Abstract
The prevalence of obesity is increasing rapidly worldwide, which is cause for concern because obesity increases the risk of cardiovascular disease and diabetes, reduces life expectancy and impairs quality of life. A better understanding of the risk factors for obesity is therefore a critical global health concern and human biologists can play an important role in identifying these risk factors in various populations. The objective of this review is to present the evidence that inadequate sleep may be a novel risk factor associated with increased vulnerability to obesity and associated cardiometabolic disease. Experimental studies have found that short-term sleep restriction is associated with impaired glucose metabolism, dysregulation of appetite and increased blood pressure. Observational studies have observed cross-sectional associations between short sleep duration (generally <6 hours per night) and increased body mass index or obesity, prevalent diabetes and prevalent hypertension. Some studies also reported an association between self-reported long sleep duration (generally >8 hours per night) and cardiometabolic disease. A few prospective studies have found a significant increased risk of weight gain, incident diabetes and incident hypertension associated with inadequate sleep. Given the potential link between inadequate sleep and obesity, a critical next step is to identify the social, cultural and environmental determinants of sleep, which would help to identify vulnerable populations. Future human biology research should consider variation in sleep characteristics among different populations and determine whether the associations between sleep and obesity observed in Western populations persist elsewhere.
Keywords: Sleep, body mass index, obesity, diabetes mellitus, hypertension, cardiovascular diseases
INTRODUCTION
The obesity epidemic is a global phenomenon that is affecting both adults and children. In 2005, more than 200 million men and nearly 300 million women were obese and this number is projected to increase to as many as 1.1 billion people by 2030 (Kelly et al 2008). Understanding risk factors for obesity is a critical global health objective because obesity increases the risk of cardiovascular disease and diabetes, reduces life expectancy, impairs quality of life and is associated with an increased individual and societal economic burden (Ettaro et al 2004; Fontaine et al 2003; Franco et al 2007; Solomon and Manson 1997; Wolf and Colditz 1998). Importantly, the prevalence of obesity is not distributed equally between different socioeconomic groups where those of lower socioeconomic status suffer a greater burden (Akil and Ahmad 2011; Devaux and Sassi 2011). Thus, elucidating the underlying reasons for increased vulnerability to obesity experienced by certain populations, including lower socioeconomic groups, can help to reduce health disparities in obesity and related diseases, including diabetes and cardiovascular disease.
In simplistic terms, obesity develops when energy intake is greater than energy expenditure. Although diet and physical activity play an important role in the risk of weight gain and the obesity epidemic, a potential additional factor may be inadequate sleep. Inadequate sleep can refer to either shorter sleep durations or poor sleep quality with or without the presence of a sleep disorder. This paper will review the evidence for an association between inadequate sleep and increased risk of obesity and associated cardiometabolic diseases, including diabetes, hypertension and cardiovascular diseases. It is important to note that the majority of this research has been conducted in Western countries. Unfortunately, comparative research on sleep and its association with obesity is currently lacking, but because of the potential link between sleep and both obesity and cardiometabolic diseases, consideration of variation in sleep characteristics in human biology research would be worthwhile. In addition, a better understanding of the social, cultural and environmental determinants of sleep would help to identify vulnerable populations. As such, the end of this review discusses a few potential determinants of sleep, however, much more research on human variation in sleep and the consequences for cardiometabolic health is needed.
EXPERIMENTAL STUDIES OF SLEEP RESTRICTION
Experimental sleep restriction, appetite regulation and risk of weight gain
Appetite regulation involves both peripheral signals from the body and control by specific brain regions, particularly regions of the hypothalamus that are involved in regulating food intake and energy homeostasis. Peripheral signals include leptin, which is secreted by adipose tissue and is a satiety signal, and ghrelin, which is a gut-derived signal that increases appetite (Schwartz and Porte 2005). Thus, leptin and ghrelin have opposing effects on appetite and food intake. Insulin, which is secreted by the beta cells in the pancreas in response to a meal, is another important peripheral signal that inhibits appetite and reduces food intake (Porte et al 2005; Schwartz and Porte 2005). Many of these hormones have been impacted by experimental sleep restriction.
Although several studies have examined the impact of a night of total sleep deprivation on physiology, the effects are usually temporary because total sleep deprivation cannot be maintained for very long. On the other hand, chronic partial sleep restriction is not only possible, it is quite common. In the United States, for example, the prevalence of short sleep (≤6 hours per night) is estimated to be 17–18% of adults (National Sleep Foundation 2010), which means that over 53 million people in the US are likely to be short sleepers. The first detailed laboratory study of the effects of partial sleep deprivation subjected healthy young men to 6 nights of 4 hours in bed followed by 7 nights of 12 hours in bed (Spiegel et al 1999). Mean leptin levels were 19% lower during sleep restriction compared to sleep extension despite identical caloric intake and physical activity and no change in body weight (Spiegel et al 2004a). The observed difference in leptin levels was similar to the difference observed in a study that restricted caloric intake to 70% of energy requirements for 3 days in young adults (Chin-Chance et al 2000; Spiegel et al 2005). A second experimental study of sleep restriction compared the effects of 7 days with 4 hours in bed to a 3-day sleep recovery period in 8 healthy men aged 18–25 years, and found that leptin levels were approximately 33% lower after sleep restriction (Guilleminault et al 2003). Another experimental study involved 2 days of 4 hours in bed and 2 days of 10 hours in bed and simultaneously measured leptin, ghrelin and subjective appetite (Spiegel et al 2004b). Mean leptin levels were 18% lower, mean ghrelin levels were 28% higher and subjective appetite was 23% higher in the sleep restriction condition compared to sleep extension (Spiegel et al 2004b). Although appetite for all the different food types increased, they did not increase equally. In fact, appetite for high calorie, carbohydrate-rich foods (including sweets, salty snacks, and starchy foods) increased more than for other food types (Spiegel et al 2004b). In this study, the change in the ratio of ghrelin-to-leptin between the 2 conditions was strongly correlated to the change in hunger ratings (r= 0.87, p=.01) (Spiegel et al 2004b), indicating that the changes observed in these appetite hormones was partially responsible for the increase in subjective appetite. The observed changes in appetite suggest that food intake would have increased if subjects had been allowed ad libitum food. More importantly, in addition to an increase in total caloric intake, the proportional intake of specific macronutrients, such as carbohydrates or fat, could have increased. Some have suggested that specific dietary components, especially refined sugar that leads to increased circulating insulin levels, increases risk of weight gain (Wells and Siervo 2011).
Not all studies have observed similar effects on leptin levels. One study of a single night of sleep restricted to 4.5 hours compared to one night of 7 hours observed no difference in leptin levels in 9 men aged 20–40 years (Schmid et al 2008). Fifteen healthy women aged 18–25 years spent 10 hours in bed for 2 nights followed by a single night of 3 hours in bed and leptin levels actually increased modestly in the morning after sleep restriction although subjective appetite did not change (Omisade et al 2010). These 2 studies suggest that there may be a minimum duration of exposure to sleep restriction before appetite is upregulated. Finally, a simulation of a work week and weekend consisted of 2 nights of 8 hours in bed, 5 nights of 4 hours in bed, and 2 nights of 8 hours in bed in 15 young, healthy men aged 19–29 years (van Leeuwen et al 2010). They collected fasting blood samples and subjective hunger at 7:30 a.m. after the 5 nights of sleep restriction and after 2 nights of 8 hours in bed, but observed no difference in leptin levels or satiety ratings. It is not clear if the circadian rhythm of leptin or hunger is altered due to the delayed bedtimes (Morselli et al 2010) or if indeed there was no effect on appetite regulation in this study.
Weight gain, and ultimately obesity, requires an excess of food intake relative to energy expenditure, and a few experimental studies have examined the impact of sleep restriction on food intake rather than just hormonal levels or subjective appetite. In non-overweight men and women aged 30–45 years, 6 nights of 4 hours in bed was associated with a significant increase in caloric intake, particularly from fat, without a compensatory change in energy expenditure (St-Onge et al 2011). Another experimental study that enrolled 11 healthy overweight (BMI ranged from 24 to 29 kg/m2) men and women aged 34–49 years compared 14 days of 5.5-h to 14 days of 8.5-h bedtimes with ad libitum access to palatable food and found that the bedtime restriction was associated with an increased consumption of calories from snacks but no difference in caloric intake during meals (Nedeltcheva et al 2009a). A third experimental study in women who ranged from lean to obese examined the effects of progressively increasing sleep restriction over 4 nights (7h, 6h, 6h, 4h per night) and found that caloric intake based on food dairies increased by approximately 20% after sleep restriction, despite the fact that the women slept at home and compliance to the prescribed bed times was only estimated using heart rate measures (Bosy-Westphal et al 2008). In this study, leptin levels actually increased after sleep restriction, however, the participants also increased their caloric intake and gained weight, which could lead to higher leptin levels. Another experimental study in 12 healthy, lean men found that compared to 2 nights of 8 hours in bed, average energy intake was 22% higher after 2 nights of 4 hours in bed and that during the ad libitum dinner, fat consumption increased by 98% (Brondel et al 2010). A study of men aged 20–40 years, however, observed no difference in total energy intake or subjective hunger and appetite between 2 nights of 4.25 hours in bed and 2 nights of 7.25 hours in bed (Schmid et al 2009). The results of this study suggest that a minimum duration of sleep restriction is required to observe an effect on food intake. If the increase in food intake observed in these short term studies was maintained after chronic sleep restriction, the result would be weight gain.
The other side of the energy balance equation is energy expenditure. Only a few experimental studies, however, have examined the impact of sleep restriction on energy expenditure. The Schmid et al study mentioned above, which compared 2 nights of 4.25 hours in bed and 2 nights of 7.25 hours in bed in men, observed significantly decreased physical activity during the day and less time spent in intense physical activities after the first night of sleep restriction (Schmid et al 2009). However, three of the experimental studies discussed above observed no change in estimated energy expenditure (Bosy-Westphal et al 2008; Brondel et al 2010; Nedeltcheva et al 2009b). Results from so few studies are difficult to interpret, but if inadequate sleep does increase risk of weight gain it seems more likely it does so through an upregulation of appetite and a subsequent increase in energy intake rather than through a decrease in energy expenditure.
In summary, the majority of experimental studies that restricted the time available for sleep in healthy subjects observed significant changes in hormones involved in appetite regulation in a direction that would increase food intake. Indeed subjective hunger and appetite did increase in the one study that measured it. In studies that allowed ad libitum food intake, two saw an increase in food intake, either in total calories or in the form of snacking. Nonetheless, discrepancies between the findings of these studies need to be explained. In particular, to improve our understanding of the link between inadequate sleep and obesity risk, we need to determine the minimum duration of exposure to sleep restriction that is required to impact risk of weight gain, along with the degree of sleep restriction required (i.e. amount of sleep per day) and whether the association is modified by gender, age or BMI.
Experimental sleep restriction and cardiometabolic risk
Obesity is associated with increased risk of developing cardiometabolic diseases, such type 2 diabetes, hypertension and cardiovascular disease (CVD) (Haslam and James 2005). Experimental studies of sleep restriction also provide evidence for a potential link between sleep loss and cardiometabolic diseases.
Diabetes develops due to impairments in glucose metabolism, which involves complex biochemical processes. In healthy individuals, glucose levels in the blood are carefully regulated by balancing glucose production (either from the liver or from meals) and glucose utilization by tissues. Insulin plays a key role in this process by inhibiting glucose production in the liver and by stimulating glucose uptake by insulin-sensitive tissues (i.e. muscle, liver and fat cells). Glucose tolerance refers to the ability of the body’s tissues to absorb glucose from the blood and return blood glucose levels to baseline. High blood glucose can have a number of deleterious health consequences if it not well-controlled, including blood vessel damage, kidney disease, glaucoma and nerve damage. Insulin sensitivity and insulin resistance refer to the ability (or inability) of insulin to exert its effects on target tissues and is a strong predictor of risk of diabetes.
Several experimental studies of sleep restriction observed an effect on markers of glucose metabolism. The first laboratory study of partial sleep deprivation discussed above found that glucose tolerance was 40% lower after 6 nights of 4-hour bedtimes compared to after 7 nights of 12-hour bedtimes (Spiegel et al 1999). Insulin sensitivity was also lower after sleep restriction but it was not statistically significant. The glucose tolerance values observed after sleep restriction in this study were similar to glucose tolerance values observed in older adults with impaired glucose tolerance (Garcia et al 1997), while the values observed after sleep extension were, as expected, in the range typical of healthy young subjects (Prigeon et al 1995). In a second experimental study, 20 healthy men aged 20–35 years spent 10 hours in bed for at least 8 nights followed by 5 hours in bed per night for 7 nights (Buxton et al 2010). Insulin sensitivity was reduced by 11–20% (depending on method used to estimate insulin sensitivity) and glucose tolerance was reduced by approximately 14% after sleep restriction (Buxton et al 2010). Among 9 healthy lean subjects, a single night of 4 hours in bed increased hepatic insulin resistance and decreased peripheral insulin sensitivity by 19–25% based on euglycemic clamp studies, which are the gold standard for estimating insulin sensitivity (Donga et al 2010). Two weeks of 5.5 hours in bed was associated with a 10% reduction in glucose tolerance and an 18% reduction in insulin sensitivity compared to 2 weeks of 8.5 hours in bed, even though weight gain was comparable in both conditions (Nedeltcheva et al 2009a). The study that simulated a work week and weekend in 15 young, healthy men found that the 5 days of sleep restriction was associated with an increase in morning fasting insulin levels with no change in glucose levels, which suggests reduced insulin sensitivity (van Leeuwen et al 2010). Levels returned to baseline after 2 nights of 8 hours in bed, suggesting some recovery may be possible on weekends, at least in young, healthy men. An experimental study in women, however, which involved progressively increasing sleep restriction over 4 nights, observed no changes in glucose or insulin levels (Bosy-Westphal et al 2008). Unfortunately, this study did not require participants to spend the night in the laboratory and they returned to the laboratory in the morning at varying times and the length of fasting could not be verified. Nonetheless, most of these studies suggest that a few days of bedtime restriction could increase the risk of developing impaired glucose tolerance or diabetes if these alterations in glucose metabolism persist with longer periods of sleep restriction.
A few laboratory studies of short-term sleep deprivation have also observed effects on blood pressure. Thirty-six men and women ranging in age from 34 to 68 years who were recently diagnosed with hypertension spent 2 separate nights in the laboratory, one had 8 hours in bed and the other only 4 hours in bed (Lusardi et al 1996). Both systolic and diastolic blood pressure was elevated, particularly in the early evening and morning, during the 4-hour bedtime condition. A study of 18 male technical workers aged 23 to 48 years compared 24-hour blood pressure levels throughout a day with normal sleep (mean 8 hours) and a day with insufficient sleep (mean 3.6 hours) and found significantly higher systolic and diastolic blood pressure after one night of insufficient sleep (Tochikubo et al 1996). Finally, in a small study of 8 healthy adults, mean blood pressure increased after one night of total sleep deprivation compared to after 2 nights of undisturbed sleep (Kato et al 2000). Thus, chronically reduced sleep duration could have an adverse impact on blood pressure regulation and promote the development of hypertension (Schroeder et al 2003).
In summary, experimental studies of sleep restriction have observed substantial and clinically significant changes in appetite regulation, hunger, food intake, glucose metabolism and blood pressure control. One important limitation of laboratory studies is that they are short-term, lasting a few weeks at most. This raises the question of whether these effects will persist outside the laboratory when sleep restriction is chronic.
OBSERVATIONAL STUDIES OF SLEEP
Epidemiologic studies provide important insight into the associations between sleep, obesity and cardiometabolic diseases outside of the laboratory. Below, observational studies that have examined the relationship between sleep duration or quality and the presence or development of obesity, diabetes, hypertension and CVD are reviewed.
Habitual Sleep and BMI/Obesity
Several observational studies have examined the association between sleep and obesity or BMI. At least 65 published articles have found a significant cross-sectional association between short sleep duration (generally <6 hours per night) and increased prevalence of obesity or higher BMI in both adults and children from various countries (see (Knutson and Van Cauter 2008; Marshall et al 2008; Patel and Hu 2008) for reviews). Some of these studies also observed higher mean BMI associated with longer sleep durations (generally >8 hours per night), suggesting a U-shaped association between self-reported sleep duration and BMI. Studies that examined subjective sleep quality have found worse sleep quality was also associated with higher BMI (Asplund and Aberg 2001; Jennings et al 2007). Two recent meta-analyses analyzed data from some of these cross-sectional studies. Cappuccio et al (Cappuccio et al 2008) found that short sleep duration (<5 hours per night for adults, <10h per night for children) significantly predicted obesity in adults (pooled odds ratio [OR] was 1.55, 95% confidence interval [CI]: 1.43–1.68) and in children (pooled OR was 1.89, 95% CI: 1.46–2.43). A second meta-analysis examined short sleep and obesity in children only and the pooled OR was 1.58 (95% CI: 1.26–1.98), which means that children who were short sleepers had 58% greater odds of being obese (Chen et al 2008). Thus, both meta-analyses confirmed that short sleep duration was associated with higher odds of being obese.
In summary, most studies have found a significant cross-sectional association between inadequate sleep and higher BMI or the presence of obesity. Of note, however, is that the association between sleep and BMI appears stronger at younger ages (Danielsen et al 2010), which suggests that children and adolescents may be more vulnerable to the effects of inadequate sleep. An important limitation of cross-sectional studies is that causal direction cannot be inferred, and indeed the association between sleep and BMI could be bidirectional. This is important because if obese individuals are more likely to have inadequate sleep, then they will be more vulnerable to the cardiometabolic impairments caused by inadequate sleep. It is well-known that obesity is a strong risk factor for obstructive sleep apnea, a sleep disorder that is associated with increased risk of cardiometabolic diseases (see (Pamidi et al 2010) and (Pack and Gislason 2009) for reviews).
A few prospective studies have examined sleep and weight gain in both adults and children. Some found no statistically significant association between sleep duration and change in body size (Gangwisch et al 2005; Lauderdale et al 2009; Stranges et al 2008a). Many studies, however, did report a significant association between shorter sleep and greater weight gain in adult men and/or women (Chaput et al 2008; Lopez-Garcia et al 2008; Patel et al 2006b; Watanabe et al 2010). A recent study among 2 minority groups in the U.S., African-Americans and Hispanic adults, found that among those aged 18–39 years who reported sleeping ≤5 hours per night experienced a larger increase in BMI, visceral fat and subcutaneous fat over 5 years compared to those who reported sleeping 6–7 hours per night (Hairston et al 2010). In this same study, no association between sleep and change in BMI or fat was observed for those aged 40 years or older (Hairston et al 2010). Among children, a few studies observed increased risk of obesity or weight gain associated with shorter sleep durations (Reilly et al 2005; Snell et al 2007; Taveras et al 2008). These prospective studies together suggest that sleep duration is associated with changes in body size, which could increase the risk of developing obesity and associated cardiometabolic disease. However, several of these studies suggest that there may be important age or gender differences in these associations, which require further examination.
Habitual sleep, appetite regulation and dietary behavior
A few observational studies have examined the relationship between habitual sleep characteristics and either hormonal levels and/or dietary behavior. For example, in cross-sectional analysis of data from 740 men and women the Quebec Family Study, leptin levels in those sleeping 5–6 hours per night were approximately 15–17% lower than predicted based on body fat alone (Chaput et al 2007). The Wisconsin Sleep Cohort Study, which enrolled state employees aged 30–60 years, found that total sleep time from one night of polysomnography was inversely associated with ghrelin levels (beta = −0.69, p=0.008) and average habitual sleep duration was positively associated with leptin levels independently of BMI (beta = 0.11, p=0.01) (Taheri et al 2004). Although these two epidemiologic studies are consistent with the laboratory studies, two studies in women did not observe similar associations. Participants in the Nurse’s Health Study returned a blood sample through the mail for assessment of leptin, and no association was found between self-reported sleep duration and leptin levels (Williams et al 2007). A study among 173 obese, sedentary postmenopausal women aged 50–74 year also did not observe a significant cross-sectional association between self-reported sleep duration and leptin or total ghrelin levels (Littman et al 2006). These discrepant results may be due to differences in the association between sleep and appetite regulation in women, particularly obese older women, or may be due to methodological issues such as self-reported sleep duration or sample collection.
A few studies examined the relationship between habitual sleep patterns and dietary intake. The Women’s Health Initiative study in the U.S. examined the relationship between sleep and dietary intake based on food frequency questionnaires in 459 postmenopausal women aged 50–81 years (Grandner et al 2010a). They found that average nocturnal sleep duration from one week of wrist actigraphy was negatively correlated with dietary fat intake and total calories even after adjusting for BMI and physical activity, which suggests that for a given level of BMI and physical activity, short sleepers have a greater food intake particularly in the form of fat, which would increase their risk of weight gain. A study in adolescents found no association between hunger ratings and average nocturnal sleep from 7-day sleep diaries, however, those who slept 3 hours or more during the daytime reported greater caloric intake and food cravings and this association did not appear to be confounded by nocturnal sleep duration (Landis et al 2009). A Finnish study of 1,265 children aged 9–11 years reported that among both boys and girls, shorter sleep durations during school nights were associated with an increased consumption of high calorie foods (Westerlund et al 2009). Given the paucity of data in this area, more research is required to determine whether habitual inadequate sleep is truly associated with greater appetite and greater food intake.
Habitual sleep and cardiometabolic disease risk
Several observational studies have examined habitual sleep duration and the prevalence or incidence of cardiometabolic diseases, including diabetes, hypertension and cardiovascular disease. Several have found cross-sectional associations between inadequate sleep and greater prevalence of diabetes or impaired glucose tolerance (see (Knutson and Van Cauter 2008) for a review). Among those who already have diabetes, sleep quality is generally worse based on both subjective and objective estimates (Trento et al 2008). Furthermore, there is some evidence that among persons with diabetes, poorer glycemic control is associated with worse sleep quality and increased sleep disordered breathing (Aronsohn et al 2010; Knutson et al 2006; Knutson et al 2011). Indeed, obstructive sleep apnea seems to be highly prevalent among patients with type 2 diabetes, with estimates ranging from 58 to 86% (Pamidi et al 2010). Although the results of these studies indicate that inadequate sleep is associated with diabetes, the direction of causality cannot be determined. Insufficient sleep may increase the risk of developing diabetes, as the laboratory studies suggest, or, conversely, having diabetes could impair sleep quality. Furthermore, the potential impact of inadequate sleep on glucose control among patients with diabetes is particularly important as understanding this association could help identify methods to improve disease management and reduce the risk of complications.
Prospective studies provide better evidence for causal link between inadequate sleep and the development of cardiometabolic diseases. Many studies reported increased odds of diabetes associated with short sleep duration (≤5h and/or ≤6h) and some observed a U-shaped association (Ayas et al 2003a; Chaput et al 2009; Gangwisch et al 2007; Xu et al 2010; Yaggi et al 2006). Furthermore, impaired sleep quality, such as difficulty initiating or maintaining sleep, has been associated with increased odds of developing diabetes in multiple studies (Beihl et al 2009; Hayashino et al 2007; Kawakami et al 2004; Mallon et al 2005; Meisinger et al 2005; Nilsson et al 2004). Note that one study did not find a significant association between sleep and incident diabetes (Bjorkelund et al 2005), while one study observed a significant association in men only (Mallon et al 2005). A meta-analysis of 10 prospective studies examined the association between the incidence of diabetes and either short self-reported sleep duration, long self-reported sleep duration or subjective sleep disturbances (Cappuccio et al 2009). The estimated pooled OR for short sleep was 1.28 (95% CI 1.03–1.6), however, there was a significant gender difference. The OR was 2.07 (95% CI 1.16–3.72) for men and 1.07 (95% CI 0.90–1.28) for women, indicating a stronger association among men. The pooled OR for long sleep was 1.48 (95% CI 1.13–1.96), indicating that overall there is a significant U-shaped association between self-reported sleep duration and incident diabetes. Finally, both difficulty initiating sleep (pooled OR 1.57, 95% CI 1.25–1.97) and difficulty maintaining sleep (pooled OR 1.84, 95% CI 1.39–2.43) significantly predicted incident diabetes. Overall, prospective epidemiologic studies suggest that subjective short or long sleep duration and poor sleep quality predict the development of diabetes.
In cross-sectional analyses, self-reported short sleep durations or subjectively poor sleep quality have been associated with higher average blood pressure or higher prevalence of hypertension (Cappuccio et al 2007; Choi et al 2008; Gottlieb et al 2006; Houyez et al 1990; Kawabe and Saito 2008; Kotani et al 2007; Stang et al 2008). Some also observed higher blood pressure among long sleepers (Choi et al 2008; Gottlieb et al 2006) and two of these studies observed a significant association only in women (Cappuccio et al 2007; Stang et al 2008). However, a few studies found no association between habitual sleep and blood pressure, including two among elderly adults (Lima-Costa et al 2008; van den Berg et al 2007) and one among children aged 3–10 years (Bayer et al 2009), which suggests that associations between sleep and blood pressure may be modified by age. Two studies used wrist actigraphy, one of which found that low sleep quality, but not sleep duration, was significantly associated with prevalent prehypertension among adolescents (Javaheri et al 2008). In a study among adults aged 35–50 years, shorter sleep duration and lower sleep quality based on wrist actigraphy were both associated with higher blood pressure (Knutson et al 2009). Thus, cross-sectional studies generally support a relationship between inadequate sleep and higher mean blood pressure, but the causal direction cannot be determined and the strength of these associations may be modified by gender or age.
Several prospective epidemiologic studies have examined cardiovascular outcomes in relation to habitual sleep. The National Health and Nutrition Examination Survey (NHANES) in the US reported that short sleepers (≤5 hours per night) had increased odds of incident hypertension over 8–10 years (OR 1.32, 95% CI 1.02–1.71) compared to those reporting sleeping 7–8 hours per night after adjusting for numerous potential confounders (Gangwisch et al 2006). In the study of adults aged 35–50 years, sleep duration estimated by actigraphy was significantly associated with incident hypertension over 5 years (OR 0.72; 95% CI: 0.55, 0.95) (Knutson et al 2009) and incident coronary artery calcification (OR = 0.67 per hour, p=.011; 95% CI 0.49–0.91), which is a predictor of the development of coronary heart disease (King et al 2008). The Nurses’ Health Study found a significantly increased risk of incident coronary heart disease over 10 years associated with short (≤5 hours per night) self-reported sleep duration (risk ratio 1.45; 95%CI: 1.1–1.92) and long (≥9 hours per night) self-reported sleep duration (risk ratio 1.38; 95%CI: 1.03–1.86) compared to those sleeping 8 hours per night (Ayas et al 2003b). A few studies have examined cardiovascular disease mortality in relation to self-reported sleep duration, but none found a significant association in fully adjusted models (Chien et al 2010; Heslop et al 2002; Mallon et al 2002). Poor subjective sleep quality was also associated with increased coronary artery disease mortality in men (RR: 3.1, 95%CI 1.5–6.3) (Mallon et al 2002) A study in the US of over 3400 men and women 35 years of age or older reported a significant increase of cardiovascular disease events in those who complained of insomnia every day compared to those without any insomnia complaint (RR: 1.78, 95%CI 1.03–3.08) (Chien et al 2010). Overall, there is some evidence that inadequate sleep is associated with cardiovascular disease and hypertension, but more rigorous studies are required to fully understand whether inadequate sleep actually leads to cardiovascular disease.
LIMITATIONS
Although most of the observational studies described above reported consistent significant associations between inadequate sleep and cardiometabolic disease, there are important methodological limitations of these studies to consider. First, the majority relied on a self-reported measure of sleep duration, which may not be very accurate. Recent analysis comparing sleep durations estimated from wrist actigraphy to self-reported sleep duration in 600 middle-aged adults indicated only moderate agreement between these measures (r=0.47) (Lauderdale et al 2008). In addition, there may be important confounders that are not taken into account in these analyses, including race, socioeconomic status, physical activity, alcohol and caffeine consumption, and psychological disorders (Lauderdale et al 2008; Magee et al 2008). Finally, these studies have been predominantly conducted in Western countries (the U.S. and Europe), with the exception of Japan. Future studies need to examine the potential human variation in sleep and its association with cardiometabolic diseases by conducting research in other parts of the world. Research should incorporate objective measures of sleep, include detailed measures of potential confounding variables and consider interventional studies that can address causality.
POTENTIAL MECHANISMS
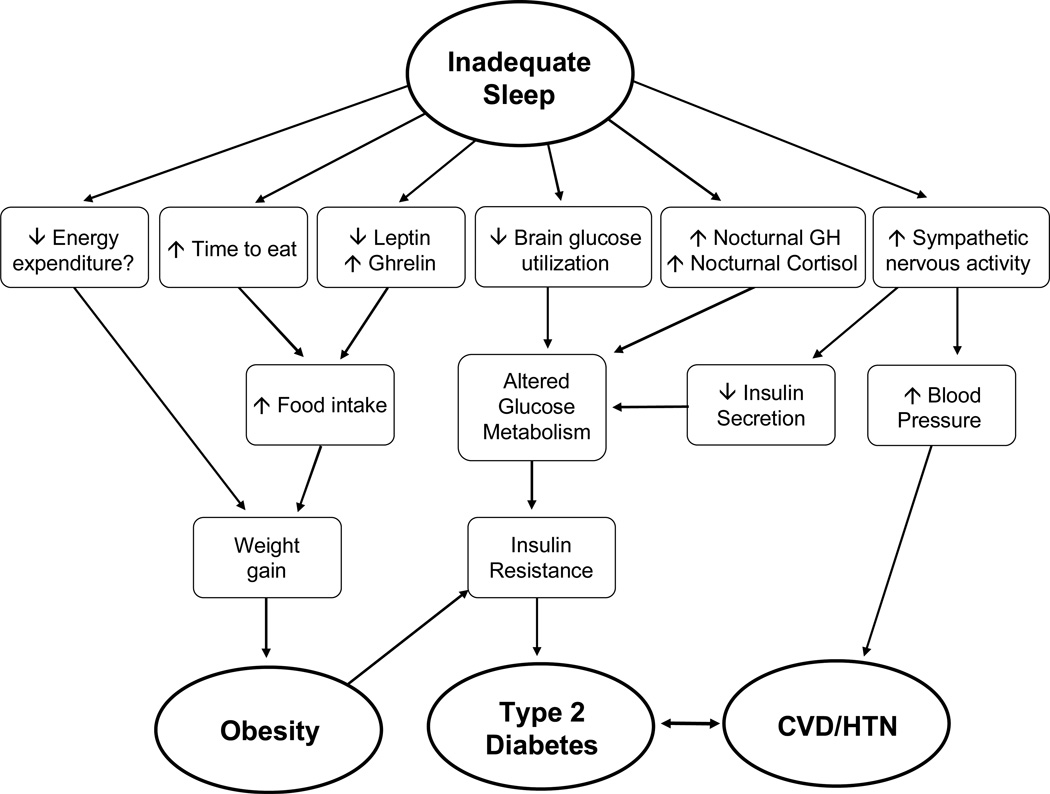
The mechanisms underlying the associations between inadequate sleep, obesity and associated cardiometabolic disease are still not fully understood. Nonetheless, laboratory studies have provided some suggestive evidence for mechanisms and Figure 1 outlines some of the potential pathways. Note that multiple pathways are likely involved in these associations and additional pathways may remain unidentified.
Figure 1.
Schematic representation of possible mechanistic pathways linking inadequate sleep to obesity, diabetes, cardiovascular disease (CVD) and hypertension (HTN) (modified from (Knutson 2010).
The left side of the figure presents three pathways that link inadequate sleep to risk of weight gain and ultimately obesity. Decreased energy expenditure could lead to weight gain, but very few studies have examined the association between sleep and energy expenditure and only one observed significantly reduced physical activity after sleep loss. The second pathway simply acknowledges that people awake for more of the day have more opportunity to eat. The third pathway involves alterations in levels of appetite-regulating hormones, such as leptin and ghrelin, which lead to increased appetite and subsequently increased food intake. If the increased food intake was not compensated for by increased physical activity, weight gain would result.
The final three pathways link inadequate sleep to the cardiometabolic diseases, which are also associated with obesity. Inadequate sleep may lead to decreased brain glucose utilization, which would promote reduced glucose tolerance. Studies of total sleep deprivation that used positron emission tomography (PET) did in fact observe decreased brain glucose utilization after sleep deprivation (Thomas et al 2000). The second pathway involves alterations in two hormones related to glucose metabolism, specifically growth hormone (GH) and cortisol. A laboratory study of sleep restriction observed both an extended duration of elevated nighttime GH concentrations (Spiegel et al 2000) and an increase in evening cortisol levels (Spiegel et al 1999). Increased levels of GH can induce transient insulin resistance in muscle cells, resulting in decreased glucose uptake, elevated blood glucose levels and subsequent increases in insulin resistance in other tissues. Elevated evening cortisol concentrations can lead to reduced insulin sensitivity the following morning, an alteration that can further impair glucose tolerance following sleep restriction (Van Cauter et al 1997). However, not all experimental studies of sleep restriction have observed changes in cortisol and/or GH levels (Donga et al 2010; Nedeltcheva et al 2009a), thus further research is required to test whether this is a valid pathway linking inadequate sleep to diabetes risk. The final pathway indicates that inadequate sleep is associated with increased sympathetic nervous activity, which has been observed in laboratory studies of sleep restriction (Spiegel et al 2004a; Spiegel et al 1999). Increased sympathetic nervous activity at the level of the pancreas could result in a reduction of insulin secretion from pancreatic beta-cells, but this has not yet been directly assessed in any sleep restriction study. Deleterious alterations in glucose metabolism, can lead to the development of insulin resistance, a risk factor for the development of type 2 diabetes. Finally, increased sympathetic nervous activity could lead to increased blood pressure and predispose individuals to the development of hypertension and cardiovascular disease if these alterations remained chronic.
As described above, many observational studies reported a significant association between long self-reported sleep duration and obesity, diabetes or hypertension. However, to date, no biological mechanisms have been identified to explain this association. Thus far, the studies that observed a U-shaped association included self-reported sleep duration. Therefore, it is not clear if long sleepers are actually obtaining more physiologic sleep or if they are just spending more time in bed. One study that recruited individuals who self-reported sleep duration was ≥9 hours per night supports the latter explanation because they found that these “long sleepers” only obtained an average of 7–7.5 hours of sleep per night based on actigraphy (Zielinski et al 2008). Potential confounders of the association between long self-reported sleep and morbidity or mortality risk, include sleep disorders (insomnia or obstructive sleep apnea), depression and lower socioeconomic status (Patel et al 2006a). Furthermore, the possible link between increased time in bed and decreased physical activity or increased isolation, particularly among elderly individuals, needs to be examined. Given the large number of studies that have found an association between long sleep duration and morbidity or mortality risk, it is important that more research into possible mechanisms or explanations be conducted.
IMPACT OF BIOLOGY, CULTURE AND ENVIRONMENT ON SLEEP
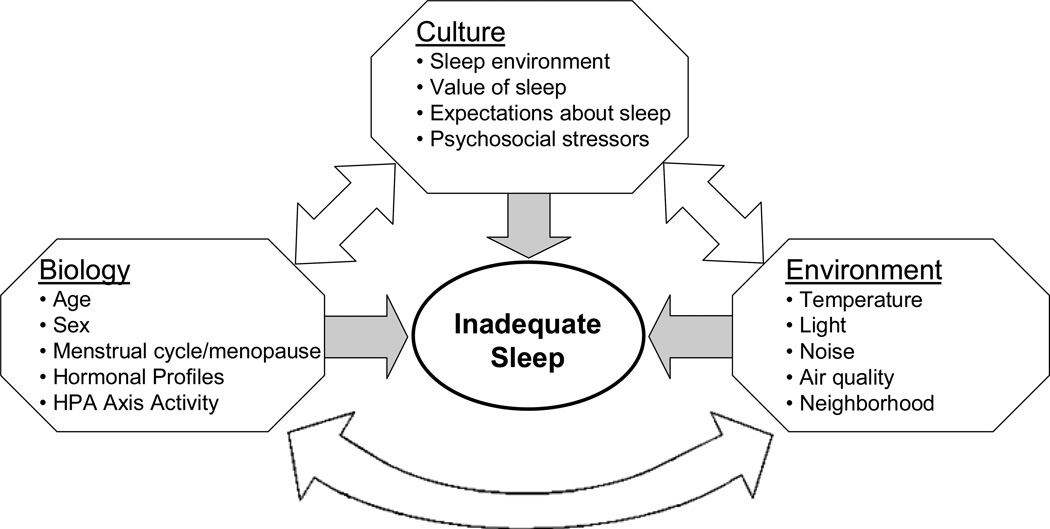
As reviewed above, there is substantial evidence suggesting that inadequate sleep may increase the risk of obesity and, importantly, the cardiometabolic diseases associated with obesity, including diabetes and hypertension. As such, a critical next step is to understand better who is at risk of inadequate sleep, thereby increasing their vulnerability to obesity and associated diseases. One approach is to consider the biological, cultural and environmental factors that may have an impact on sleep duration or quality. Figure 2 posits a few characteristics within each of these domains that may be important. Identification of these characteristics, and their interaction with each other, can provide important insight into the risk of inadequate sleep.
Figure 2.
Factors associated with biology, culture and environment that can impact and interact with sleep to increase vulnerability to obesity and its health consequences.
Several biological factors have been associated with sleep, particularly age and gender. For example, many studies in the United States have reported a decline in sleep duration and quality with advancing age (Hornung et al 2005; Unruh et al 2008; Van Cauter et al 2000). A meta-analysis of 65 studies with over 3,500 subjects aged 5 to 102 years found that total sleep time, and several measures of sleep quality significantly decreased with age (Ohayon et al 2004). However, some have argued that the decline in sleep with aging is not inevitable, but is only observed among those with declining health (Vitiello 2009; Vitiello et al 2002). More work is necessary to determine which aspects of sleep decline with increasing age independently of co-morbidities, as well as whether sleep disturbances can exacerbate existing health problems in older adults. In addition, age-related changes in health measures, including hearing loss and blood pressure, are not necessarily universal (Dickson 1968; Stevenson 1999), and therefore sleep may not demonstrate the same associations with age outside the Western world.
Gender differences in sleep duration and quality have also been reported. Based on estimates from wrist actigraphy, habitual sleep duration is longer and sleep quality is higher on average in women than in men (Jean-Louis et al 2000; Lauderdale et al 2006). Contrary to the objective findings, however, subjective reports of sleep quality tend to be lower in women than men (van den Berg et al 2009) and prevalence of insomnia is higher in women (Ohayon 2002). This contradiction has not been fully explained and may be related to cultural factors associated with gender roles and expectations, including willingness to admit to sleep problems, or to increased anxiety and depression among women (Voderholzer et al 2003). Furthermore, among women, the menstrual cycle and menopause have been associated with changes in sleep quality. Subjective sleep complaints increase during the few days preceding and during menses, although sleep stages remain stable (Baker and Driver 2007), which raises the question of why many women subjectively feel they sleep worse during menses despite no change in objective measures. Possible explanations include interactions between specific hormonal changes, symptoms of the menstrual cycle, such as pain, and feelings of fatigue. Several studies have also reported an increase in sleep disturbances, particularly subjective complaints, during the menopausal transition (see Minarik 2009 for a review). The increased sleep disturbances during menopause may be due to vasomotor symptoms, including hot flashes, or it may simply be due to confounding by age or co-morbidities and not menopause per se (Minarik 2009). The potential gender differences in sleep duration or quality and their underlying causes, whether due to biological differences such as hormones or to sociocultural factors related to gender roles and expectations, require further examination to elucidate potential gender differences in disease risk.
Other biological factors that impact sleep include certain hormones. A common example is melatonin, which is secreted by the pineal gland only during darkness at the subjective night (i.e. when the brain thinks it is night) (Dubocovich 2007). Melatonin therefore serves as a signal to the brain that it is nighttime and promotes appropriate nocturnal activities, which in humans is sleep. As such, many have proposed that the secretion of melatonin may help to promote sleep onset (Dubocovich 2007; Shochat et al 1998). A lack of melatonin, such as experienced with jet lag, could therefore impair one’s ability to fall asleep. Hormones involved in the hypothalamo-pituitary- adrenocortical (HPA) axis are also implicated in sleep regulation. In particular, cortisol levels exhibit a complex relationship between sleep stage transitions across the night (Steiger 2007). In pathological conditions associated with high cortisol levels, such as Cushing’s disease, sleep disturbances have been reported (Steiger 2007), suggesting that excessive cortisol can impair sleep quality. There are several other hormones that appear to affect sleep architecture, but a comprehensive list is beyond the scope of this article (for a review see Van Cauter 1995 or Steiger 2007). The endocrinology of sleep is a complex phenomenon because some hormones can impact sleep quality, while other hormones are affected by sleep quality. In some cases, such as with cortisol, the association is bidirectional indicating a possible reinforcing cycle between impaired sleep and altered hormonal profiles.
Environmental factors that can impair sleep include extended periods of light, such as those experienced in northern regions during the summer months, or elevated light levels in one’s bedroom due to street lamps, internal lighting or daytime sleep. One mechanism through which exposure to light at night can impair sleep is inhibition of melatonin, as described above (Shochat et al 1998). In addition, sleep could be disturbed when the ambient temperature is too hot, too humid or too cold (Krauchi et al 2008; Okamoto-Mizuno et al 1999). Two common characteristics of large urban areas that can impair sleep include noise, which can cause arousals, and poor air quality, which could lead to breathing difficulties. Finally, aspects that can vary by neighborhood, such as crime levels, could impair sleep if it leads to increased anxiety and stress levels. Currently, insufficient research has been conducted to determine the characteristics of the neighborhood and environment that impair sleep, but such research is important particularly as it may partly explain disparities in sleep between different populations.
Cultural factors that influence sleep patterns have not yet been extensively examined and this is an important area for future human biology research. Worthman and Melby (Worthman and Melby 2002) proposed incorporating an ecological approach to examining sleep and through a review of the modest amount of available ethnographic data they convincingly argued that several factors that could impact sleep do indeed vary between cultures. In Westernized countries, the most typical ideal bedroom includes a comfortable mattress with soft pillows and bedding on which one or two individuals sleep, but this is not the norm in many other societies. Worthman and Melby (2002) provided many examples of determinants of sleep that varied between societies, including bedding type and sleeping location, number of individuals sharing a sleeping space, proximity of animals (pets, livestock or wild), presence and proximity of fire throughout the night, and nighttime rituals among others. Furthermore, developmental aspects of how, when, and where people of varying ages sleep may have important implications for sleep across the lifespan. Sleep practices common in modern, industrialized, Western populations are also probably recent developments in terms of human history, and therefore any consideration of the evolution and adaptive function of sleep should acknowledge this. Another cultural factor that could impact how and when someone sleeps includes the inherent value ascribed to sleep for health, well-being or self-worth. For example, if sleep is considered to be a lazy unproductive practice, societies that place a premium on increased work hours and economic productivity may devalue sleep. Also, sociocultural discourse about what constitutes “normal” sleep may lead to unrealistic expectations, including the idea that a healthy sleeper is unconscious for 8 continuous hours. The discrepancy between the expectation and the experience of sleep could cause anxiety that could impair sleep and increase subjective sleep complaints. Finally, sociocultural factors that increase psychosocial stress, such as discrimination or work demands, could lead to impaired sleep due to activation of the HPA axis. Several studies have reported that inadequate sleep is more common among those with lower socioeconomic status (Friedman et al 2005; Gellis et al 2005; Geroldi et al 1996; Grandner et al 2010b; Hall et al 1999; Hall et al 2009; Hunt et al 1985; Nomura et al 2010; Patel et al 2010; Stamatakis et al 2007; Stranges et al 2008b), as well as among racial minorities, particularly African Americans (Hale and Do 2007; Hall et al 2009; Lauderdale et al 2006; Mezick et al 2008; Patel et al 2010; Profant et al 2002; Stamatakis et al 2007; Stepnowsky et al 2003). The underlying reasons for these sleep disparities could involve increased psychosocial stress and decreased opportunity for sleep. There are almost certainly additional salient social or cultural factors that influence sleep and they need to be identified.
These three domains of influence do not operate independently. In fact, it is at the intersection of these factors that the most substantial effects on sleep may occur. As in the example above where the cultural expectations about normal sleep and the actual experience of sleep conflict, ensuing anxiety would interact with biological factors, particularly activation of the stress response and the HPA axis, to impair sleep. Sociocultural gender roles may interact with the endocrinology of the reproductive cycle and age to influence subjective estimates of sleep quality. Food availability, which is partly determined by the environment, and food preference, which is influenced by culture, could also mediate the association between sleep and obesity risk because an increase in appetite may not necessarily lead to increased intake of obesity-promoting foods such as those laden with refined sugars. In addition, several potential effect modifiers of determinants of sleep have been discussed, including differences by age, gender and health status. The influences and interactions of biology, culture and environment may hold a wealth of novel information about risk factors for insufficient or impaired sleep, and consequently for obesity, but this area is currently understudied. This deficiency in research needs to be rectified, in part because it will help us to understand human variation in sleep as well as variation in associated conditions, including obesity, diabetes and cardiovascular diseases.
SUMMARY
The accumulated evidence from experimental and observational studies suggests that inadequate sleep may play a role in the risk of obesity as well as vulnerability to associated cardiometabolic diseases, such as diabetes and cardiovascular disease. Potential pathways through which inadequate sleep could lead to the development of these conditions include impairments in appetite regulation, glucose metabolism and sympatho-vagal balance. However, more research is required to better understand how sleep impacts cardiometabolic risk and, importantly, whether strategies to improve sleep will alleviate risk. In addition, the social, cultural, biological and environmental determinants of inadequate sleep need to be identified as they inform our understanding and identification of at-risk groups. Obesity, diabetes and cardiovascular disease have enormous negative impacts on quality of life, life expectancy and financial burden and a better understanding of the factors that increase vulnerability to these conditions could help improve the lives of millions of people around the world.
Acknowledgments
Financial Support: This work was partly supported by a grant (1 P30 HL101859-01) from the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH)
References Cited
- Akil L, Ahmad HA. Effects of socioeconomic factors on obesity rates in four southern states and Colorado. Ethnicity and Disease. 2011;21(1):58–62. [PMC free article] [PubMed] [Google Scholar]
- Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. American Journal of Respiratory and Critical Care Medicine. 2010;181(5):507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund R, Aberg H. Sleep complaints in women of ages 40–64 years in relation to sleep in their parents. Sleep Med. 2001;2(3):233–237. doi: 10.1016/s1389-9457(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003a;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003b;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Bayer O, Neuhauser H, von Kries R. Sleep duration and blood pressure in children: a cross-sectional study. Journal of Hypertension. 2009;27(9):1789–1793. doi: 10.1097/HJH.0b013e32832e49ef. [DOI] [PubMed] [Google Scholar]
- Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19(5):351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bjorkelund C, Bondyr-Carlsson D, Lapidus L, Lissner L, Mansson J, Skoog I, Bengtsson C. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005;28(11):2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1(5):266–273. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. American Journal of Clinical Nutrition. 2010;91(6):1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009 doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med. 2009;10(8):919–924. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15(1):253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31(4):517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16(2):265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33(2):177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Chance C, Polonsky KS, Schoeller D. Twenty-four hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85:2685–2691. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32(7):1091–1097. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- Danielsen YS, Pallesen S, Stormark KM, Nordhus IH, Bjorvatn B. The relationship between school day sleep duration and body mass index in Norwegian children (aged 10–12) Int J Pediatr Obes. 2010 doi: 10.3109/17477160903473739. [DOI] [PubMed] [Google Scholar]
- Devaux M, Sassi F. Social inequalities in obesity and overweight in 11 OECD countries. Eur J Public Health. 2011 doi: 10.1093/eurpub/ckr058. [DOI] [PubMed] [Google Scholar]
- Dickson R. The normal hearing of Bantu and Bushmen: A pilot study. Journal of Laryngology and Otology. 1968;82(6):505–522. doi: 10.1017/s0022215100069061. [DOI] [PubMed] [Google Scholar]
- Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. Journal of Clinical Endocrinology and Metabolism. 2010;95(6):2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8 Suppl 3:34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Ettaro L, Songer TJ, Zhang P, Engelgau MM. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. 2004;22(3):149–164. doi: 10.2165/00019053-200422030-00002. [DOI] [PubMed] [Google Scholar]
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. Jama. 2003;289(2):187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167(11):1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs R, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep Duration as a Risk Factor for Diabetes Incidence in a Large US Sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate Sleep as a Risk Factor for Obesity: Analyses of the NHANES I. Sleep. 2005;28(10):1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- Garcia G, Freeman R, Supiano M, Smith M, Galecki A, Halter J. Glucose metabolism in older adults: a study including subjects more than 80 years of age. J Am Geriatr Soc. 1997;45:813–817. doi: 10.1111/j.1532-5415.1997.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Gellis LA, Lichstein KL, Scarinci IC, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Socioeconomic status and insomnia. Journal of Abnormal Psychology. 2005;114(1):111–118. doi: 10.1037/0021-843X.114.1.111. [DOI] [PubMed] [Google Scholar]
- Geroldi C, Frisoni G, Rozzini R, De Leo D, Trabucchi M. Principal lifetime occupation and sleep quality in the elderly. Gerontology. 1996;42:163–169. doi: 10.1159/000213788. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010a;11(2):180–184. doi: 10.1016/j.sleep.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, Gooneratne N. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010b;11(5):470–478. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Powell NB, Martinez S, Kushida C, Raffray T, Palombini L, Philip P. Preliminary observations on the effects of sleep time in a sleep restriction paradigm.[see comment] Sleep Medicine. 2003;4(3):177–184. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33(3):289–295. doi: 10.1093/sleep/33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Bromberger J, Matthews K. Socioeconomic status as a correlate of sleep in African-American and Caucasian women. Annals of New York Academy of Sciences. 1999;896:427–430. doi: 10.1111/j.1749-6632.1999.tb08161.x. [DOI] [PubMed] [Google Scholar]
- Hall MH, Matthews KA, Kravitz HM, Gold EB, Buysse DJ, Bromberger JT, Owens JF, Sowers M. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health. 2007;7:129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop P, Smith G, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Medicine. 2002;3:305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Danker-Hopfe H, Heuser I. Age-related changes in sleep and memory: commonalities and interrelationships. Exp Gerontol. 2005;40(4):279–285. doi: 10.1016/j.exger.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Houyez F, Degoulet P, Cittee J, Fouriaud C, Jacquinet-Salord MC, Lang T, Aime F. Sommeil et hypertension artérielle. Arch Mal Coeur Vaiss. 1990;83(8):1085–1088. [PubMed] [Google Scholar]
- Hunt S, McEwen J, McKenna S. Social inequalities and perceived health. Effective Health Care. 1985;2(4):151–159. [PubMed] [Google Scholar]
- Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biological Psychiatry. 2000;47(10):921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30(2):219–223. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35(5):1173–1175. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- Kawabe H, Saito I. Does short sleep duration in daily life affect morning home blood pressure? Evaluation in Japanese people. Clinical and Experimental Hypertension. 2008;30(3):183–190. doi: 10.1080/10641960802064575. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27(1):282–283. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Archives of Internal Medicine. 2009;169(11):1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K, Saiga K, Sakane N, Mu H, Kurozawa Y. Sleep status and blood pressure in a healthy normotensive female population. Int J Cardiol. 2007;125(3):425–427. doi: 10.1016/j.ijcard.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Krauchi K, Gasio PF, Vollenweider S, Von Arb M, Dubler B, Orgul S, Flammer J, Stutz EZ. Cold extremities and difficulties initiating sleep: evidence of co-morbidity from a random sample of a Swiss urban population. Journal of Sleep Research. 2008;17(4):420–426. doi: 10.1111/j.1365-2869.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- Landis AM, Parker KP, Dunbar SB. Sleep, hunger, satiety, food cravings, and caloric intake in adolescents. J Nurs Scholarsh. 2009;41(2):115–123. doi: 10.1111/j.1547-5069.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. American Journal of Epidemiology. 2009;170(7):805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. American Journal of Epidemiology. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- Lima-Costa MF, Peixoto SV, Rocha FL. Usual sleep duration is not associated with hypertension in Brazilian elderly: The Bambui Health Aging Study (BHAS) Sleep Med. 2008;9(7):806–807. doi: 10.1016/j.sleep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Littman AJ, Vitiello MV, Foster-Schubert K, Ulrich CM, Tworoger SS, Potter JD, Weigle DS, McTiernan A. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2006;31(3):466–475. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87(2):310–316. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9(5):503–505. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- Magee CA, Iverson DC, Huang XF, Caputi P. A link between chronic sleep restriction and obesity: methodological considerations. Public Health. 2008;122(12):1373–1381. doi: 10.1016/j.puhe.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251(3):207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28(11):2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12(4):289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Heier M, Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48(2):235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Strollo PJ, Jr., Buysse DJ, Kamarck TW, Owens JF, Reis SE. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70(4):410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minarik PA. Sleep disturbance in midlife women. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2009;38(3):333–343. doi: 10.1111/j.1552-6909.2009.01031.x. [DOI] [PubMed] [Google Scholar]
- Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24(5):687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. 2010 Sleep in America Poll. Washington, DC: 2010. [Google Scholar]
- Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. Journal of Clinical Endocrinology and Metabolism. 2009a;94(9):3242–3250. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009b;89(1):126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27(10):2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- Nomura K, Yamaoka K, Nakao M, Yano E. Social determinants of self-reported sleep problems in South Korea and Taiwan. Journal of Psychosomatic Research. 2010;69(5):435–440. doi: 10.1016/j.jpsychores.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Okamoto-Mizuno K, Mizuno K, Michie S, Maeda A, Iizuka S. Effects of humid heat exposure on human sleep stages and body temperature. Sleep. 1999;22(6):767–773. [PubMed] [Google Scholar]
- Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiology and Behavior. 2010;99(5):651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Progress in Cardiovascular Diseases. 2009;51(5):434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24(5):703–715. doi: 10.1016/j.beem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NP, Grandner MA, Xie D, Branas CC, Gooneratne N. "Sleep disparity" in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010;10:475. doi: 10.1186/1471-2458-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006a doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between Reduced Sleep and Weight Gain in Women. Am J Epidemiol. 2006b;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D, Jr., Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54(5):1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- Prigeon RL, Kahn SE, Porte D., Jr. Changes in insulin sensitivity, glucose effectiveness, and B-Cell function in regularly exercising subjects. Metabolism. 1995;44:1259–1263. doi: 10.1016/0026-0495(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? American Journal of Human Biology. 2002;14(3):321–326. doi: 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- Reilly J, Armstrong J, Dorosty A, Emmett P, Ness A, Rogers I, Steer C, Sherriff A. Early life risk factors for obesity in childhood: cohort study. British Medical Journal. 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17(3):331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. American Journal of Clinical Nutrition. 2009;90(6):1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42(6):1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307(5708):375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Shochat T, Haimov I, Lavie P. Melatonin - the key to the gate of sleep. Annals of Medicine. 1998;30:109–114. doi: 10.3109/07853899808999392. [DOI] [PubMed] [Google Scholar]
- Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78(1):309–323. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66(4 Suppl):1044S–1050S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Colecchia EF, L'Hermite-Baleriaux M, Nie Z, Copinschi G, Van Cauter E. Adaptation of the 24-h growth hormone profile to a state of sleep debt. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2000;279(3):R874–R883. doi: 10.1152/ajpregu.2000.279.3.R874. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev P, Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology and Metabolism. 2004a;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels and increased hunger and appetite. Annals of Internal Medicine. 2004b;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, Roychoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. American Journal of Clinical Nutrition. 2011;94(2):410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Annals of Epidemiology. 2007;17(12):948–955. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A, Moebus S, Mohlenkamp S, Erbel R, Jockel KH. Gender-specific associations of short sleep duration with prevalent hypertension. Hypertension. 2008;51(3):e15–e16. doi: 10.1161/HYPERTENSIONAHA.107.108456. author reply e17. [DOI] [PubMed] [Google Scholar]
- Steiger A. Neurochemical regulation of sleep. Journal of Psychiatric Research. 2007;41(7):537–552. doi: 10.1016/j.jpsychires.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Stepnowsky CJ, Jr., Moore PJ, Dimsdale JE. Effect of ethnicity on sleep: complexities for epidemiologic research. Sleep. 2003;26(3):329–332. doi: 10.1093/sleep/26.3.329. [DOI] [PubMed] [Google Scholar]
- Stevenson D. Blood Pressure and Age in Cross-Cultural Perspective. Human Biology. 1999;71(4):529–551. [PubMed] [Google Scholar]
- Stranges S, Cappuccio FP, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008a;167(3):321–329. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Shipley MJ, Kandala NB, Trevisan M, Miller MA, Donahue RP, Hovey KM, Ferrie JE, Marmot MG, et al. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. Am J Epidemiol. 2008b;168(12):1353–1364. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short Sleep Duration Is Associated With Reduced Leptin, Elevated Ghrelin, And Increased Body Mass Index (BMI) Sleep. 2004;27:A146–A147. doi: 10.1371/journal.pmed.0010062. (Abstract Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162(4):305–311. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27(6):1318–1324. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, Passera P, Tibaldi P, Tomelini M, Cavallo F, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45(4):225–229. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- Unruh ML, Redline S, An MW, Buysse DJ, Nieto FJ, Yeh JL, Newman AB. Subjective and objective sleep quality and aging in the sleep heart health study. Journal of the American Geriatrics Society. 2008;56(7):1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- Van Cauter E. Hormones and sleep. In: Kales A, editor. The Pharmacology of Sleep. Berlin: Springer Verlag; 1995. pp. 279–306. [Google Scholar]
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine Reviews. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32(10):1367–1775. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg JF, Tulen JH, Neven AK, Hofman A, Miedema HM, Witteman JC, Tiemeier H. Sleep duration and hypertension are not associated in the elderly. Hypertension. 2007;50(3):585–589. doi: 10.1161/HYPERTENSIONAHA.107.092585. [DOI] [PubMed] [Google Scholar]
- van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV. Recent Advances in Understanding Sleep and Sleep Disturbances in Older Adults: Growing Older Does Not Mean Sleeping Poorly. Current Directions in Psychological Science. 2009;18(6):316–320. [Google Scholar]
- Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. Journal of Psychosomatic Research. 2002;53(1):555–559. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- Voderholzer U, Al-Shajlawi A, Weske G, Feige B, Riemann D. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depression and Anxiety. 2003;17(3):162–172. doi: 10.1002/da.10101. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33(2):161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC, Siervo M. Obesity and energy balance: is the tail wagging the dog? European Journal of Clinical Nutrition. 2011 doi: 10.1038/ejcn.2011.132. [DOI] [PubMed] [Google Scholar]
- Westerlund L, Ray C, Roos E. Associations between sleeping habits and food consumption patterns among 10–11-year-old children in Finland. British Journal of Nutrition. 2009;102(10):1531–1537. doi: 10.1017/S0007114509990730. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30(5):1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6(2):97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Worthman C, Melby M. Toward a Comparative Developmental Ecology of Human Sleep. In: Carskadon M, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. New York: Cambridge University Press; 2002. pp. 69–117. [Google Scholar]
- Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33(1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Kline CE, Kripke DF, Bogan RK, Youngstedt SD. No effect of 8-week time in bed restriction on glucose tolerance in older long sleepers. J Sleep Res. 2008;17(4):412–419. doi: 10.1111/j.1365-2869.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]