Abstract
Introduction
Flow cytometry is often used to measure in vivo platelet activation in critically-ill patients. Variability in blood sampling techniques, which may confound these measurements, remains poorly characterized.
Materials and Methods
Platelet activation was measured by flow cytometry performed on arterial and venous blood from 116 critically-ill patients. We determined how variability in vascular sampling site, processing times, and platelet counts influenced levels of platelet-monocyte aggregates (PMA), PAC-1 binding (for glycoprotein (GP) IIbIIIa), and P-selectin (P-SEL) expression.
Results
Levels of PMA, but not PAC-1 binding or P-SEL expression, were significantly affected by variability in vascular sampling site. Average PMA levels were approximately 60% higher in whole blood drawn from an arterial vessel compared to venous blood (16.2±1.8% vs. 10.7±1.2%, p<0.05). Levels of PMA in both arterial and venous blood increased significantly during ex vivo processing delays (1.7% increase for every 10 minute delay, p<0.05). In contrast, PAC-1 binding and P-SEL expression were unaffected by processing delays. Levels of PMA, but not PAC-1 binding or P-SEL expression, were correlated with platelet count quartiles (9.4±1.6% for the lowest quartile versus 15.4±1.6% for the highest quartile, p<0.05).
Conclusions
In critically-ill patients, variability in vascular sampling site, processing times, and platelet counts influence levels of PMA, but not PAC-1 binding or P-SEL expression. These data demonstrate the need for rigorous adherence to blood sampling protocols, particularly when levels of PMA, which are most sensitive to variations in blood collection, are measured for detection of in vivo platelet activation.
Keywords: Platelets, Platelet-Monocyte Aggregate, Sepsis, Acute Lung Injury/Acute Respiratory Distress Syndrome, P-selectin, Flow Cytometry
INTRODUCTION
Critical illnesses, such as sepsis and acute lung injury/acute respiratory distress syndrome (ALI/ARDS), are associated with alterations in normal platelet number, function, and reactivity which contributes to organ failure, tissue injury, and thrombosis [1–3]. Although changes in platelet number (e.g. thrombocytopenia) are well-recognized complications of critical illnesses and may correlate with outcomes, changes in platelet reactivity remain incompletely understood.
To study in vivo platelet activation in critical illnesses, whole blood flow cytometry is commonly used to measure levels of platelet-monocyte aggregates (PMA), to detect GP IIbIIIa (designated as PAC-1 binding), and to measure platelet surface P- selectin (P- SEL) expression. P-SEL, traditionally considered the “gold standard” for detecting platelet activation, is contained within the α-granules of resting platelets and upon activation is rapidly translocated to the platelet surface[4, 5]. Within approximately two hours, the surface P-SEL is shed from activated platelets, although these platelets may continue to circulate and function[6]. In comparison to P-SEL, PMAs also form quickly yet are detectable for longer periods of time than P-SEL following ex vivo activation, even when platelets no longer express P-SEL and may be a more sensitive index of in vivo platelet activation[7].
Clinical studies have shown that platelets are activated in many illnesses, including sepsis[3, 8], acute coronary syndromes and cardiovascular disease[9–11], and burn injuries[12]. The degree of activation reported (e.g. versus unstimulated whole blood from healthy subjects) varies considerably, making comparisons across studies difficult. These differences likely reflect heterogeneity in patient populations, illness severity and duration, and whole blood collection and processing techniques.
Of these factors, deviation from standard protocols for obtaining blood from patients and assessing platelet activation by flow cytometry may strongly influence measured levels of PMA, PAC-1 binding, and P-SEL expression yet remain poorly characterized. In the present study, we prospectively studied in vivo platelet activation in a large cohort of critically-ill patients to determine how variability in vascular sampling sites, processing times, and clinical variables influence measurements of PMAs, PAC-1 binding, and P-SEL expression.
MATERIALS AND METHODS
Patient Enrollment and Whole Blood Collection
The Institutional Review Board approved this study and all patients (or a legally-authorized representative) provided written, informed consent. Adult (age ≥ 21 years) men and women admitted to the intensive care unit (ICU) with a principal diagnosis of sepsis, severe sepsis, septic shock, or ALI/ARDS, based on consensus criteria[13, 14], were eligible for study participation. Whole blood was collected within 24 hours of ICU admission and then every 48(±24) hours while patients remained in the ICU. The first 3mls of blood were discarded and samples with gross hemolysis or clotting were not used. The source of whole blood (e.g. arterial or venous) and the time of collection was carefully recorded for all patients.
Blood samples were drawn into 8.6mL sterile acid-citrate-dextrose (ACD; 1.4 mL ACD/8.6 mL blood) vacutainer® tubes (Becton Dickinson, Franklin Lakes, NJ, USA), inverted to ensure adequate mixing of whole blood with ACD, then carefully transported at room air temperature to the laboratory for flow cytometry. The elapsed time from when the whole blood was drawn from patients to when the whole blood was fixed for flow cytometry (e.g. processing time) was recorded for each sample. Platelet activation was measured both in unstimulated and TRAP-activated (thrombin-receptor activating peptide, Sigma, St. Louis, MO, USA, 5μm final concentration) conditions. TRAP is a synthetic peptide that elicits a robust platelet activation response, through the proteinase-activated receptor-1 (PAR-1), and is commonly used as a platelet agonist[15].
Whole Blood Flow Cytometry
Flow cytometry was performed using a FACScan Analyzer (BD Biosciences, San Jose, CA, USA) with CellQuest software for analysis (Becton Dickinson, Franklin Lakes, NJ, USA) within 12 hours of fixation. The flow cytometer was calibrated daily and cleaned carefully before each sample acquisition. All antibodies were obtained from BD Biosciences (San Jose, CA, USA).
For detection of PMA formation, whole blood (800μl) was diluted in 2.4mL HEPES/Tyrode’s (HT) buffer (10mM HEPES/137mM NaCl/2.8mM KCl/1mM MgCl2/12mM NaHCO3/0.4mM Na2PO4/0.35% BSA, 5.5mM Glucose with pH 7.4) and left alone or treated with TRAP (5μM) for 15 minutes at room temperature (20–25°C). FACS lysis buffer (5mL, BD Biosciences, San Jose, CA, USA) was subsequently added to the blood for an additional 10 minutes at room temperature in the presence of CD41-phycoerythrin (CD41-PE; a marker for platelets) and CD14- fluorescein isothiocyanate (CD14-FITC; a marker for human monocytes). The sample was then fixed by adding 250μl of FACS buffer. Monocytes were selected by live gating a total of 1,500 CD41+ events on a two-parameter dot plot displaying side scatter (SSC) versus CD14-FITC. Single platelets were excluded using a combination of FSC and SSC and positive anti-CD14 fluorescence. The percentage of platelet-marker-positive monocyte conjugates was measured to represent the percentage of monocytes with ≥1 adherent platelet.
For detection of unstimulated and TRAP-activated PAC-1 binding and P-SEL expression, freshly drawn whole blood (20μl) was first diluted in HT buffer (180μl) and then co-stained (10μl) with the CD41-PE and either the FITC anti-human mouse monoclonal antibodies PAC-1, which recognizes GP IIbIIIa, or the FITC human anti-CD62P for P-SEL expression. Isotypic IgG1-FITC and IgG2-PE antibodies were used to control for non-specific fluorescence. Blood samples were incubated at room temperature (20–25°C) in the dark for 10 minutes and then fixed with the addition of 250ul of FACS buffer (BD Biosciences, San Jose, CA, USA). For measurement of PAC-1 binding and P-SEL expression, platelets were selected by gating a total of 10,000 CD41+ events on a two-parameter dot plot displaying both side (SSC) and forward-scatter (FSC). The percentage of platelets binding to PAC-1 or expressing P-SEL was adjusted for any non-specific binding.
Clinical Measurements and Outcomes
All patients were followed prospectively during their ICU course. APACHE II (Acute Physiology and Chronic Health Evaluation) scores were calculated according to published criteria[16] upon study entry and then on every day whole blood was collected. The receipt of medications, such as anti-platelet agents and heparinoids, which could affect platelet function, was carefully recorded. Clinical laboratory tests, such as platelet counts, were determined from EDTA anti-coagulated blood samples that were drawn in parallel with the ACD samples used for platelet activation studies. Platelet counts were measured through a reference laboratory according to established protocols and with the appropriate quality controls. In-hospital, all-cause mortality was captured prospectively for all patients.
Statistical Analyses
Statistical analyses were performed by using the STATA program (version 11.0, StataCorp, College Station, TX 77845). For all analyses, continuous variables were assessed for normality with skewness and kurtosis tests. Parametric two-tailed t-tests or ANOVA were used for continuous variables and the chi-square and Fisher’s exact test for categorical variables. To control for the potential influence of confounders such as APACHE II score, gender, age, and ICU day on PMA formation, PAC-1 binding, and P-SEL expression, mixed effects regression analyses were used to test differences between groups. Logistic linear regression analyses were used in post-hoc analyses to identify any correlations between processing times and PMA formation, PAC-1 binding, and P-SEL expression. Significance was pre-determined at p<0.05. Values are expressed as mean ± SEM, unless otherwise indicated.
The coefficient of variance (CV) for each flow cytometry assay was prospectively determined in control subjects (n=5). For PMA formation, the intra-assay CV was 0.75% and 0.60% and the inter-assay CV was 0.66% and 0.61% in baseline and TRAP-activated conditions, respectively. For PAC-1 binding, the intra-assay CV was 0.66% and 6.41% and the inter-assay CV was 0.12% and 4.57% in baseline and TRAP-activated conditions, respectively. For P-SEL, the intra-assay CV was 0.36% and 2.82% and the inter-assay CV was 0.73% and 1.52% in baseline and TRAP-activated conditions, respectively.
RESULTS
Demographics and patients characteristics upon ICU admission are shown in Table 1. The average (±SD) age of ICU patients was 51.4 (±19.1) years and 58.6% were female. The average (±SD) APACHE II score was 18.1 (±6.8) and 80% (n=93) met consensus criteria for severe sepsis or septic shock. As expected, more than 20% of patients had a history of diabetes. The incidence of pre-existing cardiovascular disease was low (12.9%) and only a small percentage of patients were taking aspirin (n=7, 6%) prior to ICU admission. The in-hospital all-cause mortality rate was 18.1% (n=21).
Table 1.
Characteristics of the study population (all values represent the mean±SD unless otherwise specified
| Demographic | Critically Ill Subjects (n=116) |
|---|---|
| Age | 51.4 (±19.1) |
| Female Gender, n (%) | 68 (58.6%) |
| APACHE II Score | 18.1 (±6.8) |
| Platelet Count (K/uL) | 181 (±106) |
| Hemoglobin (mg/dL) | 10.5 (±2.0) |
| White Blood Cell Count (K/uL) | 12.6 (±6.0) |
| Fibrinogen (mg/dL) | 570 (±206) |
| Admission Diagnosis | |
| Sepsis, n (%) | 16 (13.8%) |
| Severe Sepsis, n (%) | 54 (46.6%) |
| Septic Shock, n (%) | 39 (33.6%) |
| ALI/ARDS, n (%) | 7 (6.0%) |
| Medical History | |
| Diabetes Mellitus, n (%) | 25 (21.6%) |
| Congestive Heart Failure, n (%) | 6 (5.2%) |
| Cardiovascular Diseaseγ, n (%) | 15 (12.9%) |
includes stroke, transient ischemic attack, and myocardial infarction.
ALI: acute lung injury; APACHE: Acute Physiology and Chronic Health Evaluation; ARDS: adult respiratory distress syndrome).
Levels of Platelet-Monocyte Aggregates are Affected by Vascular Sampling Site
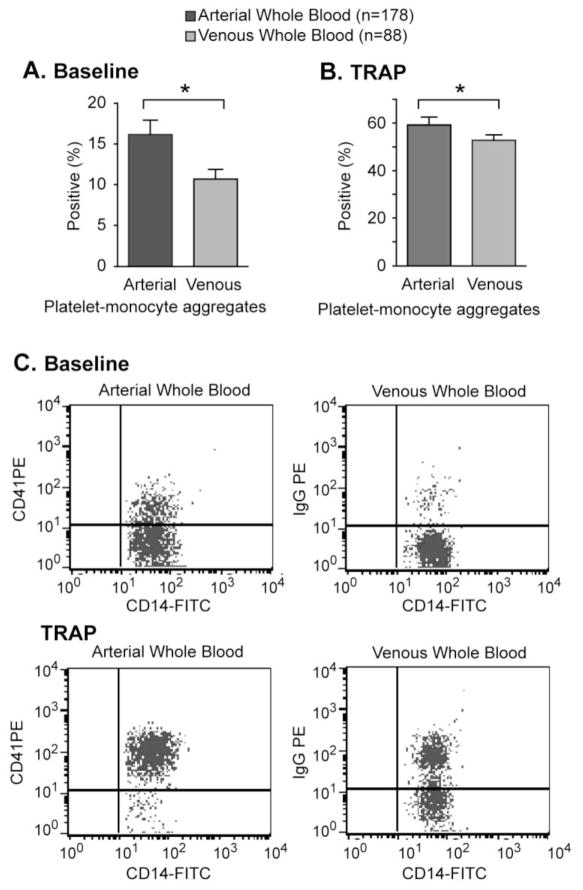
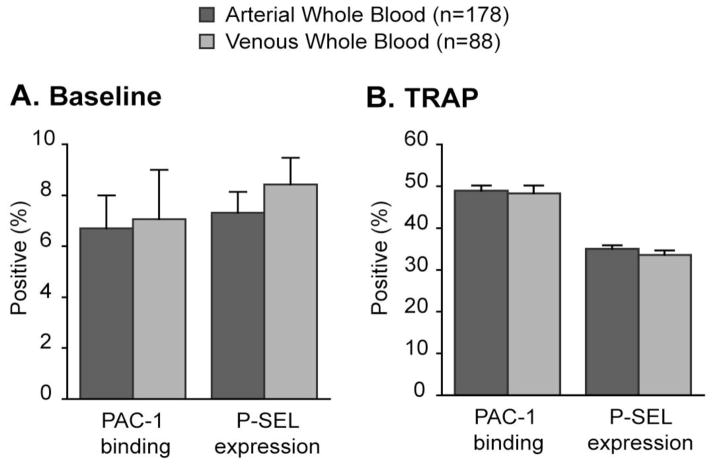
During the study period, 266 whole blood samples from the 116 critically ill patients were collected (average of 2.3 blood samples per patient). Of these, 178 (66.9%) were arterial and 88 (33.1%) were venous blood samples. As illustrated in Figure 1, levels of PMA were approximately 60% higher in arterial compared to venous blood samples. These differences were observed in both baseline (i.e. unstimulated) and TRAP-activated conditions. In comparison, PAC-1 binding and P-SEL expression did not differ between arterial and venous whole blood samples (Figure 2).
Figure 1.
Platelet-monocyte aggregates are higher in arterial whole blood from critically-ill patients. Platelet-monocyte aggregates were assessed in (A) baseline (e.g. unstimulated) or (B) TRAP-activated arterial or venous whole blood. Panel C shows a representative flow cytometric analysis of unstimulated and TRAP-activated platelet-monocyte aggregates measured in arterial (left) and venous (right) whole blood samples (*p<0.05 versus venous whole blood samples in multivariate comparisons after controlling for APACHE II score, processing time, ICU day, age, gender, and platelet count).
Figure 2.
PAC-1 binding and P-SEL expression does not differ between (A) baseline or (B) TRAP-activated arterial and venous whole blood in critically-ill patients (p=NS all comparisons in multivariate comparisons after controlling for APACHE II score, processing time, ICU day, age, gender, and platelet counts).
Heterogeneity in centrally-placed versus peripherally-placed catheters may influence measured indices of platelet activation[17]. Thus, in a subgroup of patients we compared platelet activation in blood drawn from a central (n=63) or peripheral venous catheter (n=25) already placed as part of patients standard medical care. Consistent with our findings in arterial blood (Figure 1), baseline (e.g. unstimulated) levels of PMA were higher in blood from a central versus peripheral venous catheter but the absolute increase in PMA levels was not as marked and differences did not reach statistical significance (16.9% versus 12.6%, Supplemental Figure 1A; p=0.12). In TRAP-stimulated blood, there were no differences in levels of PMA between central and peripheral venous catheters (Supplemental Figure 1B). In comparison and also consistent with our observations in arterial blood (Figure 2), PAC-1 binding and P-SEL expression were similar in blood drawn from central and peripheral venous catheters (Supplemental Figures 1A and 1B).
Levels of Platelet-Monocyte Aggregates Increased with Delayed Processing
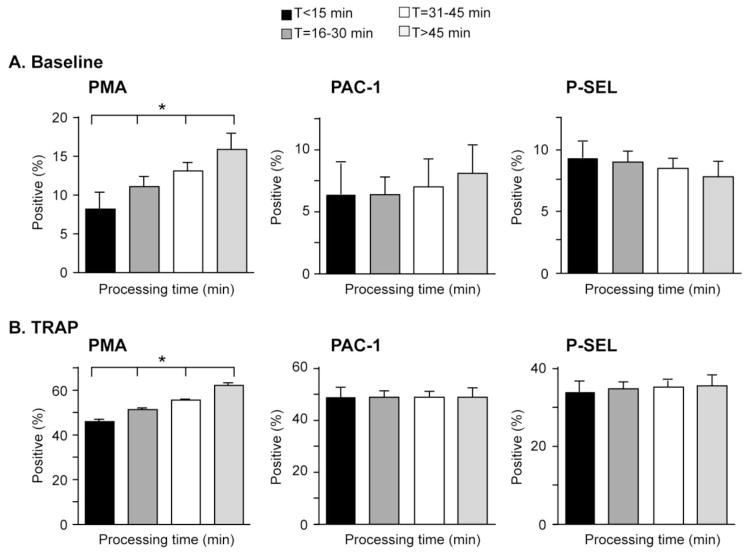
Platelet auto-activation may occur ex vivo following whole blood collection potentially causing misleadingly high values of PMA, PAC-1 binding, and P-SEL expression. We analyzed the influence of processing times on these indices of platelet activation in whole blood from critically-ill patients. Our average (±SD) processing time (e.g. from blood collection to fixation for flow cytometry) was 31.2 (±11.8) minutes (range 3–70 minutes).
As illustrated in figure 3, there was no correlation between processing times and baseline PAC-1 binding and P-SEL expression (Figures 3A and 3B, middle and right graphs). Furthermore, longer processing times did not result in prolonged or blunted platelet activation responses to TRAP-stimulation (Figures 3A and 3B, middle and right graphs).
Figure 3.
Delayed processing times were associated with increased levels of platelet-monocyte aggregates (PMA), but not PAC-1 binding or P-SEL expression. Whole blood was left alone (baseline, Panel A) or stimulated with TRAP (Panel B) for 15 minutes. (*p<0.05 for trend in multivariate comparisons after controlling for APACHE II score, age, gender, vascular sampling site, ICU day, and platelet counts).
In comparison, processing times did correlate with levels of PMA (Figure 3). Overall, baseline (i.e. unstimulated) levels of PMA increased (1.7% absolute increase for every 10 minute processing delay ex vivo) in stepwise fashion (Figure 3A, left graph). For example, levels of PMA in blood where processing was delayed by 45 minutes or longer were two-fold higher compared to blood processed within 15 minutes of collection (8.2% versus 15.9%, p<0.05; Figure 3A, left graph). In TRAP-activated whole blood, levels of PMA also correlated with processing times, consistent with ex vivo auto-activation (Figure 3B, left graph). The magnitude of these differences were statistically significant yet also highly relevant for investigators with levels of PMA being approximately 30% higher when blood sample processing was delayed by 45 minutes or longer compared to when processing occurred within 15 minutes of blood sample collection (62.1% vs 46.1%, p<0.05; Figure 3B, left panel).
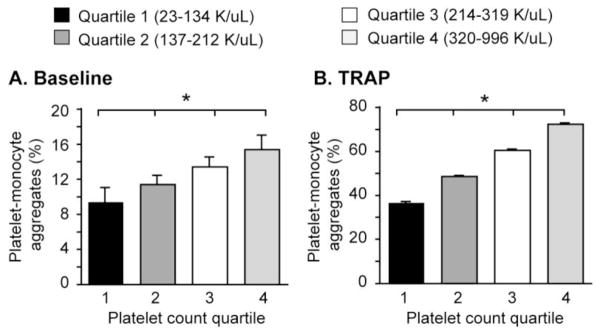
Levels of Platelet-Monocyte Aggregates Correlate with the Number of Circulating Platelets
Dynamic changes in the circulating number of platelets capable of responding to activating signals, bacterial agonists, and circulating toxins to bind monocytes may influence total levels of PMA. Consistent with this hypothesis, we found that levels of PMA in unstimulated whole blood correlated with systemic platelet counts. We found that baseline levels of PMA correlated significantly with platelet counts obtained in parallel on the same blood sample (15.4% vs. 9.4%, p<0.05; Figure 4A). As expected, levels of PMA were also higher in TRAP-activated whole blood with higher numbers of platelets (36.6% vs. 72.4%, p<0.05; Figure 4B), consistent with a larger circulating pool of platelets able to respond to agonist stimulation. In comparison, PAC-1 binding and P-SEL expression did not correlate with platelet counts (data not shown).
Figure 4.
Levels of PMA correlate with the number of circulating platelets. Whole blood was left alone (baseline, Panel A) or stimulated with TRAP (Panel B) for 15 minutes (*p<0.05 for trend in multivariate analyses after controlling for APACHE II score, age, gender, ICU day, vascular sampling site, and processing time).
DISCUSSION
Whole blood flow cytometry is commonly used to detect in vivo platelet activation in health and disease[9, 10, 15, 18]. Technical variation in blood collection methods, delays in specimen transport and processing, and heterogeneity in clinical features may confound these measurements and impede accurate comparisons between patient groups and across studies. In our prospectively studied cohort of critically-ill patients, levels of PMA were significantly higher in both unstimulated and TRAP-activated arterial blood compared to venous blood. In contrast, PAC-1 binding and P-SEL expression were unaffected by differences in vascular sampling sites.
Although there is limited published data on how vascular sampling site influences measurements of platelet activation, our data are consistent, in part, with prior observations. Rubens and colleagues examined platelet activation in eight patients undergoing elective cardiac surgery[19]. Overall, levels of GP IIbIIIa (i.e. PAC-1) and GMP-140 (i.e. P-SEL) did not differ significantly between arterial and venous whole blood. However, when these assays were performed in triplicate on two patients, levels of GP IIbIIIa and GMP-140 were higher in arterial whole blood[19].
Our study extends this published work to a larger cohort of patients not previously studied in this setting. The underlying mechanism(s) responsible for the increased levels of PMA in arterial blood from critically-ill patients with sepsis and ALI/ARDS is not entirely clear but several explanations are plausible. Although not well characterized, during septic syndromes, platelets have been suggested to undergo sequestration in venous vascular beds and thus may not as readily interact with and bind to monocytes to form heterotypic PMAs[20].
An alternative hypothesis that is supported with both in vitro and in vivo data is that the septic milieu alters normal platelet reactivity to make them more susceptible to activation from shear forces. Shear forces in arteries are often greater than forces in veins [21] and play a pivotal role in platelet activation, adhesion, and aggregation[22]. For example, in vitro studies demonstrate that platelet-leukocyte aggregation and P-SEL expression are increased in whole blood subject to shear stress [23]; an effect that may be reversed by blocking P-SEL[24]. Similarly, in stable patients with coronary artery disease, intracoronary shear stress induced platelet activation as evidenced by up-regulation of PMA formation without platelet activation of glycoprotein IIb/IIIa [25], which can be detected through PAC-1 binding. Our findings are consistent with these published in vitro and in vivo observations as we found that PMA formation, but not PAC-1 binding, was increased in arterial blood. Although we did not observe increased P-SEL expression in arterial whole blood, in patients with sepsis, platelet P-selectin may be rapidly translocated to the cell surface and shed even though platelets continue to circulate and function[6]. This rapid shedding of P-SEL, which likely occurred early during the onset of sepsis (and perhaps before patients were admitted to the ICU), may have prevented us from detecting differences.
Ex vivo processing time delays greater than 45 minutes resulted in levels of PMA that were 2-fold higher compared to levels in whole blood that was processed within 15 minutes of collection. In comparison, delays in processing time did not affect PAC-1 binding or P-SEL expression in either unstimulated or TRAP-activated whole blood. These data parallel published reports in healthy subjects. For example, Harding et al[17] observed a 2.8% increase in levels of PMA for every 10 minute delay prior to processing for whole blood flow cytometry, a trend very similar to our observations in critically-ill subjects (i.e. 1.7% absolute increase for every 10 minute delay prior to processing). Our findings build on Harding and colleague’s report by demonstrating that delays in processing time also influence TRAP-stimulated levels of PMA. Additionally, our data support the importance of timely processing of whole blood for flow cytometry when measuring PMA formation in critically-ill patients[15].
Since platelets undergo auto-activation in whole blood ex vivo, once drawn, blood should be promptly utilized for measurements of platelet activation by whole blood flow. PMAs, suggested to be a very sensitive marker of platelet activation[7], may offer advantages for the detection of in vivo platelet activation. Nevertheless, since PMAs are also influenced by the source of blood, processing time, and platelet counts, any studies using PMAs as a marker of platelet activation should follow strict procedures when drawing and processing blood to minimize confounding. In situations where facilities for the flow cytometry preparation are not located in close proximity to clinical care units and thus delays in processing are likely, measuring PAC-1 binding or P-SEL expression may be advantageous as these markers of platelet activation appear to be less sensitive to processing delays.
Finally, these data also demonstrate that in unstimulated whole blood from critically ill patients, PMA formation, but not PAC-1 binding or P-SEL expression, increased with greater numbers of circulating platelets. Not surprisingly, upon stimulation with TRAP, levels of PMA also increased in patients with higher platelet counts, perhaps reflecting the presence of a larger population of platelets responding to ex vivo activating signals.
The strengths of our study include the large number of patients, the prospective study design, and rigorous data collection methods. Potential confounders were well controlled for in multivariate regression analyses, minimizing bias and reducing the likelihood that our findings were due solely to chance. In addition, as variability in vascular sampling site, processing time, and platelet counts selectively influenced levels of PMA but not PAC-1 binding or P-SEL expression, our findings are unlikely to be due solely to chance. Rather, they suggest intrinsic differences between these indices of platelet activation in response to agonists commonly present during sepsis.
Although we could not collect blood from all patients simultaneously from both an arterial and venous blood vessel, we did measure levels of PMA, PAC-1 binding, and P-SEL expression, in unstimulated arterial and venous blood drawn simultaneously from septic patients who were prospectively studied (n=4). Consistent with the findings of our larger study cohort (Figure 1), we found that levels of PMA were higher in arterial whole blood (Supplemental Figure 2A), although the sample size was too small to demonstrate significant differences. Also consistent with our observations in the larger cohort (Figure 2), there was no difference in PAC-1 binding or P-SEL expression (Supplemental Figure 2B).
We also did not study a group of healthy, control subjects in parallel and this may be considered a limitation. Nevertheless, the objective of our study was to prospectively examine how variability in blood collection and processing influences measurements of in vivo platelet activation. Additional studies are necessary to determine if our findings are also true in healthy subjects as well as hospitalized patients without ALI/ARDS (e.g. elective or urgent surgery, heart failure, acute myocardial infarction, etc).
Heparin, often used to maintain the patency of arterial catheters, may inadvertently cause platelet activation[26, 27]. However, this did not influence the findings of our study as all the arterial and venous lines were maintained with normal saline rather than heparin. Furthermore, we also performed subgroup analyses to confirm that levels of PMA, PAC-1 binding, and P-SEL expression did not differ between septic patients receiving heparinoids and those not receiving heparinoids (data not shown). Thus, ex vivo activation due to heparinoids cannot account for our observations. Anti-platelet agents, such as aspirin, may attenuate platelet activation responses and confound measurements. Nevertheless, published and unpublished studies have demonstrated that aspirin does not influence platelet-leukocyte aggregation or P-SEL expression([28] and Rondina et al, unpublished data). Furthermore, only a small number of patients in our study were taking aspirin at the time of ICU admission (6%, n=7). In pre-specified analyses where these patients were excluded, differences in levels of PMA (but not PAC-1 binding or P-SEL expression) between arterial and venous whole blood persisted. Moreover, these differences were also apparent in analyses restricted to only the patients taking aspirin at the time of admission. Thus, based on these secondary analyses, we do not think that concomitant aspirin treatment affected the results of our study.
Finally, FACS lysis buffer, which we used to measure PMA levels, may increase P-SEL expression by lysing red blood cells[29]. However, as our incubation times with lysis buffer remained constant, regardless of the vascular sampling site, processing times, or platelet counts, this was unlikely to have contributed to the findings of our study.
CONCLUSION
Levels of PMAs are significantly affected by heterogeneity in vascular sampling site, delays in processing times, and circulating platelet counts in septic patients. In contrast, PAC-1 binding and P-SEL expression are not influenced by variance in these factors. The detection of platelet activation in critical illness may be improved by systematic whole blood collection with careful attention to these factors. In addition, since PMAs are very sensitive to variations in vascular sampling site, processing times, and platelet counts, investigations measuring PMA should follow strict procedures when drawing and processing blood to minimize confounding.
Supplementary Material
Supplemental Figure S1. Indices of platelet activation in critically-ill patients are similar between central and peripheral venous whole blood. Whole blood was left alone (Panel A) or stimulated with TRAP (Panel B) for 15 minutes (p=NS for all comparisons in multivariate regression analysis after controlling for APACHE II scores, processing time, ICU day, age, gender, and platelet count; PMA: platelet-monocyte aggregates; P-SEL: platelet surface P-selectin).
Supplemental Figure S2. Platelet-monocyte aggregates (PMA, panel A), but not PAC-1 binding or P-SEL expression (Panel B) are higher in arterial blood from critically-ill patients compared to venous blood drawn simultaneously from the same patient.
Acknowledgments
Financial Support: Work in this report was supported by grants from the N.I.H. (HL091754, HL066277, and K23HL092161) and public health service grant ULI-RRO25764 from the National Center for Research Resources.
We thank Diana Lim for her assistance with figure preparation, Brittany Patterson for her assistance with manuscript preparation, and Diana Kastendieck and Saritha Kalva for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwertz H, Weyrich AS, Zimmerman GA. Cellular interactions of platelets, leukocytes and endothelium in systemic inflammatory responses and sepsis. In: Castro Faria Neto H, Marcus D, editors. Sepsis: From Bench to Bedside. Rio de Janerio: Revinter Press; 2007. pp. 107–120. [Google Scholar]
- 2.Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ. Platelet function in septic multiple organ dysfunction syndrome. Intensive care medicine. 1997;23(4):379–385. doi: 10.1007/s001340050344. [DOI] [PubMed] [Google Scholar]
- 3.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest. 1995;25(11):843–851. doi: 10.1111/j.1365-2362.1995.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 4.Michelson AD, Furman MI. Laboratory markers of platelet activation and their clinical significance. Curr Opin Hematol. 1999;6(5):342–348. doi: 10.1097/00062752-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Michelson AD. Flow cytometry: a clinical test of platelet function. Blood. 1996;87 (12):4925–4936. [PubMed] [Google Scholar]
- 6.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996;93(21):11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104(13):1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 8.Harris ES, Rondina MT, Schwertz H, Weyrich AS, Zimmerman GA. Pathogenesis of Sepsis and Sepsis-Induced Acute Lung Injury. In: Choi AMK, editor. Acute Respiratory Distress Syndrome. Informa Healthcare; 2010. pp. 369–419. [Google Scholar]
- 9.Shanker J, Gasparyan AY, Kitas GD, Kakkar VV. Platelet Function and Antiplatelet Therapy in Cardiovascular Disease: Implications of Genetic Polymorphisms. Current vascular pharmacology. 2011 doi: 10.2174/157016111796197224. [DOI] [PubMed] [Google Scholar]
- 10.Alstrom U, Granath F, Oldgren J, Stahle E, Tyden H, Siegbahn A. Platelet inhibition assessed with VerifyNow, flow cytometry and PlateletMapping in patients undergoing heart surgery. Thromb Res. 2009;124(5):572–577. doi: 10.1016/j.thromres.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Freedman JE, Loscalzo J. Platelet-monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105(18):2130–2132. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- 12.Lupia E, Bosco O, Mariano F, Dondi AE, Goffi A, Spatola T, Cuccurullo A, Tizzani P, Brondino G, Stella M, et al. Elevated thrombopoietin in plasma of burned patients without and with sepsis enhances platelet activation. J Thromb Haemost. 2009;7(6):1000–1008. doi: 10.1111/j.1538-7836.2009.03348.x. [DOI] [PubMed] [Google Scholar]
- 13.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9(1):72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 14.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20 (6):864–874. [PubMed] [Google Scholar]
- 15.Michelson AD, Linden MD, Barnard MR, Furman MI, Frelinger AL. Flow Cytometry. In: Michelson AD, editor. Platelets. 2. Elsevier; 2007. pp. 545–563. [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 17.Harding SA, Din JN, Sarma J, Jessop A, Weatherall M, Fox KA, Newby DE. Flow cytometric analysis of circulating platelet-monocyte aggregates in whole blood: methodological considerations. Thrombosis and haemostasis. 2007;98 (2):451–456. [PubMed] [Google Scholar]
- 18.Mobarrez F, Antovic J, Egberg N, Hansson M, Jorneskog G, Hultenby K, Wallen H. A multicolor flow cytometric assay for measurement of platelet-derived microparticles. Thromb Res. 2010;125(3):e110–116. doi: 10.1016/j.thromres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Rubens FD, Labow RS, Waghray G, Robblee J. The importance of sampling site in the measurement of whole-blood platelet flow cytometry. J Cardiothorac Vasc Anesth. 1998;12(3):309–313. doi: 10.1016/s1053-0770(98)90012-x. [DOI] [PubMed] [Google Scholar]
- 20.Sigurdsson GH, Christenson JT, el-Rakshy MB, Sadek S. Intestinal platelet trapping after traumatic and septic shock. An early sign of sepsis and multiorgan failure in critically ill patients? Critical care medicine. 1992;20 (4):458–467. doi: 10.1097/00003246-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JN, Bergeron AL, Yu Q, Sun C, McIntire LV, Lopez JA, Dong JF. Platelet aggregation and activation under complex patterns of shear stress. Thrombosis and haemostasis. 2002;88(5):817–821. [PubMed] [Google Scholar]
- 22.de Groot PG, Sixma JJ. Perfusion Chambers. In: Michelson AD, editor. Platelets. New York: Elsevier; 2007. pp. 575–585. [Google Scholar]
- 23.Hu H, Varon D, Hjemdahl P, Savion N, Schulman S, Li N. Platelet-leukocyte aggregation under shear stress: differential involvement of selectins and integrins. Thromb Haemost. 2003;90(4):679–687. doi: 10.1160/TH03-05-0274. [DOI] [PubMed] [Google Scholar]
- 24.Konstantopoulos K, Neelamegham S, Burns AR, Hentzen E, Kansas GS, Snapp KR, Berg EL, Hellums JD, Smith CW, McIntire LV, et al. Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin. Circulation. 1998;98(9):873–882. doi: 10.1161/01.cir.98.9.873. [DOI] [PubMed] [Google Scholar]
- 25.Yong AS, Pennings GJ, Chang M, Hamzah A, Chung T, Qi M, Brieger D, Behnia M, Krilis SA, Ng MK, et al. Intracoronary shear-related up-regulation of platelet P-selectin and platelet-monocyte aggregation despite the use of aspirin and clopidogrel. Blood. 2011;117(1):11–20. doi: 10.1182/blood-2010-04-278812. [DOI] [PubMed] [Google Scholar]
- 26.Kuhne T, Hornstein A, Semple J, Chang W, Blanchette V, Freedman J. Flow cytometric evaluation of platelet activation in blood collected into EDTA vs. Diatube-H, a sodium citrate solution supplemented with theophylline, adenosine, and dipyridamole. Am J Hematol. 1995;50(1):40–45. doi: 10.1002/ajh.2830500108. [DOI] [PubMed] [Google Scholar]
- 27.Hirsh J. Heparin. N Engl J Med. 1991;324(22):1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Hu H, Hjemdahl P. Aspirin treatment does not attenuate platelet or leukocyte activation as monitored by whole blood flow cytometry. Thromb Res. 2003;111(3):165–170. doi: 10.1016/j.thromres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie JL, Alexander HD, Rea IM. Flow cytometry analysis of platelet P-selectin expression in whole blood--methodological considerations. Clinical and laboratory haematology. 2000;22(6):359–363. doi: 10.1046/j.1365-2257.2000.00339.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Indices of platelet activation in critically-ill patients are similar between central and peripheral venous whole blood. Whole blood was left alone (Panel A) or stimulated with TRAP (Panel B) for 15 minutes (p=NS for all comparisons in multivariate regression analysis after controlling for APACHE II scores, processing time, ICU day, age, gender, and platelet count; PMA: platelet-monocyte aggregates; P-SEL: platelet surface P-selectin).
Supplemental Figure S2. Platelet-monocyte aggregates (PMA, panel A), but not PAC-1 binding or P-SEL expression (Panel B) are higher in arterial blood from critically-ill patients compared to venous blood drawn simultaneously from the same patient.