Abstract
Growing evidence suggests that Huntington’s disease (HD), a neurodegenerative movement disorder caused by the mutant huntingtin (htt) with an expanded polyglutamine (polyQ) repeat, is associated with the altered intracellular trafficking and synaptic function. GABAA receptors, the key determinant of the strength of synaptic inhibition, have been found to bind to the huntingtin associated protein 1 (HAP1). HAP1 serves as an adaptor linking GABAA receptors to the kinesin family motor protein 5 (KIF5), controlling the transport of GABAA receptors along microtubules in dendrites. In this study, we found that GABAAR-mediated synaptic transmission is significantly impaired in a transgenic mouse model of HD expressing polyQ-htt, which is accompanied by the diminished surface expression of GABAA receptors. Moreover, the GABAAR/HAP1/KIF5 complex is disrupted and dissociated from microtubules in the HD mouse model. These results suggest that GABAAR trafficking and function is impaired in HD, presumably due to the interference of KIF5-mediated microtubule-based transport of GABAA receptors. The diminished inhibitory synaptic efficacy could contribute to the loss of the excitatory/inhibitory balance, leading to increased neuronal excitotoxicity in HD.
Keywords: huntingtin, GABAA receptor, IPSC, KIF5, microtubule, trafficking
Introduction
Huntington’s disease (HD) is a devastating neurological disorder characterized by uncontrolled movements, which is associated with the dysfunction and eventually degeneration of striatal medium spiny neurons (MSNs, Vonsattel et al., 1985; Vonsattel and DiFiglia, 1998). The GABAergic MSNs account for >90% neuronal population in the striatum, a key area in basal ganglia whose main function is the movement control. Genetic studies have found that HD is caused by an abnormally elongated polyglutamine (polyQ) tract in the large protein huntingtin (htt, Mangiarini et al., 1996), however, both the normal function of htt in neurons and the molecular mechanism by which the expanded polyQ sequence in htt causes selective neurodegeneration remain elusive. In addition to roles in regulating apoptosis and transcription (Ross, 2002), polyQ-htt may in part mediate its neurotoxic action in HD by altering neuronal membrane trafficking and synaptic function (Fan and Raymond, 2007; Smith et al., 2005). Several htt-interacting proteins implicated in intracellular transport have been identified (Harjes and Wanker, 2003), one of which is huntingtin-associated protein 1 (HAP1) (Li et al., 1995). HAP1 interacts more tightly with polyQ-htt than wild-type htt (Li et al., 1995), and may act as a key mediator of pathological alterations in membrane trafficking by mutant htt (Gauthier et al., 2004; Rong et al., 2006; Li and Li, 2005).
HAP1 associates with kinesin or dynein microtubule motor proteins (Engelender et al., 1997; Li et al., 1998; Gauthier et al., 2004; McGuire et al., 2006; Twelvetrees et al., 2010). Growing evidence suggests that mutant huntingtin impairs the HAP1/motor-dependent anterograde or retrograde transport of neuronal cargos along microtubules (Gauthier et al., 2004; Rong et al., 2006). Deficits in these neuronal transport systems have been suggested to underlie the pathogenesis of a number of neurodegenerative diseases (Goldstein, 2003).
Fast inhibitory neurotransmission mediated by GABAA receptors (GABAARs) plays a critical role in regulating neuronal excitability. The trafficking of GABAARs underlies dynamic changes in synaptic receptor numbers and inhibitory postsynaptic current amplitudes, providing an effective mechanism for regulating the strength and plasticity of synaptic inhibition (Jacob et al., 2008). One critical determinant for GABAAR trafficking and inhibitory transmission is the kinesin family member KIF5 motor protein, which associates with HAP1 (Twelvetrees et al., 2010). Based on studies in transfected neuronal cultures, it has been found that HAP1 interacts with GABAARs, facilitating the recycling of internalized GABAARs back to synapses (Kittler et al., 2004), and suppressing HAP1 expression attenuates GABAAR trafficking and synaptic inhibition (Twelvetrees et al., 2010). In this study, we sought to determine whether synaptic inhibition is impaired in a mouse model of HD, and whether it results from the loss of GABAAR transport along microtubules due to the disruption of the HAP1/KIF5/GABAAR multiprotein complex in vivo. The diminished strength of inhibitory synaptic transmission could contribute to the loss of the excitatory/inhibitory balance, leading to increased neuronal excitotoxicity.
Material and Methods
Animals
All experiments were performed with the approval of State University of New York at Buffalo Animal Care Committee. The transgenic mouse model of HD, N171-82Q, which expresses a mutant N-terminal fragment of huntingtin (the first 171 aa of human htt with 82Q, Schilling et al., 1999), was purchased from Jackson Lab. Experiments were conducted at the symptomatic stage (3–5 months old) unless otherwise stated.
Electrophysiological recordings in slices
Mice were first anesthetized by inhaling Halothane (Sigma) for ~30 sec and decapitated quickly. Brains were removed and cut into coronal slices (300 μm) using Vibratome (Leica VP1000S) in the presence of a low Ca2+, HEPES-buffered salt solution (in mM: 140 Na isethionate, 2 KCl, 4 MgCl2, 0.1 CaCl2, 23 glucose, 15 HEPES, pH =7.4, 300–305 mOsm). Slices were then incubated for 1–4 hrs at room temperature (20–22°C) in a NaHCO3-buffered saline bubbled with 95% O2, 5% CO2.
Whole-cell voltage-clamp technique was used to record GABAAR-IPSC in slices (Zhong et al., 2003; Chen et al., 2006; Yuen et al., 2011). The internal solution contained (in mM): 100 CsCl, 30 N-methyl-d-glucamine (NMG), 10 HEPES, 4 NaCl, 1 MgCl2 5 EGTA, 2.2 QX-314, 12 phosphocreatine, 5 MgATP, 0.5 Na2GTP, pH 7.2–7.3, 265–270 mosM. Slices was perfused with ACSF (in mM: 130 NaCl, 26 NaHCO3, 3 KCl, 5 MgCl2, 1.25 NaH2PO4, 1 CaCl2, 10 Glucose, pH 7.4, 300 mOsm) bubbled with 95% O2 and 5% CO2 containing APV-5 (20 μM) and CNQX (25 μM). The calculated chloride reversal potential is about −8 mV. Neurons were visualized with a 40 × water-immersion lens and illuminated with near infrared IR light. All recordings were performed using a Multiclamp 700A amplifier. Tight seals (2–10 GΩ) were first generated by negative pressure, followed by additional suction to obtain the whole-cell configuration. IPSC was evoked by delivering pulses with a series of intensities (50–90 μA) from a stimulation isolation unit controlled by a S48 pulse generator (Grass Technologies, West Warwick, RI). A bipolar stimulating electrode (FHC, Inc., Bowdoinham, ME) was positioned ~100 μm from the neuron under study. Neurons were held at −70mV throughout the recording. For miniature IPSC recording, TTX (0.5 μM) was added in the ACSF. Striatal medium spiny neurons and cortical pyramidal neurons were recorded. Data analyses were performed with Clampfit (Axon instruments), Mini Analysis Program (Synaptosoft, Leonia, NJ) and Kaleidagraph (Albeck Software). Student t tests or ANOVA tests were performed for the analysis of statistical significance.
Whole-cell recordings in acutely dissociated neurons
Whole-cell ionic current in acutely dissociated neurons was recorded as previously described (Yan and Surmeier, 1997; Wang et al., 2002). The internal solution contained (in mM): 180 N-methyl-D-glucamine (NMG), 40 HEPES, 4 MgCl2, 0.1 BAPTA, 12 phosphocreatine, 3 Na2ATP, 0.5 Na2GTP, and 0.1 leupeptin, pH 7.2–7.3, 265–270 mOsm. The external solution contained (in mM): 127 NaCl, 20 CsCl, 1 MgCl2, 10 HEPES, 5 BaCl2, 12 glucose, 0.001 TTX, pH 7.3–7.4, 300–305 mOsm. Neurons were held at −40mV and GABA (100 μM) was applied for 2 s every 30 s via a gravity-fed ‘sewer pipe’ system. The array of application capillaries (ca. 150 μm i.d.) was positioned a few hundred microns from the cell under study. Solution changes were performed by the SF-77B fast-step solution stimulus delivery device (Warner Instrument). Currents through the voltage-dependent calcium channel (VDCC) were recorded with a ramp depolarization protocol (from −80 mV to +60 mV).
Biochemical measurement of surface-expressed receptors
Surface receptors were measured with Sulfo-NHS-LC-Biotin (Pierce Chemical Co.) as previously described (Yuen et al., 2009). Slices were incubated with ACSF containing sulfo-NHS-LC-Biotin (1 mg/mL, 40 min, on ice). After rinsing in TBS to quench the biotin reaction, slices were homogenized in 500 μL modified RIPA buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mMNaPO4, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM PMSF, and 1 mg/mL leupeptin). The homogenates were centrifuged at 14,000 × g for 15 min at 4°C, and supernatant fractions were collected. To measure total expression, 15 μg proteins in the supernatant were removed. For surface expression, 150 μg proteins in the supernatant were incubated with 100 μL of 50% Neutravidin agarose (Pierce Chemical Co.) overnight at 4°C, and bound proteins were resuspended in SDS sample buffer and boiled. Quantitative Western blots were performed on both total and biotinylated (surface) proteins. Primary antibodies used include: anti-GABAAR β2/3 (1:500, Millipore, MAB341), anti-NR2A (1:500, Upstate, 07–632), anti-actin (1: 1000, Santa Cruz, sc1616), anti-MAP2 (1: 500, Santa Cruz, sc20172) and anti-synaptophysin (1:1000, Sigma, S5768).
Co-immunoprecipitation
Slices were homogenized in 0.5% NP-40 lysis buffer (0.5% NP-40, 10% glycerol, 50 mM Tris, pH 7.6, 150 mM NaCl, 50mM NaF, 0.1mM EDTA, and 0.1mM Na3VO4, 1mM phenylmethylsulfonyl fluoride, and protease inhibitor tablet), then lysates were ultracentrifuged (200,000 × g) at 4°C for 60 min. Supernatant fractions were incubated with anti-α-tubulin (15 μg, Sigma, T6199) or anti-GABAAR β2/3 (15 μg, Millipore, MAB341) for overnight at 4°C, followed by incubation with 50 μl of protein A/G plus agarose (Santa Cruz Biotechnology) for 1 hr at 4°C. Immunoprecipitates were washed three times with lysis buffer containing 0.2 M NaCl, then boiled in 2x SDS loading buffer for 5 min, and separated on 7.5% SDS-polyacrylamide gels. Western blotting experiments were performed with anti-GABAAR β2/3 (1:500, Millipore, MAB341), anti-tubulin (1:1000, Sigma, T6199), anti-KIF5 heavy chain (1:500, SUK4, Twelvetrees et al., 2010), anti-HAP1 (1:200, Santa Cruz Biotechnology, sc-32257), or anti-htt (1:1000, Millipore, MAB2166).
Results
GABAAR-mediated inhibitory transmission is disrupted in HD
Since GABAAR-mediated synaptic response is impaired by transfected polyQ-htt in neuronal cultures (Twelvetrees et al., 2010), we hypothesize that mice with in vivo expression of mutant huntingtin might show altered GABAergic transmission. To test this, we examined GABAAR-mediated inhibitory postsynaptic current (IPSC) in a transgenic mouse model of HD, N171-82Q, which expresses a mutant N-terminal fragment of huntingtin. N171-82Q mice develop behavioral abnormalities resembling HD, including loss of coordination, tremors, hypokinesis and abnormal gait (Schilling et al., 1999). Both pyramidal neurons in frontal cortex and medium spiny neurons in dorsal striatum were examined at the symptomatic stage (3–5 months old) of N171-82Q mice.
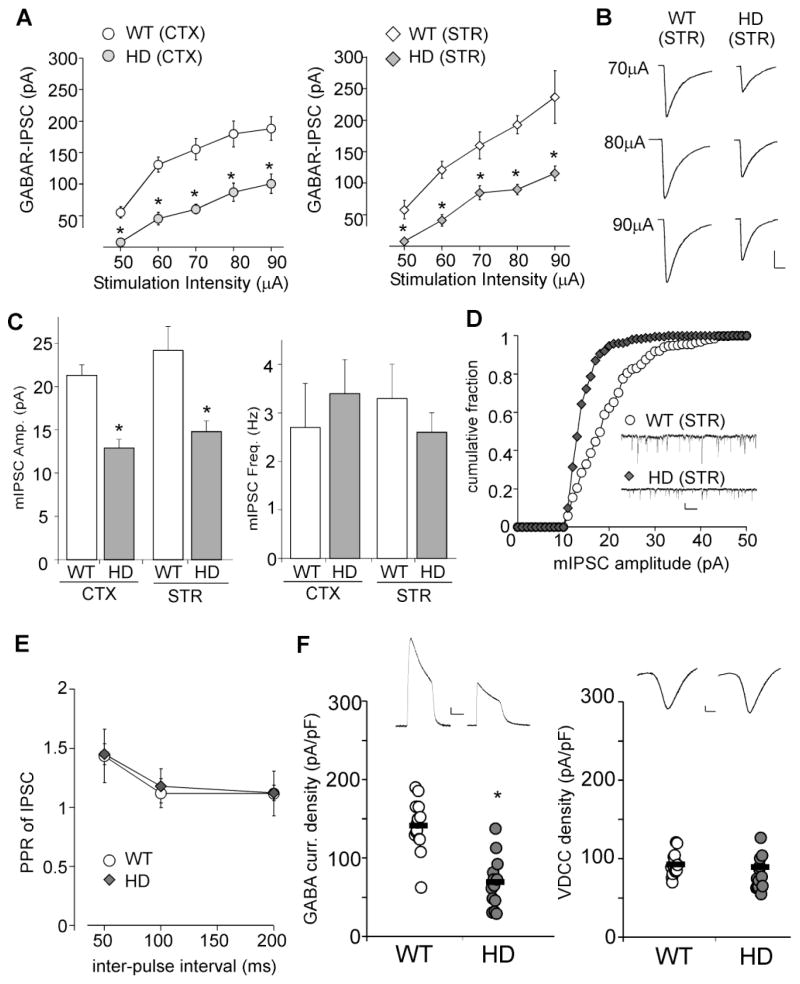
Compared to age-matched WT mice, GABAAR-IPSC evoked by a series of stimulus intensities was markedly smaller in both types of neurons from HD mice (Figure 1A, cortex: 46%–61% reduction, striatum: 48%–53% reduction, p<0.001, ANOVA, n=7–10 per group from 5–6 pairs of animals). Figure 1B shows the representative examples of eIPSC recorded in striatal neurons from WT and HD mice. Membrane capacitance (Cm) was not significantly altered in either type of neurons from the HD mouse model (WT cortex: 68.4 ± 2.9pF, n=9; HD cortex: 65.7 ± 2.1pF, n=7; WT striatum: 53.2 ± 3.1pF, n=15; HD striatum: 52.7 ± 2.7pF, n=13).
Figure 1. HD mice show impaired synaptic inhibition at the symptomatic stage.
A, Summarized input-output plot showing the amplitude of GABAAR-IPSC evoked by a series of stimulation intensities in cortical pyramidal neurons and striatal medium spiny neurons (MSN) taken from N171-82Q mice (~4 months old) vs. age-matched wild-type (WT) mice. B, Representative eIPSC traces in striatal MSNs. Scale bar: 50pA, 20ms. C, Bar graph summary of mIPSC amplitude and frequency in cortical or striatal neurons taken from WT vs. HD mice (~4 months old). D, Cumulative distribution of mIPSC amplitudes in representative striatal MSNs. Inset: Representative mIPSC traces. Scale bar: 20pA, 1s. E, Paired-pulse ratio of eIPSC with various inter-pulse intervals in cortical neurons from WT vs. HD mice (~4 months old). F, Dot plots showing GABAAR current density (left) or voltage-dependent calcium channel (VDCC) current density (right) in acutely dissociated cortical neurons from WT vs. HD mice (~4 months old). Inset: Representative ionic current traces. Scale bars: 100pA, 1s (GABA current); 100pA, 5ms (VDCC). *: p<0.001.
Next, we measured miniature IPSC (mIPSC), a response from quantal release of single GABA vesicles. As shown in Figure 1C, the mIPSC amplitude was significantly decreased in both cortical and striatal neurons from HD mice (WT cortex: 21.3 ± 1.2pA, n=6; HD cortex: 12.9 ± 1.0pA, n=7; WT striatum: 24.2 ± 2.7pA, n=7, HD striatum: 14.8 ± 1.2pA, n=7, p<0.001, t test), while mIPSC frequency was not significantly changed (WT cortex: 2.7 ± 0.9Hz, n=6; HD cortex: 3.4 ± 0.7Hz, n=7; WT striatum: 3.3 ± 0.7Hz, n=7; HD striatum: 2.6 ± 0.4Hz, n=7, p>0.05). Representative examples further showed a leftward shift towards lower amplitudes in the cumulative distribution plot of mIPSC in striatal neurons from HD mice (Figure 1D).
To test the pre- vs. post-synaptic nature of the effect on GABA responses, we measured the ratio of GABAAR-IPSC evoked by paired-pulses (PPR), a readout that is affected by presynaptic transmitter release (Manabe et al., 1993). As shown in Figure 1E, PPR was not significantly different in cortical neurons from WT vs. HD mice (PPR at 50ms interval: WT: 1.4 ± 0.22, n=8; HD: 1.45 ± 0.08, n=8).
Furthermore, we recorded whole-cell ionic currents in acutely isolated cortical neurons (pure post-synaptic preparations). As shown in Figure 1F, neurons from HD mice had a significantly decreased GABAAR current density (pA/pF) (WT: 140.8 ± 8.7, n=14; HD: 65.8 ± 9.1, n=14, p<0.001, t test). The reduced GABA response in HD mice is not a nonspecific effect resulting from the unhealthy condition of neurons, as voltage-dependent calcium channel (VDCC) current density was not altered (WT: 87.1 ± 2.7, n=14; HD: 84.7 ± 7.1, n=14, p>0.05, t test). Taken together, these lines of evidence suggest that the depression of GABAergic transmission in symptomatic HD mice is likely due to altered postsynaptic GABAA receptors but not presynaptic GABA release.
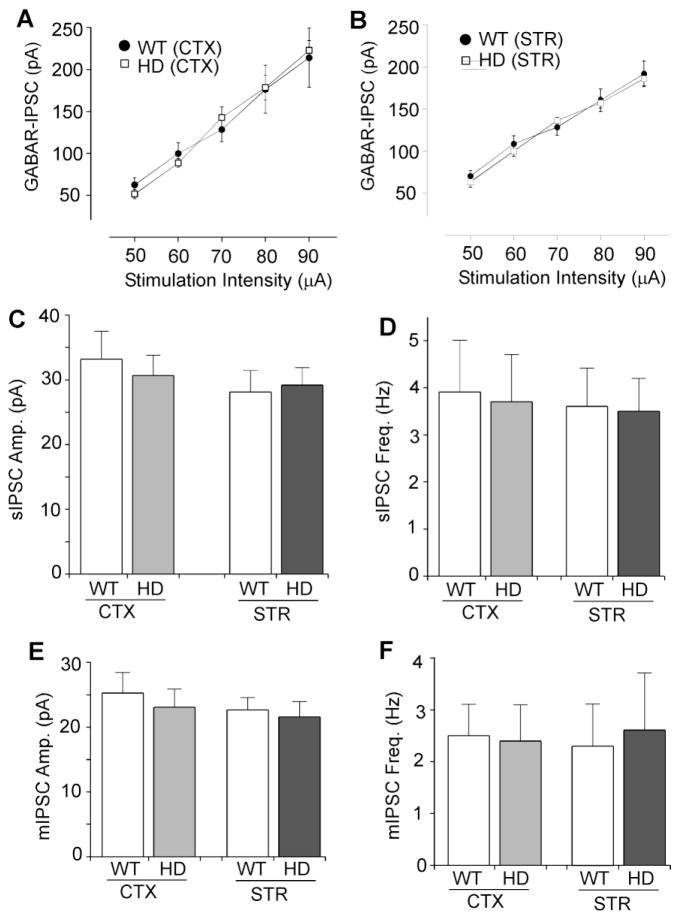
To test whether the impaired GABAergic synaptic transmission in HD mice is a consequence of early altered neurotransmission, we also examined IPSC in presymptomatic (1–2 months old) N171-82Q mice. As shown in Figure 2A and 2B, GABAergic synaptic strength, as measured by the input/output curves of evoked IPSC, was unchanged in cortical or striatal neurons from the presymptomatic HD mice (WT cortex: n=7; HD cortex: n=7; WT striatum: n=7; HD striatum: n=8, p>0.05). The spontaneous IPSC (sIPSC) amplitude or frequency was also not significantly changed (Figure 2C and 2D, WT cortex: 33.2 ± 4.3pA, 3.9 ± 1.1Hz, n=6; HD cortex: 30.7 ± 3.1pA, 3.7 ± 1.1Hz, n=7, p>0.05; WT striatum: 28.1 ± 3.3pA, 3.6 ± 0.8Hz, n=7; HD striatum: 29.2 ± 2.7pA, 3.5 ± 0.7Hz, n=7, p>0.05). Moreover, no significant alteration was found in the mIPSC amplitude or frequency of presymptomatic N171-82Q mice (Figure 2E and 2F, WT cortex: 25.3 ± 3.1pA, 2.5 ± 0.6Hz, n=7; HD cortex: 23.1 ± 2.8pA, 2.4 ± 0.7Hz, n=6, p>0.05; WT striatum: 22.8 ± 1.6pA, 2.3 ± 0.8Hz, n=7; HD striatum: 21.7 ± 2.3pA, 2.6 ± 1.1Hz, n=7, p>0.05). These data suggest that synaptic inhibition is normal in the HD mouse model at the presymptomatic stage.
Figure 2. HD mice show normal synaptic inhibition at the presymptomatic stage.
A, B, Summarized input-output plot showing the amplitude of GABAAR-IPSC evoked by a series of stimulation intensities in cortical pyramidal neurons (A) and striatal medium spiny neurons (B) taken from N171-82Q mice (1–2 months old) vs. age-matched wild-type (WT) mice. C–F, Bar graph summary of sIPSC (C, D) and mIPSC (E, F) amplitude and frequency in cortical or striatal neurons taken from WT vs. HD mice (1–2 months old).
Surface GABAAR expression is diminished in HD
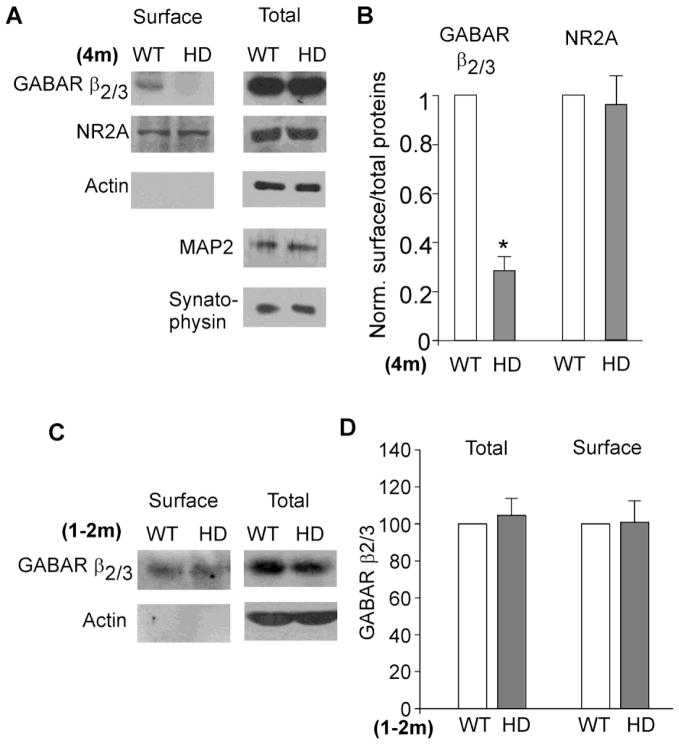
Since the reduced GABAergic transmission in HD mice is likely through a postsynaptic mechanism, we next performed surface biotinylation and Western blotting experiments to detect the level of surface and total GABAARs in striatal slices. As shown in Figure 3A and 3B, HD mice (~4 months old) showed a significant decrease in the surface GABAAR β2/3 subunits (70 ± 6% decrease, n=5, p<0.01, t test), while the total GABAAR β2/3 remained unchanged. No change was detected in the level of surface NMDAR NR2A subunits (4 ± 10% decrease, n=3, p>0.05, t test). The expression of actin, MAP2 (a dendritic marker) or synatophysin (a presynaptic marker) was also unchanged. HD mice at the presymptomatic stage (1–2 months old) showed the normal level of surface GABAAR β2/3 subunits (Figure 3C and 3D). These data suggest that GABAARs at the cell surface were selectively reduced in HD mice at the symptomatic stage, which may underlie the disrupted GABAergic transmission.
Figure 3. HD mice show reduced surface GABAAR expression at the symptomatic stage.
A, Immunoblots showing the surface and total GABAAR β2/3 and NR2A subunits in striatal lysates from WT vs. HD mice (~4 months old). The expression of actin, MAP2 and synaptophysin is also shown. The lack of actin (an intracellular protein) in the surface pool has indicated the specificity of this approach. B, Quantitation showing the level of surface GABAAR β2/3 or NR2A in the striatum of WT vs. HD mice (~4 months old). *: p<0.01. C, D, Immunoblots and quantitation showing the level of surface and total GABAAR β3 subunits in striatal lysates from WT vs. HD mice (1–2 months old).
The KIF5-mediated microtubule-based transport of GABAARs is impaired in HD
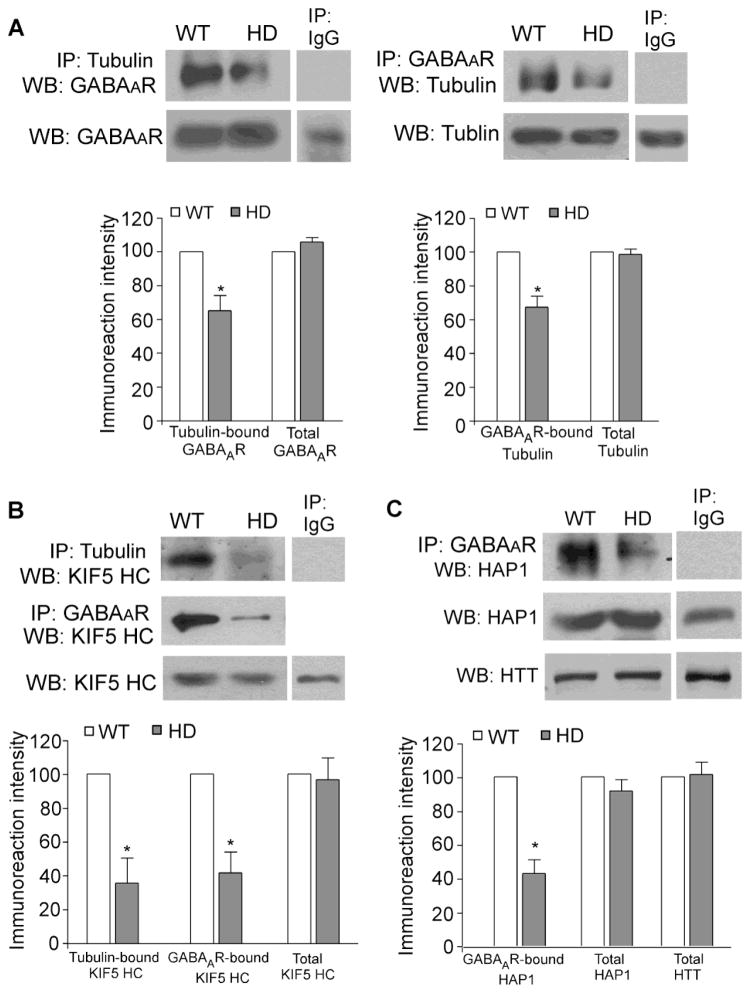
Next, we tried to figure out why HD mice exhibit the disrupted GABAAR membrane trafficking. It is known that HAP1 interacts with GABAARs (Kittler et al., 2004) and the kinesin motor protein KIF5 (McGuire et al., 2006; Twelvetrees et al., 2010). PolyQ-htt binds to HAP1 with a higher affinity, compared to WT-htt (Li et al., 1995). Thus, we hypothesize that the aberrant interaction of polyQ-htt/HAP1 may disrupt the kinesin-dependent GABAAR transport along microtubules (MT) in HD mice, leading to impaired GABAAR surface expression and GABAergic transmission. To test this, we performed co-immunoprecipitation assays to analyze the htt/HAP1/KIF5/GABAAR/MT complex in striatal lysates from WT vs. N171-82Q mice (3–5 months old). As shown in Figure 4A, the GABAARs bound to tubulin were markedly reduced in HD mice (tubulin-bound GABAAR: 65.3±8.8% of WT; GABAAR-bound tubulin: 67.4±6.6% of WT; n=4 pairs, p<0.05). The KIF5 heavy chain (HC) also lost the interaction with tubulin (Figure 4B, tubulin-bound KIF5 HC: 35.5 ± 14.8% of WT, n=5 pairs, p<0.05) or GABAARs (Figure 4B, GABAAR-bound KIF5 HC: 41.5 ± 12.3% of WT, n=3 pairs, p<0.05). Furthermore, a strong decrease was found with the HAP1 bound to GABAARs in HD mice (Figure 4C, 42.9 ± 8.3% of WT, n=4 pairs, p<0.05). None of these proteins show significant changes in their expression in HD mice (Figure 4A–C). These data suggest that polyQ-htt causes a dissociation of the KIF5/GABAAR complex from microtubules, and a dissociation of the cargo GABAAR from the motor protein KIF5, which may lead to the disrupted transport of GABAARs in HD.
Figure 4. The KIF5/GABAAR/MT complex is disrupted in HD mice.
A, Co-immunoprecipitation blots and quantification showing the interaction between GABAAR and tubulin from striatal slices of WT vs. HD mice (3–5 months old). B, Co-immunoprecipitation blots and quantification showing the KIF5 motor protein that binds to tubulin or GABAAR from striatum of WT vs. HD mice. C, Co-immunoprecipitation blots and quantification showing the interaction between HAP1 and GABAAR from striatum of WT vs. HD mice. Each experiment was repeated in 3–5 pairs of mice. *: p<0.05.
Discussion
It is known that GABAergic transmission plays a key role in the neuronal communication in striatal or cortical circuits. GABAAR dysfunction is implicated in multiple neurological diseases, such as epilepsy, anxiety disorders, fragile X syndrome, and schizophrenia (Benarroch, 2007; Rudolph and Mohler, 2004; D’Hulst and Kooy, 2007; Lewis and Gonzalez-Burgos, 2006). In HD studies, GABAergic transmission in cortical and striatal neurons has been examined in different mouse models, such as R6/2 mice (expressing exon 1 of human htt gene with ~150 CAG repeats, Cepeda et al., 2004; Centonze et al. 2005; Cummings et al., 2009), YAC128 mice (expressing full-length mutant htt, Cummings et al., 2009; 2010), CAG140 knock-in mice (expressing chimeric mouse/human htt in normal mouse genome, Cummings et al., 2009; 2010), BAC HD transgenic mice (Spampanato et al., 2008), and conditional HD mice (expressing mutant htt exon1 in discrete neuronal populations, Gu et al., 2005). Spontaneous IPSC (sIPSC) frequency in cortical pyramidal neurons is found to be decreased in some HD mouse models after they display the overt behavioral phenotype (Gu et al., 2005; Spampanato et al., 2008; Cummings et al., 2009), but is found to be increased in other HD mice at the symptomatic stage (Cummings et al., 2009). Increased sIPSC frequency has also been reported in striatal neurons from HD mice (Cepeda et al., 2004; Centonze et al. 2005; Cummings et al., 2010). Symptomatic R6/2 HD mice show significantly reduced mIPSC amplitude and frequency in cortical neurons (Cummings et al., 2009), suggesting contributions of both presynaptic and postsynaptic components. In this study, we show that GABAergic synaptic strength, as indicated by the input/output curve of evoked IPSC, is significantly diminished in both cortical and striatal neurons of the symptomatic mouse model of HD, N171-82Q. This electrophysiological phenomenon is likely mediated by a postsynaptic mechanism, because of the lack of changes in mIPSC frequency and paired-pulse ratio. The significant reduction of whole-cell GABAAR current density in acutely isolated neurons from N171-82Q mice further suggests that the decreased synaptic inhibition in the HD model is likely due to the loss of postsynaptic GABAARs.
It is known that the regulation of GABAAR trafficking is an essential determinant for the efficacy of synaptic inhibition (Kittler and Moss, 2003; Jacob et al., 2008). Under basal conditions, synaptic GABAARs undergo constitutive clathrin-dependent endocytosis (Kittler et al., 2000; Herring et al., 2003). The internalized receptors are either rapidly recycled back to the cell surface or targeted for lysosomal degradation. The GABAAR endocytic sorting is regulated by a direct interaction of GABAARs with HAP1 (Kittler et al., 2004). Overexpression of HAP1 in neurons inhibits GABAAR degradation and consequently increases receptor recycling (Kittler et al., 2004). Furthermore, HAP1 overexpression increases steady-state surface levels of GABAARs and mIPSC amplitude (Kittler et al., 2004). It suggests that HAP1 may play an important role in controlling synaptic inhibition by regulating the membrane trafficking of GABAARs. The impact of HAP1 regulation of GABAARs is further shown in hypothalamus, where downregulation of HAP1 results in decreased GABAAR levels, causing decreased food intake and weight loss (Sheng et al., 2006). In this study, we provide biochemical data showing that the level of surface GABAARs is markedly reduced in the HD mouse model at the symptomatic stage, which may underlie the impaired GABAergic transmission.
Emerging evidence suggests that HD is associated with disrupted HAP1 transport of cargos that are critical for maintaining neuronal functions, such as BDNF and TrkA, along microtubules (Gauthier et al., 2004; Rong et al., 2006; Li and Li, 2005). HAP1 interacts with the kinesin microtubule motor protein KIF5 light chain (McGuire et al., 2006) and heavy chains (Twelvetrees et al., 2010). Moreover, suppressing HAP1 expression inhibits the kinesin-dependent transport of amyloid precursor protein vesicles (McGuire et al., 2006) and GABAAR-containing vesicles (Twelvetrees et al., 2010) in transfected cultures. In this study, we demonstrate that the association of kinesin (motor) with GABAARs (cargo) and with microtubules (track) is severely lost in the HD mouse model, which may underlie the disrupted GABAAR trafficking to synaptic membrane.
The polyQ-htt-dependent alteration of GABAAR trafficking may cause a prolonged and potentially deleterious down-regulation of synaptic inhibition in HD. In agreement with this, a greater propensity to develop seizures has been found in a mouse model of HD (Mangiarini et al., 1996), and in juvenile HD, a prominent symptom is epileptic seizures (Mangiarini et al 1996; Gambardella et al., 2001). The deficits in GABAergic inhibition, which is caused by disrupted GABAAR trafficking to synapses, along with the previously reported polyQ-htt-dependent enhancement in NMDAR function in striatal neurons (Zeron et al., 2002; Fan et al., 2007), could contribute to disruption of the excitatory/inhibitory balance, leading to increased neuronal excitotoxicity.
Conclusion
In summary, our results show that the HAP1/KIF5-mediated anterograde transport of GABAARs along dendritic microtubules is impaired by the mutant htt in HD conditions. PolyQ-htt alters GABAAR vesicle transport, resulting in reduced surface delivery and accumulation of GABAARs at inhibitory synapses and ensuing reduced inhibitory synaptic response. Blocking polyQ-htt disruption of the machinery underlying HAP1/KIF5-facilitated trafficking of GABAARs to synapses may be a therapeutic approach for restoring aberrant synaptic functions in Huntington’s disease.
Highlights.
We compared synaptic inhibition in wild-type and a transgenic mouse model of HD.
We examined GABAA receptor surface expression in the HD mouse model.
We examined the GABAAR/KIF5/microtubule complex in the HD mouse model.
We find that GABAAR-mediated synaptic inhibition is impaired in HD.
We find that KIF5-mediated microtubule-based transport of GABAARs is disturbed in HD.
Acknowledgments
We thank Xiaoqing Chen for excellent technical support. This work was supported by NIH R21 grant NS069929 to Z.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benarroch EE. GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology. 2007;68:612–4. doi: 10.1212/01.wnl.0000255669.83468.dd. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, André VM, Ariano MA, Levine MS. Increased GABAergic function in mouse models of Huntington’s disease: reversal by BDNF. J Neurosci Res. 2004;78:855–67. doi: 10.1002/jnr.20344. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Prosperetti C, Tscherter A, Bernardi G, Maccarrone M, Calabresi P. Abnormal sensitivity to cannabinoid receptor stimulation might contribute to altered gamma-aminobutyric acid transmission in the striatum of R6/2 Huntington’s disease mice. Biol Psychiatry. 2005;57:1583–9. doi: 10.1016/j.biopsych.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABA(A) receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci. 2006;26:2511–9. doi: 10.1523/JNEUROSCI.4712-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, André VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington’s disease. J Neurosci. 2009;29:10371–86. doi: 10.1523/JNEUROSCI.1592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Cepeda C, Levine MS. Alterations in striatal synaptic transmission are consistent across genetic mouse models of Huntington’s disease. ASN Neuro. 2010;2:e00036. doi: 10.1042/AN20100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30:425–31. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur EL, Ross CA. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–12. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- Fan MM, Fernandes HB, Zhang LY, Hayden MR, Raymond LA. Altered NMDA receptor trafficking in a yeast artificial chromosome transgenic mouse model of Huntington’s disease. J Neurosci. 2007;27:3768–79. doi: 10.1523/JNEUROSCI.4356-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog Neurobiol. 2007;81:272–93. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pagès M, Dompierre JP, Rangone H, Cordelières FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–38. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Goldstein LS. Do disorders of movement cause movement disorders and dementia? Neuron. 2003;40:415–25. doi: 10.1016/s0896-6273(03)00630-5. [DOI] [PubMed] [Google Scholar]
- Gu X, Li C, Wei W, Lo V, Gong S, Li SH, Iwasato T, Itohara S, Li XJ, Mody I, Heintz N, Yang XW. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–44. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Harjes P, Wanker EE. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem Sci. 2003;28:425–33. doi: 10.1016/S0968-0004(03)00168-3. [DOI] [PubMed] [Google Scholar]
- Gambardella A, Muglia M, Labate A, Magariello A, Gabriele AL, Mazzei R, Pirritano D, Conforti FL, Patitucci A, Valentino P, Zappia M, Quattrone A. Juvenile Huntington’s disease presenting as progressive myoclonic epilepsy. Neurology. 2001;57:708–11. doi: 10.1212/wnl.57.4.708. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–43. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–7. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004;101:12736–41. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–22. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Li XJ, Li SH. HAP1 and intracellular trafficking. Trends Pharmacol Sci. 2005;26:1–3. doi: 10.1016/j.tips.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Li XJ, Li SH, Sharp AH, Nucifora FC, Jr, Schilling G, Lanahan A, Worley P, Snyder SH, Ross CA. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- Li SH, Gutekunst CA, Hersch SM, Li XJ. Interaction of huntingtin-associated protein with dynactin P150Glued. J Neurosci. 1998;18:1261–9. doi: 10.1523/JNEUROSCI.18-04-01261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–9. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- McGuire JR, Rong J, Li SH, Li XJ. Interaction of huntingtin-associated protein-1 with kinesin light chain: Implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–9. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- Rong J, McGuire JR, Fang ZH, Sheng G, Shin JY, Li SH, Li XJ. Regulation of intracellular trafficking of huntingtin-associated protein-1 is critical for TrkA protein levels and neurite outgrowth. J Neurosci. 2006;26:6019–30. doi: 10.1523/JNEUROSCI.1251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA. Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron. 2002;35:819–22. doi: 10.1016/s0896-6273(02)00872-3. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–98. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- Sheng G, Chang GQ, Lin JY, Yu ZX, Fang ZH, Rong J, Lipton SA, Li SH, Tong G, Leibowitz SF, Li XJ. Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat Med. 2006;12:526–33. doi: 10.1038/nm1382. [DOI] [PubMed] [Google Scholar]
- Smith R, Brundin P, Li JY. Synaptic dysfunction in Huntington’s disease: a new perspective. Cell Mol Life Sci. 2005;62:1901–12. doi: 10.1007/s00018-005-5084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Gu X, Yang XW, Mody I. Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington’s disease. Neuroscience. 2008;157:606–20. doi: 10.1016/j.neuroscience.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci. 2002;22:9185–93. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABAA currents in striatal cholinergic interneurons through a protein kinase A/protein phosphatase 1 cascade. Neuron. 1997;19:1115–26. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–9. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–70. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Zhong P, Gu Z, Wang X, Jiang H, Feng J, Yan Z. Impaired modulation of GABAergic transmission by muscarinic receptors in a mouse transgenic model of Alzheimer’s disease. J Biol Chem. 2003;278:26888–96. doi: 10.1074/jbc.M302789200. [DOI] [PubMed] [Google Scholar]