Abstract
A tumor’s dependence on angiogenesis for survival and growth has led to the advancement of a variety of blood vessel directed anticancer treatment strategies. Over expression of angiopoietin-2 (Ang-2) in tumor vasculature and its crucial role in angiogenesis, i.e. the destabilization of endothelial/peri-endothelial cell interactions, now raises the possibility of additional novel anti-angiogenic therapeutics. The present study utilized a co-culture sphere model to (i) demonstrate the destabilizing effect of Ang-2 on endothelial/smooth muscle cell contact and (ii) evaluate the impact of the investigational Ang-2 antibody MEDI3617 on endothelial/smooth muscle cell dissociation. Real time imaging of spheres showed both exogenous Ang-2 and PMA induced endogenous Ang-2 secretion resulting in sphere destabilization (loss of endothelial cells from smooth muscle cell core). The presence of MEDI3617 inhibited this process. To assess the anti-angiogenic potential of MEDI3617 in vivo, nude mice were injected intradermally with human renal cell carcinoma cells (Caki-1, Caki-2) and the number of blood vessels induced over a 3 day period was scored. MEDI3617 (2, 10, 20 mg/kg) significantly reduced the initiation of blood vessels for both tumor models at all doses investigated. These data indicate that MEDI3617 treatment significantly impairs the initiation of angiogenesis by inhibiting the Ang-2 mediated disruption of endothelial/muscle cell interaction associated with blood vessel destabilizing and thereby reduces tumor cell induced angiogenesis. The results support the notion that targeting the angiopoietin/Tie2 axis may offer novel anti-angiogenic strategies for cancer treatment.
Keywords: Angiopoietin-2, Angiogenesis, Anti-angiogenic therapy, Antibody therapy, Spheroids
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing ones, is a hallmark of cancer (Folkman, 1990; Hanahan and Weinberg, 2000). Normal vasculature consists of a single layer of endothelial cells surrounded by peri-endothelial mural cells (smooth muscle cells and pericytes) which form stable vessel networks via tight associations through adhesion molecules (Armulik et al., 2005; Dejana, 2004). The dynamic nature of endothelial cells allows for rapid vascular responses to environmental changes that occur under normal and pathological conditions (Cines et al., 1998). Angiogenesis requires two distinct events consisting of the disruption of the vascular structure followed by activation of the endothelium. First, the tight association between endothelial-endothelial and endothelial-peri-endothelial cells is disrupted in order to allow for vessel permeability and exposure of endothelial cells to proangiogenic cytokines (Augustin et al., 2009; Bergers and Benjamin, 2003). Second, pro-angiogenic factors activate the endothelium to proliferate, migrate and establish tubular networks to form new blood vessels (Bergers and Benjamin, 2003; Ferrara, 2002).
The angiopoietin/Tie2 system is central to the initial phase of angiogenesis, the disruption of endothelial and peri-endothelial cell interactions (Augustin et al., 2009; Davis et al., 1996; Lauren et al., 1998; Maisonpierre et al., 1997; Partanen et al., 1992; Schnurch and Risau, 1993). Angiopietin-1 (Ang-1) and angiopoietin-2 (Ang-2) are secreted proteins that interact with the Tie2 receptor either in a paracrine (Ang-1) or autocrine (Ang-2) manner; Ang-1 is expressed and secreted by peri-endothelial mural cells while Ang-2 is expressed and secreted by endothelial cells (Augustin et al., 2009; Scharpfenecker et al., 2005). Although both angiopoietins bind Tie2 with similar affinities on the same receptor site, they have opposing functions (Davis et al., 2003; Fiedler et al., 2003); Ang-1 stabilizes and Ang-2 destabilizes the vasculature (Augustin et al., 2009; Davis et al., 1996).
In the adult, Ang-1 is found in tissues throughout the body and continuously secreted at low levels (Augustin et al., 2009; Davis et al., 1996). Ang-1-Tie2 association leads to tyrosine kinase phosphorylation of the Tie2 receptor and downstream signaling maintaining tight adhesion molecule interactions in the vascular cellular networks, between endothelial and peri-endothelial cells (Fukuhara et al., 2008; Gavard et al., 2008; Saharinen et al., 2008). Ang-2 is localized in Weibel-Palade Bodies (WPB) of endothelial cells and upon stimulation (ie. inflammation, hypoxia, shear stress) at sites of active angiogenesis the WPBs are exocytosed (Fiedler et al., 2004; Huang et al., 2002; Krikun et al., 2000; Lowenstein et al., 2005; Rondaij et al., 2006; Scharpfenecker et al., 2005; Tait and Jones, 2004). Secreted Ang-2 destabilizes the vasculature by autocrine interaction with Tie2, antagonizing Ang-1 signaling, leading to disruption of adhesion molecule interactions between cells and turning the angiogenic switch towards a proangiogenic phenotype (Augustin et al., 2009; Scharpfenecker et al., 2005; Tait and Jones, 2004).
Elevated Ang-2 levels have been associated with disease progression in many cancers (Helfrich et al., 2009; Lind et al., 2005; Tait and Jones, 2004; Tsutsui et al., 2006). Several reports also implicate an integrin-Ang-2 interaction in metastatic disease (Carlson et al., 2001; Hu et al., 2006; Imanishi et al., 2007). Increased circulating Ang-2 serum levels correlate to poor patient survival or advanced disease (Anargyrou et al. 2008; Sie et al. 2009). Furthermore, in the presence of Ang-2 tumor associated macrophages (TAMs) express Tie2 and show pro-angiogenic activity (De Palma et al. 2010; De Palma and Naldini 2011). The observations of high Ang-2 levels in tumor microenvironments coupled with its role in the initial vessel destabilization phase of angiogenesis, has made Ang-2 an attractive new target for anti-angiogenic cancer therapy (Hu and Cheng, 2009).
MEDI3617 is a fully human antibody which binds Ang-2 at the fibrinogen domain with high selectivity (KD value = 42 pM) and potently blocks Ang-2 binding to the Tie2 receptor (EC50 = 0.510 nM) (Leow et al. 2012). Initial preclinical studies have shown anti-tumor effects in human colon, ovarian, renal and hepatocellular xenografts (Leow et al. 2012). MEDI3617 is a more potent Ang-2 binding antibody then its precursor 3.19.3 (KD value = 86 pM) which previously had shown vascular and antitumor effects in several tumor models (Brown et al. 2010; Mazzieri et al. 2011). The goals of the present investigation were to directly assess the impact of MEDI3617 on the vascular destabilizing effects of Ang-2 using real time imaging of an endothelial/muscle cell co-culture sphere model and to evaluate the effects of MEDI3617 on tumor cell induced angiogenesis in vivo.
Materials and Methods
Reagents
Phorbol 12-Myristate 13-Acetate (PMA) (Sigma), thrombin (MP Biomedicals, LLC), and human recombinant angiopoietin-2 (R&D Systems®) were dissolved in DMSO, water and PBS + 0.1% BSA respectively. Human Ang-2 Quantikine ELISA Kits were purchased from R&D Systems® and stored at 4°C until use. Methylcellulose (viscosity 4000 cP) was obtained from Sigma; stock solutions were dissolved in basal medium (endothelial cell or smooth muscle cell) and ultra centrifuged to a clear solution (3500 rpm, 4°C, 3h) and the top 45% of supernatants stored at 4°C up to 3 months. CellTracker™ Orange CMRA, CellTrace™ Oregon Green® 488 Carboxylic Acid Diacetate Succinimidyl Ester, and CellTracker™ Violet BMQC (Molecular Probes – Invitrogen) were dissolved in DMSO and stored at −20°C.
Drug preparation
MEDI3617 was kindly provided by MedImmune, LLC. For in vitro investigations 5 mg/ml stock solutions were diluted to 10 µM working solutions in sodium citrate buffer before subsequent dilutions in sterile saline. For in vivo studies the stock solution (5 mg/ml) was diluted to working concentrations in sodium citrate buffer solution. Stock solutions were kept at −80°C and working concentrations at 4°C.
Cell culture
Human umbilical vein endothelial cells (HUVEC) and human umbilical artery smooth muscle cells (HUASMC) were purchased from Clonetics®. HUVEC were cultured in Nutrient mixture F-12 Ham, Kaighn’s supplemented with 0.03 mg/ml Endothelial Cell Growth Supplement (ECGS) and 0.1 mg/ml heparin (Sigma) with 10% fetal bovine serum (FBS). HUASMC were cultured in SmGM-2 (Clonetics®). The human clear cell renal cell carcinoma Caki-1 and Caki-2 cell lines were originally received as a gift from Dr. Susan Knox (Stanford University). Caki-1 and Caki-2 were grown in Dulbecco’s modified minimum essential medium (D-MEM, Invitrogen) supplemented with 10% FBS (Invitrogen), 1% penicillin-streptomycin (Invitrogen), and 1% 200-mmol/L L-glutamine (Invitrogen). Cells were kept at 37°C, 5% CO2. HUVEC and HUASMC were used between passages 2 and 4. Caki-1 and Caki-2 cells were used between passages 2 and 10.
Endothelial-smooth muscle cell co-culture sphere formation
Co-culture spheres of HUVEC and HUASMC were generated as previously reported (Korff et al., 2001; Scharpfenecker et al., 2005). Confluent monolayer cultures of HUVEC were traced with 1 µg/ml CellTracker™ Orange CMRA in serum free media for 15 min. Confluent monolayer culture of HUASMC were traced with either 1 µg/ml CellTrace™ Oregon Green® 488 Carboxylic Acid Diacetate Succinimidyl Ester (imaging with Nikon eclipse TS100 microscope) or 1 µg/ml CellTracker™ Violet BMQC (imaging with Leica SP5 confocal microscope) in serum free media for 15 min. A 1:1.5 ratio of HUVEC to HUASMC (1000 cells per spheroid) were suspended in a 1:1 ratio of endothelial and smooth muscle cell complete medium in the presence of 4.2% carboxymethylcellulose into a non-adherent round-bottom 96 well plate (Sigma). 48 hr later the spheres were selected for experiments.
Confocal imaging of endothelial-smooth muscle cell co-culture spheres
Confocal microscopy was used to evaluate co-culture sphere orientation. HUVEC were tagged with CellTracker™ Orange CMRA and HUASMC were tagged with CellTracker™ Violet BMQC prior to sphere formation and imaged with Leica SP5 confocal microscope with an environmental chamber for live imaging at 20× magnification. Spheres were imaged on FluoroDish, FD35-100 (World Precision Instruments, Inc.). 405 Diode laser was used to image HUASMC with CellTracker™ Violet BMQC and HeNe 543 laser was used to visualize HUVEC with CellTracker™ Orange CMRA. LAS AF software was used to obtain confocal images of spheroids. Coronal view of spheroids was obtained from a series of 10 z-stack images at 20.42 µm per step. 3 dimensional images were compiled from a series of 30 z-stack images, at 6.19 µm per step, using LAS AF software and were processed as 3D projections.
Imaging co-culture sphere formation and destabilization
Co-culture sphere formation and destabilization, endothelial cell loss, was imaged with a Nikon eclipse TS100 inverted microscope. HUVEC were tagged with CellTracker™ Orange CMRA and HUASMC were tagged with CellTrace™ Oregon Green® 488 Carboxylic Acid Diacetate Succinimidyl Ester prior to sphere formation. HUVEC were fluorescently visualized using TRIC filter and HUASMC were viewed using FITC filter with a Nikon Intensilight C-HGFI lamp. Spheroids were imaged at t=0 and t=4hr at 10× magnification and NIS Elements D 3.2 software was used to obtain individual and merged images.
Human Angiopoietin-2 ELISA
HUVEC were cultured in 60 mm dishes and grown to confluence. Following media removal and washing 2 ml of media containing either 50 ng/ml PMA or 1 unit/ml thrombin were added. At various times thereafter the media was collected, centrifuged (1000 rpm, 4°C, 10 min) and 1.5 ml of supernatant collected and stored at -20°C until analysis. Cells were trypsinized, harvested and counted for each sample. A Human Angiopoietin-2 Quantikine ELISA Kit was used to analyze Angiopoietin-2 secretion. ELISA measurements were made according to manufacturer protocols. Triplicates of each sample were run and secreted Ang-2 (pg/ml) was normalized to 105 cell number and the baseline was set to 0 based on basal level readings.
Stimulation of endothelial-smooth muscle cell co-culture spheres
Endothelial and smooth muscle cells were allowed to form spheres for 48 hr and were then transferred to a non-adhesive round bottom 96 well plate (1 sphere per well) and were live imaged (Nikon eclipse TS100 microscope) at t=0 prior to any stimulation. Stimulants, human recombinant angiopoietin-2, thrombin or PMA, were then added into the wells and spheres were live imaged at t=4 hr. The number of fluorescent tagged HUVEC that detached from the smooth muscle cell cores was counted in the field of view. Statistical significance between stimulated groups and control (unstimulated) spheres was determined using the Mann-Whitney U test at P<0.05. In each experimental group 12 spheres (1 sphere per well) were analyzed.
Intradermal angiogenesis assay
All in vivo procedures were carried out in agreement with a protocol approved by the University of Florida Institutional Animal Care and Use Committee. Athymic nu/nu mice were injected intradermally with 105 Caki-1 or Caki-2 cells in a volume of 10 µl at four sites on the ventral surface. One drop of 0.4% trypan blue was added to the cell suspension, which making it lightly colored, simplified subsequent location of the sites of injection. MEDI3617 (0, 2, 10, 20 mg/kg) was administered via the intraperitoneal route (IP) on the same day as tumor cell inoculation. Three days later the mice were euthanized and skin flaps removed. Vessels growing into tumor nodules were counted using a dissecting microscope, Leica MZ8, at 2× original magnification. Images were captured with Leica DFC320 camera and Leica Application Suite software. Statistical significance between control and MEDI3617 treated groups was determined using the Mann-Whitney U test at P<0.05.
Results
Endothelial and smooth muscle cells form co-culture spheres
When combined, endothelial (HUVEC) and smooth muscle cells (HUASMC) form spheroids which mimic the vascular association of these cell types (Korff et al., 2001; Scharpfenecker et al., 2005). Live imaging of HUVEC and HUASMC with red and green cell trackers respectively, was used to demonstrate the time course of endothelial/muscle cell co-culture spheroid formation (Fig. 1A). Confocal imaging of these spheroids and its 3 dimensional reconstruction shows an inside-out orientation of spheres with smooth muscle cells found at the core and endothelial cells on the periphery of the sphere (Fig. 1B).
Figure 1. Endothelial and smooth muscle cells form co-culture spheres.
A) Formation of an endothelial-smooth muscle cell sphere over a 48 hr time period. Smooth muscle cells (HUASMC) are shown in green (CellTrace Oregon Green) and endothelial cells (HUVEC) are shown in red (CellTracker Orange CMRA). Merged images are also shown. Images were taken with Nikon eclipse TS100. Scale bar represents 100 µm B) Coronal and 3-dimensional view of co-culture sphere with smooth muscle cells located in the core and endothelial cells on the periphery. Live images were taken with a Leica SP5 confocal microscope. Scale bar represents 100 µm.
MEDI3617 inhibits exogenous Ang-2 dependent sphere destabilization
Ang-2 interacts with Tie2 receptor on the endothelial cells and leads to loss of endothelial cell contact with surrounding endothelial and peri-endothelial cells (Augustin et al., 2009; Scharpfenecker et al., 2005). The addition of human recombinant Ang-2 (0.5 ng/ml) to co-culture spheres for 4 hr (Fig. 2A) led to a 5-fold increase in endothelial cell loss from the spheres (P<0.0001). Administration of MEDI3617 (0.5 nM) significantly reduced (~ 3.5-fold) the endothelial cell loss as compared to Ang-2 treated spheres (P<0.0001). Fig. 2B illustrates the live imaging of co-culture spheroids and demonstrates the endothelial cell loss upon exogenous Ang-2 stimulation and its inhibition in the presence of MEDI3617.
Figure 2. Exogenous Ang-2 causes sphere destabilization, MEDI3617 inhibits this response.
A) Stimulation of HUVEC with exogenous human recombinant Ang-2 (0.5 ng/ml) for 4 hr led to the loss of endothelial cells from the smooth muscle cell core. The presence of MEDI3617 (0.5 nM) impairs this loss. Column, mean; bars, SD (n=12 spheres per experimental group). ***, P<0.0001; Mann-Whitney U test. B) Images demonstrating control, Ang-2 (0.5 ng/ml) induced destabilization of co-culture spheres and its inhibition in the presence of MEDI3617 (0.5 nM) at 4 hr. Images as in Figure 1.
MEDI3617 inhibits endogenous Ang-2 dependent sphere destabilization
Stimulation of HUVEC with PMA or thrombin led to endogenous Ang-2 secretion as measured by ELISA (Fig. 3A). PMA exposure led to detectable Ang-2 secretion at 1 hr and increased to 2.8 and 7.8-fold above control at 6 and 24 hr respectively (Fig. 3A). Ang-2 secretion was not detectable after 1 hr of thrombin stimulation and even at 6 and 24 hr exposure the Ang-2 levels were significantly lower than those observed following PMA treatment (Fig. 3A). Treatment of spheres for 4 hr with 0.5, 5, 50 ng/ml PMA (Fig. 3B) resulted in 3.4, 6.2, and 6.8-fold increase in endothelial cell loss from spheres respectively (P<0.0001). Administration of MEDI3617 to PMA (50 ng/ml) treated spheres significantly decreased the number of endothelial cells lost from the spheres (Fig. 3C). Live imaging of co-culture spheres (Fig. 3D) illustrates the disruption of endothelial/muscle cell interaction upon PMA stimulation and its inhibition in the presence of MEDI3617.
Figure 3. PMA induced endogenous Ang-2 causes sphere destabilization, MEDI3617 inhibits this response.
A) Stimulation of HUVEC with PMA (50 ng/ml) or thrombin (1 unit/ml) led to Ang-2 secretion over time. Ang-2 secretion was measured by ELISA and data were normalized to 105 cells, baseline readings from basal levels were set at 0. Column, mean; bars, SD of three independent experiments. B) Stimulation of HUVEC with PMA for 4 hr resulted in loss of endothelial cells from the smooth muscle cell core in a dose dependant manner. Column, mean; bars, SD (n=12 spheres per experimental group). ***, P<0.0001; Mann-Whitney U test. C) Ang-2 antibody, MEDI3617, inhibited co-culture sphere destabilization when stimulated with PMA (50 ng/ml) for 4 hr. Column, mean; bars, SD (n=12 spheres per experimental group). *, P<0.05: **, P<0.01: ***, P<0.0001; Mann-Whitney U test. D) Images demonstrating control, PMA (50 ng/ml) induced destabilization of co-culture spheres and its inhibition in the presence of MEDI3617 (50 nM) at 4 hr. Images as in Figure 1.
MEDI3617 inhibits tumor induced angiogenesis in vivo
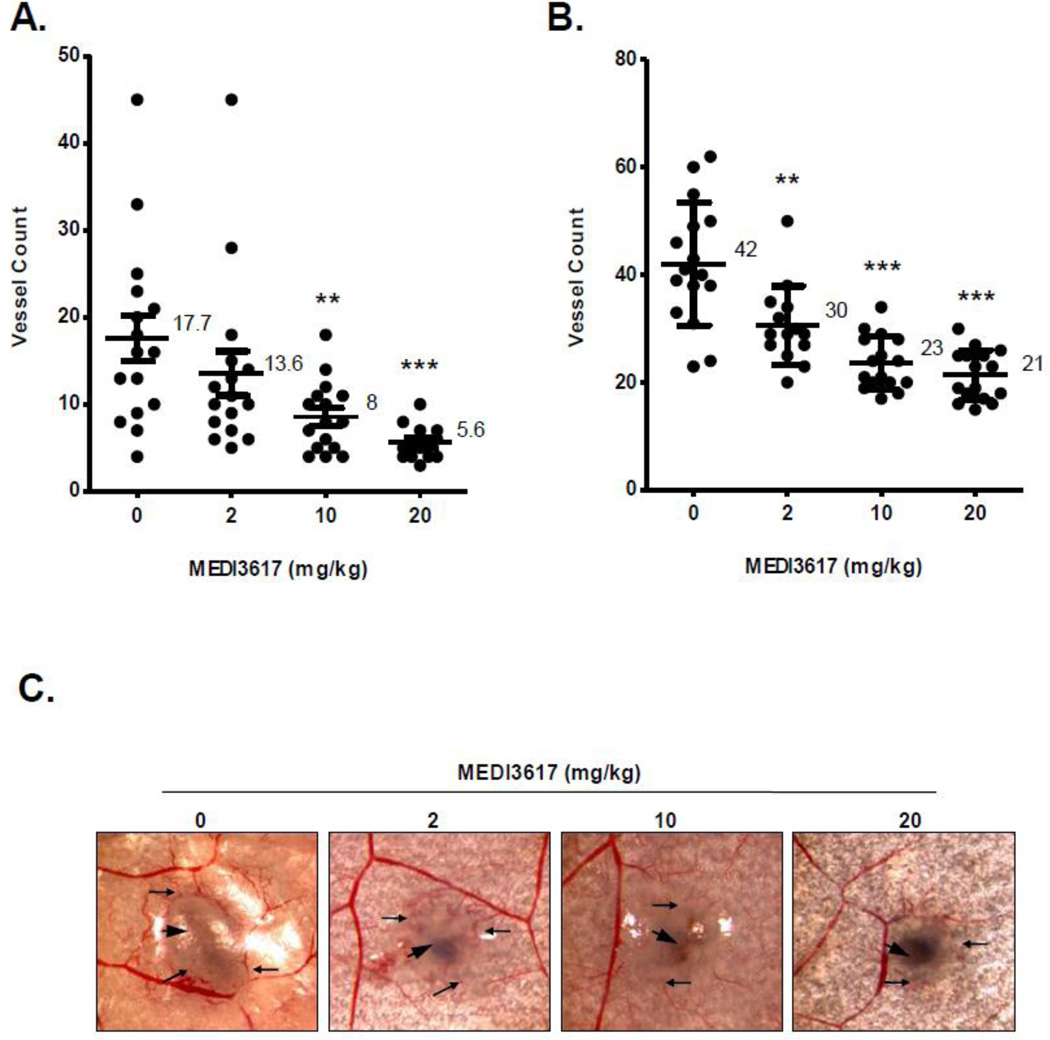
To evaluate the effects of MEDI3617 on in situ angiogenesis, nude mice were inoculated intradermally with either Caki-1 (normal VHL) or Caki-2 (mutated VHL) human renal cell carcinoma cells (1×105) and treated with increasing doses of MEDI3617. The results (Fig. 4) showed that Ang-2 antibody treatment reduced the number of tumor cell induced blood vessels in a dose dependent manner in both cell lines. In the Caki-1 tumor model (Fig. 4A), 2, 10, 20 mg/kg doses of MEDI3617 decreased the number of tumor induced blood vessels ~1.3, 2.2, and 3.1-fold as compared to control mice, respectively. Similar results were seen in the Caki-2 tumor model (Fig. 4B, C). MEDI3617 doses of 2, 10, 20 mg/kg reduced the number of tumor cell induced blood vessels by ~ 1.4, 1.8, and 2-fold, respectively.
Figure 4. MEDI3617 inhibits tumor induced blood vessel formation in vivo.
Mice were treated with various doses of MEDI3617 on the day of tumor cell inoculation. The number of blood vessels initiated by (A) Caki-1 and (B) Caki-2 tumor cells were determined at the end of a 3 day period. Lines, mean; bar, SD (n=16). **, P<0.01: ***, P<0.001; Mann-Whitney U test. C) Representative images of blood vessels induced by Caki-2 tumor cells using a dissecting microscope at 2× magnification. Large arrow head, tumor nodule; small arrows, tumor induced blood vessels.
Discussion
The importance of angiogenesis in the growth of solid tumors has led to the active pursuit of anti-angiogenic anticancer therapies (Folkman, 1971). Although such agents have demonstrated some efficacy in the clinic, concerns about lack of treatment response and the potential for resistance to therapy have been raised (Bergers and Hanahan, 2008; Jain et al., 2006). Resistance to anti-angiogenic therapy may occur when inhibition of a particular signaling pathway is overcome by the activation of another to sustain tumor blood vessel growth (Bergers and Hanahan, 2008; Jain et al., 2006). One possible approach to overcoming such concerns is to target the angiopoietin/Tie2 axis since it is considered to be the sole pathway responsible for maintaining blood vessel integrity. (Augustin et al., 2009; Hu and Cheng, 2009). While Ang-1 binding to Tie2 receptor stabilizes the vasculature, Ang-2 binding primes the vasculature for angiogenesis by freeing endothelial cells from peri-endothelial cells, thus making them sensitive to stimulation by pro-angiogenic factors (Augustin et al., 2009). Minimizing the dissociation of endothelial and peri-endothelial cells by targeting Ang-2 therefore would hinder tumor-induced angiogenesis by blocking the access of pro-angiogenic factors to endothelial cells, thus making this ligand a desirable target for vascular directed anticancer therapeutic approaches.
The present study examined the destabilizing effects of Ang-2 on endothelial/smooth muscle cell contacts in the presence or absence of the investigational Ang-2 antibody MEDI3617 using a co-culture sphere model and real time imaging (Fig. 1). The results showed that Ang-2 initiates endothelial-peri-endothelial cell contact loss (Fig. 2); a finding consistent with the previous report that direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates the response to VEGF (Korff et al., 2001). Although the model is an inside-out orientation of the natural appearance of the vasculature, it nonetheless mimics the interaction between the two cell types essential in the make-up of blood vessels. Even though smooth muscle cells are usually found on larger arteries while pericytes coat endothelial cells of smaller arterioles, venules, or capillaries, these two cell types share a common lineage and interact with endothelial cells via N-Cadherin, a major adhesion molecule between endothelial and peri-endothelial cells that is broken upon Ang-2-Tie2 association during angiogenesis (Armulik et al., 2005; Dejana, 2004).
Consistent with previous reports on the endothelial cell/peri-endothelial cell interactions (Scharpfenecker et al., 2005), exogenously applied Ang-2 was found to result in a loss of endothelial cells from co-culture spheroids. The current study employed real time imaging of spheres before and after stimulation with Ang-2 (Fig. 2) and demonstrated that even 400-fold lower concentrations of Ang-2 (0.5 ng/ml) than previously reported (Scharpfenecker et al., 2005), led to a significant increase in the number of endothelial cells dissociating from the unaffected smooth muscle cell core (Fig. 2). Because the in situ destabilization of endothelial/peri-endothelial cell interactions is modulated by the endogenous release of Ang-2 from endothelial cells, experiments also were undertaken to assess whether such Ang-2 release would affect the co-culture spheres. Ang-2 is stored in Weibel-Palade Bodies in endothelial cells and is rapidly secreted during both physiological and pathological angiogenic stimulus (Fiedler et al., 2004; Huang et al., 2002; Krikun et al., 2000; Lowenstein et al., 2005; Rondaij et al., 2006). Chemical stimulation of endothelial cells with PMA leads to Weibel-Palade Bodies exocytosis and results in Ang-2 secretion (Fiedler et al., 2004); a finding confirmed in the present investigation by ELISA analysis of endogenous Ang-2 secretion from endothelial cells following PMA stimulation (Fig. 3). When co-culture spheroids were treated with PMA (50 ng/ml), the number of endothelial cells released was found to be increased ~ 6-fold without an effect on the smooth muscle cell core (Fig. 3). A similar result was noted when spheroids were treated with the clotting factor thrombin (data not shown).
The current studies focused on real time imaging of Ang-2 initiated endothelial cell dissociation from smooth muscle cell contacts and the ability of the investigational Ang-2 antibody MEDI3617 to inhibit that dissociation. Endothelial/smooth muscle co-culture spheres exposed to either exogenous Ang-2 (Fig. 2) or agents to initiate endogenous Ang-2 expression (Fig. 3) in the presence or absence of the antibody. Loss of endothelial cells from the co-culture spheres was monitored using cell trackers. The results showed that treatment with low dose MEDI3617 (0.5 nM) counteracted the effects of exogenous Ang-2 and inhibited the dissociation of endothelial from smooth muscle cells (Fig. 2). Similarly, endothelial cell loss from the coculture spheroids exposed to PMA could be significantly impaired (~ 3-fold) by MEDI3617 treatment (Fig. 3). In a recent in vivo study Mazzieri et al. (2011) reported that treatment with the Ang-2 antibody 3.19.3 reduced the number of tumor blood vessels but the remaining tumor vasculature was heavily coated with pericytes. It should be noted however that the observation was made at the end of an extended treatment period; i.e. at the end of prolonged Ang-2 depletion which ultimately favors an Ang-1 based stabilization of the endothelial cell/pericyte interaction. The present investigation demonstrates that Ang-2 antibody treatment blocks the early phases of the angiogenesis process by inhibiting the initial destabilization of the endothelial/smooth muscle cell contacts. Therefore this study demonstrates that Ang-2 targeting with MEDI3617 may have beneficial anti-angiogenic effects by blocking the initial phase of angiogenesis, the dissociation of endothelial and smooth muscle cells in a stable blood vasculature.
Importantly, when administered to mice implanted with human renal cell carcinoma cells, MEDI3617 treatment effectively reduced the number blood vessels induced by both the moderately (Caki-1) and highly (Caki-2) angiogenic tumor models at very low doses of the antibody (Fig. 4). Renal cell carcinoma is a highly vascularized tumor and anti-angiogenic therapy is a common treatment option for patients with this disease (emedicine.medscape.com, cancer.gov). The Von Hippel-Lindau (VHL) gene is frequently mutated in this cancer yielding an even more densely vascularized tumor as compared to wild-type VHL tumors (emedicine.medscape.com). The positive results of these in situ angiogenesis experiments clearly are encouraging, and have led to the initiation of anti-tumor activity studies of MEDI3167 in established renal cell carcinoma xenografts.
The presence of Ang-2 in the tumor microenvironment and circulation has been associated with disease progression (Helfrich et al., 2009; Sie et al. 2009; Anargyrou et al. 2008; Lind et al., 2005; Tait and Jones, 2004; Tsutsui et al., 2006) and enhanced metastatic potential (Carlson et al., 2001; Hu et al., 2006; Imanishi et al., 2007). In the angiogenic process, the angiopoietin/Tie2 axis is currently considered to be a non-redundant pathway involved in maintaining blood vessel integrity. Consequently, Ang-2 has been considered as a novel anticancer target. The current study supports the rationale for Ang-2 targeting as a therapeutic intervention in the angiogenic process. Experiments using fluorescent imaging of co-culture endothelial/smooth muscle cell spheres and an in vivo intradermal assay clearly demonstrate a significant impact of the human monoclonal Ang-2 antibody, MEDI3617, on Ang-2 dependent destabilization of the endothelial/muscle cell interaction in vitro and tumor cell induced blood vessel formation in vivo. The present results support the concept that this antibody has inhibitory effects on new blood vessel formation via inhibition of Ang-2 induced endothelial/peri-endothelial cell contact loss. Since minimizing the dissociation of endothelial and peri-endothelial cells by targeting Ang-2 could hinder tumor-induced angiogenesis by blocking the endothelial cells from the influence of pro-angiogenic factors, MEDI3617 warrants further investigation as a vascular directed anticancer therapy.
Highlights.
Exogenous Ang-2 stimulation of spheroids results in endothelial cell loss
Ang-2 antibody, MEDI3617, inhibits Ang-2 dependant endothelial cell loss
PMA stimulates endothelial cell loss of spheres in dose dependant manner
MEDI3617 able to decrease PMA induced endothelial cell loss even at a small dose
MEDI3617 reduces renal cell induced blood vessels in an in vivo intradermal assay
Supplementary Material
Acknowledgements
The authors thank MedImmune (Gaithersburg, MD, USA) for providing the Ang-2 antibody, Steve McClellan (Interdisciplinary Center for Biotechnology Research, University of Florida and Shands Cancer Center) for assistance with confocal microscopy, and Dr. Lori Rice, Sharon Lepler and Chris Pampo for the technical assistance.
Financial Support
These studies were supported in part by a grant from the National Cancer Institute (Public Health Service Grant CA089655) and National Institutes of Health (T32 Training Grant 5 T32 CA009126-33).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- Anargyrou K, et al. Normalization of the serum angiopoietin-1 to angiopoietin-2 ratio reflects response in refractory/resistant multiple myeloma patients treated with bortezomib. Haematologica. 2008;93:451–454. doi: 10.3324/haematol.11852. [DOI] [PubMed] [Google Scholar]
- Armulik A, et al. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Augustin HG, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther. 2010;9:145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- Carlson TR, et al. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Davis S, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Davis S, et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- De Palma M, et al. Angiopoietin-2 regulates gene expression in Tie2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- De Palma M, Naldini L. Angiopoietin-2 Ties up macrophages in tumor angiogenesis. Clin Cancer Res. 2011;17:1–7. doi: 10.1158/1078-0432.CCR-10-0171. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Fiedler U, et al. Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J Biol Chem. 2003;278:1721–1727. doi: 10.1074/jbc.M208550200. [DOI] [PubMed] [Google Scholar]
- Fiedler U, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- Gavard J, et al. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Helfrich I, et al. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res. 2009;15:1384–1392. doi: 10.1158/1078-0432.CCR-08-1615. [DOI] [PubMed] [Google Scholar]
- Hu B, Cheng SY. Angiopoietin-2: development of inhibitors for cancer therapy. Curr Oncol Rep. 2009;11:111–116. doi: 10.1007/s11912-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, et al. Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res. 2006;66:775–783. doi: 10.1158/0008-5472.CAN-05-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YQ, et al. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood. 2002;99:1646–1650. doi: 10.1182/blood.v99.5.1646. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, et al. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007;67:4254–4263. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- Korff T, et al. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. Faseb J. 2001;15:447–457. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- Krikun G, et al. Expression of angiopoietin-2 by human endometrial endothelial cells: regulation by hypoxia and inflammation. Biochem Biophys Res Commun. 2000;275:159–163. doi: 10.1006/bbrc.2000.3277. [DOI] [PubMed] [Google Scholar]
- Lauren J, et al. Is angiopoietin-2 necessary for the initiation of tumor angiogenesis? Am J Pathol. 1998;153:1333–1339. doi: 10.1016/S0002-9440(10)65717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow CC, et al. MEDI3617, a human anti-Angiopoietin 2 monoclonal antibody, inhibits angiogenesis and tumor growth in human xenograft models. Int J Oncol. 2012 doi: 10.3892/ijo.2012.1366. [DOI] [PubMed] [Google Scholar]
- Lind AJ, et al. Angiopoietin 2 expression is related to histological grade, vascular density, metastases, and outcome in prostate cancer. Prostate. 2005;62:394–399. doi: 10.1002/pros.20163. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, et al. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–308. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Mazzieri R, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Partanen J, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondaij MG, et al. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- Saharinen P, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cellcell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- Scharpfenecker M, et al. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–968. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- Sie M, et al. The angiopietin 1/angiopoietin 2 balance as a prognostic marker in primary glioblastoma multiforme. J Neurosurg. 2009;110:147–155. doi: 10.3171/2008.6.17612. [DOI] [PubMed] [Google Scholar]
- Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, et al. Angiopoietin 2 expression in invasive ductal carcinoma of the breast: its relationship to the VEGF expression and microvessel density. Breast Cancer Res Treat. 2006;98:261–266. doi: 10.1007/s10549-005-9157-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.