Abstract
Major histocompatibility complex (MHC) class I and class II are crucial for the function of the human adaptive immune system. An NLR protein, CIITA (MHC class II transactivator), is a master regulator of MHC class II gene expression as well as of some of the genes involved in MHC class II antigen presentation. It has recently been discovered that another member of the NLR protein family, NLRC5, transcriptionally activates MHC class I genes, and thus acts as “CITA” (MHC class I transactivator), a counterpart to CIITA. In addition to MHC class I genes, NLRC5 can induce the expression of β2M, TAP1 and LMP2, essential components of MHC class I antigen presentation. These findings indicate that NLRC5 and CIITA are transcriptional regulators that orchestrate the concerted expression of critical components in the MHC class I and MHC class II pathways, respectively.
Keywords: MHC class I, NLR proteins, CIITA
1. Introduction
Major histocompatibility complex (MHC) class I and class II play essential roles in antigen presentation to T lymphocytes. Antigen presentation by MHC class II is essential for the activation of CD4 T cells whereas MHC class I molecules are required for the activation of CD8 T cells [1]. Both MHC class I and class II molecules are highly inducible and their expression is crucial for the induction and maintenance of adaptive immune responses. It has now become clear that two NLR (nucleotide-binding domain, leucine-rich repeat) proteins play critical roles in the induction of MHC class I and class II gene expression. While it has been well established that CIITA is essential for MHC class II expression [2-4], it has recently been discovered that another NLR family member, NLRC5, is important for the expression of MHC class I genes [5]. We will attempt to discuss the function of NLRC5 as CITA or MHC class I transactivator, comparing its roles and molecular mechanisms of action with those of CIITA. Other possible functions of NLRC5 will also be addressed in later sections.
2. MHC class I antigen presentation pathway
MHC class I genes encode a family of proteins that forms the basis of the adaptive immune surveillance system. MHC class I molecules are composed of MHC-encoded heavy chains and the invariant subunit β2-microglobulin (β2M) [6]. Humans have three classical MHC class Ia molecules (HLA-A, HLA-B and HLA-C), as well as three non-classical MHC class Ib molecules (HLA-E, HLA-F and HLA-G), which have immune regulatory functions [7, 8]. MHC class Ia is important for initiating immune responses against intracellular pathogens such as viruses by presenting antigen-derived peptides to MHC-restricted CD8 T cells [9, 10]. Peptides are mostly produced from the degradation of cytoplasmic proteins by the proteasome; during infection, a specialized “immunoproteasome” containing several IFN-γ-inducible subunits, such as LMP2 and LMP7, is used to generate optimal MHC class I peptides [11]. Peptide loading onto MHC class I is carried out by the peptide loading complex (PLC), which includes the MHC class I heavy chain, β2M, tapasin, ERp57, calreticulin and TAP1/TAP2, a transporter that translocates peptides from the cytoplasm into the ER [11, 12]. In addition to detection and elimination of viruses, MHC class Ia molecules are essential for immune surveillance of cancerous and transplanted cells. MHC class Ia and Ib molecules can also suppress NK cell function as ligands for killer inhibitory receptors (KIRs) [13, 14]. Unlike MHC class II, which is found mainly in antigen-presenting cells, MHC class I genes are ubiquitously expressed in almost all nucleated cells [2, 6].
3. NLR family of proteins
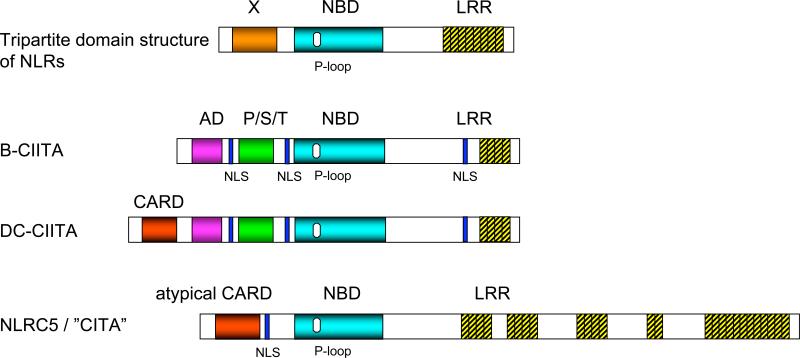
NLR proteins constitute a diverse protein family associated with the innate immune system, consisting of 22 members in humans and 34 in mice. NLR proteins are prevalent in a wide variety of cells, including immune and epithelial cells [15, 16]. Although there are exceptions, NLR proteins are mostly localized to the cytoplasm of the cells, and play an important role in the recognition of and host defense against pathogens [17]. NLR proteins are characterized by a tripartite domain structure [15, 16] (Fig. 1). They possess a centrally located NBD (nucleotide binding oligomerization domain), which is involved in oligomerization and activation of NLRs [18, 19]. At the C-terminus they contain leucine-rich repeats (LRRs), generally believed to act as sensors of danger signals or ligands of microbial origin. At the N-terminus they display a variable effector domain, which can consist of either a caspase recruitment domain (CARD), a pyrin domain (also known as PAAD, PYD or DAPIN) [20-26], an acidic domain, or a baculovirus inhibitor repeat (BIR) domain [17, 27]. The major function of the NLR proteins is to initiate innate immune responses upon recognition of components of infecting pathogens [28]. For example, the CARD-carrying NLR NOD2 can recognize muramyl dipeptide (MDP), the active peptidoglycan moiety in bacterial cell walls, and stimulate immune responses in antigen presenting cells and intestinal epithelial cells [29]. Another NLR, NLRP3, belongs to the pyrin domain containing subfamily of NLRs (NLRP subfamily). Upon activation, NLRP3 participates in a protein complex called the inflammasome to activate the cysteine protease caspase-1, which promotes the processing and secretion of the pro-inflammatory cytokine IL-1β [30]. A subgroup of NLR genes, such as NLRP5, is expressed in oocytes and is required for embryonic development [31].
Figure 1. NLRC5 has a typical tripartite domain structure characteristic of the NLR protein family.
The schematic structures of CIITA (B-CIITA), the DC-specific isoform of CIITA (DC-CIITA), and NLRC5 are shown. X: variable N-terminal effector domain. NBD: nucleotide binding domain. LRR: leucine-rich repeats. AD: acidic domain or activation domain. P/S/T domain: proline-serine-threonine-rich domain. NLS: nuclear localization signal. CARD: caspase recruitment domain.
4. CIITA, the master regulator of MHC class II genes
CIITA (MHC class II transactivator) was the first member of the NLR family of proteins and was discovered before other NLRs were identified as regulators of innate immunity [16, 32, 33]. The function of CIITA is distinct from other NLRs, and it was originally isolated as a molecule required for MHC class II gene expression. A group led by Bernard Mach cloned CIITA using a complementation approach by introducing a cDNA expression library into a bare lymphocyte syndrome patient cell line, which lacks the expression of MHC class II [32]. Although CIITA displays the tripartite molecular structure characteristic of the NLR family, CIITA has additional structural motifs [33](Fig. 1). CIITA contains an acidic domain (AD) and a PST (proline/serine/threonine rich) domain at the N-terminus, which serve as protein interaction domains (Fig. 1) and play an important role in the recruitment of components of the general transcription machinery [34]. The centrally located NBD is crucial for the function of CIITA; mutations of the NTP binding site, also called the Walker A motif, abolish the function of CIITA [35]. At the C-terminus, CIITA contains LRRs similar to other NLRs. The LRRs have been implicated in homodimerization and recruitment to a transcription factor complex at the MHC class II gene locus [34]. CIITA contains both nuclear localization signals (NLS) and nuclear exportation signals (NES) [36-38](Fig. 1). Indeed, unlike other NLR proteins that are mostly localized in the cytoplasm, or associated with mitochondria or the plasma membrane, CIITA can shuttle into the nucleus [36-38]. The most striking function of CIITA is its control of MHC class II gene expression, which is induced in B cells and dendritic cells as a function of developmental stage, as well as by IFN-γ in most cell types [39-41]. Both constitutive and inducible expression of MHC class II are regulated by CIITA, and consequently CIITA has been referred to as a “master regulator” of MHC class II [3]. CIITA is required for the expression of not only conventional MHC class II (HLA-DR, -DP and -DQ) molecules, but also of regulatory molecules involved in the class II antigen presentation pathway: the invariant chain, HLA-DM and HLA-DO, which are accessory proteins required for intracellular trafficking and peptide loading of MHC class II molecules [34].
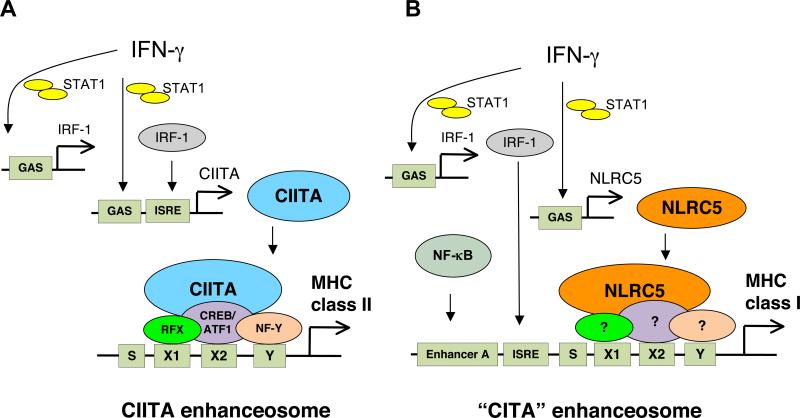
Interestingly, CIITA itself does not possess a DNA-binding domain, and thus regulates the transcription of MHC class II by associating with other transcription factors [42, 43]. Both MHC class I and class II genes are highly inducible by IFN-γ stimulation and contain similar cis-regulatory elements in their promoters, termed W/S, X1, X2 and Y-box motifs, suggesting that similar transcription factor complexes are recruited to both promoters [44, 45]. These transcription factors include the X-box binding trimeric RFX protein complex (composed of RFX5, RFXAP and RFXANK), the X2-box binding CREB/ATF1, and the Y-box binding NF-Y protein complex (composed of NF-YA, NF-YB and NF-YC)[3, 4, 46] (Fig. 2). The factor binding to the W/S motif has not yet been identified, but it has been suggested that RFX proteins may also associate with the W/S cis-regulatory element [4]. These transcription factors are constitutively expressed but they are not sufficient to activate the MHC class II promoter. CIITA interacts with these transcription factors bound to the corresponding cis-regulatory elements to form a macromolecular nucleoprotein complex termed the MHC enhanceosome [43]. In addition to associating with the transcriptional regulators that form the CIITA enhanceosome, CIITA can also participate in chromatin remodeling and recruit general transcription factors to initiate the transcription of MHC class II genes [3, 34].
Figure 2. Model of transcriptional regulation of MHC class I and class II genes by the “CITA” and the CIITA enhanceosome, respectively.
A) MHC class II gene regulation by CIITA: IFN-γ stimulation activates STAT1, which induces the expression of CIITA, directly and/or indirectly via the transcription factor IRF-1. CIITA associates with the DNA binding protein complexes RFX, CREB/ATF1 and NF-Y to form the CIITA enhanceosome complex on the conserved WXY motif in the MHC class II gene promoters to transactivate MHC class II genes.
B) MHC class I gene regulation by CITA or NLRC5: IFN-γ stimulation induces NLRC5 expression via direct binding of activated STAT1 homodimers to the GAS sites in the NLRC5 promoter. Subsequently, NLRC5 may generate a “CITA” enhanceosome on the MHC class I promoter together with transcription factors bound to the WXY motif. The promoters of most MHC class I genes also contain binding sites for NF-κB (Enhancer A) and IRF-1 (ISRE), which may further modulate NLRC5-mediated MHC class I gene expression.
CIITA appears to also have a role in the transactivation of MHC class I genes, at least in vitro [2, 44-47]. The expression of CIITA, however, is generally restricted to lymphocytes and professional antigen-presenting cells, and is thus unlikely to account for the ubiquitous expression of MHC class I genes [2, 3]. Furthermore, a subgroup of BLS patients who carry mutations in the CIITA gene display reduced MHC class II expression but retain normal expression levels of MHC class I [32, 48]. Similarly, in mice deficient for CIITA, both constitutive and IFN-γ-induced expression of MHC class I molecules is intact [49-51]. Therefore, other factors or mechanisms that are involved in the regulation of MHC class I gene expression have long been sought after.
5. NLRC5, a new member of the NLR gene family
NLRC5 (NLR family, CARD domain containing 5 or NOD27/CLR16.1), a recently characterized member of the NLR gene family, is located at the 16q13 locus in the human genome, spanning a region of approximately 96 kbp [52]. Similar to other NLR proteins, NLRC5 has a characteristic tripartite domain structure; it consists of an N-terminal CARD, a centrally located NBD and C-terminal LRRs [5, 52-54]. Like other CARD-containing NLRs, the CARD of NLRC5 consists of repeated alpha helices as suggested by structure predicting algorithms, but is structurally distinct, and may thus be referred to as an atypical CARD [55]. NLRC5 consists of 1866 amino acids with a predicted size of 204 kDa, mostly due to its unusually long stretch of C-terminal leucine rich repeats; NLRC5 has 27 leucine rich repeats that are encoded by 43 exons [54] (Fig. 1). As a result, NLRC5 is the largest NLR protein; other NLR proteins are typically 80-120 kDa in size. Phylogenetically, NLRC5 is most closely related to CIITA, which also contains a CARD in the DC isoform and thus can also be considered as an NLRC subfamily protein [5, 54, 56].
Although it has been reported that NLRC5 is expressed widely in various tissues in both humans and mice, NLRC5 is predominantly expressed in hematopoietic cells [52, 54]. Neerincx et al. showed that NLRC5 is highly expressed in the spleen, lymph nodes, and bone marrow, and particularly in lymphocytes (CD4+, CD19+, CD8+ cells), as well as in other leukocytes, CD14+ cells and mononuclear cells [53]. There are several isoforms of NLRC5 that display shorter LRRs [53], but the function of these NLRC5 isoforms are currently unknown. A striking feature of the NLRC5 gene is its high inducibility by IFN-γ stimulation [5, 52-54]. It has also been reported that NLRC5 is induced modestly by LPS, poly(IC), IFN-β and/or virus infection [53, 54]. The promoter of NLRC5 contains two predicted STAT1 binding sites (GAS or IFN-γ activated sequence), including one that overlaps with a predicted NF-κB binding site [52], suggesting that STAT1 homodimers induced by IFN-γ stimulation via JAK1/JAK2 kinases directly bind to the NLRC5 promoter to activate the transcription of NLRC5. Indeed, a JAK inhibitor suppresses NLRC5 promoter activation upon IFN-γ stimulation [52]. The discrepancy in NLRC5 expression levels in various tissues among different reports may be due to the IFN-γ-mediated inducibility of NLRC5 expression as tissue samples with a higher inflammation status may have greater expression of NLRC5.
Unlike most other NLR proteins, NLRC5 is a nuclear protein [5, 54]. Upon overexpression, NLRC5 tends to localize to the cytoplasm, possibly due to artificial aggregation. However, careful observation of NLRC5 cellular localization revealed that NLRC5 is found in both the cytoplasm and the nucleus [5, 54]. It has been demonstrated that CIITA, which also displays a steady-state, heterogeneous distribution, shuttles between the nucleus and the cytosol via importin-α-mediated nuclear import and CRM1-dependent nuclear export [36, 37, 57]. Similar to CIITA, NLRC5 is trapped in the nucleus upon treatment with the CRM1 inhibitor leptomycin B (LMB), indicating that NLRC5 also shuttles between the cytosol and nucleus [5, 54]. Due to its large size, NLRC5 requires active import and export mechanisms to shuttle into and out of the nucleus. Indeed, similar to CIITA, which has three nuclear localization signals (NLSs), NLRC5 also contains a bipartite NLS between the CARD and NBD, and point mutations within the NLS prevent the import of NLRC5 into the nucleus [5](Fig. 1).
6. NLRC5 is a MHC class I transactivator (CITA)
Through a collaboration with Dr Peter van den Elsen's laboratory at Leiden University, we recently identified NLRC5 as a MHC class I transactivator [5], which is reminiscent of the role of CIITA in regulating MHC class II gene expression. The discovery was made by genome-wide gene expression profiling of NLRC5-expressing stable cell lines [5]. Although the function of NLRC5 was not known at the time, it was predicted that, like other NLR proteins, NLRC5 requires the P-loop in the NBD for its function. Therefore, we generated cell lines expressing either wild-type or mutant forms of NLRC5 harboring mutations in the NBD, including: Walker A (deficient in nucleotide binding), Walker B (deficient in nucleotide hydrolysis), and the double mutant Walker AB, which carries both mutations. Clustering analysis grouped the active forms of NLRC5 (WT and Walker B) and inactive forms (Walker A, Walker AB), and identified a limited set of genes that were differentially expressed between groups. Amongst the genes most upregulated by the active forms of NLRC5 were the various MHC class I genes as well as other genes involved in class I antigen presentation and processing, such as β2M, LMP2 and TAP1 [5]. We confirmed that NLRC5 upregulates the mRNA transcript and protein levels of conventional MHC class I genes (HLA-A, B, C), non-conventional MHC class I genes (HLA-E), as well as β2M, LMP2 and TAP1 [5]. The impact of NLRC5 is specific to MHC class I genes, as NLRC5 has no effect on the expression of MHC class II genes [5]. We also demonstrated that NLRC5 can associate with and transactivate promoters of MHC class I genes, indicating that NLRC5 can directly transactivate MHC class I genes [5]. Using a chromatin immunoprecipitation assay (ChIP), we showed that NLRC5 specifically associates with the promoters of the HLA-A, and HLA-B genes but not with the promoter of MHC class II (HLA-DRA) or an unrelated gene. In luciferase-based reporter gene assays, NLRC5 activated promoters of HLA-A, -B, -C, -F, -G, and β2M but not promoters of any of the MHC class II genes (HLA-DRA, -DQA, -DPA), whereas CIITA strongly activated MHC class II gene promoters [5]. Interestingly, the induction of NLRC5 and CIITA follow similar kinetics after IFN-γ stimulation. The induction of CIITA expression by IFN-γ precedes the upregulation of MHC class II [40](Fig. 2). Similarly, NLRC5 transcript levels are upregulated early upon IFN-γ stimulation, while MHC class I gene expression is induced 12-24 hours after IFN-γ stimulation. Although both NLRC5 and CIITA are responsive to IFN-γ stimulation, NLRC5 appears to play a dominant role in the transactivation of MHC class I genes, as siRNA-mediated knockdown of NLRC5 expression impaired IFN-γ-induced upregulation of MHC class I even though the induction of CIITA expression was intact. Moreover, knockdown of NLRC5 expression had no effect on the expression of MHC class II. Therefore, upregulation of NLRC5 is critical for the efficient induction of MHC class I by IFN-γ stimulation [5].
Our study highlighted the function of NLRC5 as a MHC class I transactivator or CITA. Since the discovery of CIITA by Bernard Mach's group in 1993, it had been postulated that a similar protein regulates MHC class I genes. As mentioned above, there are many structural and functional similarities between CIITA and NLRC5. First, both NLRC5 and CIITA belong to the CARD containing NLR subfamily and phylogenetically they are most similar among all NLR proteins [5, 56]. Second, both carry NLSs and can translocate into the nucleus (Fig. 1) [5, 36, 37, 57]. Third, both molecules are highly inducible upon IFN-γ stimulation via STAT1 activation [5, 40, 52, 58-61]. Fourth, similar to other NLR protein family members, both NLRC5 and CIITA require an active NBD domain for their function; the NTP binding motif in NLRC5 and CIITA is required for transactivation of MHC class I and MHC class II genes, respectively [5, 35, 62, 63]. Fifth, despite the lack of a DNA-binding domain, both NLRC5 and CIITA can associate with and transactivate MHC gene promoters [5, 47, 64]. Finally and most strikingly, both NLRC5 and CIITA orchestrate the concerted expression of sets of functionally related genes critical for antigen presentation. CIITA, in addition to the classical MHC class II genes, induces the invariant chain Ii, and the non-classical MHC class II genes HLA-DM, HLA-DO [41]. NLRC5, in contrast, can upregulate β2M, TAP1 and LMP2 in addition to MHC class I genes; NLRC5 is thus a key transcriptional regulator of genes involved in the MHC class I antigen presentation pathway.
The striking similarities between the two molecules suggest that the mechanism of NLRC5-mediated transactivation of MHC class I genes may also be similar to that of CIITA. CIITA is known to associate with a set of transcription factor complexes to form an ‘MHC enhanceosome’ on the WXY motif of the MHC class I and class II gene promoters. Since NLRC5 has a similar protein structure and function, it is likely that NLRC5 may use a comparable platform to activate MHC class I gene promoters. The following is a model of NLRC5 transactivation of MHC class I genes. Upon IFN-γ stimulation, activated STAT1 homodimers induce CIITA expression by two independent mechanisms; STAT1 can directly transactivate CIITA by binding to the GAS site in its promoter, or upregulate IRF-1, which then induces CIITA expression (Fig. 2A). Subsequently, CIITA activates the promoters of MHC class II genes by forming the MHC enhanceosome on the conserved WXY motif in the MHC promoters, which includes the RFX, CREB/ATF1 and NF-Y protein complexes (Fig. 2A). IFN-γ also induces the expression of MHC class I genes through a similar mechanism. IFN-γ induces both, IRF-1 and NLRC5 through STAT1 binding to the GAS site in their promoters (Fig. 2B). While IRF-1 directly associates with the ISRE cis-regulatory element in MHC class I gene promoters, NLRC5 is likely to assemble a “CITA enhanceosome” on the MHC class I promoter, similar to the MHC class II enhanceosome (Fig. 2B). However, unlike the CIITA enhanceosome, the CITA enhanceosome is specific to promoters of MHC class I and other related genes.
7. Other possible function of NLRC5
There have been several reports suggesting that NLRC5 may have functions other than being a transcriptional activator of MHC class I genes. Although the proposed functions are intriguing, these reports have had conflicting conclusions, and have not been supported by data obtained from the analysis of NLRC5-deficient mice and cells. Therefore, careful reexamination will be required to clarify the proposed functions of NLRC5.
7.1 NLRC5 as a negative regulator of Toll-like receptor (TLR) signaling
It has recently been proposed that NLRC5 is a negative regulator of TLR4 signaling that directly interacts with IKKα and IKKβ and thus prevents NF-κB activation [65]. In agreement with this observation, Benko et al. showed that Nlrc5 knockdown in a murine macrophage cell line resulted in enhanced responses to LPS stimulation, including augmented production of IL-6, TNF-α and IL-1β [54]. In addition, a third group observed that overexpression of NLRC5 can suppress NF-κB activation in reporter gene assays when co-transfected with expression vectors for various components of the TLR signaling pathway [66]. Together, these data suggest that NLRC5 can act as a negative regulator of TLR signaling under certain experimental settings. Contrary to the former reports, however, Kumar et al. observed that NF-κB activation was not suppressed by NLRC5 in IKKβ transfected cells [66]. Moreover, cells from NLRC5-deficient mice produced pro-inflammatory cytokines at a level similar to that of cells from wild-type mice, as shown at both protein and mRNA levels upon TLR2, TLR4 and TLR9 stimulation [66]. It is difficult to draw a single conclusion from these seemingly contradicting data. Experiments using NLRC5-deficient mice clearly demonstrated that NLRC5 is dispensable for TLR responses, at least in mice [66]. However, it remains possible that NLRC5 may have an inhibitory effect on TLR signaling under limited experimental conditions.
7.2 NLRC5 as a negative regulator of RIG-I-like receptor (RLR) signaling
There are mixed reports regarding the involvement of NLRC5 in antiviral responses. Cui et al. found that the CARD of NLRC5 directly interacts with RIG-I, and proposed that NLRC5 is a negative regulator of RLR signaling by inhibiting NF-κB and IRF-3 activation [65]. On the contrary, other groups have shown that siRNA-mediated silencing of NLRC5 results in reduced production of type I IFN upon infection with cytomegalovirus (CMV) and Sendai virus (SeV), suggesting an antiviral function for NLRC5[52, 53]. The analysis of cells derived from NLRC5-deficient mice did not support either of these findings [66]. Wild-type and NLRC5-deficient cells produce similar levels of IFN-β, IL-6 and TNF-α, and induce comparable levels of IFN-β, IL-6, TNF-α and RANTES mRNA transcripts after infection with NDV, which is recognized by RIG-I [66]. NLRC5-deficient cells are also capable of responding to cytoplasmic dsDNA: induction of IFN-β is normal in these cells upon stimulation with B-DNA (poly [dA:dT]) or calf thymus DNA, as well as after infection with Listeria monocytogenes, which is known to introduce DNA into the cytosol [66]. Similarly, infection with HSV-1, a DNA virus, resulted in normal production of proinflammatory cytokines and IFN-β [66]. Consequently, NLRC5 is dispensable for RIG-I signaling and cytoplasmic DNA-induced innate immune responses. Data from our group support this conclusion; NLRC5 overexpression does not activate the promoters of IFN-α, IFN-β, ISRE, IRF3, NF-κB and AP-1[5]. Also NLRC5 did not inhibit ISRE and NF-κB promoter activity induced by transfection of RIG-I, MDA-5 or MAVS/IPS-1 (unpublished data).
The previously observed inhibitory effect of NLRC5 on antiviral responses may thus reflect cell type-specific differences or depend on specific experimental conditions.
7.3 NLRC5 as an activator of the inflammasome
NLRC5 has also been proposed to be an activator and component of the inflammasome, a protein complex which acts as a platform for caspase-1 activation and inflammatory cytokine maturation [67]. Inflammasome activation is required for the cleavage of the proforms of IL-1β and IL-18 by active caspase-1[30]. Davis et al. showed that NLRC5 associates with the inflammasome components NLRP3 and ASC when all three proteins are overexpressed in 293T cells [67]. Moreover, shRNA-mediated silencing of NLRC5 in the human monocytic cell line THP1 resulted in reduced caspase-1 activation and decreased IL-1β production upon Escherichia coli infection[67]. Similarly, human primary monocytes transfected with siRNA for NLRC5 also showed reduced IL-1β production upon E. coli infection. Reduced IL-1β production was also observed upon infection with Staphylococcus aureus and Shigella flexineri, as well as upon stimulation with TLR ligands (for TLR2 and TLR4) and NLRP3 activating crystals (alum and monosodium urate), but not upon stimulation with NLRP3 activating pore forming toxins (Nigericin and α-hemolysin), indicating that there is ligand-specificity for the requirement of NLRC5 for NLRP3 inflammasome activation [67]. Interestingly, both NLRP3 and NLRC5 knockdown almost completely abolished IL-1β production, suggesting that cooperation between NLRC5 and NLRP3 is required for caspase-1 activation. Another group also observed that overexpression of NLRC5 in 293T cells increased caspase-1 activation and subsequent IL-1β release [66]. However, the same group demonstrated that peritoneal cells, bone marrow-derived macrophages and dendritic cells from NLRC5-deficient mice produced IL-1β at levels similar to that of wild-type mice in response to NLRP3 activating stimuli including LPS +ATP, nigericin, zymosan, and curdlan. NLRC5-deficient cells also produced normal levels of IL-1β when stimulated with poly (dA:dT) or Francisella tularensis, which both activate the AIM2 inflammasome. Moreover, caspase-1 activation was similar between wild-type and NLRC5-deficient cells upon infection with Salmonella typhimurium, which induces activation of the NLRC4 and NLRP3 inflammasomes. In summary, studies using NLRC5-deficient mice demonstrate that NLRC5 is not required for activation of the NLRP3, NLRC4 or AIM2 inflammasomes. It is, however, possible that NLRC5 is activated by a yet unidentified ligand that is required to observe its effects on inflammatory signaling. Therefore, at the current state of investigation, the involvement of NLRC5 in the inflammasome activation remains controversial.
8. Concluding remarks
A transcriptional regulator of MHC class I has been sought since the discovery of CIITA in 1993, and the recent identification of NLRC5 as CITA helps enrich our understanding of the transcriptional regulation of MHC class I. NLRC5 has remarkably similar characteristics to CIITA, but strictly activates MHC class I and related genes involved in MHC class I-dependent antigen presentation. Clearly, more research is required to elucidate MHC class I gene activation by NLRC5. Similar to CIITA, NLRC5 does not possess a DNA binding domain. This indicates that NLRC5 may associate and cooperate with other transcription factors on MHC class I promoters, although the components of this ‘CITA enhanceosome’ and its assembly mechanism are currently unknown. Additionally, it remains unclear if CIITA and NLRC5 cooperate at the MHC class I promoter. In vitro experiments indicate that CIITA can activate and induce MHC class I genes, but CIITA deficiency in human BLS patients or knockout mice has no effect on MHC class I gene expression. This could suggest that CIITA and NLRC5 may have non-redundant functions in vivo, especially since CIITA expression is mostly limited to hematopoietic cells, whereas NLRC5 is widely expressed in various cells and tissues upon INF-γ stimulation. Finally, given the importance of MHC class I antigen presentation in directing the activity of CD8 T lymphocytes and NK cells, NLRC5 should play a major role in the function of these cells. Future studies using in vivo models of NLRC5 deficiency are required to explore the role of NLRC5 in CD8 T and NK cell responses. Further exploration of the role of NLRC5, or CITA, in regulating MHC class I and other related genes will contribute to a broad area of HLA biology, including transplantation, infectious disease and tumor immunology.
Acknowledgments
This work was supported by grants from the NIH and the Crohn's and Colitis Foundation of America (K.S.K.). K.S.K. is a recipient of the Investigator Award from the Cancer Research Institute and the Claudia Adams Barr Award and T.B.M has been a recipient of the EMBO Long Term fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- 1.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 2.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 3.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109(Suppl.):S21–33. doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 4.Choi NM, Majumder P, Boss JM. Regulation of major histocompatibility complex class II genes. Curr. Opin. Immunol. 2011;23:81–87. doi: 10.1016/j.coi.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, Bayir E, Iliopoulos D, van den Elsen PJ, Kobayashi KS. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. USA. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pamer E, Cresswell P. Mechanisms of MHC class I--restricted antigen processing. Annu. Rev. Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 7.Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr. Opin. Immunol. 1999;11:100–108. doi: 10.1016/s0952-7915(99)80018-1. [DOI] [PubMed] [Google Scholar]
- 8.Le Bouteiller P, Solier C. Is antigen presentation the primary function of HLA-G? Microbes Infect. 2001;3:323–332. doi: 10.1016/s1286-4579(01)01386-7. [DOI] [PubMed] [Google Scholar]
- 9.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 10.Oldstone MB. Virus-lymphoid cell interactions. Proc. Natl. Acad. Sci. USA. 1996;93:12756–12758. doi: 10.1073/pnas.93.23.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shastri N, Cardinaud S, Schwab SR, Serwold T, Kunisawa J. All the peptides that fit: the beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol. Rev. 2005;207:31–41. doi: 10.1111/j.0105-2896.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 12.Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu. Rev. Cell. Dev. Biol. 2008;24:343–368. doi: 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 13.Lanier LL. Natural killer cell receptors and MHC class I interactions. Curr. Opin. Immunol. 1997;9:126–131. doi: 10.1016/s0952-7915(97)80169-0. [DOI] [PubMed] [Google Scholar]
- 14.Moretta A, Biassoni R, Bottino C, Pende D, Vitale M, Poggi A, Mingari MC, Moretta L. Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol. Rev. 1997;155:105–117. doi: 10.1111/j.1600-065x.1997.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 15.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J. Leukoc. Biol. 2008;83:13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koonin EV, Aravind L. The NACHT family - a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem. Sci. 2000;25:223–224. doi: 10.1016/s0968-0004(00)01577-2. [DOI] [PubMed] [Google Scholar]
- 19.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 20.Bertin J, DiStefano PS. The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ. 2000;7:1273–1274. doi: 10.1038/sj.cdd.4400774. [DOI] [PubMed] [Google Scholar]
- 21.Inohara N, Nunez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene. 2001;20:6473–6481. doi: 10.1038/sj.onc.1204787. [DOI] [PubMed] [Google Scholar]
- 22.Martinon F, Hofmann K, Tschopp J. The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr. Biol. 2001;11:R118–120. doi: 10.1016/s0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 23.Fairbrother WJ, Gordon NC, Humke EW, O'Rourke KM, Starovasnik MA, Yin JP, Dixit VM. The PYRIN domain: a member of the death domain-fold superfamily. Protein Sci. 2001;10:1911–1918. doi: 10.1110/ps.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlowski K, Pio F, Chu Z, Reed JC, Godzik A. PAAD - a new protein domain associated with apoptosis, cancer and autoimmune diseases. Trends Biochem. Sci. 2001;26:85–87. doi: 10.1016/s0968-0004(00)01729-1. [DOI] [PubMed] [Google Scholar]
- 25.Weber CH, Vincenz C. The death domain superfamily: a tale of two interfaces? Trends Biochem. Sci. 2001;26:475–481. doi: 10.1016/s0968-0004(01)01905-3. [DOI] [PubMed] [Google Scholar]
- 26.Staub E, Dahl E, Rosenthal A. The DAPIN family: a novel domain links apoptotic and interferon response proteins. Trends Biochem. Sci. 2001;26:83–85. doi: 10.1016/s0968-0004(00)01717-5. [DOI] [PubMed] [Google Scholar]
- 27.Birnbaum MJ, Clem RJ, Miller LK. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J. Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Biswas A, Petnicki-Ocwieja T, Kobayashi KS. Nod2: a key regulator linking microbiota to intestinal mucosal immunity. J. Mol. Med. doi: 10.1007/s00109-011-0802-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 2000;26:267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- 32.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 33.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 34.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 35.Bewry NN, Bolick SC, Wright KL, Harton JA. GTP-dependent recruitment of CIITA to the class II major histocompatibility complex promoter. J. Biol. Chem. 2007;282:26178–26184. doi: 10.1074/jbc.M611747200. [DOI] [PubMed] [Google Scholar]
- 36.Spilianakis C, Papamatheakis J, Kretsovali A. Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol. Cell. Biol. 2000;20:8489–8498. doi: 10.1128/mcb.20.22.8489-8498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cressman DE, O'Connor WJ, Greer SF, Zhu XS, Ting JP. Mechanisms of nuclear import and export that control the subcellular localization of class II transactivator. J. Immunol. 2001;167:3626–3634. doi: 10.4049/jimmunol.167.7.3626. [DOI] [PubMed] [Google Scholar]
- 38.Raval A, Weissman JD, Howcroft TK, Singer DS. The GTP-binding domain of class II transactivator regulates its nuclear export. J. Immunol. 2003;170:922–930. doi: 10.4049/jimmunol.170.2.922. [DOI] [PubMed] [Google Scholar]
- 39.DeSandro A, Nagarajan UM, Boss JM. The bare lymphocyte syndrome: molecular clues to the transcriptional regulation of major histocompatibility complex class II genes. Am. J. Hum. Genet. 1999;65:279–286. doi: 10.1086/302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 41.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 42.Beresford GW, Boss JM. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat. Immunol. 2001;2:652–657. doi: 10.1038/89810. [DOI] [PubMed] [Google Scholar]
- 43.Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 2000;14:1156–1166. [PMC free article] [PubMed] [Google Scholar]
- 44.van den Elsen PJ, Gobin SJ, van Eggermond MC, Peijnenburg A. Regulation of MHC class I and II gene transcription: differences and similarities. Immunogenetics. 1998;48:208–221. doi: 10.1007/s002510050425. [DOI] [PubMed] [Google Scholar]
- 45.van den Elsen PJ, Peijnenburg A, van Eggermond MC, Gobin SJ. Shared regulatory elements in the promoters of MHC class I and class II genes. Immunol. Today. 1998;19:308–312. doi: 10.1016/s0167-5699(98)01287-0. [DOI] [PubMed] [Google Scholar]
- 46.Boss JM, Jensen PE. Transcriptional regulation of the MHC class II antigen presentation pathway. Curr. Opin. Immunol. 2003;15:105–111. doi: 10.1016/s0952-7915(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 47.Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/s1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- 48.Benichou B, Strominger JL. Class II-antigen-negative patient and mutant B-cell lines represent at least three, and probably four, distinct genetic defects defined by complementation analysis. Proc. Natl. Acad. Sci. USA. 1991;88:4285–4288. doi: 10.1073/pnas.88.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 50.Itoh-Lindstrom Y, Piskurich JF, Felix NJ, Wang Y, Brickey WJ, Platt JL, Koller BH, Ting JP. Reduced IL-4-, lipopolysaccharide-, and IFN-gamma-induced MHC class II expression in mice lacking class II transactivator due to targeted deletion of the GTP-binding domain. J. Immunol. 1999;163:2425–2431. [PubMed] [Google Scholar]
- 51.Williams GS, Malin M, Vremec D, Chang CH, Boyd R, Benoist C, Mathis D. Mice lacking the transcription factor CIITA--a second look. Int. Immunol. 1998;10:1957–1967. doi: 10.1093/intimm/10.12.1957. [DOI] [PubMed] [Google Scholar]
- 52.Kuenzel S, Till A, Winkler M, Hasler R, Lipinski S, Jung S, Grotzinger J, Fickenscher H, Schreiber S, Rosenstiel P. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J. Immunol. 2010;184:1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- 53.Neerincx A, Lautz K, Menning M, Kremmer E, Zigrino P, Hosel M, Buning H, Schwarzenbacher R, Kufer TA. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J. Biol. Chem. 2010;285:26223–26232. doi: 10.1074/jbc.M110.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benko S, Magalhaes JG, Philpott DJ, Girardin SE. NLRC5 limits the activation of inflammatory pathways. J. Immunol. 2010;185:1681–1691. doi: 10.4049/jimmunol.0903900. [DOI] [PubMed] [Google Scholar]
- 55.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Nickerson K, Sisk TJ, Inohara N, Yee CS, Kennell J, Cho MC, Yannie PJ, 2nd, Nunez G, Chang CH. Dendritic cell-specific MHC class II transactivator contains a caspase recruitment domain that confers potent transactivation activity. J. Biol. Chem. 2001;276:19089–19093. doi: 10.1074/jbc.M101295200. [DOI] [PubMed] [Google Scholar]
- 57.Cressman DE, Chin KC, Taxman DJ, Ting JP. A defect in the nuclear translocation of CIITA causes a form of type II bare lymphocyte syndrome. Immunity. 1999;10:163–171. doi: 10.1016/s1074-7613(00)80017-5. [DOI] [PubMed] [Google Scholar]
- 58.Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J. Exp. Med. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muhlethaler-Mottet A, Di Berardino W, Otten LA, Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 60.Piskurich JF, Wang Y, Linhoff MW, White LC, Ting JP. Identification of distinct regions of 5' flanking DNA that mediate constitutive, IFN-gamma, STAT1, and TGF-beta-regulated expression of the class II transactivator gene. J. Immunol. 1998;160:233–240. [PubMed] [Google Scholar]
- 61.Piskurich JF, Linhoff MW, Wang Y, Ting JP. Two distinct gamma interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor beta. Mol. Cell. Biol. 1999;19:431–440. doi: 10.1128/mcb.19.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright KL, Chin KC, Linhoff M, Skinner C, Brown JA, Boss JM, Stark GR, Ting JP. CIITA stimulation of transcription factor binding to major histocompatibility complex class II and associated promoters in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:6267–6272. doi: 10.1073/pnas.95.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 64.Gobin SJ, Peijnenburg A, Keijsers V, van den Elsen PJ. Site alpha is crucial for two routes of IFN gamma-induced MHC class I transactivation: the ISRE-mediated route and a novel pathway involving CIITA. Immunity. 1997;6:601–611. doi: 10.1016/s1074-7613(00)80348-9. [DOI] [PubMed] [Google Scholar]
- 65.Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ, Wang RF. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar H, Pandey S, Zou J, Kumagai Y, Takahashi K, Akira S, Kawai T. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J. Immunol. 2011;186:994–1000. doi: 10.4049/jimmunol.1002094. [DOI] [PubMed] [Google Scholar]
- 67.Davis BK, Roberts RA, Huang MT, Willingham SB, Conti BJ, Brickey WJ, Barker BR, Kwan M, Taxman DJ, Accavitti-Loper MA, Duncan JA, Ting JP. Cutting edge: NLRC5-dependent activation of the inflammasome. J. Immunol. 2011;186:1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]