briefly, glucose was converted to 6-phosphogluconate, the by-product NADH was quantified spectrophotometrically, and trehalose levels were calculated after subtracting background levels of glucose observed in the ΔotsBA strain (Cánovas et al., 2001). There were 1.7 x 10−15 moles of trehalose per wild-type cell, consistent with levels observed previously in bacteria exposed to salt stress (Table 1). The ΔopuA-D mutant strain accumulated 15% more trehalose per cell than the wild-type strain (Figure 3D). An intact otsBA locus was required for trehalose accumulation in ΔopuA-D mutants and in all strains examined in both low and high salt. Complementation of ΔotsBA with otsBA expressed from a plasmid restored trehalose production (Figure 4D), although the presence of the plasmid pRB3-273c reduced glucose accumulation in all strains by approximately one third (Figure 4). In conclusion, two different methods demonstrate otsBA-dependent accumulation of similar levels of trehalose in S. Typhimurium ΔopuA-D mutant strains exposed to stress.
Table 1.
Accumulated Trehalose in E. coli and Salmonella enterica.
| Bacteria | NaCl conc. (molar) | Trehalose conc. (moles trehalose/cell) | Reference |
|---|---|---|---|
| E. coli – WT | 0.1 | not detectable | Record, Jr. 1998 |
| E. coli – WT | 1.0 | 13x10−15 | Record, Jr. 1998 |
| S. enterica – WT | 0.01 | not detectable | This study |
| S. enterica – ΔopuA-D | 0.01 | not detectable | This study |
| S. enterica – WT | 0.4 | 1.7x10−15 | This study |
| S. enterica – ΔopuA-D | 0.4 | 2.0x10−15 | This study |
ΔopuA-D mutants are resistant to low pH in an otsBA-dependent manner
Trehalose confers microbial resistance to low physiological pH (Carvalho et al., 2011). Upon oral inoculation, Salmonellae encounter low pH in the stomach and again in macrophage vesicles after traversing the gut lumen (Knodler and Steele-Mortimer, 2003; McConnell et al., 2008). To establish whether deletion of opuA-D increases S. Typhimurium resistance to low pH, equivalent numbers of wild-type and mutant bacteria were inoculated separately into pH 5.0 M9 and grown overnight at 37°C. As anticipated, the bacteria became saturated for growth at a lower OD600 (<0.4) than at neutral pH (Stokes and Bayne, 1957; Huhtanen, 1975; Russell and Dombrowski, 1980). Nevertheless, the ΔopuA-D mutant strain grew faster than the wild-type strain and the ΔopuA-D, ΔotsBA double mutant lagged behind the wild-type strain (Figure 5A). Thus, the phenotype of enhanced resistance to low pH observed in ΔopuA-D mutants requires the otsBA locus. Complementation of the ΔopuA-D, ΔotsBA double mutant with otsBA on a plasmid restored growth at low pH (Figure 5B). Indeed, all strains carrying the complementing plasmid grew better than corresponding strains caring only the vector, suggesting that increased trehalose is sufficient to confer resistance to low pH. Overall, the data show that ΔopuA-D mutants have increased resistance to low pH that is dependent upon otsBA.
Figure 5. Growth advantage of ΔopuA-D strains at low pH requires otsBA.
Strains (as noted) were grown in M9 pH5 (A-B) and OD600 nm was monitored for 17 hours. Error bars are SD, N > 5 experiments. P < 0.01 from six to eight hours, for all strains compared to wild-type, as determined using an one-way ANOVA with a Tukey post-test (Smith and Bidochka, 1998).
ΔopuA-D mutants are resistant to hydrogen peroxide in an otsBA-dependent manner
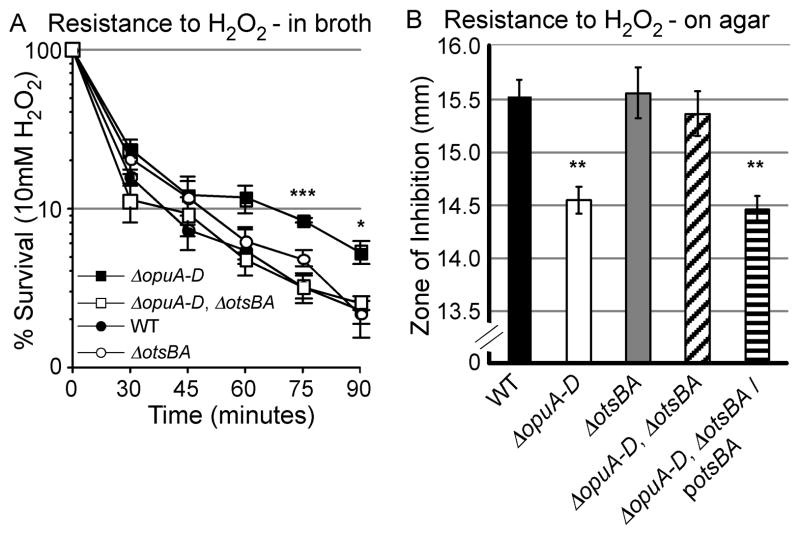
Trehalose production protects microbes from oxidative stress, including exposure to hydrogen peroxide, which forms in macrophage phagosomes upon S. Typhimurium infection (Alvarez-Peral et al., 2002). To determine whether ΔopuA-D strains have increased resistance to hydrogen peroxide in liquid culture, equivalent numbers of mutant and wild-type bacteria were exposed to 10 mM hydrogen peroxide, a lethal concentration, in M9 broth for 90-minutes. Bacteria were periodically removed and plated on LB agar to allow for enumeration of CFU (Figure 6A). Though viability decreased for all strains, ΔopuA-D had prolonged survival compared to wild-type (P < 0.001). Survival of ΔopuA-D mutants in hydrogen peroxide also required an intact otsBA locus indicating a role for trehalose production in resistance to oxidative stress.
Figure 6. OpuABCD confers resistance to hydrogen peroxide in an otsBA-dependent manner.
A) Strains were grown in M9 with 10 mM hydrogen peroxide. Error bars are SD, N > 3 experiments. Asterisks indicate P < 0.05 (*) and 0.001 (***) compared to WT. B) Filter Disc inhibition assay. Error bars are SEM, N = 3. Asterisks indicate P < 0.01 (**) compared to WT.
To establish whether ΔopuA-D mutants grown on agar are similarly resistant to hydrogen peroxide in an otsBA-dependent manner, filter disc assays were performed. A bacterial lawn was spread onto M9 agar and filter discs treated with 150 mM hydrogen peroxidewere overlaid. The zone of growth inhibition around each disc was measured after 18 hours (Figure 6B). Consistently smaller zones of inhibition were observed for the ΔopuA-D strains than wild-type (P < 0.001), confirming the mutant is resistant to hydrogen peroxide. Hydrogen peroxide resistance required otsBA and was restored in ΔopuA-D, otsBA double mutants upon complementation with otsBA. These observations indicate that in the absence of OpuA-D, otsBA is necessary and sufficient for S. Typhimurium increased resistance to hydrogen peroxide.
ΔopuA-D mutants resist killing by macrophages in an otsBA-dependent manner
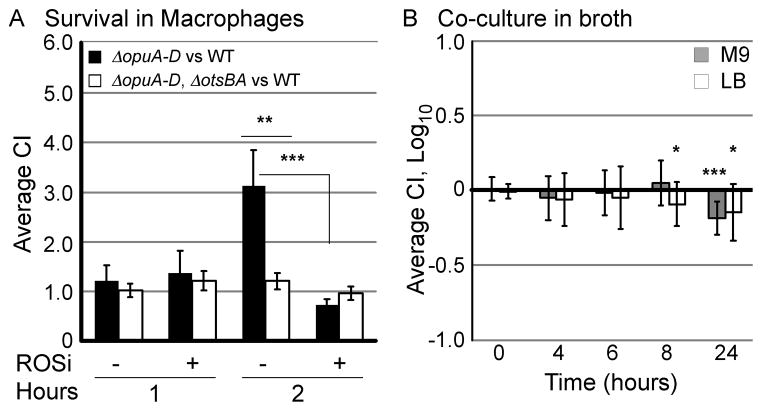
Upon phagocytosis by macrophages, bacteria are exposed to oxidative species generated by the respiratory burst including hydrogen peroxide and superoxide. S. Typhimurium has mechanisms by which it resists oxygen radicals, but approximately 90% of the bacteria are nevertheless killed within two hours of phagocytosis by primary mouse macrophages (Vazquez-Torres et al., 2000). Reactive oxygen species limit bacterial growth by damaging, for instance, enzymes within the Krebs cycle (Richardson et al., 2011). To establish whether ΔopuA-D mutants have improved survival in macrophages relative to wild-type, bone marrow derived mouse macrophages were isolated and activated with INF-γ and LPS. Mixed infection experiments were performed because they are highly sensitive based on the presence of an internal wild-type control (Beuzón and Holden, 2001). After infection with equivalent numbers of ΔopuA-D and wild-type bacteria, macrophages were lysed and CFU enumerated. Within two hours, ΔopuA-D mutants out-competed the wild-type strain, a phenotype dependent upon otsBA (Figure 7A). Treatment of cells with MDL 72527, an inhibitor of spermine oxidase that blocks the production of hydrogen peroxide (Chaturvedi et al., 2004), abrogated the increased survival of ΔopuA-D mutants. The spermine oxidase inhibitor had no significant effect on macrophage viability under these conditions (data not shown). As a control, equivalent numbers of ΔopuA-D mutant and wild-type strains were co-inoculated into M9 or LB and monitored by plating for CFU over a 24hr-period. Under these circumstances, ΔopuA-D mutants did not out-compete wild-type strains (Figure 7B). These results, in combination with hydrogen peroxide resistance observations, suggest that in the absence of opuA-D, S. Typhimurium have increased resistance to macrophage reactive oxygen species and possibly reactive nitrogen species that is dependent upon the otsBA trehalose production operon.
Figure 7. OpuABCD confers resistance to the macrophage respiratory burst in an otsBA-dependent manner.
A) Macrophages treated with and without ROS inhibitor (ROSi), MDL-72527, were infected with equivalent numbers of WT and ΔopuA-D (black) or ΔopuA-D, ΔotsBA (white) strains. Macrophages were lysed and CI values calculated. Error bars are SEM, N=3. Asterisks indicate P < 0.01 (**) and 0.001 (***). B) Co-culture of a 1:1 mixture of WT and ΔopuA-D in M9 (gray) and LB broth (white). Each bar represents the average log CI; error bars are SD, N > 3. Asterisks indicate P < 0.05 (*) and 0.001 (***).
ΔopuA-D mutants out-compete wild-type strains in mice, a phenotype that requires otsBA
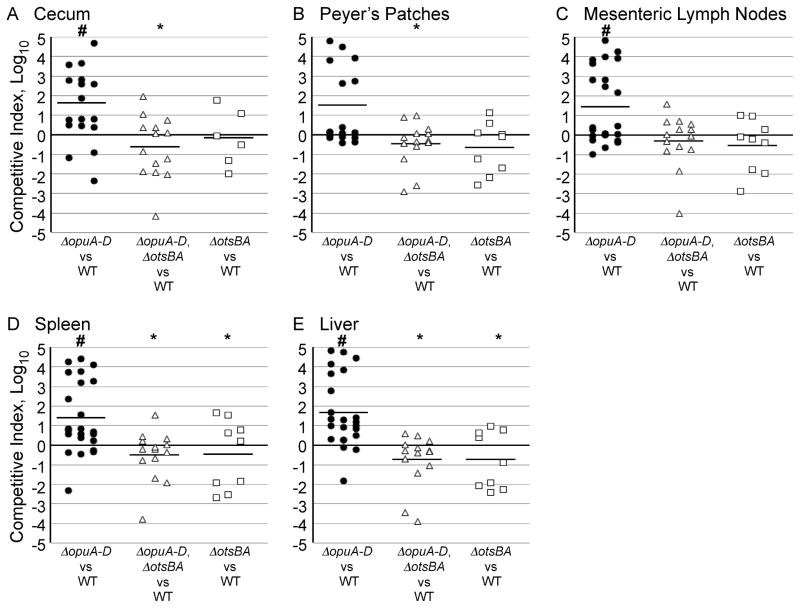
Data suggest that S. Typhimurium strains lacking the opu locus are resistant to several stressors, including high concentrations of salt, low pH and oxidative compounds. Each of these stressors may be encountered by bacteria during systemic infection of mammals, suggesting that ΔopuA-D mutants may have increased virulence in mice. To test this notion, mice were intragastrically inoculated with wild-type or an ΔopuA-D strain. An LD50, as defined by the dose at which 50% of the population dies within 28 days, was established. Nramp1−/− (BALB/C) mice were chosen for these studies because they develop fatal S. Typhimurium infections upon intragastric inoculation (Tsolis et al., 2011). The ΔopuA-D strain had only a three-fold lower LD50 than wild-type, a statistically insignificant difference (4.2x106 versus 1.3x107, for ΔopuA-D and wild-type, respectively; 95% confidence intervals: 4.2 x 105 – 4.2 x 107 and 3.4 x 106 – 4.6 x 107). These data indicate that the absence of opuA-D has little to no effect on the oral LD50 of mice. However, genes that limit virulence may have little effect on animal survival and yet impact pathogenesis as determined by more sensitive methods, such as tissue colonization (Gal-Mor et al., 2008). To establish whether ΔopuA-D mutants out-compete wild-type strains in tissue, animal mixed-infection experiments were performed. The mouse strain used was Sv129S6 (Nramp1+/+), which tolerates greater than 1010 orogastric CFU of S. Typhimurium. Mice fasted for 9–12 hours were inoculated orogastrically with equivalent numbers of differentially-marked mutant and wild-type bacteria. Three weeks post-infection, tissues were harvested and CFU enumerated. In the cecum, spleen, and liver, the mutant bacteria out-competed the wild-type by 14 – 17-fold (Figure 8A-E, 9A). An opuA-D, otsBA double-mutant strain competed equivalently with the wild-type strain, suggesting the increased colonization phenotype of ΔopuA-D mutants requires the trehalose production operon. An otsBA mutant also competed equivalently with wild-type for colonization in mice, indicating that trehalose production genes are not needed during systemic colonization. These data suggest that strains lacking opuA-D out-compete wild-type S. Typhimurium for tissue colonization in mice, and require otsBA to do so. Overall, the data indicate that the OpuA-D locus plays a role in limiting virulence.
Figure 8. ΔopuA-D strains require the otsBA locus to out-compete wild-type strains in mice.
Mice were orally infected with a 1:1 mixture of the strains indicated on the x-axes. After three weeks, mice were euthanized and their organs (cecum (C), Peyer’s patches (PP), mesenteric lymph nodes (MLN), spleen (S), and liver (L)) immediately collected for analysis. Bacteria were enumerated after plating homogenized A) cecum, B) Peyer’s patches, C) mesenteric lymph nodes, D) spleen, and E) liver. Each symbol represents one mouse, N = 6–23. P values are # < 0.01 compared to the null hypothesis and * < 0.05 compared to ΔopuA-D vs WT.
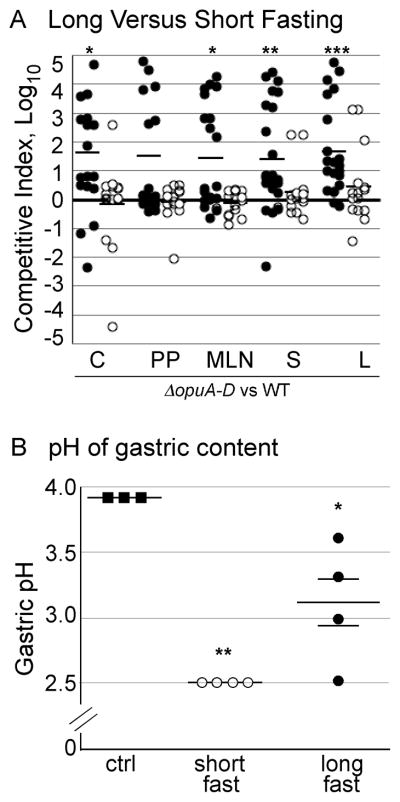
Figure 9. Fasting time affects gastric pH and colonization of ΔopuA-D strains in mice.
A) Mice were fasted for 9–12 hours (closed circles) or 2–4 hours (open circle) prior to oral inoculation with a 1:1 mixture of the ΔopuA-D and wild-type strains. After three weeks, mice were euthanized and their organs (cecum (C), Peyer’s patches (PP), mesenteric lymph nodes (MLN), spleen (S), and liver (L)) immediately collected for analysis. Each symbol represents one mouse, N=13–23. Asterisks indicate P < 0.01 (*), 0.001 (**) or 0.001 (***) compared to the null hypothesis. B) The pH of the gastric content was measured for mice fasted for three hours (short) and 11 hours (long); control (ctrl) mice were not fasted. Error bars are SD, N= 3–4 mice. Asterisks indicate P < 0.05 (*) and 0.01 (**) compared with control.
A long fast prior to inoculation is required for ΔopuA-D mutants out-compete wild-type strains in mice
While, a 9–12 hour fast is standard in the field, members of the University of Colorado Institutional Animal Care and Use Committee encouraged the testing of shorter fasts to reduce animal stress. Therefore, another set of infections was performed in which food was withheld for only 2–4 hours prior to infection. No differences were observed in tissue colonization between ΔopuA-D mutants and wild-type strains (Figure 9A). These data suggest that a physiologic consequence(s) of a long fast is needed for ΔopuA-D mutants to out-compete wild-type strains in mice.
The gastric pH of long-fasted mice is not lower than that of short-fasted mice
Given that ΔopuA-D mutant strains withstand low pH better than wild-type, it was possible that the pH of the gastric contents of the mice was lower after a long versus a short fast. To test this hypothesis, gastric content pH was measured in mice after a 3-and 11-hour fast. Control mice were not fasted and had a mean gastric pH of 3.9 (Figure 9B). After a short fast the mean gastric pH was 2.5, greater than 10-fold more acidic than that of control mice. After a long fast the gastric pH was variable and ranged from 2.5 – 3.6. These data indicate that a long fast does not consistently increase gastric acidity, nor result in lower gastric pH than a short fast. Therefore, gastric acidity is not the dominant stress that results in increased tissue colonization by ΔopuA-D mutant strains relative to wild-type strains.
DISCUSSION
Data within indicate that in the absence of the OpuABCD predicted glycine betaine importer, exposure to stress results in increased trehalose production via otsBA, and the bacteria develop enhanced resistance to multiple insults. The otsBA operon is activated by the stress-response sigma factor RpoS (Fang et al., 1996; Hengge-Aronis et al., 1991). RpoS is activated in M9 minimal media in high salt (Dong and Schellhorn, 2009), conditions under which significant trehalose production occurs. Upon infection of macrophages or mice, strains lacking opuA-D out-compete wild-type strains, a phenotype that requires otsBA. Pathogens do have access to glycine betaine in animals, which is present at approximately 35 μM in human blood and at higher concentrations in tissues (Lever and Slow, 2010). Only 20 μM glycine betaine is sufficient to restore the growth of P. syringae opuA-D mutants in the presence of 0.4 M sodium chloride (Chen and Beattie, 2007), suggesting that the concentration of glycine betaine in human blood is physiological with respect to bacterial stress responses. In contrast mammals do not make trehalose (Richards et al., 2002). These observations together suggest that during infection the S. Typhimurium OpuABCD predicted glycine betaine importer limits trehalose production, perhaps to maintain the balance between host and pathogen necessary for chronic infection.
Trehalose is produced by bacteria, archea, fungi, plants and invertebrates in response to environmental stress and is hypothesized to stabilize proteins and lipids by replacing or trapping water at the surface of macromolecules (Fedorov et al., 2010). However, multiple laboratories have demonstrated that in S. Typhimurium, trehalose production is dispensable for pathogenicity. In BALB/C (Nramp1−/−) mice, which do not survive infection with S. Typhimurium, otsA mutants are fully virulent with regard to lethality and tissue colonization upon intraperitoneal and intragastric inoculation (Fang et al., 1996; Howells et al., 2002). In Sv129S6 (Nramp1+/+) mice, which survive acute infection and become chronically infected with S. Typhimurium, the otsBA operon plays no detectable role in tissue colonization. While apparently unnecessary for S. Typhimurium virulence, in other microbes trehalose is a demonstrated virulence determinant. In the fungal pathogen Candida albicans, trehalose production defends the wild-type yeast against reactive oxygen species produced by macrophages (Martínez-Esparza et al., 2007). In another fungal pathogen Cryptococcus gattii, trehalose production genes are important in broth for melanin synthesis, capsule formation, and cell wall integrity. In mice, C. gattii requires trehalose production genes for colonization of the lung and brain (Ngamskulrungroj et al., 2009). Thus, trehalose accumulation can protect microbes from stress encountered in the host. The amount of trehalose produced by a pathogen may depend on the evolutionarily determined optimal tissue colonization level for that pathogen.
It was surprising that the colonization advantage of strains lacking the OpuABCD putative glycine betaine importer was a function of the period of time for which mice had fasted prior to oral gavage, as ΔopuA-D strains out-competed wild-type bacteria in mice fasted for 9–12, but not 2–4 hours. One possibility was that gastric pH was lower after a long fast than after a short fast. However, this idea was not supported by data, suggesting that stomach pH is not the major contributing factor in the colonization advantage of ΔopuA-D. An alternative hypothesis is that long fasts may trigger inflammatory responses in mice. This notion has some support in the literature, as within one day of fasting, levels of the cytokine IL-12 increase in the liver and mononuclear cells increase expression of superoxide dismutase, which generates hydrogen peroxide(Shen et al., 2009). Inoculation after a 9–12 hour fast may thus expose S. Typhimurium to macrophages that are more aggressively activated and/or have higher levels of oxygen radicals, resulting in trehalose production and increased survival in ΔopuA-D mutant strains. However, a considerable body of work will be required to test this and other hypotheses. Finally, long fasts may not be unnatural, as animals in the wild, including the house mouse, eat significantly less food over time than they prefer (Boutin, 1990). Thus, while members of an Institutional Animal Care and Use Committee may prefer the use of 2–4 hour fasting periods, a 9–12 hour fast may be a physiologically realistic timeframe.
Our data demonstrate that ΔopuA-D glycine betaine importer mutant strains out-compete wild-type strains in tissues by more than 10-fold, which is consistent with the role of OpuABCD as an antivirulence locus, as defined by the ability to limit one or more aspect of pathogenicity (Ho and Slauch, 2001). Previously described loci that limit virulence include ZirTS, a two-partner secretion system. The absence of functional ZirTS, like Opu, has a small and insignificant effect on the mouse survival. However, zirTS mutant strains out-compete wild-type strains in mixed-infection experiments by 3-to 13-fold (Gal-Mor et al., 2008). The mechanism(s) by which ZirTS limits virulence remains unknown. The S. Typhimurium global regulator of transcription leucine-responsive regulatory protein (LRP) also limits virulence. Strains lacking LRP out-compete wild-type by 3- to 9-fold during mixed infection. It has been suggested that LRP functions indirectly to curtail colonization, as it negatively regulates type three secretion systems required for virulence (Baek et al., 2009). Thus, LRP mutants may have increased virulence due to increased or ectopic expression of virulence determinants.
Despite its potential role in limiting colonization in mice, an opu locus is present throughout the six subspecies of S. enterica at the orthologous position in the genome, including the human pathogens Typhi and Paratyphi A – C. However, other Enterobacteriaceae family members including S. bongori, E. coli, Shigella, and Klebsiella do not encode genes resembling an opu locus in the orthologous regions of their genomes. This suggests that an opu cassette was acquired by S. enterica after divergence from S. bongori. One possibility is that during systemic infection, which is generally associated with S. enterica but not S. bongori, there is selective pressure for Salmonellae to keep colonization of host tissues under a threshold defined by the host response to the pathogen. For instance, S. Typhimurium fitness may depend upon preventing excessive tissue damage that threatens survival of the host and thereby the bacteria. A smaller population of microbes may also be better able to establish and maintain chronic infection by eliciting a more modest host immune response. Selective pressures to retain the trehalose synthesizing genes otsA and otsB may reflect the importance of trehalose in withstanding diverse non-host environmental stress, such as desiccation and fluctuations in temperature. Overall, the data indicate that pathogens carefully regulate responses to stress encountered within the host environment.
EXPERIMENTAL PROCEDURES
Bacterial strains and culture conditions
Strains (Table 2) were derived from Salmonella enterica serovar Typhimurium strain SL1344 (Smith et al., 1984). Bacteria were grown aerobically in Luria-Bertani (LB) medium or M9 minimal media (M9) (1 mM magnesium sulfate, 2% dextrose, 0.002% L-histidine, 0.05% casamino acids, and 20% M9 5x salts (211.3 mM disodium hydrogen phosphate, 110 mM monopotassium phosphate, 93.4 mM ammonium chloride, 42.77 mM sodium chloride)), pH 7.4 at 37°C, with antibiotics as indicated: streptomycin, 30 μg mL−1; ampicillin, 50 μg mL−1; kanamycin 30 μg mL−1; and chloramphenicol 20 μg mL−1. For growth curves, overnight cultures were diluted to an OD600 of 0.01 in 200 μL of M9 at pH 7, pH 5, or with 0.4 M added sodium chloride. Bacteria were grown in a 96-well plate shaking in a Synergy2 plate reader (BioTek) at 37°C and the OD600 was recorded at 20-minute intervals.
Table 2.
Bacterial strains used in this study related to Experimental Procedures.
| Strain | Genotype / plasmid | Reference |
|---|---|---|
| SL1344 | hisG xyl rpsL (wild-type) | Smith et al (1984) |
| 14028 | ATCC | |
| csd221 | 14028s / pKD46 | Detweiler et al (2003) |
| kde444 | STM1491–1494 (termed: ΔopuA-D)::cm | Erickson, Detweiler (2006) |
| nds692 | otsBA::kan (termed: ΔotsBA) | This study |
| mcp783 | SL1344 / pRB3-273c | This study |
| mcp786 | SL1344 / potsBA | This study |
| mcp787 | otsBA::kan / pRB3-273c | This study |
| mcp790 | otsBA::kan / potsBA | This study |
| mcp809 | kde444 / pRB3-273c | This study |
| mcp813 | kde444, otsBA::kan | This study |
| mcp854 | mcp813 / pRB3-273c | This study |
| mcp855 | mcp813 / potsBA | This study |
| mcp856 | kde444 / potsBA | This study |
Strain and plasmid construction
Deleted genes were replaced with kanamycin or chloramphenicol resistance markers (Datsenko and Wanner, 2000). Oligos used for generating otsBA::kan are otsBA-P1 (CGTTTGTGAGTCTCAATATGATGATAAGGAGGAGACCAGGGTGTAGGCTGGAGCT GCTTC), and otsBA-P2 (TGGTGCCCTTAGCGGGCGACTAGTCGCCGCTCGCGATATTCATATGAATATCCTC CTTA). Underline denotes P1 or P2 primer. Mutations were made in the 14028 background strain (source ATCC), verified by PCR (otsBA-5′ (ACTTACATGACTAATGAGAC) and otsBA-3′ (CAGCCAGGTAGATGTGTTGC)), and transduced into SL1344 using standard P22 phage transduction. Transductants were verified by growth on LB agar containing antibiotic and by PCR. Genomic DNA from strain SL1344 was used as a template to amplify otsB and otsA (−228 from otsB ORF to +1481 from otsA ORF) with 5′ primer #874 (TCTAAAGCTTAGCCAGGTAGATGTGTTGCT) and 3′ primer #875 (TTGGATCCGGCTGAATCCTTCTGACAAC). Underline denotes added sites for restriction endonucleases. The otsBA PCR product was digested with HindIII and BamHI and ligated into the same sites of pRB3-273c (Berggren et al., 1995) to construct potsBA. This plasmid was transformed into Salmonella by electroporation.
Carbohydrate extraction and quantification
Strains were grown overnight in 100 mL M9 minimal media containing 0.01 M or 0.4 M NaCl. Bacteria were pelleted and re-suspended in water to 4.6 x 108 bacteria per μL. Carbohydrates were extracted by incubation at 95°C for 20 hours. Debris was pelleted and aqueous extracts (0.5 μL) were spotted onto a silica gel TLC plate and separated using an ethyl acetate-acetic acid-water-pyridine (26:14:7:2 [vol/vol/vol/vol]) solvent system. Resolved sugars were stained with 0.1% potassium permanganate in 1 M NaOH. Integrated densitometry analysis was performed with Image J software version 1.44 (NIH). Glucose content of aqueous extracts was additionally quantified with a Glucose (HK) Assay Kit (Sigma) according to the manufacturer’s protocol. Trehalose levels were calculated by subtracting the glucose content of ΔotsBA mutant strains from wild-type and ΔopuA-D mutant strains and then dividing by two.
Hydrogen peroxide resistance
Overnight cultures were centrifuged at 4,000x g for 10 minutes and cell pellets were re- suspended in equal volume PBS. Cultures were diluted 1/10 in M9 broth and grown at 37°C for one hour. Broth Resistance Assay - Cultures were further diluted to an OD600 of 0.05 in 200 μL M9 broth with 10 mM hydrogen peroxide, corresponding to 1 nMol of hydrogen peroxide per bacterium and in accordance with previous studies (Fang et al., 1992; Tu et al., 2006). Bacteria were grown shaking in a 96-well plate at 37°C for 90 minutes. Bacterial survival was determined by plating on selective LB agar plates for CFU. Exposure of the strains to 0.1 mM hydrogen peroxide revealed no differences in strain survival. Filter Disc Inhibition assay - 1x106 bacteria were spread on an LB streptomycin plate and four filter discs treated with either water or 150 mM H2O2 were overlayed. Zones of inhibition were measured after incubation at 37°C overnight.
Bacterial infection of cultured bone marrow derived macrophages (BMDMs)
BMDMs isolated as described (Silva-Herzog and Detweiler, 2010) were seeded at 1.5x105 cells/well in 24-well tissue culture plates for at four hours before treatment with 2 ng mL−1 recombinant murine IFN-y (Peprotech, Rocky Hill, NJ), 20 μg/mL LPS from S. Typhimurium (Sigma). Where indicated, cells were treated with 25 μmol L−1 of MDL 72527, a spermine oxidase inhibitor (Sigma). MDL 72527 had no significant affect on macrophage viability under these conditions as determined by trypan blue staining and cell counting. After 18 to 20 hours at 37°C in 5% CO2, infection experiments were performed in triplicate. Bacteria were grown aerobically in Luria-Bertani (LB) medium overnight. The overnight cultures were diluted 1/5 in M9 minimal medium and exposed to 0.4 M sodium chloride for one hour. The bacteria were then opsonized with 20% normal mouse serum (Sigma) and added to BMDMs at a multiplicity of infection (MOI) of 10–13 for each strain (total MOI of approximately 25), determined by plating for CFU. Infections were synchronized by centrifugation at 50x g for five minutes. After 30 minutes, gentamicin (100 μg mL−1) was added to kill extracellular bacteria, 90 minutes later, 10 μg mL−1 gentamicin was added to prevent extracellular replication of bacteria. At one and two hours post-infection, cells were washed twice with PBS, incubated with 0.1% Triton X-100 for five minutes, and lysed. Serial dilutions were plated to quantify CFU and the competitive indexes (CIs) were calculated: (CFUmutant/CFUwild-type) output / (CFUmutant/CFUwild-type) input (Beuzón and Holden, 2001). Levels of ROS were determined using H2DCFDA (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol (data not shown).
Determination of the lethal dose50 (LD50)
Six to eight week old, female, BALB/c mice were intragastrically inoculated with wild- type or ΔopuA-D strains at concentrations from 103 to 108 bacteria. Mice were monitored daily for 28 days and deaths were recorded as days post-infection. The LD50 was calculated as previously described (Beuzón and Holden, 2001). The confidence interval was calculated by logit analysis (Reed and Muench, 1938).
Competition assays
Mice - Seven-week old SV129S6 mice (Taconic Laboratory, Hudson, NY) were orogastrically inoculated with equivalent numbers (1x109) of two bacterial strains (2x109 total), as verified by plating for CFU on selective LB agar. Three weeks after inoculation, tissues (cecum, liver, spleen, MLN, and Peyer’s patches) were collected in 1 mL PBS, homogenized with a TissueMiser (Fisher Scientific, Pittsburgh, PA) and diluted in PBS for plating on selective LB agar plates. CFU were enumerated and CI values were calculated as described above. Research protocols were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Colorado Institutional Biosafety and Animal Care and Use Committees. Broth - The ΔopuA-D and wild-type strains were grown overnight in LB medium at 37°C. Experiments were performed in triplicate. Overnight bacterial cultures were centrifuged at 4,000 x g for 10 minutes and cell pellets were re-suspended in equal volume phosphate buffered saline (PBS). Equal numbers, 2x109 total bacteria, (verified by plating for CFU) of the ΔopuA-D and wild-type strains were inoculated in LB broth or M9 minimal medium and incubated at 37°C for 24 hours. At varying time points, cells were diluted and plated on selective LB agar plates. CIs were calculated as described above.
Gastric pH
After fasting for three or eleven hours, or not fasting control mice, mice were euthanized and their stomachs dissected. The stomach was cut laterally and the stomach content was scraped into an eppendorf tube. The gastric content was centrifuged at 4,000 x g for 5 minutes and the supernatant was applied to a pH indicator strip pH2.5–4.5 (EMD Chemicals Inc, Gibbstown, NJ).
Statistics
Data were analyzed using InStat version 3.1a (GraphPad Software, San Diego CA). Two-way comparisons were analyzed with a students t-test (parametric), Mann-Whitney (non-parametric), one sample t test (parametric, comparison to null hypothesis) or Wilcoxon rank sum test (non-parametric, comparison to null hypothesis). Multiple comparisons were examined by analysis of variance (ANOVA) with a Tukey (parametric) or Dunn (non-parametric) post-test, respectively (Smith and Bidochka, 1998).
Acknowledgments
We thank M.W. McCoy, and N.R. Pace for discussions, K. Erickson and T. Fenn for the preliminary observation that ΔopuA-D mutants out-compete wild-type and the LD50 data. We thank N. Sanchez for help with ΔotsBA infection experiments and strain construction. This work was supported by AI072492 and AI095395 to CD.
References
- Alvarez-Peral FJ, Zaragoza O, Pedreno Y, Argüelles JC. Protective role of trehalose during severe oxidative stress caused by hydrogen peroxide and the adaptive oxidative stress response in Candida albicans. Microbiology (Reading, Engl) 2002;148:2599–2606. doi: 10.1099/00221287-148-8-2599. [DOI] [PubMed] [Google Scholar]
- Baek CH, Wang S, Roland KL, Curtiss R. Leucine-responsive regulatory protein (Lrp) acts as a virulence repressor in Salmonella enterica serovar Typhimurium. J Bacteriol. 2009;191:1278–1292. doi: 10.1128/JB.01142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren RE, Wunderlich A, Ziegler E, Schleicher M, Duke RC, Looney D, Fang FC. HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:489–495. [PubMed] [Google Scholar]
- Beuzón CR, Holden DW. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 2001;3:1345–1352. doi: 10.1016/s1286-4579(01)01496-4. [DOI] [PubMed] [Google Scholar]
- Biemans-Oldehinkel E, Mahmood NABN, Poolman B. A sensor for intracellular ionic strength. Proc Natl Acad Sci USA. 2006;103:10624–10629. doi: 10.1073/pnas.0603871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JM, Searle S, Mohamed H, White JK. Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol Lett. 2003;85:197–203. doi: 10.1016/s0165-2478(02)00231-6. [DOI] [PubMed] [Google Scholar]
- Botsford JL, Alvarez M, Hernandez R, Nichols R. Accumulation of glutamate by Salmonella typhimurium in response to osmotic stress. Appl Environ Microbiol. 1994;60:2568–2574. doi: 10.1128/aem.60.7.2568-2574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S. Food supplementation experiments with terrestrial vertebrates: patterns, problems, and the future. Canadian Journal of Zoology. 1990;68:203–220. [Google Scholar]
- Cánovas D, Fletcher SA, Hayashi M, Csonka LN. Role of trehalose in growth at high temperature of Salmonella enterica serovar Typhimurium. J Bacteriol. 2001;183:3365–3371. doi: 10.1128/JB.183.11.3365-3371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AL, Cardoso FS, Bohn A, Neves AR, Santos H. Engineering trehalose synthesis in Lactococcus lactis for improved stress tolerance. Appl Environ Microbiol. 2011;77:4189–4199. doi: 10.1128/AEM.02922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayley S, Lewis BA, Record MT. Origins of the osmoprotective properties of betaine and proline in Escherichia coli K-12. J Bacteriol. 1992;174:1586–1595. doi: 10.1128/jb.174.5.1586-1595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Cheng Y, Asim M, Bussière FI, Xu H, Gobert AP, Hacker A, Casero RA, Jr, Wilson KT. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- Chen C, Beattie GA. Characterization of the osmoprotectant transporter OpuC from Pseudomonas syringae and demonstration that cystathionine-beta-synthase domains are required for its osmoregulatory function. J Bacteriol. 2007;189:6901–6912. doi: 10.1128/JB.00763-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke FJ, Wain J. The emergence of antibiotic resistance in typhoid fever. Travel Med Infect Dis. 2004;2:67–74. doi: 10.1016/j.tmaid.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detweiler CS, Monack DM, Brodsky IE, Mathew H, Falkow S. virK, somA and rcsC are important for systemic Salmonella enterica serovar Typhimurium infection and cationic peptide resistance. Mol Microbiol. 2003;48:385–400. doi: 10.1046/j.1365-2958.2003.03455.x. [DOI] [PubMed] [Google Scholar]
- Domínguez-Bernal G, Pucciarelli MG, Ramos-Morales F, García-Quintanilla M, Cano DA, Casadesús J, García-del Portillo F. Repression of the RcsC-YojN-RcsB phosphorelay by the IgaA protein is a requisite for Salmonella virulence. Mol Microbiol. 2004;53:1437–1449. doi: 10.1111/j.1365-2958.2004.04213.x. [DOI] [PubMed] [Google Scholar]
- Dong T, Schellhorn HE. Control of RpoS in global gene expression of Escherichia coli in minimal media. Mol Genet Genomics. 2009;281:19–33. doi: 10.1007/s00438-008-0389-3. [DOI] [PubMed] [Google Scholar]
- Erickson KD, Detweiler CS. The Rcs phosphorelay system is specific to enteric pathogens/commensals and activates ydeI, a gene important for persistent Salmonella infection of mice. Mol Microbiol. 2006;62:883–894. doi: 10.1111/j.1365-2958.2006.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC, Chen CY, Guiney DG, Xu Y. Identification of sigma S-regulated genes in Salmonella typhimurium: complementary regulatory interactions between sigma S and cyclic AMP receptor protein. J Bacteriol. 1996;178:5112–5120. doi: 10.1128/jb.178.17.5112-5120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC, Libby SJ, Buchmeier NA, Loewen PC, Switala J, Harwood J, Guiney DG. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC, Libby SJ, Castor ME, Fung AM. Isocitrate lyase (AceA) is required for Salmonella persistence but not for acute lethal infection in mice. Infect Immun. 2005;73:2547–2549. doi: 10.1128/IAI.73.4.2547-2549.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov MV, Goodman JM, Nerukh D, Schumm S. Self-assembly of trehalose molecules on a lysozyme surface: the broken glass hypothesis. Phys Chem Chem Phys. 2010 doi: 10.1039/c0cp01705a. [DOI] [PubMed] [Google Scholar]
- Foreman-Wykert AK, Miller JF. Hypervirulence and pathogen fitness. Trends Microbiol. 2003;11:105–108. doi: 10.1016/s0966-842x(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Fortier A, Min-Oo G, Forbes J, Lam-Yuk-Tseung S, Gros P. Single gene effects in mouse models of host: pathogen interactions. J Leukoc Biol. 2005;77:868–877. doi: 10.1189/jlb.1004616. [DOI] [PubMed] [Google Scholar]
- Fritsche G, Nairz M, Werner ER, Barton HC, Weiss G. Nramp1- functionality increases iNOS expression via repression of IL-10 formation. Eur J Immunol. 2008;38:3060–3067. doi: 10.1002/eji.200838449. [DOI] [PubMed] [Google Scholar]
- Gal-Mor O, Gibson DL, Baluta D, Vallance BA, Finlay BB. A novel secretion pathway of Salmonella enterica acts as an antivirulence modulator during salmonellosis. PLoS Pathog. 2008;4:e1000036. doi: 10.1371/journal.ppat.1000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever HM, Styrvold OB, Kaasen I, Strøm AR. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988;170:2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JS. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 2008;16:284–290. doi: 10.1016/j.tim.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R, Klein W, Lange R, Rimmele M, Boos W. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol. 1991;173:7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincha DK, Hagemann M. Stabilization of model membranes during drying by compatible solutes involved in the stress tolerance of plants and microorganisms. Biochem J. 2004;383:277–283. doi: 10.1042/BJ20040746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TD, Slauch JM. Characterization of grvA, an antivirulence gene on the gifsy-2 phage in Salmonella enterica serovar typhimurium. J Bacteriol. 2001;183:611–620. doi: 10.1128/JB.183.2.611-620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells AM, Bullifent HL, Dhaliwal K, Griffin K, García de Castro A, Frith G, Tunnacliffe A, Titball RW. Role of trehalose biosynthesis in environmental survival and virulence of Salmonella enterica serovar typhimurium. Res Microbiol. 2002;153:281–287. doi: 10.1016/s0923-2508(02)01321-9. [DOI] [PubMed] [Google Scholar]
- Huhtanen CN. Use of pH gradient plates for increasing the acid tolerance of salmonellae. Appl Microbiol. 1975;29:309–312. doi: 10.1128/am.29.3.309-312.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappes RM, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappes RM, Kempf B, Kneip S, Boch J, Gade J, Meier-Wagner J, Bremer E. Two evolutionarily closely related ABC transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol Microbiol. 1999;32:203–216. doi: 10.1046/j.1365-2958.1999.01354.x. [DOI] [PubMed] [Google Scholar]
- Knodler LA, Steele-Mortimer O. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic. 2003;4:587–599. doi: 10.1034/j.1600-0854.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- Larsen PI, Sydnes LK, Landfald B, Strøm AR. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987;147:1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- Lever M, Sizeland PC, Bason LM, Hayman CM, Chambers ST. Glycine betaine and proline betaine in human blood and urine. Biochim Biophys Acta. 1994;1200:259–264. doi: 10.1016/0304-4165(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010;43:732–744. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Luzardo MC, Amalfa F, Nuñez AM, Díaz S, Biondi De Lopez AC, Disalvo EA. Effect of trehalose and sucrose on the hydration and dipole potential of lipid bilayers. Biophys J. 2000;78:2452–2458. doi: 10.1016/s0006-3495(00)76789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud SA, Nagahisa K, Hirasawa T, Yoshikawa K, Ashitani K, Shimizu H. Effect of trehalose accumulation on response to saline stress in Saccharomyces cerevisiae. Yeast. 2009;26:17–30. doi: 10.1002/yea.1646. [DOI] [PubMed] [Google Scholar]
- Martínez-Esparza M, Aguinaga A, González-Párraga P, García-Peñarrubia P, Jouault T, Argüelles JC. Role of trehalose in resistance to macrophage killing: study with a tps1/tps1 trehalose-deficient mutant of Candida albicans. Clin Microbiol Infect. 2007;13:384–394. doi: 10.1111/j.1469-0691.2007.01663.x. [DOI] [PubMed] [Google Scholar]
- McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008;60:63–70. doi: 10.1211/jpp.60.1.0008. [DOI] [PubMed] [Google Scholar]
- McDougall J, Kaasen I, Strøm AR. A yeast gene for trehalose-6-phosphate synthase and its complementation of an Escherichia coli otsA mutant. FEMS Microbiol Lett. 1993;107:25–30. doi: 10.1016/0378-1097(93)90348-6. [DOI] [PubMed] [Google Scholar]
- Nairz M, Fritsche G, Crouch MLV, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009;11:1365–1381. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamskulrungroj P, Himmelreich U, Breger JA, Wilson C, Chayakulkeeree M, Krockenberger MB, Malik R, Daniel HM, Toffaletti D, Djordjevic JT, et al. The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect Immun. 2009;77:4584–4596. doi: 10.1128/IAI.00565-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam A. Immunology and Biotechnology. New Delhi, India: Tata McGraw-Hill; 2007. Lab Manual in Biochemistry. [Google Scholar]
- Nix RN, Altschuler SE, Henson PM, Detweiler CS. Hemophagocytic Macrophages Harbor Salmonella enterica during Persistent Infection. PLoS Pathog. 2007;3:e193. doi: 10.1371/journal.ppat.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler SW. The principles and practice of medicine. New York: D. Appleton and Company; 1892. [Google Scholar]
- Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- Record MT, Jr, Courtenay ES, Cayley DS, Guttman HJ. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem Sci. 1998;23:143–148. doi: 10.1016/s0968-0004(98)01196-7. [DOI] [PubMed] [Google Scholar]
- Reed L, Muench H. A simple method of estimating fifty percent endpoints. The American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- Richards AB, Krakowka S, Dexter LB, Schmid H, Wolterbeek APM, Waalkens-Berendsen DH, Shigoyuki A, Kurimoto M. Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem Toxicol. 2002;40:871–898. doi: 10.1016/s0278-6915(02)00011-x. [DOI] [PubMed] [Google Scholar]
- Richardson AR, Payne EC, Younger N, Karlinsey JE, Thomas VC, Becker LA, Navarre WW, Castor ME, Libby SJ, Fang FC. Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar typhimurium. Cell Host Microbe. 2011;10:33–43. doi: 10.1016/j.chom.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JB, Dombrowski DB. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980;39:604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Ren H, Tomiyama-Miyaji C, Watanabe M, Kainuma E, Inoue M, Kuwano Y, Abo T. Resistance and augmentation of innate immunity in mice exposed to starvation. Cell Immunol. 2009;259:66–73. doi: 10.1016/j.cellimm.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, Mastroeni P. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol. 2003;5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- Silva-Herzog E, Detweiler CS. Salmonella enterica Replication in Hemophagocytic Macrophages Requires Two Type Three Secretion Systems. IAI. 2010;78:3369–3377. doi: 10.1128/IAI.00292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BP, Reina-Guerra M, Hoiseth SK, Stocker BA, Habasha F, Johnson E, Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984;45:59–66. [PubMed] [Google Scholar]
- Smith MA, Bidochka MJ. Bacterial fitness and plasmid loss: the importance of culture conditions and plasmid size. Can J Microbiol. 1998;44:351–355. [PubMed] [Google Scholar]
- STOKES JL, BAYNE HG. Growth rates of Salmonella colonies. J Bacteriol. 1957;74:200–206. doi: 10.1128/jb.74.2.200-206.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Kingsley RA, Townsend SM, Ficht TA, Adams LG, Bäumler AJ. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv Exp Med Biol. 1999;473:261–274. [PubMed] [Google Scholar]
- Tsolis RM, Xavier MN, Santos RL, Bäumler AJ. How to become a top model: The impact of animal experimentation on human Salmonella disease research. Infect Immun. 2011 doi: 10.1128/IAI.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Young GM, Solnick JV, Bäumler AJ. From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol. 2008;6:883–892. doi: 10.1038/nrmicro2012. [DOI] [PubMed] [Google Scholar]
- Tu X, Latifi T, Bougdour A, Gottesman S, Groisman EA. The PhoP/PhoQ two-component system stabilizes the alternative sigma factor RpoS in Salmonella enterica. Proc Natl Acad Sci USA. 2006;103:13503–13508. doi: 10.1073/pnas.0606026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler R, Wolfrom M. Methods in carbohydrate chemistry 1980 [Google Scholar]