Abstract
Pancreatic neuroendocrine tumors (PanNETs) with prominent stromal fibrosis are often clinically, radiographically, and grossly indistinguishable from ductal adenocarcinoma. We recently described a small series of fibrotic PanNETs that express serotonin. In order to understand better the relationship between histopathological patterns and serotonin expression, we reviewed 361 PanNETs to identify those with prominent stromal fibrosis exceeding 50% of total tumor area. We identified 52 cases, and immunolabeled these neoplasms with antibodies to serotonin and Ki-67. Two predominant histologic subtypes were identified: 14 of 52 (26.9%) had a trabecular or trabecular-glandular cellular pattern with interspersed fibrosis, while 38 of 52 (73.1%) had solid architecture. Fourteen of the 52 (26.9%) PanNETs showed at least focal serotonin immunoreactivity. Tumors with predominantly trabecular architecture were significantly more likely to express serotonin than those with solid architecture (P < 0.01). Only 2 of 34 PanNETs with fibrosis less than 30% of total tumor area expressed serotonin. The 14 serotonin-expressing tumors were less likely to have lymph node metastases (P = 0.016) and more likely to involve large pancreatic ducts (P < 0.01) than were the 38 serotonin-negative tumors. The serotonin-expressing tumors were also found in a younger patient population (P < 0.01). There was no significant association of serotonin immunoreactivity with Ki-67 proliferation index, tumor size, or distant metastases. Our data demonstrate a strong correlation between trabecular architecture and serotonin immunoreactivity in PanNETs with stromal fibrosis. Serotonin-expressing tumors are also less likely to have lymph node metastases and more likely to involve large pancreatic ducts.
Introduction
Pancreatic neuroendocrine tumors (PanNETs) are, according to the 2010 WHO classification, well-differentiated neuroendocrine neoplasms [1–4]. These uncommon neoplasms may produce various hormones that are orthotopic or ectopic to the normal pancreas. Histologically, they may show solid, glandular and/or trabecular patterns and a variable degree of fibrosis. Despite their rarity, it is critical to distinguish PanNETs from pancreatic ductal adenocarcinoma, because PanNETs have a more favorable prognosis and the therapy for PanNETs differs significantly from that of ductal adenocarcinomas [5]. Although most PanNETs are discrete, solid cellular neoplasms, which are easily distinguished from ductal adenocarcinomas both radiographically and histologically, a minority are hypocellular and associated with dense stromal fibrosis, a feature more typically associated with ductal adenocarcinomas.
Recently, we described a series of small PanNETs associated with pancreatic duct stenosis that was caused by the tumor’s location and prominent tumoral stromal fibrosis [6]. Due to the similarity of the stromal fibrosis found in these tumors with that frequently seen in serotonin-expressing NETs (i.e. carcinoids) of the ileum, we tested the series for serotonin immunoreactivity. We found a significant association between prominent fibrosis and the expression of serotonin [6].
In the current study, in order to understand the association between stromal fibrosis and serotonin immunoreactivity, we undertook a retrospective analysis of all PanNETs resected at our institution from 1984 to 2009 and identified those with prominent stromal fibrosis. We correlated stromal fibrosis with histopathological features and serotonin immunoreactivity, as well as Ki-67 proliferative index, tumor size, and lymph node and distant metastases at time of resection.
Materials and methods
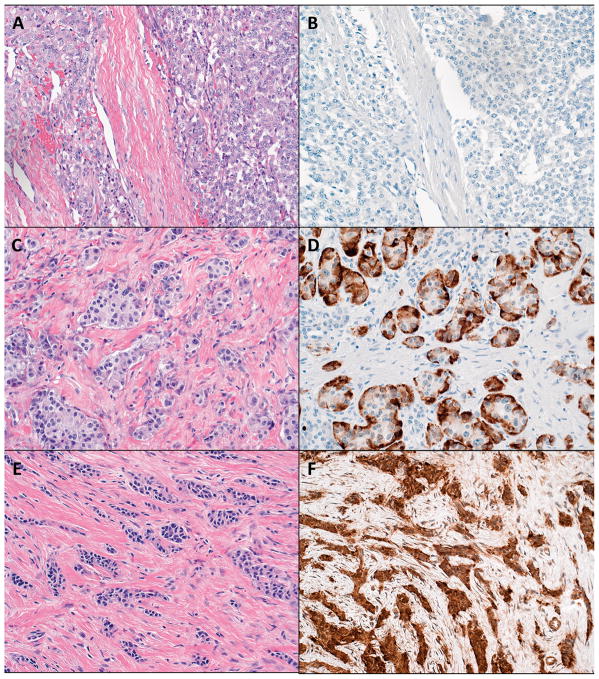
We reviewed the Surgical Pathology files of the Johns Hopkins Hospital Department of Pathology for patients with a final diagnosis of PanNETs from 1984 to 2009. This query yielded 361 PanNETs with slides available for review. Slide review further revealed 52 PanNETs with stromal fibrosis occupying more than 30% of the total tumor area (Figure 1). These cases were further subclassified according to the histopathological architecture of the neoplastic cells: solid sheets of cells with surrounding stromal fibrosis and/or trabecular or trabecular-glandular architecture with interspersed stromal fibrosis (Figure 1) [5]. The first type of PanNETs rich in fibrosis was characterized by solid nests and some sheets of neoplastic cells separated by fibrous tissue bands. The thickness of the fibrous bands differed from area to area and the total amount of fibrosis was generally less than 50% of the total tumor area (Figure 1a). The second type of PanNETs rich in fibrosis was characterized by small trabecular-glandular or purely trabecular arrangements of neoplastic cells that are surrounded and spaced apart by diffusely developed fibrotic tissue. The total amount of fibrosis generally exceeded 50% of the total tumor area (Figure 1c and e).
Figure 1. Fibrotic pancreatic neuroendocrine tumors display a range of histological architectures, which correlate with serotonin immunoreactivity.
a) Solid tumor nests with surrounding fibrosis (hematoxylin and eosin, 100 × original magnification).
b) Negative serotonin immunoreactivity and solid architecture (serotonin immunohistochemical stain, 100 × original magnification).
c) Trabecular-glandular architecture with interspersed fibrosis (hematoxylin and eosin, 100 × original magnification).
d) Positive serotonin immunoreactivity and trabecular-glandular architecture (serotonin immunohistochemical stain, 100 × original magnification).
e) Trabecular architecture with interspersed fibrosis (hematoxylin and eosin, 100 × original magnification).
f) Positive serotonin immunoreactivity and trabecular architecture (serotonin immunohistochemical stain, 100 × original magnification).
Representative formalin-fixed paraffin-embedded tissue blocks from each case with stromal fibrosis were selected. Sections of each block were immunolabeled for Ki-67 and serotonin. Serotonin-positive tumors were also immunolabeled for insulin, glucagon, somatostatin, and gastrin. Standard automated immunohistochemistry using the Ventana Benchmark XT system was performed for Ki-67, CDX2, insulin, somatostatin, gastrin, glucagon, and serotonin (monoclonal antibody, clone 5HTH209, DakoCytomation, Glostrup, Denmark, 1:40).
As control group we randomly selected 34 PanNETs from our series of 361 cases for serotonin immunolabeling. In none of these tumors did stromal fibrosis exceed 30% of the tumor area. Serotonin immunoreactivity was scored as indicated in Table 1. Positivity for serotonin was compared with histopathological patterns, patient gender, and lymph node and distant metastases present at time of resection using Fisher’s exact test. Analysis of patient age and Ki-67 proliferation index was performed using Student’s t-test or the Kruskal-Wallis test. Patient survival data and clinical information were obtained from our surgery clinical database. Analysis of patient survival data was performed using a Kaplan-Meier curve and log-rank test. Analysis was conducted using STATA 11.
Table 1.
Serotonin-Positive Case Characteristics
| Case No. | Tumor Size (cm) | Ki-67 index (%)a | Mitoses per 10 HPF | Lymph Node Metastases (+/total LNs) | Distant Metastasis | TNM Stage | Serotoninb | Large Duct Involvement | Perineural Invasion | Small Vessel Involvement |
Large Vessel Involvement |
Necrosis | Major histological architecturec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.2 | 0 | 0 | 0/1 | N | T2N0M0 | + | N | N | N | N | N | TG |
| 2 | 4.5 | 4 | 0.2d | 4/14 | Liver | T3N1M1 | + | Y | Y | Y | N | N | TG |
| 3 | 2.5 | 3 | 0 | 2/12 | --e | T3N1Mx | ++ | Y | N | N | N | Y | S |
| 4 | 2.5 | 3 | 0 | 0/0 | -- | T2NxMx | ++ | --f | N | Y | N | N | S |
| 5 | 1.5 | 1 | 0 | 0/10 | -- | T3N0Mx | ++ | N | Y | Y | Y | N | S |
| 6 | 1.5 | 1 | 0 | 0/25 | N | T1N0M0 | ++ | Y | Y | N | N | N | TG |
| 7 | 3 | 2 | 0 | 0/22 | N | T2N0M0 | ++ | Y | Y | Y | N | N | T |
| 8 | 1.5 | 1 | 0 | 0/16 | N | T1N0M0 | ++ | Y | N | N | N | N | S |
| 9 | 3 | 16 | 0.2d | 3/27 | -- | T3N1Mx | ++ | Y | Y | N | N | Y | S |
| 10 | 2.4 | 3 | 0 | 0/9 | N | T2N0M0 | ++ | Y | N | N | N | N | T |
| 11 | 1 | 1 | 0 | 0/12 | N | T1N0M0 | +++ | Y | N | N | N | N | S |
| 12 | 1.5 | 1 | 0 | 0/30 | Liver | T1N0M1 | +++ | Y | Y | N | N | N | T |
| 13 | 6 | 2 | 0 | 0/17 | N | T3N0M0 | +++ | Y | Y | N | N | N | T |
| 14 | 2 | 10 | 0 | 1/16 | -- | T2N1Mx | +++ | Y | N | N | N | N | TG |
Minimum 200 cells counted.
+ = <15% positive; ++ = 15–70% positive; +++ = >70% positive.
Histological architecture abbreviations: S: Solid; TG: trabecular-glandular; T: trabecular
1 mitotic figure in 50 high-power fields counted.
Information on distant metastases unavailable on 5 cases.
Slides unavailable
We obtained appropriate institutional approval for this study.
Results
Fifty-two of the 361 PanNETs in our case files from 1984 to 2009 had significant stromal fibrosis. Their clinicopathological data are listed in Table 2. These fibrotic neoplasms had three distinct histological patterns of growth (Figure 1): solid nests/sheets of cells surrounded by fibrosis, a trabecular and glandular pattern interspersed by fibrosis, and/or a purely trabecular pattern of cells spaced apart by fibrosis. Of the 52 PanNETs, 38 were predominantly solid, 9 were predominantly trabecular-glandular, and 5 were predominantly trabecular (see Tables 1 and 2).
Table 2.
Summary of Findings
| Serotonin Negative | Serotonin Positive | P value | ||
|---|---|---|---|---|
| Number | 38 | 14 | -- | |
| Median age (Range) | 57 (36–90) | 49 (23–66) | 0.046a | |
| Sex ratio M:F | 20:18 | 4:10 | 0.21b | |
| Median tumor size (Range) | 3.2 cm (1–7) | 2.3 cm (1–6) | 0.39a | |
| Tumor locationc: | Head | 23 of 37 (62%) | 10 of 14 (71%) | 0.74b |
| Body or tail | 14 of 37 (38%) | 4 of 14 (29%) | ||
| Median mitoses per 10 HPF (Range) | 0.2 (0–11) | 0 (0–0.2) | <0.01i | |
| Average Ki-67 proliferation indexd | 5.48% | 3.34% | 0.17a | |
| Tumor grade (by Ki-67%)e: | G1 | 13 of 37 (35%) | 8 of 14 (57%) | 0.21b,f |
| G2 | 23 of 37 (62%) | 6 of 14 (43%) | ||
| G3 | 1 of 37 (3%) | 0 of 14 | ||
| Lymph node metastasesg | 25 of 34 (74%) | 4 of 13 (31%) | 0.016b | |
| Known distant metastasesh | 15 of 29 (52%) | 2 of 9 (22%) | 0.15b | |
| Large duct involvement | 15 of 37 (41%) | 11 of 13 (85%) | <0.01b | |
| Infiltrative growth | 32 of 38 (84%) | 14 of 14 (100%) | 0.17b | |
| Necrosis | 7 of 38 (18%) | 2 of 14 (14%) | 1b | |
| Perineural invasion | 26 of 38 (68%) | 7 of 14 (50%) | 0.33b | |
| Small vessel invasion | 20 of 37 (54%) | 4 of 14 (29%) | 0.13b | |
| Large vessel invasion | 3 of 38 (8%) | 1 of 14 (7%) | 1b | |
| Trabecular or trabecular-glandular architecture | 6 of 38 (16%) | 8 of 14 (57%) | <0.01b | |
| CDX2 positive | 9 of 33 (27%) | 5 of 12 (42%) | 0.47b | |
Student’s t-test, two-tailed.
Fisher’s exact test.
One case had no location recorded in the anatomic pathology report.
One serotonin-negative case had no tissue remaining for Ki-67 immunohistochemical staining.
G1 = <2%; G2 = 2–20%; G3 = >20%
G1 vs. G2/G3
Five cases had no lymph nodes submitted.
14 cases had no information on distant metastases.
Kruskal-Wallis equality-of-populations rank test
We then examined each neoplasm for serotonin immunoreactivity. Of the 52 PanNETs, 14 (27%) were at least focally serotonin-immunoreactive (Figure 1 and Table 1). Two were less than 15% positive; 8 were 15–70% positive; 4 were greater than 70% positive. There was a significant association between serotonin expression and trabecular or trabecular-glandular architecture: 8 of the 14 positive PanNETs (57%) had trabecular or trabecular-glandular architecture, while only 6 of the 38 (16%) negative PanNETs had similar histological patterns (P < 0.01). By contrast, only 2 of 34 control PanNETs at least focally expressed serotonin. Examining these controls further, 8 of 34 (24%) showed stromal fibrosis occupying 20 to 25% of the tumor area, and both of the serotonin-positive controls were in the fibrotic group.
After finding an association between serotonin expression and trabecular architecture in strongly fibrotic PanNETs, we next determined whether other clinical and pathological features of these PanNETs correlate with serotonin expression. First, among the 52 fibrotic PanNETs, the serotonin-expressing tumors were found in a significantly younger patient population (median age 47 versus 59 years) (P = 0.046), while serotonin status did not correlate with gender (see Table 2). All 52 tumors were clinically non-functional with the exception of two serotonin-negative tumors: one glucagonoma and one VIPoma. One serotonin-negative tumor was found in a patient with documented multiple endocrine neoplasia type 1 (MEN-1). Serum serotonin levels were not available, and no patient had clinically-documented carcinoid syndrome. Overall survival was better in the serotonin-positive tumors than serotonin-negative, but this trend did not meet statistical significance (P = 0.096) (Figure 2).
Figure 2.
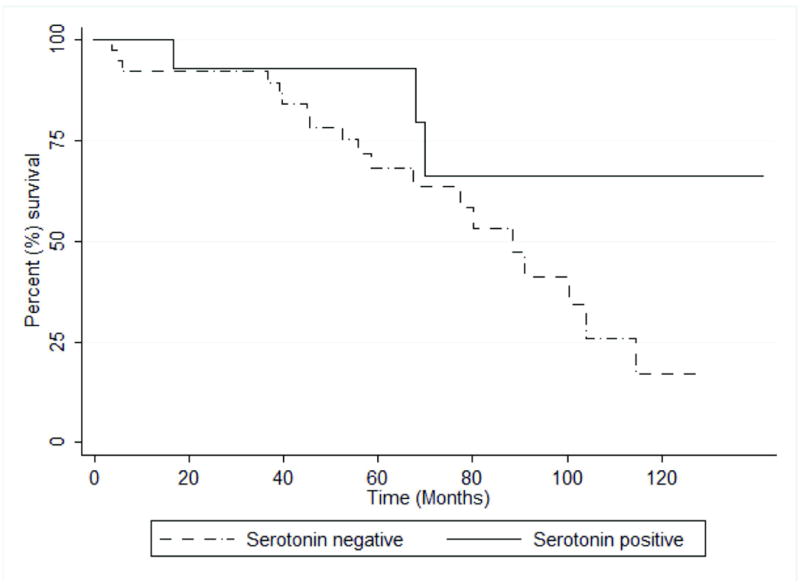
Serotonin immunoreactivity does not significantly correlate with overall patient survival. Kaplan-Meier overall survival curves. P = 0.096 (Log-rank test).
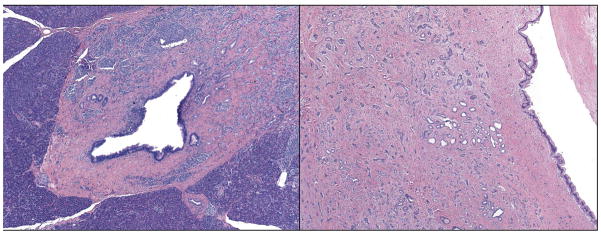
Serotonin-positive tumors had a significantly lower mitotic rate than those without serotonin expression (P < 0.01). In resections that included lymph nodes, there was a significant negative correlation between the presence of lymph node metastases and expression of serotonin: 25 of 34 serotonin-negative tumors (71%) had lymph node metastases while only 4 of 13 serotonin-expressing tumors (31%) had the same finding (P = 0.016). Furthermore, where data on distant metastases were available, there were fewer distant metastases in the serotonin-expressing tumors (2 of 9, 22%) than in serotonin-negative tumors (15 of 29, 52%), though this did not reach statistical significance (P = 0.15). Serotonin immunoreactivity also correlated strongly with large pancreatic duct involvement (Figure 3): of tumors where all slides were available for review, 11 of 13 (85%) of serotonin-expressing tumors involved large ducts compared with 15 of 37 (41%) of the serotonin negative tumors (P < 0.01). Serotonin status did not correlate with tumor size or the presence of infiltrative growth, necrosis, perineural invasion, or small or large vessel vascular invasion (see Table 2).
Figure 3.
Two examples of large pancreatic duct involvement in serotonin-positive PanNETs.
We next sought to characterize the serotonin-expressing tumors further by immunolabeling with Ki-67 and CDX2. In tumors where at least 200 neoplastic cells were available to count, we found that serotonin-expressing tumors had an average Ki-67 proliferative index of 3.34%. Eight of 14 (57%) were WHO grade G1 (<2%); 6 of 14 (43%) were G2 (2–20%). By contrast, the serotonin-negative tumors had an average Ki-67 of 5.48% (G1 (<3%): 13 of 37 (35%), G2 (3–20%): 23 of 37 (62%), G3 (>20%): 1 of 37 (3%)) [1]. These differences between serotonin expressing and non-expressing tumors did not reach statistical significance, either average Ki-67 (P = 0.17) or grade 1 versus higher grade (P = 0.21). Since the observed expression of serotonin associated with a trabecular pattern of growth with stromal fibrosis is similar to that seen in ileal carcinoids, which typically express the marker CDX2 [7], we immunolabeled each tumor for CDX2. We did not find a statistically significant association with CDX2 expression in comparing the 45 cases for which the stain was interpretable: 5 of 12 (42%) of serotonin-expressing tumors showed at least weak labeling for CDX2, while 9 of 33 (27%) of serotonin-negative tumors were positive (P = 0.42). Our CDX2 labeling percentage is consistent with the 30% value reported in the literature for “pancreatic carcinoids” [7], as well as the 23% recently reported for serotonin-expressing PanNETs [8].
Finally, as serotonin expression in PanNETs has also been found in tumors with other endocrine hormone expression [8,9], we examined whether the serotonin-positive PanNETs in our series also demonstrated immunoreactivity to other hormones. Of the 11 tumors for which tissue was available, one demonstrated reactivity to gastrin in approximately 10% of tumor cells (Table 3). One additional tumor showed individual (<5%) gastrin-positive neoplastic cells, two showed individual somatostatin-positive neoplastic cells, and one tumor showed individual neoplastic cells positive for somatostatin, glucagon, and insulin. Of note, while we concluded that there were rare, individual sneoplastic cells positive for hormones other than serotonin in some cases, we frequently noted entrapped normal islets at the edge of the tumors, which were associated with the appearance of multihormonality in PanNETs in a previous study [10].
Table 3.
Hormone Expression in Serotonin-Positive Cases
| Case No. | Insulin | Glucagon | Somatostatin | Gastrin |
|---|---|---|---|---|
| 1 | – | – | – | NDa |
| 2 | – | – | – | – |
| 3 | – | – | <5% | – |
| 4 | – | – | – | 10% |
| 5 | – | – | <5% | – |
| 6 | – | – | – | – |
| 7 | – | – | – | – |
| 8 | ND | ND | ND | ND |
| 9 | – | – | – | – |
| 10 | – | <5% | – | – |
| 11 | – | – | – | ND |
| 12 | – | – | – | – |
| 13 | <5% | <5% | <5% | – |
| 14 | – | – | – | – |
ND = not determined due to tumoral tissue not remaining in the paraffin block.
Discussion
In this study, in a large series of fibrotic PanNETs, we extended our previous observation that serotonin immunoreactivity in PanNETs is associated with prominent stromal fibrosis [6]. Review of each case revealed three distinct histologic architectures: solid, trabecular-glandular, and trabecular. We then immunolabeled each tumor for serotonin, and found that serotonin immunoreactivity correlated with trabecular or trabecular-glandular architecture. Consistent with our previous study, we found that serotonin-expressing tumors were much more likely to involve a large pancreatic duct [6]. We also noted a significant association of serotonin immunoreactivity with a younger patient population, fewer mitotic figures, and fewer lymph node metastases.
In addition, we also examined the relationship of serotonin immunoreactivity with tumor size, Ki-67 proliferative index, and the presence of known distant metastases. In each case, the serotonin-expressing tumors trended in a more benign direction—smaller tumors with lower Ki-67 proliferative indices and fewer distant metastases. However, none of these data reached statistical significance. This raises the possibility that differences in stage and age simply reflect the earlier detection of serotonin-positive tumors, perhaps because of duct obstruction and subsequent symptoms. With a larger series, it may be possible to discern whether serotonin immunoreactivity significantly correlates with these outcomes, though the rarity of these tumors may make such studies difficult.
Our results stand in contrast to those reported in a meta-analysis of 43 serotonin-positive PanNETs reported in the literature up to 1998, where the majority of cases were large and metastatic at the time of diagnosis [11]. A key distinction is that the meta-analysis included unresectable cases diagnosed by the presence of 5-hydroxyindoleacetic acid (5-HIAA) in the urine or elevated serotonin in the serum. Our current results are only from resected tumors, which are typically smaller and lower stage, which makes direct comparison of our data with the meta-analysis difficult.
Our previous case series was prompted by the clinical observation that some small pancreatic neuroendocrine tumors were associated with pancreatic duct stenosis and marked dilatation of the main pancreatic duct and/or marked atrophy of the upstream pancreas [6]. Five of the six tumors associated with pancreatic duct stenosis in the earlier series were serotonin-positive. In the literature, there are five other reports (twelve total cases) of duct obstruction secondary to serotonin-positive PanNETs, further confirming the potential link between duct obstruction/stenosis and serotonin expression [12–16]. Two cases reported by Hamada et al show a histological pattern consistent with the “trabecular” subtype in our series [12]. A single case reported by Kim et al shows a trabecular-glandular architectural pattern [13]. Four of five cases of PanNETs associated with the pancreatic duct in a recent series by Walter et al demonstrate a trabecular growth pattern (“carcinoid-like” in the report) and serotonin expression [14]. Finally, four additional fibrotic, serotonin-positive, duct-associated, trabecular or trabecular-glandular PanNETs were recently described by colleagues in our radiology department [15]. Although the retrospective nature of the current series does not allow us to ascertain whether the current series of serotonin-positive tumors were also detected because they produced pancreatic duct dilatation or pancreatic atrophy, it should be noted that most of them did involve a larger pancreatic duct.
In addition, a very recent report by La Rosa et al supports our conclusions. They described in detail the immunohistochemical and histological features of a series of 15 serotonin-expressing PanNETs [8]. 6 of the 15 tumors in this study showed “abundant” fibrous stroma and four showed “intermediate” levels of fibrosis; all tumors except one showed either a trabecular or “solid nested” pattern of growth similar to our findings. Interestingly, all of the tumors in their study had diffusely positive serotonin expression, even the five without significant stromal fibrosis. In our control set of 34 PanNETs, we did not identify any serotonin-expressing tumors without significant fibrosis; the discrepancy between our results and La Rosa et al may simply reflect the much larger sample size (362 PanNETs immunolabeled for serotonin) in their study.
Moreover, serotonin has been linked to fibrosis through several lines of evidence: it promotes fibroblast growth [17], has been shown to be important in the development of hepatic fibrosis [18,19], and most recently, a serotonin inhibitor reduced growth factor synthesis (transforming growth factor beta 1 [TGFβ1], connective tissue growth factor [CTGF] and fibroblast growth factor [FGF2]) by a neuroendocrine tumor cell line and concomitantly reduced proliferation of co-cultured fibroblasts [20]. Furthermore, the carcinoid syndrome has long been known to be associated with fibrosis at sites distant from the primary tumor, which may be secondary to serotonin release into the serum [21]. Our finding that serotonin expression correlates with extensive intratumoral fibrosis within trabecular architecture is consistent with these reports. Conversely, La Rosa et al found that none of the serotonin-expressing PanNETs in their study demonstrated immunoreactivity for acidic fibroblast growth factor (aFGF), and only weak CTGF expression was found in 4 of 15 tumors, compared with strong expression of each growth factor in ileal carcinoid controls [8]. They conclude that serotonin-positive PanNETs are likely to arise from a different type of enterochromaffin (EC) cell than ileal or appendiceal carcinoids, which is also supported by their and our CDX2 expression data. However, it is unclear from their results whether the weak CTGF expression correlated with the presence or extent of stromal fibrosis, and there was abundant fibrous stroma in only a minority of their cases. Taken together, our data and that from La Rosa et al suggest that while trabecular architecture with interspersed fibrosis strongly correlates with serotonin immunoreactivity, serotonin immunoreactivity may not be exclusive to PanNETs with stromal fibrosis.
In summary, in PanNETs, we found a clear correlation between serotonin immunoreactivity and a trabecular architecture with interspersed fibrosis, large pancreatic duct involvement, fewer lymph node metastases, and a younger patient population. Our data also show that the serotonin-positive PanNETs clearly differ from the serotonin-positive NETs of the ileum by their special histopathological pattern and less frequent positivity for CDX2. This suggests that they may derive from a cell lineage different from that of the intestinal EC cell. Finally, our results lend more support to the hypothesis that serotonin may be important to the development of fibrosis in some neuroendocrine tumors, and define serotonin-positive sclerotic tumors with trabecular growth as a distinct subset of fibrotic PanNETs which trend toward more benign biological features.
Acknowledgments
Funding disclosure: This work is funded in part by NIH grant P50-CA62924 (RHH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klimstra DS, Arnold R, Capella C, et al. Neuroendocrine neoplasms of the pancreas. In: Bosman F, Carneiro F, Hruban RH, Theise N, editors. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press; 2010. pp. 322–6. [Google Scholar]
- 2.Capelli P, Martignoni G, Pedica F, et al. Endocrine neoplasms of the pancreas: pathologic and genetic features. Arch Pathol Lab Med. 2009;133:350–64. doi: 10.5858/133.3.350. [DOI] [PubMed] [Google Scholar]
- 3.Klöppel G, Rindi G, Anlauf M, Perren A, Komminoth P. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007;451 (Suppl 1):S9–27. doi: 10.1007/s00428-007-0461-0. [DOI] [PubMed] [Google Scholar]
- 4.Verbeke CS. Endocrine tumours of the pancreas. Histopathology. 2010;56:669–682. doi: 10.1111/j.1365-2559.2010.03490.x. [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH, Bishop Pitman M, Klimstra DS. Tumors of the Pancreas. Washington, DC: Armed Forces Institute of Pathology; 2007. Endocrine neoplasms; pp. 251–304. [Google Scholar]
- 6.Shi C, Siegelman SS, Kawamoto S, et al. Pancreatic duct stenosis secondary to small endocrine neoplasms: a manifestation of serotonin production? Radiology. 2010;257:107–14. doi: 10.1148/radiol.10100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saqi A, Alexis D, Remotti F, Bhagat G. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol. 2005;123:394–404. doi: 10.1309/UKN6-PVRK-XHG4-22DA. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa S, Franzi F, Albarello L, et al. Serotonin-producing enterochromaffin cell tumors of the pancreas: clinicopathologic study of 15 cases and comparison with intestinal enterochromaffin cell tumors. Pancreas. 2011;40:883–95. doi: 10.1097/MPA.0b013e31822041a9. [DOI] [PubMed] [Google Scholar]
- 9.van der Sluys Veer JS, Choufour JC, Querido A, van der Huel RO, Hollander CF, van Rijssel T. Metastasising islet-cell tumour of the pancreas associated with hypoglycaemia and carcinoid syndrome. Lancet. 1964;1:1416–9. doi: 10.1016/s0140-6736(64)91985-3. [DOI] [PubMed] [Google Scholar]
- 10.Kapran Y, Bauersfeld J, Anlauf M, Sipos B, Klöppel G. Multihormonality and entrapment of islets in pancreatic endocrine tumors. Virchows Arch. 2006;448:394–8. doi: 10.1007/s00428-005-0147-4. [DOI] [PubMed] [Google Scholar]
- 11.Mao C, el Attar A, Domenico DR, Kim K, Howard JM. Carcinoid tumors of the pancreas. Status report based on two cases and review of the world’s literature. Int J Pancreatol. 1998;23:153–64. doi: 10.1385/IJGC:23:2:153. [DOI] [PubMed] [Google Scholar]
- 12.Hamada Y, Nakayama Y, Maeshiro K, Ikeda T, Hayashi H, Iwasaki H. Two cases of primary carcinoid tumor of the pancreas associated with marked stenosis of the main pancreatic duct. Pancreas. 2009;38:834–5. doi: 10.1097/MPA.0b013e3181b3afec. [DOI] [PubMed] [Google Scholar]
- 13.Kim HC, Park SI, Park SJ, et al. Pancreatic carcinoid tumor with obstructive pancreatitis: multislice helical CT appearance: case report. Abdom Imaging. 2005;30:601–4. doi: 10.1007/s00261-004-0285-1. [DOI] [PubMed] [Google Scholar]
- 14.Walter T, Hervieu V, Adham M, et al. Primary neuroendocrine tumors of the main pancreatic duct: a rare entity. Virchows Arch. 2011;485:537–546. doi: 10.1007/s00428-011-1067-0. [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto S, Shi C, Hruban RH, et al. Small Serotonin-Producing Neuroendocrine Tumor of the Pancreas Associated With Pancreatic Duct Obstruction. AJR Am J Roentgenol. 2011;197:W482–8. doi: 10.2214/AJR.10.5428. [DOI] [PubMed] [Google Scholar]
- 16.Nagai E, Yamaguchi K, Hashimoto H, Sakurai T. Carcinoid tumor of the pancreas with obstructive pancreatitis. Am J Gastroenterol. 1992;87:361–4. [PubMed] [Google Scholar]
- 17.Seuwen K, Magnaldo I, Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988;335:254–6. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- 18.Lesurtel M, Soll C, Graf R, Clavien P-A. Role of serotonin in the hepato-gastroIntestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65:940–52. doi: 10.1007/s00018-007-7377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Weng S-G, Leng X-S, et al. Effects of 5-hydroxytamine and its antagonists on hepatic stellate cells. Hbpd Int. 2006;5:96–100. [PubMed] [Google Scholar]
- 20.Svejda B, Kidd M, Giovinazzo F, et al. The 5-HT(2B) receptor plays a key regulatory role in both neuroendocrine tumor cell proliferation and the modulation of the fibroblast component of the neoplastic microenvironment. Cancer. 2010;116:2902–12. doi: 10.1002/cncr.25049. [DOI] [PubMed] [Google Scholar]
- 21.Modlin IM, Shapiro MD, Kidd M. Carcinoid tumors and fibrosis: an association with no explanation. Am J Gastroenterol. 2004;99:2466–78. doi: 10.1111/j.1572-0241.2004.40507.x. [DOI] [PubMed] [Google Scholar]