Abstract
Our ultimate goal is to identify and target modifiable risk factors that will reduce major cardiovascular events in African-American lupus patients. As a first step toward achieving this goal, this study was designed to explore risk factor models of preclinical atherosclerosis in a predominantly African-American group of SLE patients using variables historically associated with endothelial function in non-lupus populations.
51 subjects with SLE but without a history of clinical cardiovascular events were enrolled. At entry, a Framingham risk factor history and medication list were recorded. Sera and plasma samples were analyzed for lipids, lupus activity markers, and total 25-hydroxyvitamin D (25(OH)D) levels. Carotid ultrasound measurements were performed to determine total plaque area (TPA) in both carotids. Cases had TPA values above age-matched controls from a vascular prevention clinic population. Logistic regression and machine learning analyses were performed to create predictive models.
25(OH)D levels were significantly lower and SLE disease duration was significantly higher in cases. 25(OH)D levels inversely correlated with age-adjusted TPA. ACE-inhibitor non-use associated with case status. Logistic regression models containing ACE-inhibitor use, 25(OH)D levels, and LDL levels had a diagnostic accuracy of 84% for predicting accelerated atherosclerosis. Similar results were obtained with machine learning models, but hydroxychloroquine use associated with controls in these models.
This is the first study to demonstrate an association between atherosclerotic burden and 25(OH)D insufficiency or ACE-inhibitor non-use in lupus patients. These findings provide strong rationale for the study of ACE-inhibitors and vitamin D replenishment as preventive therapies in this high-risk population.
Keywords: Systemic lupus erythematosus, Atherosclerosis, Vitamin D deficiency, Angiotensin converting enzyme inhibitors, Hypercholesterolemia
Introduction
Systemic lupus erythematosus (SLE) is an independent risk factor for atherosclerosis and major adverse cardiovascular events (MACE) (1). African-American lupus patients have an earlier onset of cardiovascular disease hospital admission and tend to have earlier in-hospital deaths from cardiovascular disease than their Caucasian lupus counterparts (2). The mechanisms behind this racial disparity are not known. The standard Framingham risk model for cardiovascular disease is not very predictive in lupus patients, engendering the hypothesis that there are independent mechanisms driving accelerated atherosclerosis in this patient population (1). Several independent mechanisms may result in accelerated CVD in patients with SLE. The mechanisms proposed to date can ultimately lead to endothelial dysfunction, a functional characteristic common to SLE subjects with atherosclerosis (3) that is an independent risk factor for MACE in the general population and in SLE patients (4).
Due to the heterogeneity of the lupus phenotype, it is expected that no single marker will be effective in predicting CVD and ultimately MACE in SLE patients. We hypothesized that low vitamin D levels would associate with atherosclerosis for the following reasons: 1) low 25(OH)D levels are associated with MACE in non-lupus populations (5), 2) repletion of 25(OH)D in vitamin D deficient non-lupus patients improved vascular endothelial function (6), and 3) African-American lupus patients have a high prevalence of vitamin D deficiency (7). We further hypothesized that angiotensin converting enzyme (ACE) inhibitor use would associate with lower levels of atherosclerosis because of literature supporting a role for ACE inhibitors in improving endothelial function (8). Therefore, we hypothesized that multiple traditional and novel markers that are surrogates for mechanisms of endothelial cell dysfunction can be used to create a model of atherosclerosis in a largely African-American SLE patient population. Such models might support a role for the study of non-Framingham risk factors that can be modified to improve endothelial function in this population.
Materials and Methods
Study design
The goal of this study was to determine risk factor models for accelerated early atherosclerosis in a largely African-American population of lupus patients. This was a cross sectional within-lupus case-control pilot study to evaluate novel and traditional risk factors for accelerated atherosclerosis in a largely African-American SLE population. The variables considered as risk factors and co-variables for the early atherosclerosis outcome were age, traditional Framingham risk factors for atherosclerosis, measures of SLE disease activity and damage, serum 25-hydroxy vitamin D (25(OH)D) levels, and immunosuppressive, renin-angiotensin acting antihypertensive, statin, and total calculated corticosteroid medication use. Participants were considered to have accelerated atherosclerosis (cases) if their age-adjusted total carotid plaque area (TPA) was greater than the mean of historical controls in a vascular prevention clinic. The historical control population in this study was used reduce the confounding effect of age on TPA rather than to directly compare TPA in SLE and non-SLE populations.
SLE participant inclusion criteria
Participants met at least four of the 1997 revised ACR SLE criteria (9). All procedures performed were approved by the MUSC Institutional Review Board, and all subjects gave informed consent prior to initiation of any study related procedures.
SLE participant exclusion criteria
Because the goal was to determine markers of subclinical atherosclerosis, participants with a history of cardiovascular disease (CVD) endpoints such as myocardial infarction (MI), stroke (CVA), or documented peripheral, coronary, or carotid artery functionally significant narrowing were not included in the study.
Plaque area determination
Carotid total plaque area (TPA) was chosen as a surrogate for cardiovascular outcomes in this study for several reasons. First, TPA allows one to see differences between groups with far fewer subjects than IMT (10), and baseline TPA measures associate with hard cardiovascular endpoints such as five-year risk for stroke, myocardial infarction, and vascular death (11). When compared directly to IMT in prospective, longitudinal study, TPA was superior in predicting myocardial infarction (12). TPA was also more predictive of myocardial infarction in women than in men, which is ideal for studying lupus populations (13). Lupus subjects have a higher prevalence of carotid plaque than non-lupus controls, making a plaque measure more relevant for a lupus population than intima-media thickness (14). Measuring the size of plaque burden (a continuous variable) rather than measuring the presence of plaque (an ordinal variable) enhances the statistical power of the TPA measure, allowing us to reduce the sample size for this study. Finally, TPA can be age-adjusted from an historical population (11).
TPA was measured using a Phillips iU22 Ultrasound system and a L9-3 9 mHz probe in 2D mode by a single operator at each center (Charleston and London). The Charleston operator was trained by and proper techniques were confirmed by the London operator. TPA measurements were performed as previously reported (11). As a quality control measure, ten participants were randomly selected from a convenience population of volunteers in Charleston for repeated measures within a month. The intra-operator repeated measures intraclass correlation coefficient was 0.96 (95% CI = 0.84 – 0.99), while the weighted kappa statistic was 0.950 (95% CI = 0.88 - 1.00) for the Charleston operator, while the reliability of the London, Ontario operator was equivalent and was described previously (10).
Control population for age adjustment of TPA
The age adjustment controls were 4272 patients recruited from the Premature Atherosclerosis Clinic and the Stroke Prevention Clinic of the University Campus of the London Health Sciences Centre (London, Ontario) (written communication JD Spence). Participants, described previously (15), were referred to the clinic for premature atherosclerosis, stroke, transient ischemic attack, or asymptomatic narrowing of the carotid artery. These controls were used to adjust TPA values for age in the diagnostic models. The Charleston quality control population was also used to confirm similar TPA values between the Charleston, SC and London, Ontario controls.
SLE Disease Activity Index (SELENA-SLEDAI) and International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC/ACR)
Because lupus disease activity may contribute to the development of atherosclerosis in this population, we categorized disease activity by using the SELENA-SLEDAI index (16). Because SLE and medication-related damage may be a surrogate for cardiovascular risk factors, the SLICC/ACR Damage Index was used to quantify this damage (17).
Determination of traditional cardiovascular risk factors
Traditional risk factors for atherosclerosis were assessed. Historical risk factors determined by interview and chart review were: a first degree relative with history of stroke, myocardial infarction, or accelerated onset of MACE (earlier than age 50 in men and age 55 in women), history of major cardiovascular event or peripheral vascular disease, sex, number of years since SLE diagnosis, history of hypertension, diabetes, obesity, hypercholesterolemia (HC), end stage renal disease, or smoking (past or present, reported as pack*years). The following risk factors were determined at visit 1: systolic blood pressure (SBP), diastolic blood pressure (DBP), height, weight, body mass index (BMI), and waist-to-hip ratio (WHR). Medication histories were performed by interview and chart review. The following medications were recorded as either present or absent: immunosuppressants (mycophenolate mofetil, mycophenolic acid, azathioprine, or methotrexate), hydroxychloroquine (HDQ), angiotensin converting enzyme inhibitors (ACE), angiotensin receptor blockers, and statins.
Standard laboratory assessments
The Medical University of South Carolina (MUSC) Special Chemistry and Immunology Laboratories performed the following: urine creatinine, protein, and cell count and serum C3, C4, anti-double stranded DNA antibody levels (dsDNA), complete blood count, complete metabolic panel, and plasma VLDL, LDL, HDL, triglycerides (TG), and total cholesterol. Renal function (eGFR) was calculated by the abbreviated four variable Modified Diet in Renal Disease (MDRD) equation. If the eGFR was calculated to be > 60, then the Cockroft-Gault equation was used (18).
Serum total 25(OH)D and parathyroid hormone (PTH) analysis
Serum 25(OH)D levels were determined using a nonchromatographic radioimmunoassay as previously described (19). Serum PTH levels were determined using an immunoradiometric assay (DiaSorin, Stillwater, MN). The laboratory participates in the international Vitamin D External Quality Assessment Scheme (DEQAS).
Determination of total prednisone exposure
Total accumulated prednisone dose was calculated by interview and chart review and reported in grams of prednisone glucocorticoid equivalent. Accumulated exposure between clinic visits was considered to be constant unless a taper was prescribed.
Data collection and management
Study data were collected and managed using REDCap (Research Electronic Data Capture, REDCap Project), a secure, web-based application designed to support electronic data capture for research studies.
Statistical analysis
The distribution of variables was determined using Kolmogorov-Smirnov and Shapiro-Wilk tests. Comparisons between groups were made using Student's t test or Mann-Whitney U test according to the distribution of the data. For crosstab associations, a Chi-square was performed, with Fisher's Exact Test used when appropriate. For nonparametric receiver operating characteristics (ROC) analysis, continuous variables were tested against case status and reported as the area under the ROC curve (AUC) with 95% confidence interval (CI). The state variable was the case state unless a putative “protective” variable was tested. Correlations were determined using Pearson or Spearman correlation according to the distribution of the data. Backward, stepwise, logistic regression was performed using categorical and continuous variables that were significant (p ≤ 0.1) in correlation or Chi-square analyses with the exception of disease activity parameters, which are not expected to impact TPA given the waxing and waning nature of SLE disease activity. Season (March to September or October to February) was included as a variable in this analysis. The model with the greatest accuracy for predicting plaque state was reported along with the likelihood ratio (LR) and p value. Missing values (25(OH) vitamin D levels for one case and one control) were imputed as the means of cases and controls. P values ≤ 0.05 were considered significant. Statistical analysis was performed using PASW Statistics v18.0 (SPSS Inc., Chicago, IL).
Non-linear modeling
Machine learning was used to explore variables that may be important in predicting atherosclerosis and to determine if non-linear algorithms could produce models with greater diagnostic power than conventional linear models. Nearest Related Neighbor (NRN) modeling is an experimental supervised machine learning technique for multivariable analysis that utilizes a nonparametric support vector machine to segregate cases and controls based on the variable characteristics of neighboring cases and controls (20). Input variables were chosen based on historical precedent (Framingham risk factors and variables associated with either increased or decreased endothelial function) or performance in bivariate or logistic regression analysis described above. NRN was performed using an algorithm created in MatLab (The Mathworks, Natick, MA) by Dr. Jonas Almeida (21). Variables that produced the most predictive model (i.e. fewest wrong neighbors were identified) and the ROC AUC for the model were reported. A feed-forward artificial neural network (ANN) machine learning algorithm created in MatLab by Dr. Almeida was used to create a predictive model of abnormal TPA as described (22). This type of algorithm, unlike traditional linear regression, can create predictive models from large numbers of input variables. This algorithm can still perform well if there is co-linearity between input variables or if associations between inputs and outputs are non-linear (23). The same input variables were used in the ANN analysis. Eleven ANNs were trained, and the ROC AUC of the median model was reported. The input variables with the greatest relative importance (sensitivity) in predicting abnormal TPA in this median model were reported.
Results
Twenty-seven percent of SLE patients have abnormally elevated TPA
Fourteen of the 51 SLE subjects (cases) had TPAs above what was observed in age-matched controls from both the London, Ontario vascular prevention clinic population (Figure 1). The trend line for the Charleston non-lupus control population was almost identical to that seen in women in the London, Ontario vascular prevention clinic population (supplemental Figure 1). It should be noted that the vascular prevention clinic patients had approximately three times more plaque than healthy volunteers in the population-based Tromsø study (13), making the London control population a very conservative one for age adjustment.
Figure 1. Defining the lupus case population: age adjusted carotid plaque area in lupus subjects compared to a vascular prevention clinic population.
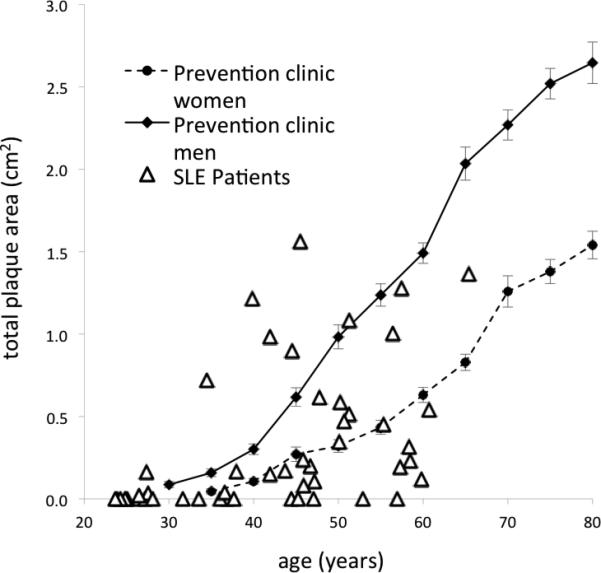
Pictured is the distribution of carotid total plaque area (TPA in cm2) by age in lupus patients (open triangles) compared to an historic population of stroke and hypertension prevention clinic patients (men - solid line, women - dashed line). TPA is presented on the y-axis, while age is represented on the x-axis. Note the lupus cases with TPA values above age-matched controls in the vascular prevention clinic.
SLE atherosclerosis cases have longer disease duration and lower 25(OH)D levels than SLE controls
25(OH)D levels were significantly lower in those with elevated TPA (Table I).; although, the mean for each group was below sufficient levels (24). Of note, all subjects with 25(OH)D levels of > 30.3 ng/ml had normal PTH levels, while 29% of those with lower levels had elevated PTH levels (data not shown). This suggests that 25(OH)D levels required to suppress PTH in other populations apply to this largely African-American population as well. Renal function was similar between cases and controls (Table I), and renal function did not correlate with 25(OH)D in this population (r=0.01, p=0.9). Framingham risk factors were not significantly different in the cases compared to the controls (supplemental Table I). LDL levels tended to be higher in those with elevated TPA, but the differences did not reach statistical significance (p = 0.07). Several lupus-related factors were different between groups. Cases had a longer SLE disease duration than controls but did not have different SLICC scores. Differences in age or the age of onset of lupus were not significant. Those with elevated TPA had lower double-stranded DNA antibody levels and SLEDAI scores and greater complement levels.
Table I.
Comparison of continuous variables between SLE cases (abnormal total plaque area (TPA)) and SLE controls (normal TPA)
| Controls (n=37) | Cases (n = 14) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | mean/median | std erra | IQRb | mean/median | std erra | IQRb | p value |
| Total Plaque Area (cm2) | 0 | 0.181 | 0.808 | 0.727 | <0.0001 | ||
| C4 (mg/dl) | 17 | 9 | 23 | 8 | 0.02 | ||
| Hemoglobin (m/mm3) | 11.4 | 0.2 | 12.4 | 0.5 | 0.02 | ||
| SLEDAI Score | 4 | 5 | 2 | 4 | 0.03 | ||
| 25(OH) Vitamin D | 19.9 | 14.3 | 13.1 | 11.9 | 0.03 | ||
| Anti-dsDNA antibodies (IU) | 94 | 229 | 8 | 30 | 0.04 | ||
| Years since SLE diagnosis | 5 | 10 | 13 | 8 | 0.04 | ||
Standard error
Interquartile range
Age and SLE disease duration correlate with TPA, while SLEDAI elements and 25(OH)D are inversely correlated with TPA
Correlations between traditional and SLE-related risk factors for cardiovascular disease and TPA were determined. None of the traditional cardiovascular risk factors correlated with TPA except for age (r = 0.58, p < 0.001). Systolic blood pressure was negatively associated with TPA (r = -0.30, p = 0.035). This is not entirely unexpected, as TPA in other studies is a more effective marker of risk for cardiovascular outcomes than hypertension (25). TPA was positively associated with the number of years since SLE diagnosis (r = 0.37, P. = 0.008) and SLE onset age (r = 0.38, p = 0.006). SLE activity measures were inversely associated with TPA (SLEDAI score r = -0.31, p = 0.03, C4 r = 0.32, p = 0.02). The number of years since SLE diagnosis was inversely associated with SLEDAI score (r = -0.48, p < 0.001), suggesting that the inverse association between SLEDAI score and TPA may relate to lower disease activity later in disease. Cumulative damage as measured by the total SLICC score or eGFR did not correlate with TPA when corrected for age. This observation suggests that SLE patients in this population need not have severe major organ involvement or renal damage to develop premature atherosclerosis. To eliminate age as a factor in the analysis, TPA was expressed as an absolute area above the mean in age-matched control subjects. The same associations were observed in this analysis (data not shown), but when age was adjusted for in this manner an inverse correlation between 25(OH)D was observed (r = -0.33, p = 0.018).
ACE-inhibitor non-use and high LDL associate with high TPA
By Chi-square analysis, those subjects who were not taking an ACE inhibitor were much more likely to have elevated TPA (LR = 9.0, p = 0.02). No subjects taking an ACE inhibitor (n = 12) had an elevated TPA, while 14 of the 39 patients not taking an ACE inhibitor had an elevated TPA. Associations between ACE inhibitor use and other potential cardiovascular risk factors were described to explore for potential confounding variables. All subjects taking an ACE inhibitor had a history of hypertension; however, those with a history of hypertension were not more or less likely to have an elevated TPA. Similarly, those with an elevated TPA did not have significantly greater amounts of proteinuria. Renal function was similar between ACE inhibitor users and nonusers (78 ± 11 vs. 88 ± 6, p = 0.4). These combined observations suggested that ACE inhibitor use was not simply a marker for hypertension or renal disease. Those with current or prior hypercholesterolemia were also more likely to have elevated TPA (LR = 3.9, p = 0.048), and baseline LDL > 100 was also a risk factor for elevated TPA (LR = 4.6, p = 0.032). Those with 25(OH)D levels < 15 ng/ml tended to be cases (LR = 3.8, P = 0.051).
25(OH)D and SLE disease activity elements discriminate between normal and abnormal TPA
To explore the combined sensitivity and specificity of individual risk factors in predicting elevated TPA, receiver operating characteristics curves (ROC) were created for all continuous variables against the diagnosis of elevated TPA. Subject 25(OH)D level at entry resulted in a significant ROC AUC (0.71 (confidence interval (CI) 0.52-0.89), p = 0.03) for elevated TPA. None of the traditional cardiovascular risk factors resulted in ROC AUC values that were significant. Of note, the lupus activity parameters associated with lack of plaque, while SLE disease duration associated with plaque (C4 level 0.70 (0.55 - 0.86), p = 0.03; anti-dsDNA levels 0.70 (0.55 - 0.85) p = 0.03; SLE duration 0.69 (0.54 - 0.84), p = 0.04).
Logistic regression model in favors ACE-inhibitor use, low 25(OHD), and hypercholesterolemia for diagnosing abnormal TPA
A backward stepwise logistic regression analysis was performed using season, 25(OH)D level, entry LDL level, ACE-inhibitor use, and hydroxychloroquine use. Season and hydroxychloroquine use did not contribute significantly to the model and were excluded. The model with the best overall accuracy contained ACE-inhibitor use, 25(OH)D levels, and history of hypercholesterolemia (Chi-square = 15.9, p = 0.001). The sensitivity for predicting abnormal TPA was 46%, while the specificity was 92%, and the overall diagnostic accuracy was 80% (Table II). The model likelihood ratio was 16.6. A similar analysis using LDL at baseline instead of entry a history of hypercholesterolemia produced a similarly robust model (data not shown). Similar results were obtained when imputed variables (25(OH)D values for one case and one control) were excluded from the analysis or when season was included in the model (data not shown).
Table II.
ROC AUC and accuracy of logistic regression, NRN, and ANN models of abnormal TPA.
| Model type | ROC AUC | Accuracy | variables in the model |
|---|---|---|---|
| Logistic regression | -- | 80% | ACE, 25(OH)D, hypercholesterolemia |
| Nearest Related Neighbor (NRN) | 0.75 | -- | Hydroxychloroquine use, 25(OH)D, ACE, statin |
| Artificial Neural Network (ANN) | 0.88 | -- | WHR, BMI, SBP, HDQ, TG, 25(OH)D, SLE onset age, DBP, LDL, age, SLEDAI, ACE, season, hypercholesterolemia, SLICC, years with SLE |
Nearest Related Neighbor (NRN) models containing hydroxychloroquine, ACE inhibitor, and statin use and 25(OH)D levels are most diagnostic of abnormal TPA
Input variables for NRN modeling were chosen either because they were significant in ROC analysis (AUC > 0.6) or because they were associated with cardiovascular disease in other studies. Season was included as an input variable but was not important in the model. The resulting exploratory NRN model had good diagnostic power, with an ROC AUC of 0.75. Hydroxychloroquine, ACE-inhibitor, and statin use and 25(OH)D levels were the variables that provided the most diagnostic power in the model (Table II).
Artificial Neural Network (ANN) Models use multiple traditional and novel variables to create a model with excellent diagnostic power
ANN analysis is well suited for large numbers of input variables with large datasets. Non-linear and co-linear associations can be included in ANN models (23). The median performing exploratory model was selected after training 11 ANN models. The median ROC AUC was 0.88. Waist-to-hip ratio, BMI, SBP, hydroxychloroquine use, triglyceride level, 25(OH)D level, SLE onset age, DPB, LDL, age, SLEDAI score, ACE inhibitor use, season, diagnosis of hypercholesterolemia, SLICC score, and years with SLE diagnosis accounted for (in that order) 89% of the sensitivity in the model (Table II).
Discussion
The current study was designed to determine potentially modifiable clinical risk factors that associate with atherosclerosis in a predominantly African-American cohort of SLE patients. In this study, ACE-inhibitor and hydroxychloroquine non-use, hypercholesterolemia, and lower 25(OH)D levels were modifiable factors significantly associated with elevated TPA.
To our knowledge, this is the first study to demonstrate an association between ACE-inhibitor non-use and abnormal atherosclerotic plaque in SLE. A possible protective effect of this medication is consistent with lower rates of atherosclerosis and cardiovascular events among ACE-inhibitor users in non-SLE at risk populations (26). ACE-inhibitors may work through restoration of endothelial cell function (8). The mechanism behind this effect is not fully understood. However, ACE-inhibitors lead to improved endothelial function in patients with vascular disease (8). Angiotensin receptor I ligation leads to superoxide generation that reduces nitric oxide levels and thus endothelial function. ACE-inhibitors may also work by inhibiting these processes (27). These hypotheses are not addressed in the current study but provide rationale for future investigation.
To our knowledge, this is also the first study to demonstrate a significant association between 25(OH)D deficiency and abnormal atherosclerotic plaque in patients with SLE. Wu et al. reported an association between 25(OH)D and cardiovascular risk factors but did not show an association with imaging markers of atherosclerosis (28). The current study may have reached significance in a smaller number of patients because of a greater prevalence of 25(OH)D deficiency in our study population (61% with levels < 15 ng/ml vs. 20% in the cohort described by Wu et al), and studies using TPA as an endpoint require fewer numbers of subjects. In a similar study by Moc et al. low 25(OH)D levels were associated with cardiovascular risk factors such as dyslipidemia but not with abnormal intima media thickness or coronary calcium (29). A lack of association in the Mok study may have occurred because there was no normalization for age or measurement of a plaque area in the atherosclerosis imaging. A possible protective effect of 25(OH)D suggested by the current study is consistent with other studies of non-SLE at risk populations. Men in the Health Professionals Follow-up Study with deficient 25(OH)D levels (≤ 15 ng/ml) at baseline had greater adjusted risk of myocardial infarction after ten years than those with sufficient levels (≥30 ng/ml) (5). In another prospective, longitudinal study, successful supplementation of deficient 25(OH)D levels (to > 30 ng/ml) was associated with reduced risk of coronary artery disease, myocardial infarction, heart failure, stroke, renal failure, and death (30).
The mechanisms through which vitamin D may prevent atherosclerosis in SLE are not known. However, the 1,25(OH)D/vitamin D receptor complex can increase the expression of vascular endothelial growth factor (VEGF) in vascular smooth muscle cells. VEGF, in turn, increases endothelial nitric oxide synthase activity in endothelial cells and increases the number of endothelial progenitor cells responsible for repair of the endothelium (31).
The increased power of machine learning models to diagnose those with abnormal plaque demonstrates their utility in a multifactorial disease such as atherosclerosis on the setting of a heterogeneous disease such as SLE. These results provide the rationale for testing machine learning modeling in larger patient populations to validate this approach.
This study is limited by its cross sectional design and limited sample size. The sample size limitation is less concerning given the greater discriminatory power of TPA when compared to traditional measures such as IMT (11). The findings of this study should be confirmed in longitudinal studies, and modeling should be qualified in an independent, external patient population. This study is unique in its focus on African-American SLE subjects. (14)
This study of a predominantly African-American population of SLE patients demonstrates an association between lower atherosclerotic burden and factors that are known to restore endothelial function in non-SLE populations or that reduce flare rates in SLE. This study provides a new rational basis for testing the effect of ACE-inhibitors and/or 25(OH)D repletion as primary atherosclerosis prevention in SLE.
Supplementary Material
Acknowledgement
Special thanks go to Lori Ann Ueberroth for coordinating this study and Marge Cappuccio for performing the carotid ultrasounds. We are ever grateful to the lupus and control patient participants for their generosity.
Source of support.
This project was supported by NIH/NCRR MUSC-SCTR Grant number UL1 RR029882, NIH/NIAMS Grant number K23 AR052364, and the MUSC General and Clinical Research Center (M01RR001070). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. Grant funding for this project also came from an award from the VA Research Enhancement Award Program and a grant from the Lupus Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Roneka L. Ravenell, Division of Rheumatology, Department of Medicine, Medical University of South Carolina, Charleston, SC..
Diane L. Kamen, Division of Rheumatology, Department of Medicine, Medical University of South Carolina, Charleston, SC..
J. David Spence, Stroke Prevention and Atherosclerosis Research Centre, Robarts Research Institute, University of Western Ontario, London, ON, Canada..
Bruce W. Hollis, Department of Pediatrics, Medical University of South Carolina, Charleston, SC.
Thomas J. Fleury, Division of Rheumatology, Department of Medicine, Medical University of South Carolina, Charleston, SC..
Michael G. Janech, Division of Nephrology, Department of Medicine, Medical University of South Carolina, Charleston, SC..
Jonas S. Almeida, Department of Bioinformatics and Computational Biology, The University of Texas M. D. Anderson Cancer Center, Houston, TX..
Stephanie R. Shaftman, Division of Biostatistics and Epidemiology, Department of Medicine, Medical University of South Carolina, Charleston, SC..
Jim C Oates, Division of Rheumatology, Department of Medicine, Medical University of South Carolina, Charleston, SC., Chief of Rheumatology, Medical Service, Ralph H. Johnson VA Medical Center, Charleston, SC..
References
- 1.Urowitz MB, Ibanez D, Gladman DD. Atherosclerotic vascular events in a single large lupus cohort: prevalence and risk factors. J Rheumatol. 2007;34:70–5. [PubMed] [Google Scholar]
- 2.Scalzi LV, Hollenbeak CS, Wang L. Racial disparities in age at time of cardiovascular events and cardiovascular-related death in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:2767–75. doi: 10.1002/art.27551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhew EY, McPherson DD, Dyer AR, Kondos GT, et al. Endothelial dysfunction (ED) correlates with plaque burden in systemic lupus erythematosus (SLE) women.. American College of Rheumatology National Meeting; San Diego, CA. 2005. [Google Scholar]
- 4.Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003;115(Suppl 8A):99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and Risk of Myocardial Infarction in Men: A Prospective Study. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarcin O, Yavuz DG, Ozben B, Telli A, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–30. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 7.Kamen DL, Cooper GS, Bouali H, Shaftman SR, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–7. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Mancini GBJ, Henry GC, Macaya C, O'Neill BJ, et al. Angiotensin-Converting Enzyme Inhibition With Quinapril Improves Endothelial Vasomotor Dysfunction in Patients With Coronary Artery Disease: The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258–65. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Spence JD. Ultrasound measurement of carotid plaque as a surrogate outcome for coronary artery disease. Am J Cardiol. 2002;89:10B–5B. doi: 10.1016/s0002-9149(01)02327-x. discussion 5B-6B. [DOI] [PubMed] [Google Scholar]
- 11.Spence JD, Eliasziw M, DiCicco M, Hackam DG, et al. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–22. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 12.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–7. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 13.Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke. 2007;38:2873–80. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 14.Roman MJ, Shanker BA, Davis A, Lockshin MD, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 15.Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke. 2010;41:1193–9. doi: 10.1161/STROKEAHA.110.577973. [DOI] [PubMed] [Google Scholar]
- 16.Buyon JP, Petri MA, Kim MY, Kalunian KC, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–62. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gladman DD, Urowitz MB. The SLICC/ACR damage index: progress report and experience in the field. Lupus. 1999;8:632–7. doi: 10.1191/096120399680411335. [DOI] [PubMed] [Google Scholar]
- 18.Group, KDCPGoCKDW. Part 5. Evaluation of Laboratory Measurementsfor Clinical Assessment of Kidney Disease. Am J Kidney Dis. 2002;39:S76–S110. [Google Scholar]
- 19.Hollis BW. Comparison of commercially available (125)I-based RIA methods for the determination of circulating 25-hydroxyvitamin D. Clin Chem. 2000;46:1657–61. [PubMed] [Google Scholar]
- 20.Bishop CM. Pattern recognition and machine learning. Springer; New York: 2006. pp. 124–127. [Google Scholar]
- 21.Almeida JS. [July 15, 2010]; BioinformaticStation.ORG September 29.2006., 2010.
- 22.Oates JC, Varghese S, Bland AM, Taylor TP, et al. Prediction of urinary protein markers in lupus nephritis. Kidney Int. 2005;68:2588–92. doi: 10.1111/j.1523-1755.2005.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida JS. Predictive non-linear modeling of complex data by artificial neural networks. Curr Opin Biotechnol. 2002;13:72–6. doi: 10.1016/s0958-1669(02)00288-4. [DOI] [PubMed] [Google Scholar]
- 24.Hollis BW. Circulating 25-Hydroxyvitamin D Levels Indicative of Vitamin D Sufficiency: Implications for Establishing a New Effective Dietary Intake Recommendation for Vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 25.Al-Shali K, House AA, Hanley AJ, Khan HM, et al. Differences between carotid wall morphological phenotypes measured by ultrasound in one, two and three dimensions. Atherosclerosis. 2005;178:319–25. doi: 10.1016/j.atherosclerosis.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 26.The Heart Outcomes Prevention Evaluation Study Investigators Effects of an Angiotensin-Converting-Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 27.Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–6. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 28.Wu PW, Rhew EY, Dyer AR, Dunlop DD, et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Care Res. 2009;61:1387–95. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok CC, Birmingham DJ, Leung HW, Hebert LA, et al. Vitamin D levels in Chinese patients with systemic lupus erythematosus: relationship with disease activity, vascular risk factors and atherosclerosis. Rheumatology (Oxford) 2011 doi: 10.1093/rheumatology/ker212. [DOI] [PubMed] [Google Scholar]
- 30.Bair T, Muhlestein, JBM HT, Horne BD, Carlquist JF, et al. Supplementing deficient vitamin D levels is associated with reduced cardiovascular risk.. American College of Cardiology Annual Meeting; Atlanta, GA. March 15; 2010. p. Presentation number 1186-111. [Google Scholar]
- 31.Cardus A, Panizo S, Encinas M, Dolcet X, et al. 1,25-Dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis. 2009;204:85–9. doi: 10.1016/j.atherosclerosis.2008.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


