Abstract
Innate immunity mediated by microglia appears to play a crucial role in initiating and propagating seizure-induced inflammatory responses. To address the role of activated microglia in the pathogenesis of childhood epilepsy, we first examined the time course of microglia activation following kainic acid induced status epilepticus (KA-SE) in Cx3cr1GFP/+ transgenic mice whose microglia are fluorescently labeled. We then determined whether this seizure-induced microglia activation primes the central immune response to overreact and to increase the susceptibility to a second seizure later in life. We used an inhibitor of microglia activation, minocycline, to block the seizure-induced inflammation to determine whether innate immunity plays a causal role in mediating the long-term epileptogenic effects of early-life seizure. First status epilepticus was induced at postnatal day (P) 25 and a second status at P39. KA-SE at P25 caused nearly a twofold increase in microglia activation within 24 hours. Significant seizure-induced activation persisted for 7 days and returned to baseline by 14 days. P39 animals with prior exposure to KA-SE not only responded with greater microglial activation in response to “second hit” of KA, but shorter latency to express seizures. Inhibition of seizure-induced inflammation by 7 day minocycline post-treatment abrogated both the exaggerated microglia activation and the increased susceptibility to the second seizure later in life. The priming effect of early-life seizures is accompanied by modified and rapidly reactivated microglia. Our results suggest that anti-inflammatory therapy after SE may be useful to block the epileptogenic process and mitigate the long-term damaging effects of early-life seizures.
Keywords: Innate Immunity, Anti-inflammatory Therapy, Status Epilepticus, Kainic Acid, Neuroinflammation
1. Introduction
There is growing recognition that neuroinflammation mediates many aspects of disease for chronic neurodegenerative disorders as well as for acute brain injury, autistic spectrum disorder and epilepsy (Choi and Koh, 2008; Vargas et al., 2005; Vezzani et al., 1999). Cytokines, free radicals, and excitatory neurotransmitters released from activated microglia provide the commonality between neuroinflammatory processes in many neurological conditions including multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (Balistreri et al., 2007; Conductier et al., 2010; McCoy and Tansey, 2008). Chronic microglial activation is an important component of these diseases and likely contributes to neuronal dysfunction, injury, loss, and hence to progression of neurological conditions. Dysregulated microglial activity may be responsible for detrimental rather than reparative central responses in various CNS diseases.
The role of brain inflammation in childhood epilepsy has long been implicated in pediatric infectious or autoimmune diseases that are often accompanied by recurrent seizures. In addition, pro- and anti-inflammatory molecules are synthesized during seizures in the brain at the sites of seizure initiation and propagation (Murashima et al., 2008). Extensive microglia activation occurs in the brain parenchyma of individuals with chronic intractable epilepsy and in animal models of seizures (Avignone et al., 2008; Beach et al., 1995; Choi et al., 2009; Drage et al., 2002; Fabene et al., 2010; Somera-Molina et al., 2009; Yang et al., 2010). Microglia, the resident macrophage in the brain parenchyma, constitute the first line of defense against pathological changes within the central nervous system (CNS) microenvironment. CNS injury triggers rapid activation of microglia with concomitant changes in distribution, morphology, immunophenotype, and metabolism. Swelling of the microglial cell body, a thickening of the proximal processes, and a reduction in the number and complexity of distal processes are key morphological signs of this activation (Buttini et al., 1996; Kloss et al., 2001). The extent to which activated microglia are involved in CNS homeostasis or diseases is a delicate balance. On the one hand, activation of the innate immune system controls infections, removes debris and promotes repair. On the other hand, these otherwise normal glial functions can sometimes result in a more severe and chronic neuroinflammatory cycle that actually promotes the shift from a physiological to a pathological inflammatory response.
We have previously developed a “two-hit” seizure model demonstrating that an early life seizure permanently decreases seizure threshold and increases the susceptibility to seizure-induced cell death in adulthood (Koh et al., 1999; Somera-Molina et al., 2007). Seizures in immature animals cause subtle functional changes and alter the response to seizures later in life (Dube et al., 2000; Holmes et al., 1998; Koh et al., 2004; Sankar et al., 1998). To elucidate the role of activated microglia “primed” by early life convulsions in later seizure susceptibility, we determined whether heightened seizure susceptibility is accompanied by exaggerated microglia activation in animals with prior exposure to early-life SE in our “two-hit” rodent seizure model. We hypothesize that the inflammatory reaction provoked by early-life seizures primes the developing brain so that microglia are modified, leading to rapid reactivation by a second seizure in adulthood. We first visualized and quantified the time course of microglia activation after kainic acid (KA)- induced status epilepticus (SE) in juvenile Cx3cr1GFP/+ transgenic mice whose parenchymal microglia are fluorescently labeled. To test the functional significance of marked activation of microglia caused by an early-life prolonged seizure, we determined whether inhibition of inflammation by minocycline, a known inhibitor of microglia activation (Henry et al., 2008; Pabreja et al., 2011), abrogates the exaggerated microglia activation and thus susceptibility to second seizure later in life.
2. Materials and Methods
2.1. Animals
We used Cx3cr1GFP/+ transgenic mice in which the fractalkine chemokine receptor has been replaced by a green fluorescent protein (GFP) reporter gene by targeted deletion via homologous recombination in embryonic stem cells (Davalos et al., 2005; Jung et al., 2000). Mice were group housed in polypropylene cages and maintained at 21°C with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Children’s Memorial Research Center Institutional Animal Care and Use Committee.
2.2. Kainic acid-induced seizures
It has long been shown that systemic injection of KA in rodents induces prolonged seizures (Ben-Ari, 1985). These seizures originate in the CA3 region of the hippocampus and can spread to other limbic structures and can be followed by neuronal loss in selected regions of the brain reminiscent of brain damage seen in patients with temporal lobe epilepsy. Here, seizures were induced by intraperitoneal (i.p.) injection of KA as previously described (Hu et al., 1998). In brief, postnatal day (P) 25 and 39 mice received an i.p. injection of either sterile phosphate buffered saline (PBS) or KA (20 mg/kg). Seizure severity and latency to the first sign of seizure were recorded. A seizure severity grade was assigned based on the maximal response achieved on a scale from 0 to V as follows: 0 — no response; I — behavioral arrest; II — staring, pawing, and head bobbing; III — clonic jerks, rearing and falling; IV — continuous grade III seizures for longer than 30 min (status epilepticus); V — death. Only mice with status epilepticus (Grade IV) were included in the study. Latency to Grade III was calculated and compared between groups; Grade 0 – II seizures were excluded from latency calculation.
2.3. Experimental Protocol
To study the time course of seizure-induced microglia activation, P25 Cx3cr1GFP/+ mice were sacrificed 1, 7 or 14 days after administration of PBS or KA. To determine the effects of early-life seizures on seizure susceptibility and microglial activation in response to the later-life “second-hit” KA, a separate group of mice were injected with PBS or KA on P25 and followed by an injection of KA on P39. On P40, 24 h after KA injections, mice were perfused transcardially.
To establish whether the tetracycline derivative, minocycline, inhibits acute seizure-induced microglia activation, P25 mice received an i.p. injection of PBS or minocycline (20 mg/kg) 3 hours after KA-SE induction and for the following six consecutive days. Minocycline (Sigma, St. Louis, MO) was dissolved in sterile water and sonicated to ensure complete solubilization. On the 7th day, 24 hour (h) after the last dose, mice were sacrificed and area of green fluorescent microglia in the hippocampus was quantified and compared between PBS and minocycline treated animals.
To determine the efficacy of minocycline in blocking the long-term effect of early-life seizures on later life seizure susceptibility and subsequent microglia activation, a separate study was conducted where P25 mice received a KA injection followed by PBS or minocycline treatment for 7 days as detailed above. Following an additional 7 days, P39 mice received an injection of KA and sacrificed 24 h later on P40.
2.4. Quantification of microglial cell activation
Mice were deeply anesthetized and perfused transcardially with PBS followed by ice-cold 4% performaldehyde/0.1M sodium phosphate buffer. Brains were harvested, post-fixed with 4% performaldehyde/30% sucrose solution over night, and mounted on a freezing microtome. 40 μm horizontal sections were cut, and every 6th section collected and mounted on slides for microscopic examination. At least 6 hippocampal sections per brain from at least 4 animals (n = 4–10) per group were selected for quantification. The anterior commissure was used as a specific landmark to match sections across experiments. Images were captured digitally at 20X magnification, converted to gray scale, and areas of positively labeled green fluorescent cells were highlighted within the hilus of the hippocampus for initial time course experiments (Figure 1) to allow consistent comparison between controls and KA animals over time. For all subsequent experiments (Figure 2–4), area directly adjacent to the fimbria (near CA3 hippocampal subregion), where microglia activation was maximal after KA-SE, was captured for quantification. Sum total spot count - defined as cell counts/0.27 mm2 unit area reflecting density of microglia - was quantified manually by including brightly fluorescent microgla with visible processes and distinct soma of at least 5 μm diameter while excluding cells out of focus with indistinct borders. Sum total spot count was quantified within the hilus where microglia were uniformly dispersed (Figure 1D, H & L). Because spot counts of individual cells within the aggregates in CA3 subregion could not be determined reliably, quantification of microglia activation was limited to % area fluorescence in Figures 2C, 3D & 4D. To control for intra/inter observer reliability, the quantification threshold was held constant for all specimens within each experimental group and quantified using ImageJ (1.43u, Public Domain, NIH) by a single observer (A.B.) who was blinded to the treatment groups.
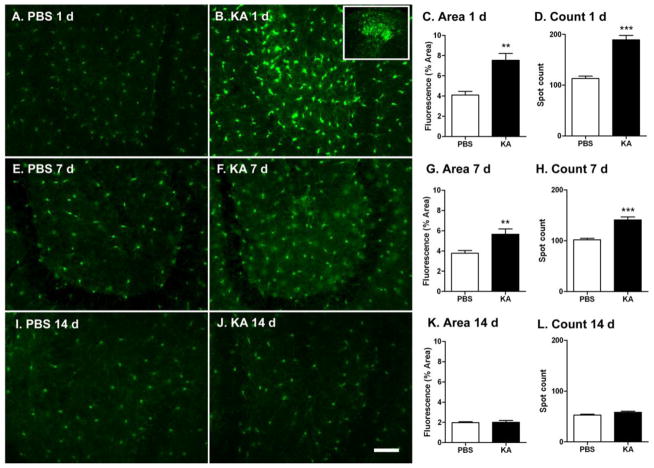
Figure 1. KA-SE induced acute and transient activation of microglia.
P25 Cx3cr1GFP/+ mice were injected i.p. with either KA (20mg/kg) or PBS and microglia activation within the hilus of the hippocampus assessed at 1(A–D), 7 (E–H) or 14 (I–L) days after administration of PBS or KA. Scale bar: 50μm. C & D 1 d PBS n=4; KA n=4; G & H, 7 d: PBS n=8, KA n=7; K & L 14 d: PBS n=7; KA n=5. (**p<0.01, ***p<0.001, Student’s t-test).
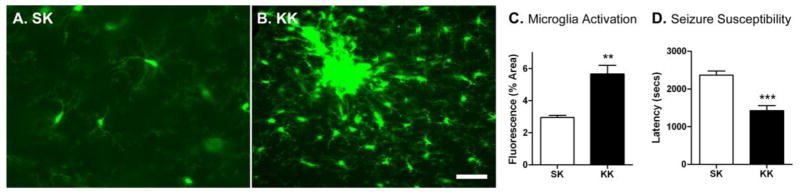
Figure 2. Early-life seizure increases microglia response and seizure susceptibility to later life seizures.
Mice were injected with PBS or KA (20mg/kg) on P25 followed by an injection of KA on P39 (A. SK vs. B. KK). Scale bar: 50μm. C. Microglia activation in the hippocampal CA3 subregion was assessed 24h after last injection. SK n=5, KK n=5. (***p<0.001, Student’s t-test). D. Seizure susceptibility defined as the latency to the onset of grade III seizure was assessed. (SK n=6, KK n=8, ***p<0.001, Student’s t-test). 9/15 SK and 12/15 KK mice experienced status epilepticus (p = 0.42, Fisher’s exact test); 3/9 SK and 4/12 KK animals died after status.
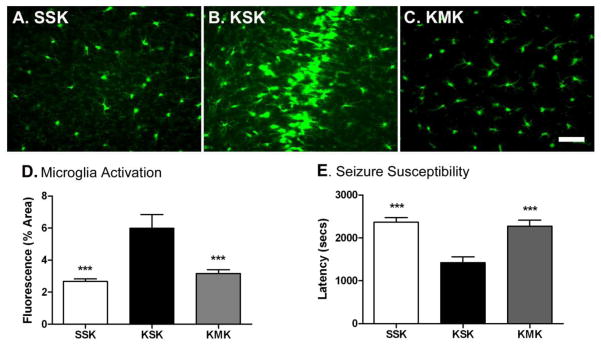
Figure 4. Minocycline post-treatment prevents the priming effect of early-life seizures.
P25 mice received an i.p. injection of PBS or minocycline (20 mg/kg) 3 hours after KA-SE induction and for the following six consecutive days. Following an additional 7 days, P39 mice received an i.p. injection of KA (A. SSK vs. B. KSK vs. C. KMK) and sacrificed 24 h later on P40. Scale bar: 50μm. D. Microglia activation was assessed 1 d after last injection. SSK n=4; KSK n=4; KM n=10, p<0.001, One-Way ANOVA with post-hoc Tukey test (***p<0.001). E. Seizure susceptibility was determined by latency (seconds) to grade III seizure. SSK n=6; KSK n=8; KM n=10, p<0.001, One-Way ANOVA with post-hoc Tukey test (***p<0.01). Comparable numbers of SSK, KSK and KMK experienced SE after KA injection at P39 (8/14, 8/9, 14/17, p>0.2, Fisher’s exact test). 6 mice from SSK, 1 from KSK and 3 from KMK had Grade II-III seizures while 2, 1 and 4 animals died, respectively.
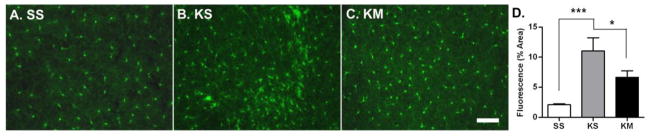
Figure 3. Minocycline post-treatment reduces early life seizure-induced microglia activation.
P25 mice were injected with PBS or KA (20mg/.kg) followed by either an i.p. injection of PBS (A. SS or B. KS) or minocycline (20 mg/kg, C. KM) for 7 consecutive days. Scale bar: 50μm. D. Microglia activation was assessed 1 d after last injection. SS n=6; KS n=5; KM n=7, p<0.001, One-Way ANOVA with post-hoc Tukey test (*p<0.05, **p<0.01).
2.5. Statistical Analysis
Student’s t-test (GraphPad Prism v. 4.0, GraphPad Software Inc., San Diego, CA) was used to compare the latency to seizure-onset and microglial activation between SS and SK. One-way analysis of variance (ANOVA) with a post hoc t-test and Tukey corrections was used to compare differences in microglia activation and seizure latency among 3 different experimental groups. Fisher exact test was used to compare number of microglia aggregates between PBS and minocycline treated animals 7 days after KA and to compare seizure severity (ratio of Grade IV seizures) between groups. Values are expressed as mean ± standard error of the mean (SEM). Significance was defined as p <0.05 for all tests.
3. Results
3.1. Transient Microglia Activation After KA-Induced Seizures
KA injections induced prolonged seizures (>1 h) in 83% (35/42) of P25 Cx3cr1GFP/+ transgenic mice; 2 subsequently died while 6/42 mice had only grade I–III seizures. KA-SE caused acute (24 h) as well as more sustained (7 days) microglia activation (Figure 1A – I). There was nearly a two-fold increase in the area of fluorescent cells within the hilus of hippocampus in KA treated mice compared to control littermates one day after SE (Figure 1C; n=4/group, p<0.01). Moreover, there was a significant increase in microglial cell number as indicated by a sum total spot count (Figure 1D; p<0.001). In addition to greater density of microglia compared to controls, in some mice (50%, 2/4), aggregates of microglia were observed over the CA3 subregion of the hippocampus, an area most vulnerable to cell injury after KA-SE in mice (Hu et al., 1998) (Figure 1B, box insert). Seven days following KA-SE, microglia remained significantly activated compared to age-matched controls (Figure 1G & H; n=8, PBS, n=7 K, p<0.01). The level of microglia activation returned to baseline two-weeks after KA-SE (Figure 1K & L). In short, KA-SE on P25 mice caused marked acute and persistent activation of hippocampal microglial cells. This activation, however, is not permanent and subsides within 14 days.
3.2. Early-life Seizure Primes Microglia Response to Later Life Seizures
An initial early-life seizure on P25 exacerbated microglia activation in response to a second seizure on P39 (Figure 2). Nearly a three-fold increase in the area of fluorescent cells was noted in “two-hit” mice (KK) with prior experience of early-life seizures compared to their “single-hit” control littermates (SK) (Figure 2C, p<0.001). This exaggerated microglia response to KA-SE was accompanied by an increase in susceptibility to the second seizure later in life. Latency to seizure onset (data expressed as seconds to grade III seizure ± SEM) was significantly reduced in mice exposed to KA both on P25 and P39 (KK) compared to mice only given KA on P39 (SK) (Figure 2D, p<0.001). Thus, heightened sensitivity to KA-SE reflected by the shortened latency to seizure onset is correlated with the modified and rapidly reactivated microglia by a second seizure in adulthood.
3.3. Minocycline post-treatment reduces early life seizure-induced microglial cell activation
Following 7 daily doses of minocycline treatment after KA-SE at P25 (first dose given on the day of KA-SE), mice were sacrificed at P32 and microglia activation quantified by comparing presence or absence of microglia aggregates and by the percent area of fluorescence (Figure 3). Similar to the above findings on the time course experiment (3.1), microglia activation remained significantly increased in KA-treated animals (KS) compared to control animals (SS) 7 days after injections (SS vs. KS; p<0.001) and this persistent microglia activation was significantly inhibited by minocycline (KS vs. KM; p<0.05). In addition, microglia aggregates were observed in 47% (14/30) of hippocampal sections from KA injected animals (n=5) 7 days after KA-SE while these aggregates were observed in less than 20% (8/42) of hippocampal sections from mice (n=7) treated with minocycline (p<0.02, Fisher exact test). In summary, daily low dose of minocycline (20mg/kg), administered for 7 days after KA-SE at P25, mitigated seizure-induced microglia activation on P32.
3.4. Minocycline post-treatment prevents the priming effect of early-life seizures
Post-treatment with minocycline following KA-SE on P25 prevented the increased seizure susceptibility and exacerbated microglial activation to a second seizure on P39 in the animals exposed to early life seizures (Figure 4). Similar to findings above (3.2), “two-hit” mice (KSK) with the experience of early-life seizures 14 days earlier compared to “single-hit” control littermates (SSK) showed exaggerated microglia response to second KA-SE. This increase was no longer apparent in animals treated with minocycline after KA exposure on P25 (KMK) (Figure 4D; p<0.001). This decrease in exaggerated microglia activation by minocycline was accompanied by an increase in seizure latency to a second-hit of KA in animals treated with minocycline (KMK) compared to saline treated controls (KSK) (Figure 4E; p<0.001); minocycline treatment returned seizure latency to that of animals only exposed to one KA-induced seizure. Thus, minocycline treatment prevented the priming effect of an initial seizure and prevented exacerbation of microglia activation as seen in KSK groups.
4. Discussion
We found that KA-induced status epilepticus caused acute activation of hippocampal microglia that persisted for as long as 7 days and subsided by 14 days post seizure in developing mice. When the animals were challenged with a second seizure 14 days later, microglia activation was markedly exaggerated in mature animals with prior experience of early-life seizures in the absence of ongoing microglia activation from the initial seizure on P14. This heightened microglia activation was accompanied by heightened susceptibility to a second seizure later in life. Both increased seizure susceptibility and exacerbated microglia activation to later-life seizure were attenuated by minocycline administered after initial seizure. Minocycline reduced initial seizure-induced microglia activation and mitigated the priming effect of early-life seizures. Taken together, our data support the hypothesis that microglia activation caused by early-life seizure renders developing animals more susceptible to subsequent seizures and seizure-induced inflammatory reaction.
Normally, microglia reside in a quiescent state, but recent evidence implicates another subset of microglia which reside in an intermediate state characterized by shortened processes and expression of cell surface markers, similar to activated microglia, but without appreciable secretion of cytokines (Cunningham et al., 2003; Frank et al., 2007; Perry et al., 2003). This microglia subset is referred to as primed due to more rapid induction and greater cytokine release upon activation compared to normally activated, non-primed microglia (Sparkman and Johnson, 2008). This phenomenon was first described in association with neurodegenerative diseases including multiple sclerosis, Alzheimer’s disease and the murine ME7 model of prion disease (Combrinck et al., 2002; Perry et al., 2002). Similar to the priming paradigm associated with neurodegenerative disease, we provide indirect evidence here that primed microglia may play a pivotal role in early-life seizures for later-life seizure susceptibility.
We found that animals “primed” with a KA-SE at P25 were more susceptible to seizures when rechallenged with KA at P39 and that treatment with minocycline for 7 days following the initial seizure prevented this increase in seizure susceptibility at P39. Seizure susceptibility in this study was operationally defined as latency to seizure onset following the systemic administration of the KA. Since the shortened latency to KA-induced seizures or heightened seizure susceptibility by early-life seizures was reversed by inhibition of seizure-induced microglia activation by minocycline, it is tempting to speculate that blocking neuroinflammation after early-life seizure may alter the ongoing disease process. Our “two-hit” study protocol, however, has limitation in providing direct evidence for the antiepileptogenic action of anti-inflammatory drugs. Such disease modifying properties of anti-inflammatory therapies may be more directly studied by considering the sequence of events that occur with time after SE such as upregulation of inflammatory genes, cytokine release, and compromised integrity of the blood-brain barrier (BBB), concomitant with continuous EEG monitoring with or without drug treatment.
One of the first responses to CNS injury is the migration of microglial cells to the site of damage triggered by substances including cytokines released at the damage site (Boissonneault et al., 2009; Lehnardt, 2010; Perry, 2010). This remarkable ability to rapidly respond to a changing external environment and to direct the response of the rest of the innate and adaptive immune system in the CNS makes microglia a prime target for therapeutic intervention in a wide variety of CNS insults. The semi-synthetic tetracycline derivative, minocycline, has potent anti-inflammatory effects independent from its microbicidal properties, as it is well known to inhibit macrophage and microglia activation (Henry et al., 2008; Pabreja et al., 2011). In our two-hit seizure model, minocycline mitigated seizure-induced microglia activation rather than completely block inflammatory response (Figure 3). Minocycline treatment after KA-SE, therefore, may have promoted the shift from a pathologic to a physiologic, homeostatic glial response. Minocycline exerts neuroprotective effects in animal models of various neurodegenerative diseases (Hu et al., 2009; Kriz et al., 2002; Thomas et al., 2003) as well as in various experimental injury paradigms including N-methyl-D-aspartate (NMDA) mediated glutamate neurotoxicity (Pi et al., 2004), ischemic injury (Tikka and Koistinaho, 2001), nitric oxide (NO) (Lin et al., 2001), and MPTP excitotoxicity (Du et al., 2001).
The anti-inflammatory effects of minocycline may be mediated by modulation of MAPK, c-JNK, AP-1, and NF-κB (Cai et al., 2010) gene transcription. Recently, in a model of neuronal transplantation, minocycline was shown to promote long-term survival of neuronal xenograft by inhibiting microglial activation and T-cell recruitment (Michel-Monigadon et al., 2010). This is of particular interest to our model of long-term affects of early-life seizures because BBB disruption has been shown to occur after acute seizures both in human and animal studies (Fabene et al., 2008; Ilbay et al., 2003; Kim et al., 2009; Leroy et al., 2003; Marchi et al., 2007; Oby and Janigro, 2006; Oztas et al., 2003). However, it is still debatable as to whether the BBB fails before, during or after seizures and this is likely due to the complexity of disease conditions associated with BBB leaks. It has been shown that cytokines may influence the transport of compounds into the brain by opening the BBB. Several studies revealed that administration of interleukins (IL)-1 and 6 as well as tumor necrosis factor alpha (TNF-α), increase endothelial permeability (Freyer et al., 1999; Laflamme et al., 1999; Nadeau and Rivest, 1999; Schilling and Wahl, 1999). If an initial early-life seizure activates microglia, which subsequently produce and release cytokines, it is reasonable to postulate that the exaggerated microglia activation by a second seizure later in life may result in a compromised BBB and infiltration of B and T lymphocytes. Thus, BBB break down may not be a mere consequence, but rather a primary cause of seizure exacerbation. We are currently investigating these and other mechanistic properties of minocycline. In conclusion, our findings from this research suggest that interventions after early-life seizures with anti-inflammatory drugs may not only modulate the acute microglial activation, but also reduce cytokine release and endothelial permeability and play an important role in reversing the deleterious long-term effects of prolonged early-life seizures.
Highlights.
Status epilepticus induces significant acute and transient microglia activation.
Early-life seizure primes microglia response to later life seizures.
Minocycline reduces early life seizure-induced microglial cell activation.
Minocycline post-treatment prevents the priming effect of early-life seizures.
Acknowledgments
We thank Dr. Jin Qi and Min-Jung Kim for providing assistance in acquisition of data, Hyokwon Chung for analysis of data and Dr. Jaime Gruzendler for providing Cx3cr1GFP/+ transgenic mice. This research was supported by National Institutes of Health (NIH) grants R01NS073768 (to S.K) and 5R01AG027855 (to J.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Avignone E, et al. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28:9133–44. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistreri CR, et al. CCR5 receptor: biologic and genetic implications in age-related diseases. Ann N Y Acad Sci. 2007;1100:162–72. doi: 10.1196/annals.1395.014. [DOI] [PubMed] [Google Scholar]
- Beach TG, et al. Reactive microglia in hippocampal sclerosis associated with human temporal lobe epilepsy. Neurosci Lett. 1995;191:27–30. doi: 10.1016/0304-3940(94)11548-1. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Boissonneault V, et al. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–92. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Buttini M, et al. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem Int. 1996;29:25–35. doi: 10.1016/0197-0186(95)00141-7. [DOI] [PubMed] [Google Scholar]
- Cai ZY, et al. Minocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat model. Neurosci Bull. 2010;26:28–36. doi: 10.1007/s12264-010-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J. 2008;49:1–18. doi: 10.3349/ymj.2008.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, et al. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J Neuroinflammation. 2009;6:38. doi: 10.1186/1742-2094-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrinck MI, et al. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Conductier G, et al. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Cunningham C, et al. Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur J Neurosci. 2003;17:2147–55. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Drage MG, et al. Hippocampal neurons and glia in epileptic EL mice. J Neurocytol. 2002;31:681–92. doi: 10.1023/a:1025747813463. [DOI] [PubMed] [Google Scholar]
- Du Y, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–74. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, et al. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–44. [PMC free article] [PubMed] [Google Scholar]
- Fabene PF, et al. The emerging role for chemokines in epilepsy. J Neuroimmunol. 2010;224:22–7. doi: 10.1016/j.jneuroim.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Fabene PF, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–83. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, et al. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Freyer D, et al. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J Immunol. 1999;163:4308–14. [PubMed] [Google Scholar]
- Henry CJ, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, et al. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Hu RQ, et al. Neuronal stress and injury in C57/BL mice after systemic kainic acid administration. Brain Res. 1998;810:229–40. doi: 10.1016/s0006-8993(98)00863-4. [DOI] [PubMed] [Google Scholar]
- Hu W, et al. PEG minocycline-liposomes ameliorate CNS autoimmune disease. PLoS One. 2009;4:e4151. doi: 10.1371/journal.pone.0004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilbay G, et al. Changes in blood-brain barrier permeability during hot water-induced seizures in rats. Neurol Sci. 2003;24:232–5. doi: 10.1007/s10072-003-0145-8. [DOI] [PubMed] [Google Scholar]
- Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JV, et al. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–5. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss CU, et al. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Koh S, et al. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adulthood. Neurology. 1999;53:915–21. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- Koh S, et al. NBQX or topiramate treatment after perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45:569–75. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- Kriz J, et al. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:268–78. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- Laflamme N, et al. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. 1999;19:10923–30. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–63. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Leroy C, et al. In the lithium-pilocarpine model of epilepsy, brain lesions are not linked to changes in blood-brain barrier permeability: an autoradiographic study in adult and developing rats. Exp Neurol. 2003;182:361–72. doi: 10.1016/s0014-4886(03)00122-5. [DOI] [PubMed] [Google Scholar]
- Lin S, et al. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61–4. doi: 10.1016/s0304-3940(01)02324-2. [DOI] [PubMed] [Google Scholar]
- Marchi N, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–42. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel-Monigadon D, et al. Minocycline promotes long-term survival of neuronal transplant in the brain by inhibiting late microglial activation and T-cell recruitment. Transplantation. 2010;89:816–23. doi: 10.1097/TP.0b013e3181cbe041. [DOI] [PubMed] [Google Scholar]
- Murashima YL, et al. Role of cytokines during epileptogenesis and in the transition from the interictal to the ictal state in the epileptic mutant EL mouse. Gene Regul Syst Bio. 2008;2:267–74. [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience. 1999;93:1449–64. doi: 10.1016/s0306-4522(99)00225-0. [DOI] [PubMed] [Google Scholar]
- Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47:1761–74. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- Oztas B, et al. Influence of hypoosmolality on the blood-brain barrier permeability during epileptic seizures. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:701–4. doi: 10.1016/S0278-5846(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Pabreja K, et al. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661:15–21. doi: 10.1016/j.ejphar.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–86. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- Perry VH, et al. Atypical inflammation in the central nervous system in prion disease. Curr Opin Neurol. 2002;15:349–54. doi: 10.1097/00019052-200206000-00020. [DOI] [PubMed] [Google Scholar]
- Perry VH, et al. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–12. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pi R, et al. Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. J Neurochem. 2004;91:1219–30. doi: 10.1111/j.1471-4159.2004.02796.x. [DOI] [PubMed] [Google Scholar]
- Sankar R, et al. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–93. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling L, Wahl M. Mediators of cerebral edema. Adv Exp Med Biol. 1999;474:123–41. doi: 10.1007/978-1-4615-4711-2_11. [DOI] [PubMed] [Google Scholar]
- Somera-Molina KC, et al. Enhanced microglial activation and proinflammatory cytokine upregulation are linked to increased susceptibility to seizures and neurologic injury in a ‘two-hit’ seizure model. Brain Res. 2009;1282:162–72. doi: 10.1016/j.brainres.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somera-Molina KC, et al. Glial activation links early-life seizures and long-term neurologic dysfunction: evidence using a small molecule inhibitor of proinflammatory cytokine upregulation. Epilepsia. 2007;48:1785–800. doi: 10.1111/j.1528-1167.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–30. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, et al. Minocycline and other tetracycline derivatives: a neuroprotective strategy in Parkinson’s disease and Huntington’s disease. Clin Neuropharmacol. 2003;26:18–23. doi: 10.1097/00002826-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–33. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- Vargas DL, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vezzani A, et al. Brain-derived neurotrophic factor immunoreactivity in the limbic system of rats after acute seizures and during spontaneous convulsions: temporal evolution of changes as compared to neuropeptide Y. Neuroscience. 1999;90:1445–61. doi: 10.1016/s0306-4522(98)00553-3. [DOI] [PubMed] [Google Scholar]
- Yang F, et al. Roles of astrocytes and microglia in seizure-induced aberrant neurogenesis in the hippocampus of adult rats. J Neurosci Res. 2010;88:519–29. doi: 10.1002/jnr.22224. [DOI] [PubMed] [Google Scholar]