Abstract
Existing evidence suggests that brain-derived neurotrophic factor (BDNF) promotes survival and proliferation of endothelial cells, stimulates mobilization of hematopoietic progenitors, and induces angiogenesis in ischemic tissues. However, the mechanisms underlying vascular protective function of BDNF are poorly understood. We hypothesized that BDNF increases antioxidant capacity of circulating angiogenic cells. Human mononuclear cells were isolated from peripheral blood of 30 healthy male volunteers (48 ± 2 years old), and cultured in endothelial growth medium-2 for 4–5 days. The attached cells (so called early endothelial progenitor cells [early EPCs], or circulating angiogenic cells) expressed BDNF receptors, tropomyosin-related kinase B and p75 neurotrophin receptor. Treatment of early EPCs with recombinant human BDNF for 24 h significantly increased manganese superoxide dismutase (MnSOD) expression, but had no effect on expression of other antioxidant enzymes including copper zinc SOD (CuZnSOD), catalase, and glutathione peroxidase-1. BDNF stimulated phosphorylation of IκB kinase (IKK)α/β and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK); however it did not activate p38, Erk, or AKT. Treatment with nuclear factor κB inhibitor, PDTC, or JNK inhibitor, SP600125, attenuated BDNF-augmented MnSOD protein expression. BDNF treatment inhibited apoptosis induced by a superoxide anion generator LY83583, and serum starvation-induced cell detachment. These findings suggest that BDNF protect EPCs by increasing expression of MnSOD thereby enhancing their antioxidant capacity.
Keywords: endothelial progenitor cells, brain-derived neurotrophic factor, manganese superoxide dismutase
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, plays a critical role in supporting neuronal survival and function during development and in adulthood (1). Under pathological conditions such as ischemic stroke, BDNF also plays an important role in the neuronal protection and neurogenesis hence has major impact on stroke outcome (2–5). In previous studies, we have reported that so called “early endothelial progenitor cells (early EPCs)” produce and release BDNF (6). This is consistent with the concept that beneficial effect of cell therapy is mediated by paracrine effects via production and release of trophic factors to the injured tissues and brain (7). Although BDNF is a trophic factor for neurons, its receptors (high-affinity receptor tropomyosin-related kinase B [TrkB], and low-affinity receptor p75 neurotrophin receptor [p75NTR]) have been found on nonneuronal cells, such as endothelial cells, vascular smooth muscle cells, epithelial cells and blood mononuclear cells (8–11).
Recently, several pro-angiogenic effects of BDNF have been described. BDNF mobilizes hemotopoietic progenitor cells and promote revascularization in ischemic injury (8, 12). In addition, it stimulates vascular endothelial growth factor (VEGF) production and endothelial proliferation (13). It has also been reported that BDNF expression is increased in the brain after cerebral ischemia (2–5), and in the ischemic muscle after hind limb ischemia injury (8). The increased levels of BDNF in the injury sites may exert beneficial effects on the mobilized or transplanted EPCs. We (14) and others (15) have demonstrated that human EPCs have higher levels of antioxidant enzymes as compared to mature endothelial cells. This phenotypic characteristic is critical for their survival under the conditions of oxidative stress. In the present study, we hypothesized that BDNF might increase antioxidant capacity in human EPCs. We provide evidence that BDNF increases expression of manganese superoxide dismutase (MnSOD) in human EPCs and protects EPCs from oxidative stress.
Materials and Methods
EPCs isolation, cell culture and phenotyping
The protocol for collection and use of human blood samples was approved by the Institutional Review Board at the Mayo Clinic. EPCs were obtained as previously described (16). Briefly, human mononuclear cells (MNC) were isolated from blood samples (provided by the Component Laboratory, Division of Transfusion Medicine, Mayo Clinic) of 30 healthy male volunteers (48 ± 2 years old) by density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences Corp.). Mononuclear cells were plated at a density of 2×107 cells/well on 6-well plates coated with human fibronectin (R & D Systems Inc.) in endothelial growth medium-2 (EGM-2, Cambrex Corp.). The attached cells [early EPCs, also referred to as circulating angiogenic cells (17)] were collected for experiments after 4–5 days of culturing. For Fluorescence-activated cell sorting (FACS) analysis, collected cells were labeled with PE-mouse anti-human CD34 or CD14 (BD Pharmigen) on ice for 1 h. An isotype-matched mouse IgG was used as negative control. For indirect staining, cells were incubated with primary antibodies [mouse anti-human CD45 (BD Biosciences), vascular endothelial growth factor receptor-2 (VEGFR-2, Sigma), or mouse IgG (control, Sigma)] on ice for 1 h, and washed with 1% bovine albumin/PBS once. Cells were then incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG. Cells were washed with 1% bovine albumin/PBS, and subjected to FACS analysis.
Western Blot Analysis
Western blotting was performed as previously described (14,16). Rabbit polyclonal antibodies against MnSOD (14), copper zinc SOD (CuZnSOD) (14), glutathione peroxidase-1 (GPX1) (Lab Frontier), and TrkB (abcam) was used at dilutions of 1:1000, 1:500, 1:250, and 1:1000, respectively. Rabbit antibodies against IkB kinase α(IKKα), IKKβ, phospho-IKKα/β(Ser180/181), Erk1/2, phospho-Erk1/2(Thr202/Tyr204), AKT, phospho-AKT(Ser473), and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) were obtained from Cell Signaling and used at dilution of 1:500. Mouse monoclonal antibodies against catalase (Sigma-Aldrich Co.), p75NTR (Millipore), phospho-JNK(Thr183/Tyr185) (Cell Signaling), p38α (Cell Signaling), and phospho-p38(Thr180/Tyr182) (Cell Signaling) were used at dilution of 1:300. Peroxidase-conjugated antibodies against rabbit or mouse IgG (Amersham Biosciences) was used at a dilutions of 1:5000 or 1:1000, respectively. Blots probed with actin (1:1000, Santa Cruz Biotechnology, Inc.) were used as loading controls. The optical densities of the bands were measured by UN-SCAN-IT (Silk Scientific Corp.). Protein expression was normalized to actin and expressed as relative densitometric units.
RT-PCR
After EPCs were incubated with EBM2 for 15 h, cells were treated with BDNF (100 ng/ml; Alomone Labs) for 7.5 h. Total RNA was isolated by Rneasy Micro Kit (Qiagen), and reverse-transcripted into cDNA using SuperScript III First-Strand Synthesis System kit (Invitrogen). The primers for amplification of cDNA are: MnSOD sense, 5'TGCATCTGTTGGTGTCCAAGGCTCAG3', MnSOD antisense, 5'ATCAATCCCCAGCAGTGGAATAAG3' (expected size of the product 139bp). GAPDH sense, 5'GTGCCAAAAGGGTCATCATCTC3', GAPDH antisense, 5'GATGGCATGGACTGTGGTCATG3' (expected size of the product 200bp). PCR was performed for 27 cycles (94°C for 30 sec, 55°C for 30sec, and 72°C for 1 min). The primers used for detection of mRNA levels of p75NRT and TrkB are (18): p75NRT (sense: 5'CCTACGGCTACTACCAGGATG3'; antisense: 5'TGGCCTCGTCGGAATACG3'; expected size of the product 147bp), TrkB (sense: 5'CCCACTCACATGAACAATGG3'; antisense: 5'TCAGTGACGTCTGTGGAAGG3'; expected size of the product 221bp). Peripheral blood mononuclear cells were served as a positive control (18). PCR products were subjected to electrophoresis on 1.5% agarose gels. Ethidium bromide-stained PCR products were visualized on Universal Hood II / Quantity One (Bio-Rad Laboratories), and quantified using UN-SCAN-IT (Silk Scientific Corporation). The measured values for MnSOD were normalized to the expression levels of GAPDH.
dUTP nick-end labeling (TUNEL) assay
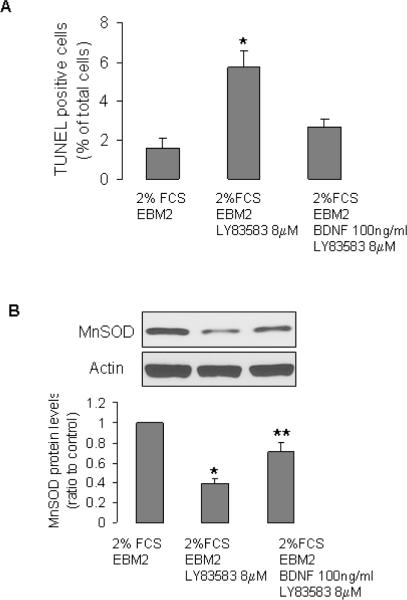
Experiments were performed as described in our previous study (14). MNC were seeded on 4-well chamber slides (7×106 /well), and cultured for 4 days. The unattached cells were removed. The remaining EPCs were treated with EBM2 containing 2% fetal calf serum (FCS) or EBM2 containing 2% FCS + BDNF (100ng/ml) for 24h. Cells were then incubated with EBM2 containing 2% FCS + LY83583 (an established generator of intracellular superoxide anion, 8 μM, Cayman Chemical) (14), EBM2 containing 2% FCS + BDNF (100ng/ml) + LY83583 (8 μM), or EBM2 containing 2% FCS (control) for 24 h. The cells were then subjected to TUNEL assay (Click-iT TUNEL Alexa fluor 488 imaging assay, Invitrogen). TUNEL positive (green) cells were detected (in 20 random fields/well), using a fluorescent microscopy. Data are presented as % of total cell number.
Statistical analysis
Data are presented as mean ± SE. Differences between mean values of multiple groups were analyzed using ANOVA followed by Tukey test (SigmaStat 3.1 for Windows). Comparison between two groups was made using unpaired Student's t-test. P<0.05 was considered statistically significant.
Results
BDNF increased expression of MnSOD
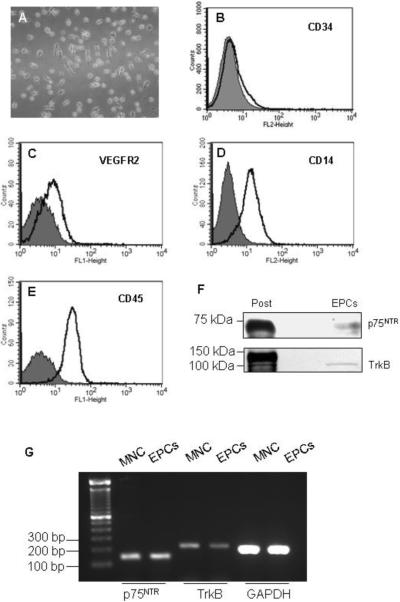
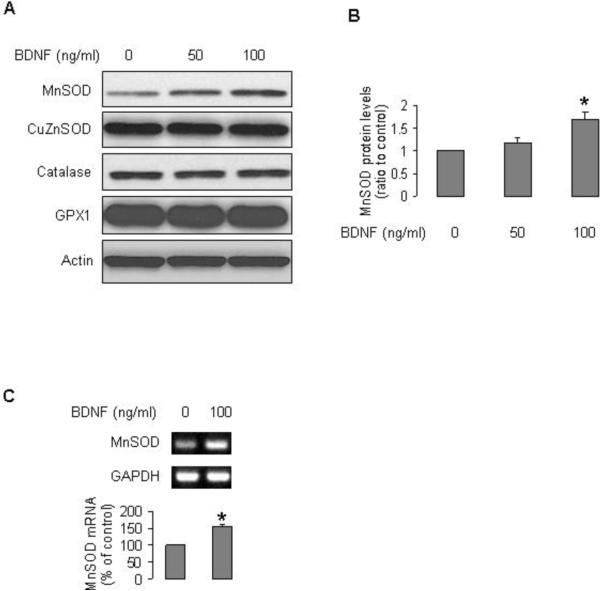
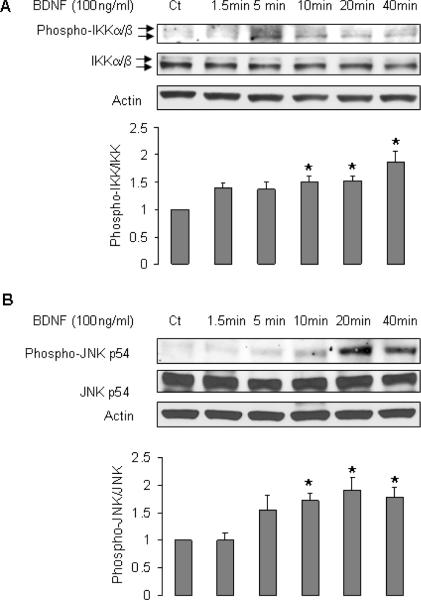
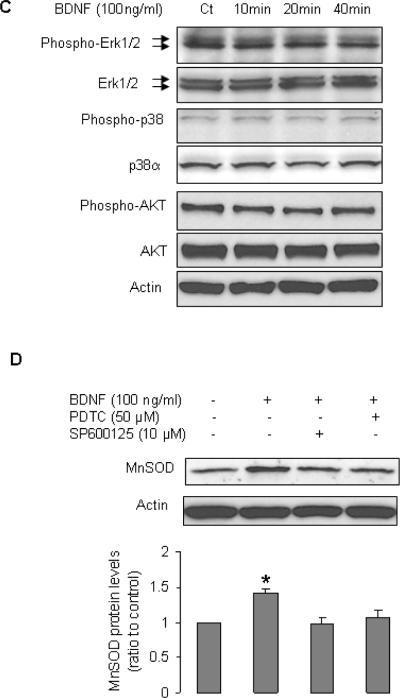
FACS analysis of EPCs indicated that the expression profile of cell surface antigens (endothelial marker VEGFR2, myelomonocytic cell marker CD14, hematopoietic cell marker CD45, and progenitor cell marker CD34 [Fig 1A–E]) was consistent with previous reports (16,17,19). EPCs expressed both receptors for BDNF, p75NTR and TrkB (Fig 1F,G). Treatment of EPCs with BDNF increased the protein and mRNA levels of MnSOD (Fig 2). However, BDNF did not affect expression of other antioxidant enzymes such as CuZnSOD, catalase, and GPX1 (Fig 2). We next investigated the cell signaling involved in the induction of MnSOD by BDNF. BDNF stimulated phosphorylation of IKKα/β, and JNK, but did not activate two other mitogen-activated protein kinases (MAPK) Erk1/2 and p38 (Fig 3A–C). BDNF did not increase phospho-AKT (Fig 3C). Inhibition of nuclear factor κB (NFκB) by pyrrolidine dithiocarbamate (PDTC) or JNK by SP600125 blocked the stimulatory effect of BDNF on expression of MnSOD (Fig 3D).
Fig 1.
Phenotyping of EPCs. Mononuclear cells were cultured on fibronectin-coated plates in EGM2 for 4 days. A: Phase contrast image of day 4 EPCs (× 20 magnification). B–E: FACS analysis of cell surface markers on EPCs. Shown are representative data from at least 3 independent experiments for each marker. The open black-lined histograms represent tested antibodies, and filled histograms represent the control IgG. F: Human EPCs expressed receptors that bind to BDNF, TrkB and p75NTR. Positive controls (Post) for p75NTR and TrkB are SK-N-MC cell lysate and mouse brain, respectively. G: mRNA expressions of TrkB and p75NTR in day 4 EPCs and circulating mononuclear cells (MNC).
Figure 2.
BDNF increased MnSOD expression in EPCs. A and B: Human EPCs were treated with BDNF (50 or 100 ng/ml) in EBM2 for 24 h. Protein samples were subjected to Western blotting. Blots are representative of 3 independent experiments. B: Optical density analysis of MnSOD protein levels; n=3, *P<0.05, compared with control. C: cells were cultured in EBM2 for 15 h, then incubated with BDNF for 7.5 h, mRNA levels were measured by RT-PCR; n=3, *P<0.05, compared to non-treatment.
Figure 3.
BDNF induced phosphorylation of IKKα/β, and JNK. A–C: EPCs were cultured in EBM-2 for 20 h, then were treated with BDNF for indicated periods. All blots are representative of at least 3 independent experiments. A: n=6–9, P<0.05 compared to control. B: n=7–11, P<0.05, compared to control. D: EPCs were pretreated with PDTC or SP600125 for 1 h, and then incubated with BDNF for 24 h; n=8, *P<0.05, compared to other 3 groups.
BDNF protected EPCs from oxidative stress
Next, we examined the protective effect of BDNF. Compared to control culture conditions, incubation of cells in EBM2 + 2%FCS + LY83583 for 24 h significantly increased number of apoptotic cells (Fig 4 A). BDNF attenuated the detrimental effect of oxidative stress (Fig 4A). Consistent with this observation, Western blotting demonstrated that LY83583 significantly reduced MnSOD protein level in EPCs (Fig 4B). BDNF treatment partially reversed the effect of LY83583 on MnSOD (Fig 4B). BDNF also decreased the serum starvation-caused cell detachment (Fig 4C).
Figure 4.
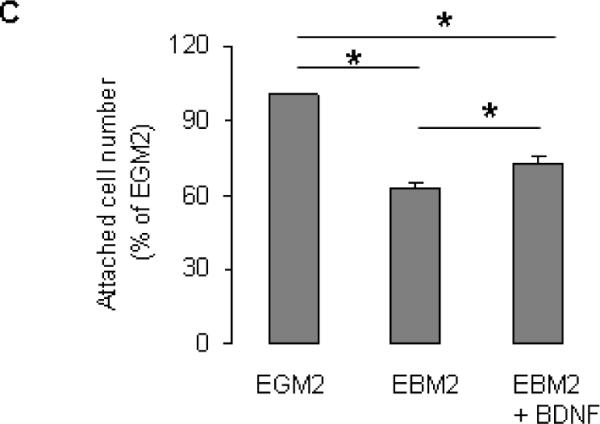
BDNF protected EPCs from oxidative stress. A and B: EPCs were incubated with indicated treatments, then were subjected to TUNEL assay (A, n=5–8, *P<0.05, compared to other 2 groups), or Western blotting (B, n=5, *P<0.05, compared to other two groups, **P<0.05, compared to control). C: MNC were seed in 6-well plates (20×106 cells/well, in duplicates) and cultured in EGM2 for 4 days. The non-adherent cells were washed away, the attached cells (EPCs) were treated with EGM2 (control), EBM2, or EBM2 +BDNF for 48 h (treatment was refreshed every 24 h). The attached cells were collected by trypsinization and cell numbers were counted using a hemocytometer. Data are presented as % to control (EGM2 alone); n=7, *P<0.05.
Discussion
In the present study, we report two novel findings: 1) BDNF increases MnSOD expression in human early EPCs; and 2) BDNF protects human EPCs from oxidative stress in vitro.
Like the other members of the neurotrophin family (including nerve growth factor, neurotrophin 3, and neurotrophin 4), BDNF is synthesized from a precursor that is proteolytically cleaved to yield the C-terminal mature protein (20). BDNF protein is widely distributed throughout the adult central nervous system, and is essential in regulation of synaptic plasticity, growth cone guidance, and long-term potentiation, thus influencing learning and memory (1,21). It has been shown that BDNF expressed by endothelial cells is neuroprotective in the neurovascular unit (22). Most importantly, BDNF also plays a key role in vascular development and in response to vascular injury (21). Indeed, BDNF is critical for survival of TrkB-expressing endothelial cells in intramyocardial vessels in the late gestational and early postnatal period (23). Moreover, BDNF has been shown to mobilize bone marrow-derived progenitor cells (8,12). However, the effects of BDNF on EPCs are largely unexplored. Our study is the first to demonstrate that treatment with BDNF increases MnSOD expression in human EPCs. Since BDNF levels are increased during ischemia in brain and limbs (2–5,8), it is conceivable that ischemic injury increases BDNF thereby enhancing resistance of mobilized or delivered EPCs to oxidative stress. This effect may improve ability of EPCs to perform regenerative program under conditions of high oxidative stress associated with ischemia/reperfusion. Interestingly, expression of other antioxidant enzymes did not change in response to BDNF treatment, thus indicating that BDNF selectively up-regulates MnSOD expression.
Both receptors for BDNF (TrkB and p75NTR) are expressed in vascular cells and mononuclear cells (8,9,11). We also detected the expression of BDNF receptors in EPCs. BDNF-induced TrkB activation stimulates pro-survival mediated by PI3K-AKT and MEK-Erk pathways (1). P75NTR (also known as CD271), a member of the tumor necrosis factor/FAS receptor family, is a low-affinity nerve growth factor receptor that has been found to be expressed on various stem cells/precursor cells, hepatic stellate cells, and cancer cells, in which p75NTR is involved in signaling response for cell proliferation and differentiation (24–30). In nervous system p75NTR can modulate the affinity and specificity of Trk receptors (31). However, it also mediates cell death when expressed in cells of the nervous system with impaired Trk signaling pathway (31). In endothelial cells and smooth muscle cells, p75NTR promotes apoptosis and regulates lesion development after vascular injury (32,33). Moreover, absence of p75NTR leads to partial perinatal lethality and defects in the vascular system including thin vessel walls, massive vessel dilations, ruptures, and blood cell leakage (34). The complexity of function of p75NTR is reflected in the ability of p75NTR to activate diverse signaling pathways. Indeed, P75NTR can activate three categories of intracellular signaling: 1) pro-apoptosis pathway including JNK pathway, 2) pro-survival pathways including NFκB, PI3K-AKT, and Ras/mitogen-activated protein kinase, and 3) pathways that modulate the cytoskeleton and cell regeneration including RhoA/Rho kinase (35). Our results demonstrated that BDNF activated NFκB and JNK pathways, but not other MAP kinases (p38 or Erk1/2) or AKT signaling. Inhibition of NFκB and JNK blocked BDNF-induced up-regulation of MnSOD. These results suggest that NFκB and JNK signaling contribute to the BDNF-induced increase in MnSOD. Whether the effect of BDNF is mediated by activation of p75NTR remains to be determined.
MnSOD is a nuclear-encoded antioxidant enzyme that localizes to the mitochondria, catalyzing conversion of superoxide anion into oxygen and hydrogen peroxide that is further degraded into water by peroxide scavenging enzymes (36). Previous studies have indicated that activity of MnSOD in EPCs is critical antioxidant responsive for the regenerative function in the conditions of oxidative stress (14,15). MnSOD gene can be positively regulated by NFκB, specificity protein1 and activating protein 1 (AP1), and negatively regulated by AP2 (37,38). Our study has demonstrated that inhibition of NFκB and JNK blocks the effect of BDNF on MnSOD expression, suggesting that BDNF may activate transcription factors NFκB and AP1 in EPCs.
Mounting evidence supports the concept that transplantation of EPCs/stem cells has beneficial effect in patients with stroke (7). EPCs/stem cells support survival of neuronal tissue and facilitate recovery of injured neurons (7). Therefore enhancement of regenerative function of EPC/stem cells in the injured site is essential for successful cell therapy. Our results suggest that treatment of angiogenic cells with BDNF improve their resistance to oxidative stress thus increasing their regenerative and therapeutic capacity
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute Grants HL-53524 and HL-91867 (ZSK); the American Heart Association (AHA) Scientist Development Grant 09SDG2190046 (TH); and the Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None.
Reference
- 1.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 2.Gao XQ, Yang CX, Chen GJ, Wang GY, Chen B, Tan SK, Liu J, Yuan QL. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J. Ethnopharmacol. 2010;132:393–399. doi: 10.1016/j.jep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Béjot Y, Prigent-Tessier A, Cachia C, Giroud M, Mossiat C, Bertrand N, Garnier P, Marie C. Time-dependent contribution of non neuronal cells to BDNF production after ischemic stroke in rats. Neurochem. Int. 2011;58:102–111. doi: 10.1016/j.neuint.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 5.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- 7.Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;41(Suppl 1):94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR, Crystal RG, Rafii S, Hempstead BL. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Invest. 2005;115:653–663. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemoto K, Fukamachi K, Nemoto F, Miyata S, Hamada M, Nakamura Y, Senba E, Ueyama T. Gene expression of neurotrophins and their receptors in cultured rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1998;245:284–288. doi: 10.1006/bbrc.1998.8418. [DOI] [PubMed] [Google Scholar]
- 10.Botchkarev VA, Metz M, Botchkareva NV, Welker P, Lommatzsch M, Renz H, Paus R. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 act as “epitheliotrophins” in murine skin. Lab. Invest. 1999;79:557–572. [PubMed] [Google Scholar]
- 11.Nassenstein C, Möhring UH, Luttmann W, Virchow J.C. Jr., Braun A. Differential expression of the neurotrophin receptors p75NTR, TrkA, TrkB and TrkC in human peripheral blood mononuclear cells. Exp. Toxicol. Pathol. 2006;57(Suppl 2):55–63. doi: 10.1016/j.etp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Li Y, Liu Y, Luo Y, Wang D, Annex BH, Goldschmidt-Clermont PJ. Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy. Am. J. Pathol. 2010;176:504–515. doi: 10.2353/ajpath.2010.081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST, Poon RT. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: implication in hepatocellular carcinoma. Clin. Cancer Res. 2011;17:3123–3133. doi: 10.1158/1078-0432.CCR-10-2802. [DOI] [PubMed] [Google Scholar]
- 14.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 15.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 16.He T, Joyner MJ, Katusic ZS. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc. Res. 2009;78:447–452. doi: 10.1016/j.mvr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassenstein C, Möhring UH, Luttmann W, Virchow J.C. Jr., Braun A. Differential expression of the neurotrophin receptors p75NTR, TrkA, TrkB and TrkC in human peripheral blood mononuclear cells. Exp Toxicol Pathol. 2006;57(Suppl 2):55–63. doi: 10.1016/j.etp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ. Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 20.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 21.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc. Med. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 24.Okumura T, Shimada Y, Imamura M, Yasumoto S. Neurotrophin receptor p75(NTR) characterizes human esophageal keratinocyte stem cells in vitro. Oncogene. 2003;22:4017–4026. doi: 10.1038/sj.onc.1206525. [DOI] [PubMed] [Google Scholar]
- 25.Moscatelli I, Pierantozzi E, Camaioni A, Siracusa G, Campagnolo L. p75 neurotrophin receptor is involved in proliferation of undifferentiated mouse embryonic stem cells. Exp. Cell Res. 2009;315:3220–3232. doi: 10.1016/j.yexcr.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, Miyachi E, Marunouchi T, Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J. Dermatol. Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- 29.Campagnolo L, Russo MA, Puglianiello A, Favale A, Siracusa G. Mesenchymal cell precursors of peritubular smooth muscle cells of the mouse testis can be identified by the presence of the p75 neurotrophin receptor. Biol. Reprod. 2001;64:464–472. doi: 10.1095/biolreprod64.2.464. [DOI] [PubMed] [Google Scholar]
- 30.Okumura T, Tsunoda S, Mori Y, Ito T, Kikuchi K, Wang TC, Yasumoto S, Shimada Y. The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin. Cancer Res. 2006;12:5096–5103. doi: 10.1158/1078-0432.CCR-05-2852. [DOI] [PubMed] [Google Scholar]
- 31.Hempstead BL. The many faces of p75NTR. Curr. Opin. Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 32.Caporali A, Pani E, Horrevoets AJ, Kraenkel N, Oikawa A, Sala-Newby GB, Meloni M, Cristofaro B, Graiani G, Leroyer AS, Boulanger CM, Spinetti G, Yoon SO, Madeddu P, Emanueli C. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ. Res. 2008;103:e15–e26. doi: 10.1161/CIRCRESAHA.108.177386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Bray P, McCaffrey T, March K, Hempstead BL, Kraemer R. p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells. Am. J. Pathol. 2000;157:1247–1258. doi: 10.1016/S0002-9440(10)64640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat. Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- 35.Geerts A. The simple truth is seldom true and never simple: dual role for p75(NTR) in transdifferentation and cell death of hepatic stellate cells. Hepatology. 2007;46:600–601. doi: 10.1002/hep.21861. [DOI] [PubMed] [Google Scholar]
- 36.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 37.St Clair DK, Porntadavity S, Xu Y, Kiningham K. Transcription regulation of human manganese superoxide dismutase gene. Methods Enzymol. 2002;349:306–312. doi: 10.1016/s0076-6879(02)49345-7. [DOI] [PubMed] [Google Scholar]
- 38.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, Simon FR. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem. J. 2004;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]