Abstract
We recently reported that the proteasomal peptidase activities are altered in the cerebellum of mice with MOG peptide-induced experimental autoimmune encephalomyelitis (EAE). To determine whether these fluctuations are caused by proteasome activation/inactivation and/or changes in the levels of individual β subunits, we characterized the proteasome subunit composition by western blotting. The results show that the rise in proteasomal peptidase activity in acute EAE correlates with an augmented expression of inducible β subunits whereas the decline in activity in chronic EAE correlates with a reduction in the amount of standard β subunits. Using pure standard (s) and immuno (i) 20S particles for calibration, we determined that the changes in the levels of catalytic subunits account for all of the fluctuations in peptidase activities in EAE. The i-20S and s-20S proteasome were found to degrade carbonylated β-actin with similar efficiency, suggesting that the amount of protein carbonyls in EAE may be controlled by the activity of both core particles. We also found an increase in proteasome activator PA28 and a decrease in inhibitor PI31 levels in acute EAE, reflecting a response to inflammation. Elevated levels of PA700 and PA28 in chronic EAE, on the other hand, may occur in response to diminished proteasomal activity in this phase. These findings are central towards understanding the altered proteasomal physiology in inflammatory demyelinating disorders.
Keywords: 20S proteasome, immunoproteasome, PA28, PA700, PI31, proteasomal dysfunction, experimental autoimmune encephalomyelitis, multiple sclerosis, oxidative stress, protein carbonylation
Introduction
Proteasomes are large protein complexes that carry out the majority of intracellular proteolysis, and therefore play an essential role in regulating cell function and maintaining homeostasis (Rechsteiner and Hill 2005). The core of these complexes, the 20S proteasome, is made of two outer rings of seven α subunits (α1-α7) and two inner rings of seven β subunits (β1-β7) (Ethen et al. 2007). Each β ring has three proteolytic sites, which display caspase-like (β1), trypsin-like (β2), and chymotrypsin-like (β5) activities (Zheng and Bizzozero 2010B). Exposure of cells to cytokines, such as interferon-γ, leads to the replacement of the catalytic β1, β2 and β5 subunits in the standard 20S particle (s-20S) for the inducible iβ1, iβ2 and iβ5 subunits, forming the immunoproteasome 20S particle (i-20S) (Aki et al. 1994). Because the relative activities of each of the inducible and constitutive β-subunits are somewhat different, the s-20S and i-20S proteasome generate different proteolytic fragments (Dahlmann et al. 2000; Klare et al. 2007). Adding complexity to this system is the recent discovery of “intermediate type” 20S particles in which one or two of the three catalytic β-subunits are replaced by the corresponding inducible subunits (Klare et al. 2007; Raijmakers et al. 2008).
Under physiological conditions a fraction of the 20S particle pool is bound to various regulators (activators and inhibitors), which greatly affect its catalytic activity and substrate specificity. For instance, the activator PA28 (a.k.a. 11S), also induced by interferon-γ, attaches to the i-20S particle forming a complex that produce peptides of the correct length for the major histocompatibility complex class 1 antigen processing (Rivett and Hearn 2004). Activation of the 20S proteasome upon binding to PA28 is blocked in the presence of PI31 (Zaiss et al. 1999), a proteasome inhibitor that also interferes with the maturation of immunoproteasome precursor complexes (Zaiss et al. 2002). PA700 (a.k.a. 19S), the other major activator, binds to the end of the 20S core particle forming the 26S proteasome, which recognizes and hydrolyzes abnormal and misfolded proteins conjugated with polyubiquitin chains (Braun et al. 1999). Degradation of carbonylated proteins is instead carried out by the 20S proteasome, mainly via its chymotrypsin-like activity, without any requirement of ATP or ubiquitin (Divald and Powell 2006). More recently, the i-20S proteasome, alone or in association with PA28, has been shown to aid in the removal of oxidized proteins suggesting an additional physiological function for the immunoproteasome (Pickering et al. 2010).
Our laboratory has recently discovered that the peptidase activity of the proteasome is altered in both multiple sclerosis (Zheng and Bizzozero 2011) and its animal model experimental autoimmune encephalomyelitis (EAE) (Zheng and Bizzozero 2010b). We found that in the acute phase of EAE, when inflammation is maximal, there is increased proteasomal chymotrypsin- and trypsin-like activity. In contrast, the chymotrypsin- and caspase-like activities are reduced in chronic EAE, which may explain the accumulation of carbonylated proteins observed in the neurodegenerative phase of the disease. Interestingly, the amount of constitutive α-subunit remains unchanged throughout the disease course, suggesting that proteasome activation/inactivation and/or changes in the β-subunit composition underlie the fluctuation in proteasomal activities observed as EAE progresses from the inflammatory to the neurodegenerative stages. To distinguish between these two possible scenarios, we decided to characterize the subunit composition of the proteasome and measure the status of various proteasome activators and inhibitors in the cerebellum of mice with MOG peptide-induced EAE. The results presented herein show for the first time that (1) fluctuations of 20S proteasome peptidase activities are largely due to changes in catalytic subunit composition; (2) both s-20S and i-20S core proteasomes, which are equally abundant in the cerebellum, degrade endogenous carbonylated proteins with similar efficiency, and (3) levels of several proteasomal regulators are altered in both acute and chronic EAE. These findings contribute to our understanding of how damaged proteins are removed in inflammatory demyelinating disorders.
Materials and Methods
Induction of experimental autoimmune encephalomyelitis (EAE)
Housing and handling of the animals as well as the euthanasia procedure were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee. Eight-week-old female C57BL/6 mice were purchased from Harlan Bioproducts (Indianapolis, IN) and housed at the UNM-animal resource facility. EAE was induced by active immunization with MOG35-55 peptide (AnaSpec, San Jose, CA) as described in our previous study (Zheng and Bizzozero 2010a). Animals were weighed and examined daily for the presence of neurological signs. Acute disease was defined as having maximal neurological symptoms of EAE without any improvement for at least three consecutive days while chronic EAE was defined arbitrarily as animals that remain in the stationary phase of the disease for 30 days. Given the variability in neurological symptoms, three animals in each the acute and chronic phase, all displaying a clinical score of 3.5 (hind limb paralysis with fore limb paresis) were selected for this study. Age-matched control animals for acute EAE (control young) and chronic EAE (control old) consisted of mice injected with complete Freund’s adjuvant alone (i.e. without the MOG peptide). EAE and control mice were euthanized by decapitation, the cerebellum was removed rapidly and was homogenized in PEN buffer (10 mM sodium phosphate pH 7.0, 1 mM EDTA and 0.1 mM neocuproine) containing 1mM 4,5-dihydroxy-1,3-benzene sulfonate and 0.5mM dithiothreitol to prevent further protein oxidation. Homogenates were kept at −80°C until use. Protein concentration was assessed with the Bio-Rad DCT protein assay using bovine serum albumin as standard.
Western blot analysis
Proteins (5 μg) from tissue homogenates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 12% gels and blotted to polyvinylidene difluoride membranes. Blots were then incubated overnight at 4°C with antibodies against β-actin (1:2,000; Sigma); Lys-48 poly-ubiquitin chain (1:2,000; Millipore Corp., Billerica, MA); 20S proteasome α-subunits (α1-3/α5-7; 1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA); β1, β2 and β5 subunits (1:1,000; Enzo); iβ1, iβ2 and iβ5 subunits (1:1,000; Enzo); PA28α (1:1,000, Enzo); PI31 (1:1,000, Enzo) and 19S proteasome Rpt4 (1:1,000, Enzo). Membranes were rinsed three times in phosphate-buffered saline solution containing 0.05% Tween-20 and were incubated for 2 h with horseradish peroxidase conjugated-conjugated anti-mouse antibody (1:2,000; Sigma) or anti-rabbit antibody (1:2,000; Sigma). Blots were developed by enhanced chemiluminescence (ECL) using the Western Lightning ECL™ kit from Perkin-Elmer (Boston, MA). Films were scanned in a Hewlett Packard Scanjet 4890 and the images were quantified using the NIH Image 1.63 imaging analysis program. Band intensities were normalized by the amount of coomassie blue staining in the corresponding lanes.
Proteasomal activity assays
The various peptidase activities present in purified s-20S and i-20S proteasomes (Enzo Life Sciences, Plymouth Meeting, PA) were determined by using fluorescence assays (Rodgers and Dean 2003). Briefly, 0.625μg of either standard proteasome or immunoproteasome (Boston Biochem Inc., Cambridge, MA) were incubated for 2h at 25°C with 50μM of the 7-aminomethyl-4-coumarin (AMC)-labeled peptide Suc-Leu-Leu-Val-Tyr-AMC (for chymotrypsin-like activity), Boc-Leu-Arg-Arg-AMC (for trypsin-like activity) or z-Leu-Leu-Glu-AMC (for caspase-like activity). The different peptidolytic activities were calculated as the fluorescence intensity at 460nm using an excitation wavelength of 380nm.
Incubation of oxidized brain proteins with s-20S and i-20S particles
Rat brain slices (400μm-thick) were prepared as described by Bizzozero et al (2006). Slices corresponding to ~80 mg of tissue were transferred to flasks containing 3 ml of Hank’s balanced salt solution supplemented with 10 mM D-glucose, and were incubated at 37°C under 95% O2/5% CO2. Tissue sections were incubated for 2h with 2mM diethyl maleate and 2 μM of the irreversible proteasome inhibitor epoxomicin. Slices were then collected by low-speed centrifugation and rinsed twice with 2 ml of ice-cold saline solution. Slices were homogenized in PEN buffer with reducing agents and centrifuged at 10,000g for 30 min. To ensure the removal of any residual epoxomicin, the supernatant was dialyzed overnight at 4°C against 25mM Hepes buffer pH 7.5 containing 4mM EDTA and 1mM dithiothreitol. Aliquots of dialyzed material (200μg protein) were incubated separately with 4μg of s-20S or i-20S for 2h at 37°C. The extent of carbonylation of individual proteins was determined by a pull-down/western blot procedure (Zheng and Bizzozero 2010a,b). Briefly, protein carbonyls were biotinylated by reaction with biotin hydrazide. A small aliquot of these protein homogenates was saved for western blotting and the rest was used to isolate the biotinylated proteins using streptavidin-agarose. Proteins were eluted from the beads with sodium dodecyl sulfate-sample buffer and analyzed by western blotting. Blots were probed with antibody against β-actin and developed by ECL as described above. Films were scanned in a Hewlett Packard Scanjet 4890 and the images were quantified using the NIH Image 1.63 imaging analysis program. The intensities corresponding to β-actin in the total and streptavidin-bound fractions were used to calculate the percentage of protein that is modified by carbonylation in the samples incubated with s-20S or i-20S.
Statistical Analysis
Results were analyzed for statistical significance by t-test utilizing the GraphPad Prism® program (GraphPad Software Inc., San Diego, CA).
Results
Distinct patterns of proteasome β and iβ subunit expression are found in acute and chronic EAE
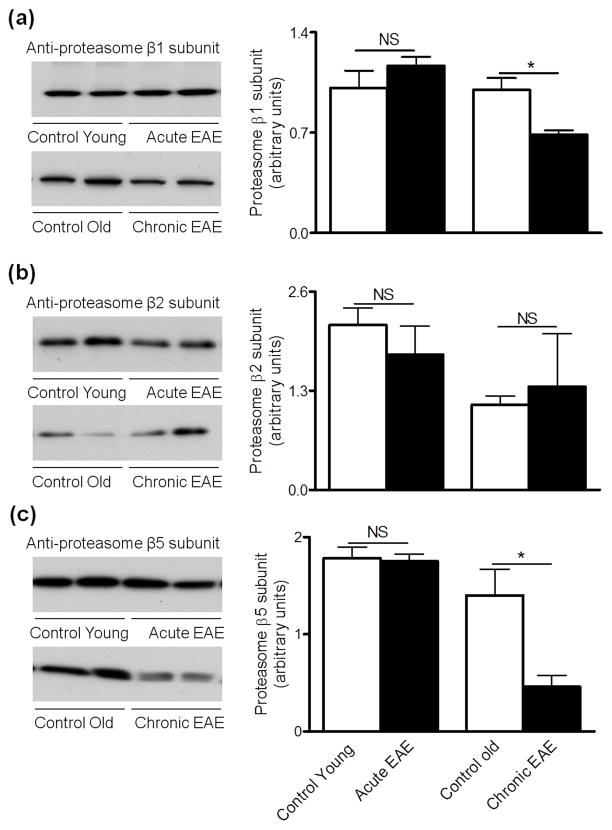
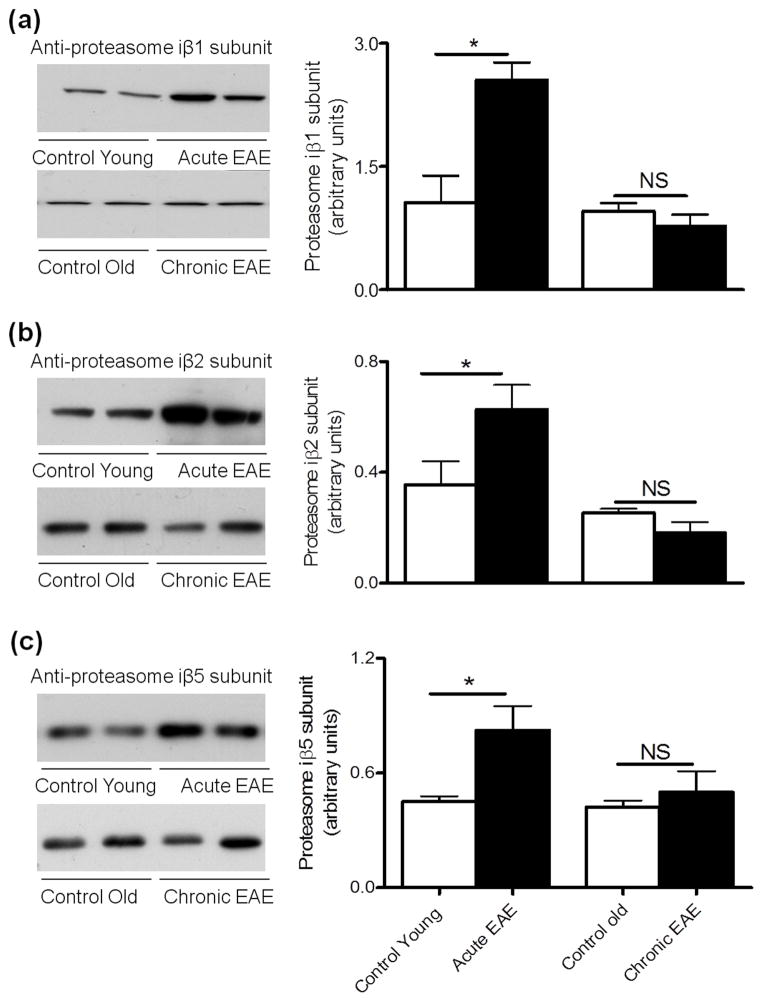
We have recently shown that cerebellum proteasomal peptidase activities in acute EAE are increased while the chymotrypsin-like and caspase-like activities in chronic EAE are decreased relative to their respective age-matched non-EAE controls (Zheng and Bizzozero 2010b; Table 1). Enzymatic assays, however, do not permit one to establish the molecular origin of these activities. For instance, an augment in chymotrypsin-like activity could represent the increased expression of proteasomes containing β5, iβ5 or both. Consequently, we sought to determine if the distinct patterns in proteasomal peptidase activities at various stages of EAE could be ascribed to changes in β subunit composition. To this end, the levels of the standard β subunits and their inducible counterparts in the cerebellum of EAE and control mice were measured by western blotting. As depicted in Fig. 1, the amount of the three standard catalytic subunits in acute EAE are the same as those in the corresponding control group, while the amount of β1 and β5 subunits decrease in the chronic phase of the disease. A different pattern of expression was found for the inducible β subunits, with the relative amounts of all three iβ chains being increased in acute EAE and unchanged in chronic EAE when compared to their respective controls (Fig. 2). An increase of active iβ chains in the acute phase of EAE was previously described in LEW-1N rats immunized with MOG1-125 peptide (Fissolo et al. 2008).
Table 1.
Peptidase activities of the 20S proteasome in EAE (FU/h/μg protein)
| Control Young | Acute EAE | Control Old | Chronic EAE | |
|---|---|---|---|---|
| Caspase-like activity | 381.3 ± 23.6 | 498.3 ± 39.2* | 328.7 ± 13.9 | 236.4 ± 29.2 * |
| Trypsin-like activity | 75.7 ± 2.2 | 108.3 ± 10.9* | 62.3 ± 6.5 | 73.0 ± 7.2 |
| Chymotrypsin-like activity | 63.4 ± 4.6 | 88.3 ± 3.8* | 77.2 ± 5.0 | 49.2 ± 6.1* |
Aliquots of cerebellum homogenates from control and EAE mice were used to determine the caspase-like, trypsin-like and chymotrypsin-like proteasomal activity as described in “Materials and Methods”. Values represent the mean ± SEM of 3 animals per experimental group. Asterisks denote values that are significantly different (p<0.05) from control. FU, fluorescence units.
Figure 1.
Levels of 20S proteasome β1 and β5 subunits are reduced in chronic EAE. Left three panels show representative immunoblots of cerebellum homogenates from control and EAE mice developed with antibodies against the β1 subunit (a), β2 subunit (b) and β5 subunit (c) of the 20S proteasome. Right panels depict the corresponding levels of each of the β subunits, which were calculated by dividing band intensity on the western blot by that of the corresponding coomassie blue stained lane. Values are the mean ± SEM of 3 animals per experimental group. *p<0.05. NS, not significant.
Figure 2.
Levels of 20S proteasome iβ1, iβ2 and iβ5 subunits are enhanced in acute EAE. Left three panels show representative immunoblots of cerebellum homogenates from control and EAE mice developed with antibodies against the iβ1 subunit (a), iβ2 subunit (b) and iβ5 subunit (c) of the 20S proteasome. Right panels depict the corresponding levels of each of the inducible β subunits, which were calculated by dividing band intensity on the western blot by that of the corresponding coomassie blue stained lane. Values are the mean ± SEM of 3 animals per experimental group. *p<0.05. NS, not significant.
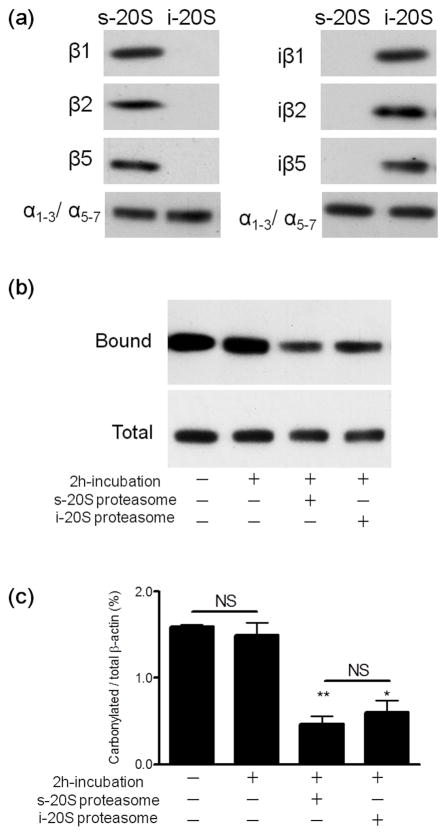
Using the intensity values obtained by western blot analysis (Figs. 1 and 2) and purified mouse s-20S and i-20S proteasomes for calibration, we estimated the absolute amounts of each catalytic subunit in the control and EAE cerebellum homogenates. The purity of the s-20S particle (positive for β1, β2 and β5 but not for their inducible counterparts) and of i-20S (positive for iβ1, iβ2 and iβ5 but not for their standard counterparts) was demonstrated by western blotting (Fig. 3A). The concentration of each subunit was then expressed in pmoles/mg protein to allow for direct comparison in subunit expression. As shown in Table 2, both control and EAE cerebellum contain a substantial amount of iβ chains as compared to the standard subunits, indicating that their expression is not restricted to immune cells. Interestingly, the molar amounts of β1, β2 and β5 subunit are different, and the same applies for the iβ subunits. However, the sum of the standard and inducible form of each catalytic subunit is similar. For example, in control cerebellum, the concentration of β1 + iβ1, β2 + iβ2, and β5 + iβ5 is 2.72, 2.98 and 2.49 pmol/mg protein, respectively. This suggests the occurrence of “intermediate type” 20S particles where one or two standard subunits are replaced by the corresponding inducible species. The average amount of hemi-proteasomes in the cerebellum (pmol/mg protein) based on the content of β and iβ subunits is 2.7 for control young, 4.0 for acute EAE, 2.2 for control old and 1.7 for chronic EAE mice.
Figure 3.
Standard and immunoproteasomes digest carbonylated β-actin in a cell-free system with similar efficacy. The purity of the s- and i-20S proteasome was verified by western blot analysis using antibodies against various catalytic subunits (a). Oxidized rat brain homogenates (200μg protein), devoid of endogenous proteasomal activity, were incubated with 4μg of either standard proteasome or immunproteasome. After 2 h, carbonylated proteins were isolated using the pull-down procedure described in “Materials and Methods”. A representative western blot of the total and streptavidin-bound fractions developed with an antibody against β-actin is shown in panel (b). Densitometric scans were obtained to calculate the proportion of carbonylated β-actin in the various conditions (c). Values represent the mean ± SEM of 5–7 experiments. Asterisks denote values that are significantly different from incubated control. * p<0.01, ** p<0.001. NS, not significant.
Table 2.
Levels of catalytic subunits of s-20S and i-20S in EAE (pmol/mg protein)
| Control young | Acute EAE | Control old | Chronic EAE | |
|---|---|---|---|---|
| β1 | 1.73 ± 0.20 | 1.99 ± 0.11 | 1.70 ± 0.14 | 1.17 ± 0.05* |
| β2 | 0.95 ± 0.10 | 0.78 ± 0.16 | 0.49 ± 0.05 | 0.59 ± 0.30 |
| β5 | 1.57 ± 0.10 | 1.55 ± 0.06 | 1.25 ± 0.24 | 0.41 ± 0.10* |
| iβ1 | 0.99 ± 0.30 | 2.37 ± 0.20* | 0.89 ± 0.09 | 0.72 ± 0.13 |
| iβ2 | 2.03 ± 0.49 | 3.59 ± 0.50* | 1.45 ± 0.08 | 1.04 ± 0.22 |
| iβ5 | 0.92 ± 0.06 | 1.69 ± 0.26* | 0.86 ± 0.07 | 1.02 ± 0.23 |
The concentration of each standard and immuno catalytic subunit in control and EAE cerebella was determined by western blot analysis (Fig. 1 and 2) using calibration curves made with increasing amounts of either purified standard proteasome or immunoproteasome. Values are the mean ± SEM of 3 animals per experimental group. Asterisks denote values that are significantly different (p<0.05) from control.
Changes in the proportion of 20S catalytic subunits are responsible for the fluctuation of peptidase activities in EAE
To convert the amount of each catalytic β-subunit present in the cerebellum of control and EAE animals into the corresponding activity, we first determined the various peptidolytic activities in isolated mouse s-20S and i-20S proteasome. In our assay conditions, the 20S particle of the immunproteasome exhibits higher trypsin-like activity and lower caspase-like activities than the standard 20S particle (Table 3). These activity values and the amount of the catalytic chains control and EAE tissue determined by western blot analysis were then used to predict the total caspase-like, trypsin-like and chymotrypsin-like peptidase activities. As shown in Table 4, the changes in peptidase activity in acute and chronic EAE predicted using the content of catalytic β-subunits are indistinguishable from those determined experimentally. These data support the notion that the fluctuations of 20S peptidase activities in EAE are due to the changes in catalytic subunit composition that occur during disease progression.
Table 3.
Peptidase activities in purified s- and i-20S proteasome (FU/h/ng proteasome)
| Standard proteasome | Immunoproteasome | |
|---|---|---|
| Caspase-like activity | 79.6 ± 9.9 | 34.3 ± 3.1* |
| Trypsin-like activity | 43.0 ± 0.6 | 64.5 ± 2.5** |
| Chymotrypsin-like activity | 63.9 ± 1.6 | 65.5 ± 0.9 |
The various peptidase activities of purified standard proteasomes or immunoproteasomes (0.625μg) were determined using the fluorescence assay described in “Materials and Methods”. Values represent the mean ± SEM of 3 separate determinations. Asterisks denote values that are significantly different form those in the standard proteasome.
p<0.05;
p<0.01; FU, fluorescence units.
Table 4.
Predicted values of proteasomal peptidase activitis in EAE (% of control)
| Acute EAE | Chronic EAE | |||
|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | |
| Caspase-like activity | 131 ± 10 | 140 ± 8 | 72 ± 9 | 71 ± 5 |
|
|
|
|||
| Trypsin-like activity | 143 ± 14 | 154 ± 22 | 117 ± 12 | 81 ± 24 |
| Chymotrypsin-like activity | 139 ± 6 | 130 ± 13 | 64 ± 8 | 69 ± 13 |
Predicted peptidase activity values were calculated using the concentration of standard and immuno catalytic β subunits in control and EAE cerebella (Table 2) and the individual enzymatic activities obtained with purified standard proteasomes and immunproteasomes (Table 3). Experimental values were calculated from the data shown in Table 1. Values represent the mean ± SEM of 3 animals per experimental group.
Both i-20S and s-20S particles are equally effective at digesting carbonylated proteins in vitro
We have proposed that the absence of protein carbonyls in acute EAE may be due to the enhancement of proteasomal activities that digest oxidized proteins (Zheng and Bizzozero 2010b). Considering that only i-20S proteasome levels are increased in acute EAE, and that this particle contributes to a significant proportion of total proteasomal peptidase activity, we thought that the immunproteasome might also participate in the degradation of carbonylated proteins. To test this idea, carbonylated brain proteins were generated by incubation of brain slices with the glutathione-depletor diethyl maleate in the presence of the irreversible proteasome inhibitor epoxomicin. After removing the excess epoxomicin, the tissue homogenate was incubated with either s-20S or i-20S and the extent of carbonylation of β-actin was quantified using a pull down-western blot procedure. This abundant cytoskeletal protein was selected for this study because (1) carbonylated β-actin is preferentially degraded by proteasomes, and (2) carbonylated βactin levels are significantly elevated in the cerebellum of mice with chronic EAE (Zheng and Bizzozero 2010a,b). The results clearly show that, the immunoproteasome is as effective as the s-20S particle at hydrolyzing the carbonylated form of β-actin (Fig. 3b,c). Thus, it is likely that the enhancement in the number of proteasomes containing iβ5 chains in acute EAE may facilitate the degradation of carbonylated proteins, leading to the lack of protein carbonyl accumulation previously reported (Zheng and Bizzozero 2010a).
Levels of various 20S proteasomal regulators are also altered in EAE
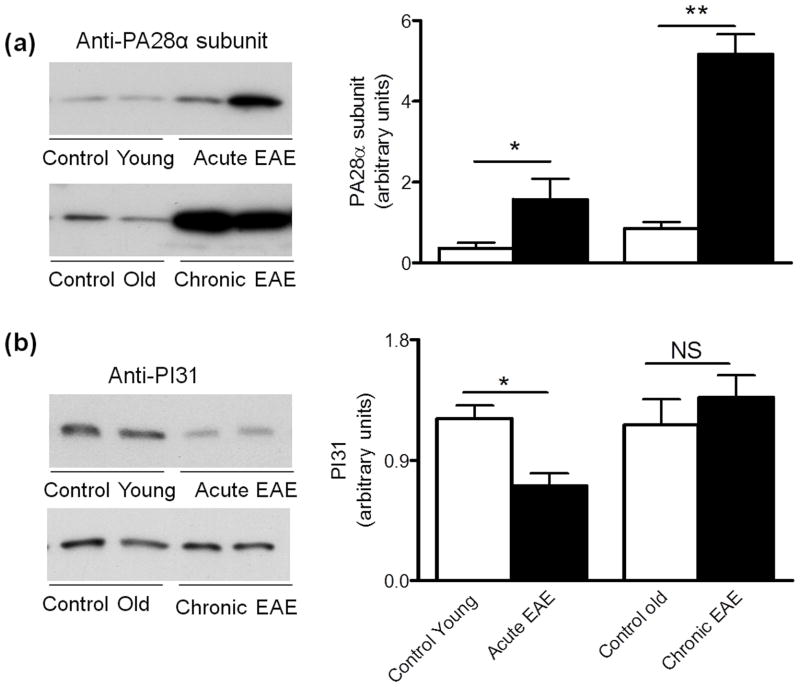
The content of two proteasomal activators (PA28 and PA700) and the proteasomal inhibitor PI31 during the course of EAE was also determined by western blot analysis. Relative to the controls, the levels of the PA28α subunit increase 4.4-fold and 6.1-fold in acute and chronic EAE, respectively (Fig. 4A). In contrast, the amount of the PA28 competitive inhibitor PI31 diminishes in acute EAE while it remains unchanged in chronic EAE (Fig. 4B). PI31 is also known to interfere with the maturation of immunoproteasome precursor complexes (Zaiss et al. 2002). Thus, the reduced level of this inhibitor in acute EAE agrees with the increased assembly of immunoproteasome that we observed at this disease stage (Fig. 2).
Figure 4.
Changes in the levels of the proteasomal activator PA28 and its competitive inhibitor PI31 in EAE. Left two panels show representative immunoblots of cerebellum homogenates from control and EAE mice developed with antibodies against the PA28α subunit of the 11S particle (a) and the proteasomal inhibitor PI31 (b). Right panels depict the corresponding levels of each of these proteins, which were calculated by dividing the band intensity on the western blot by that of the corresponding coomassie blue stained lane. Values represent the mean ± SEM of 3 animals per experimental group. *p<0.05, ** p<0.01. NS, not significant.
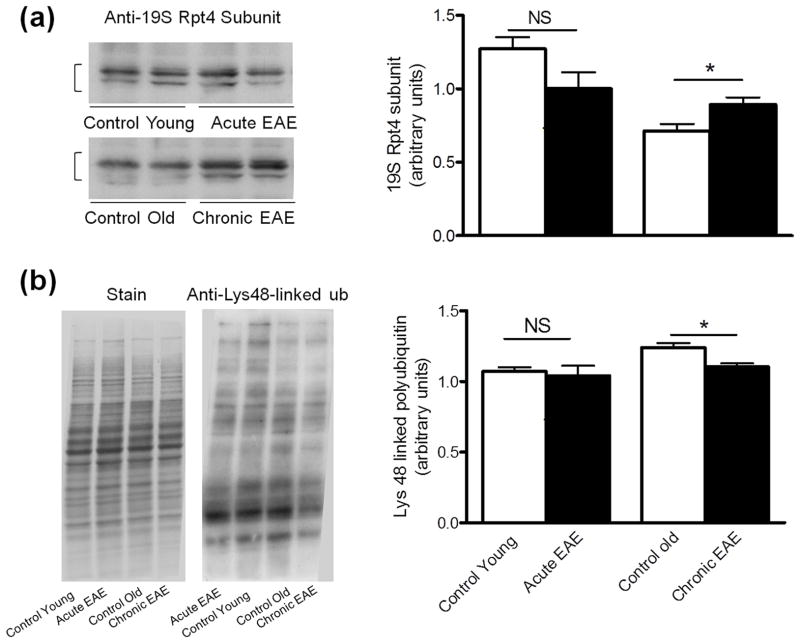
Levels of the well-known proteasomal activator PA700 (19S) were determined employing an antibody that recognizes the Rpt4/S10b subunit (ATPase subunit) in this activator. An increase in the concentration of PA700 (19S) is observed only in chronic EAE (Fig. 5A). Since the amounts of 19S and standard proteasome in chronic EAE change in opposite directions, it is difficult to predict the functional status of the 26S proteasome, which is responsible of removing ubiquitinated proteins. However, we found that the content of proteins containing Lys-48 poly-ubiquitin chains, normally targeted for proteasomal degradation, are slightly decreased in chronic EAE (Fig. 5B). These data suggest that the modest overexpression of 19S in the cerebellum of chronic EAE mice may counteract the decrease in 20S core and thus maintain a functional ubiquitin proteasome system. This finding is also consistent with the fact that the 20S core proteasome is much more abundant than the 19S particle (Brooks et al. 2000).
Figure 5.
Levels of the proteasomal activator 19S are slighlty increased in chronic EAE. Left two panels show representative immunoblots of cerebellum homogenates from control and EAE mice developed with antibodies against the Rpt4 subunit of the 19S particle (a) and Lys-48 poly-ubiquitin (b). Right panels depict the corresponding levels of each of these proteins, which were calculated by dividing the band intensity (in the case of the Rpt4 subunit) or lane intensity (in the case of Lys-48 poly-ubiquitin) on the western blot by that of the corresponding coomassie blue stained lane. Values represent the mean ± SEM of 3 animals per experimental group. *p<0.05. NS, not significant.
Discussion
In this study we investigated the mechanisms underlying the changes of proteasomal activities in the cerebellum during the course of EAE. The results clearly show that the rise in proteasome peptidase activity in the acute phase of EAE correlates with an increased expression of inducible catalytic subunits while the decline in chronic EAE correlates with a reduction in the amount of standard catalytic subunits. Moreover, the changes in the catalytic subunits of s-20S and i-20S proteasomes seem to account for all of the fluctuations of proteasomal peptidase activities during the disease course of EAE. In addition, we discovered that i-20S and s-20S are capable of digesting carbonylated β-actin in vitro with similar efficiency, suggesting that both proteasomal particles may be important for regulating the levels of protein carbonyls in EAE. Finally, the study shows for the first time an altered amount of several proteasomal regulators during the course of EAE.
While immunoproteasomes were initially thought to be present exclusively in immune cells where they participate in the generation of peptides for antigen presentation, they are now known to be expressed in most tissues and to play functions that are unrelated to the immune system (Seifert et al. 2010; Hussong et al. 2010). However, the proportion of standard proteasomes and immunoproteasomes has been found to vary greatly in different human cells and tissues. For example, s-20S/i-20S ratio is 0.02 in intestine, 0.45 in liver and 4.9 in heart (Guillaume et al. 2010). Even in the CNS, the expression level of i-20S particles varies in different areas. For instance, while all brain regions, except for the hippocampus, contain low levels of immunoproteasomes (Kremer et al. 2010; Mishto et al. 2006), inducible subunits are clearly detected in cerebellum (Mishto et al. 2006). Our results not only confirm the occurrence of inducible subunits in normal cerebellum but also show that they are as abundant as their standard counterparts. Moreover, we found that in acute EAE the majority of cerebellar neurons and oligodendrocytes are positive for both types of proteasomes (Supplemental Fig. 1 and 2). Interestingly, astrocytes express mostly standard proteasomes while microglia and T cells express mainly immunoproteasomes (supplemental Fig. 1 and 2). The presence of immunoproteasome subunits in neurons and oligodendrocytes agree with previous studies (Hussong et al. 2010) and clearly indicate that the i20S particles also play non-immune roles.
Proteolytic cleavage of propeptides to form the mature, active β chains takes place after proteasome assembly (Chen and Hochstrasser 1996). Thus, the levels of catalytic β chains shown in Table 2, which correspond to those of the mature molecular species, are an appropriate indicator of the amount of active 20S particles. Based on the content of mature catalytic β chains, the total amount of core proteasome particles (i-20S + s-20S + “intermediate type” 20S) is increased in acute EAE and decreased in chronic EAE relative to their age-matched controls. Also, the proteasome levels in cerebellum are lower in old compared to young control animals, which agrees with findings showing reduced proteasomal peptidase activity in aging (Keller et al. 2000). In contrast to changes in β subunits, the total content of 20S α subunits, measured by western blotting using the antibody against the α1-3/α5-7 chains, is not altered in the disease (Zheng and Bizzozero 2010b). This suggests the existence of a pool of α chains that is not part of mature, functional proteasomes. Indeed, we have found that the levels of α chains range between 34 and 50 pmol/mg protein (data not shown), which would correspond to 4.8–7.1 pmol hemi-proteasome/mg protein; values that exceed those calculated from the amount of mature β subunits (1.7–4.0 pmol hemi-proteasome/mg protein). Other studies have also shown an imbalance in the expression of 20S catalytic subunits and structural α subunits. For example, in the lumbar spinal cord of SOD1G93A transgenic mice, an animal model of amyotrophic lateral sclerosis, the reduction in β5 is not accompanied by an increase in amount of iβ5 subunit and yet the levels of structural 20S α chains are normal (Kabashi et al. 2008). Similarly, the up-regulation of inducible catalytic subunits in the cortex and striatum of HD94 mice, an animal model of Huntington’s disease, is not followed by an increase in α chains levels despite normal amounts of standard catalytic subunits (Díaz-Hernández et al. 2003).
Our data also suggest that both control and EAE cerebellum contain a substantial amount of “intermediate type” 20S particles where one or two standard subunits are replaced by the corresponding inducible species. This implies not only that β and iβ chains are expressed in the same cells but also that there is an imbalance in the levels of the three catalytic subunits, which may be due to changes in transcription/translation of selected chains and/or altered protein stability. It is important to note that while it has been suggested that β5 might be replaced by either iβ1, iβ2, or iβ5 to form some kind of hybrid proteasome (Raijmakers et al. 2008), there is not direct evidence showing abnormal ratios of different subunits in β rings, and knockdown of each β subunit in HEK293T cells by small interfering RNA results in arrest of the assembly process (Hirano et al. 2008).
The mechanism(s) underlying the reduced expression of standard proteasomes in chronic EAE is unclear. Abundance of proteasomes in mammalian cells is mostly regulated at the transcriptional level but there is limited information on the identity of the transcription factors (Kriegenburg 2011). It has been recently found that late-stage deletion of the transcription factor Nrf1 in neuronal cells leads to impaired proteasome function due to down-regulation of proteasomal genes that encode the catalytic subunits (Lee et al. 2011). Similarly, activation of the transcription factor Nrf2 has been shown to increase expression of standard β chains and delay senescence in human fibroblasts (Kapeta et al. 2010). While expression levels of Nrf1 and Nrf2 in chronic EAE are not known, ablation of the Nrf2 gene exacerbates EAE (Johnson et al. 2010). In addition to transcriptional regulation, several proteasome subunits are known to undergo caspase-dependent proteolysis during cell apoptosis (Adrian et al. 2004). Since the expression of various caspases, particularly caspase-3, is enhanced late in the course of EAE (Ahmed et al. 2002), the reduction in the amount of standard proteasomal subunits at this stage may be due simply to increased proteolysis. Another possibility could be the presence of oxidative modifications on the standard β chains, which may reduce antibody binding on western blots.
Abundant biochemical and immunohistochemical evidence indicates that inflammation plays a central pathophysiological role in the acute phase of EAE. Local inflammation leads to the production of several cytokines, including INF-γ, which can up-regulate both immunoproteasome subunits and the proteasome activator PA28. In line with this notion, we found that the amount of all three catalytic iβ chains rises in acute EAE, returning to normal values as disease progresses and inflammation subsides (Table 2). In contrast, the levels of PA28α continue to increase throughout the disease. This uncoordinated upregulation of PA28 and immunoproteasome expression in chronic EAE is puzzling and suggests that other mechanisms may be involved. PA28 is known to interact not only with the i-20S but also with the s-20S particle forming the respective 11S/20S complexes (Luo et al. 2010). Recent studies have shown that PA28 aids in the degradation of oxidized proteins by the core 20S particles, and that enhancement of proteasome function by PA28 overexpression protects cells against oxidative stress (Pickering et al. 2010; Li et al. 2011). Furthermore, PA28 expression augments in response to oxidative stress (Pickering et al. 2010), inhibition of proteasomal function, generation of misfolded proteins or impairment of mitochondrial function (Mcnaught et al. 2010). Thus, the increase in the levels of proteasomal activator PA28, and also PA700, as EAE progresses may reflect adaptive or compensatory mechanisms to local inflammation and oxidative injury in this disease. Future studies will address the functional consequences of such changes.
Supplementary Material
Acknowledgments
The authors thank Dr. Nora Perrone-Bizzozero (Department of Neurosciences, UNM) for critical reading of the manuscript. This work was supported by PHHS grant NS057755 from the National Institutes of Health.
Abbreviations
- β chain
standard β chain
- EAE
experimental autoimmune encephalomyelitis
- ECL
enhanced chemiluminescence
- i-20S
immuno 20S
- iβ chain
inducible β chain
- PA28
11S regulatory particle
- PA700
19S regulatory particle
- PI31
proteasome inhibitor with molecular mass of 31kDa
- s-20S
standard 20S
Footnotes
Disclosure/conflict of interest
The authors have no conflict of interest.
References
- Adrain C, Creagh EM, Cullen SP, Martin SJ. Caspase-dependent inactivation of proteasome function during programmed cell death in Drosophila and man. J Biol Chem. 2004;279:36923–36930. doi: 10.1074/jbc.M402638200. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Doward AI, Pryce G, Taylor DL, Pocock JM, Leonard JP, Baker D, Cruzner ML. A role for caspase-1 and −3 in the pathology of experimental allergic encephalomyelitis: inflammation versus degeneration. Am J Pathol. 2002;161:1577–1586. doi: 10.1016/S0002-9440(10)64436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aki M, Shimbara N, Takashina M, Akiyama K, Kagawa S, Tamura T, Tanahashi N, Yoshimura T, Tanaka K, Ichihara A. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J Biochem. 1994;115:257–269. doi: 10.1093/oxfordjournals.jbchem.a124327. [DOI] [PubMed] [Google Scholar]
- Bizzozero OA, Ziegler JL, DeJesus G, Bolognani F. Acute depletion of reduced glutathione causes extensive carbonylation of rat brain proteins. J Neurosci Res. 2006;83:656–667. doi: 10.1002/jnr.20771. [DOI] [PubMed] [Google Scholar]
- Brooks P, Fuertes G, Murray RZ, Bose S, Knecht E, Rechsteiner MC, Hendil KB, Tanaka K, Dyson J, Rivett J. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. Biochem J. 2000;346:155–161. [PMC free article] [PubMed] [Google Scholar]
- Braun B, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, Finley D, Schmidt M. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat Cell Biol. 1999;1:221–226. doi: 10.1038/12043. [DOI] [PubMed] [Google Scholar]
- Chen P, Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell. 1996;86:961–972. doi: 10.1016/s0092-8674(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Dahlmann B, Ruppert T, Kuehn L, Merforth S, Kloetzel PM. Different proteasome subtypes in a single tissue exhibit different enzymatic properties. J Mol Biol. 2000;303:643–653. doi: 10.1006/jmbi.2000.4185. [DOI] [PubMed] [Google Scholar]
- Díaz-Hernández M, Hernández F, Martín-Aparicio E, Gómez-Ramos P, Morán MA, Castanñ JG, Ferrer I, Avila J, Lucas JJ. Neuronal induction of the immunoproteasome in Huntington’s disease. J Neurosci. 2003;23:11653–11661. doi: 10.1523/JNEUROSCI.23-37-11653.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divald A, Powell SR. Proteasome mediates removal of proteins oxidized during myocardial ischemia. Free Radic Biol Med. 2006;40:156–164. doi: 10.1016/j.freeradbiomed.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Ethen CM, Hussong SA, Reilly C, Feng X, Olsen TW, Ferrington DA. Transformation of the proteasome with age-related macular degeneration. FEBS Lett. 2007;581:885–890. doi: 10.1016/j.febslet.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissolo N, Kraus M, Reich M, Ayturan M, Overkleeft H, Driessen C, Weissert R. Dual inhibition of proteasomal and lysosomal proteolysis ameliorates autoimmune central nervous system inflammation. Eur J Immunol. 2008;38:2401–2411. doi: 10.1002/eji.200838413. [DOI] [PubMed] [Google Scholar]
- Guillaume B, ChQapiro J, Stroobant V, Colau D, Van Holle B, Parvizi G, Bousquet-Dubouch MP, Théate I, Parmentier N, Van den Eynde BJ. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc. Natl Acad Sci. 2010;107:18599–18604. doi: 10.1073/pnas.1009778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Kaneko T, Okamoto K, Bai M, Yashiroda H, Furuyama K, Kato K, Tanaka K, Murata S. Dissecting beta-ring assembly pathway of the mammalian 20S proteasome. EMBO J. 2008;27:2204–2213. doi: 10.1038/emboj.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong SA, Kapphahn RJ, Phillips SL, Maldonado M, Ferrington DA. Immunoproteasome deficiency alters retinal proteasome’s response to stress. J Neurochem. 2010;113:1481–1490. doi: 10.1111/j.1471-4159.2010.06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114:237–246. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Agar JN, Hong Y, Taylor DM, Minotti S, Figlewicz DA, Durham HD. Proteasomes remain intact, but show early focal alteration in their composition in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2008;105:2353–2366. doi: 10.1111/j.1471-4159.2008.05317.x. [DOI] [PubMed] [Google Scholar]
- Kapeta S, Chondrogianni N, Gonos ES. Nuclear erythroid factor 2 (Nrf2)-mediated proteasome activation delays senescence in human fibroblasts. J Biol Chem. 2010;285:8171–8184. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neurosci. 2000;98:149–156. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Klare N, Seeger M, Janek K, Jungblut PR, Dahlmann B. Intermediate-type 20S proteasome in HeLa Cells: ‘Asymmetric’ subunit composition, diversity and adaptation. J Mol Biol. 2007;373:1–10. doi: 10.1016/j.jmb.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Kremer M, Henn A, Kolb C, Basler M, Moebius J, Guillaume B, Leist M, Van den Eynde BJ, Grottrup M. Reduced immunoproteasome formation and accumulation of immunproteasomal precursors in the brains of lymphocytic choriomeningitis virus-infected mice. J Immunol. 2010;185:5549–5560. doi: 10.4049/jimmunol.1001517. [DOI] [PubMed] [Google Scholar]
- Kriegenburg F, Poulsen EG, Koch A, Krüger E, Hartmann-Petersen R. Redox control of the ubiquitin-proteasome system: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2011;15:2265–2299. doi: 10.1089/ars.2010.3590. [DOI] [PubMed] [Google Scholar]
- Lee CS, Lee C, Hu T, Nguyen JM, Zhang J, Martin MV, Vawter MP, Huang EJ, Chan JY. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc Natl Acad Sci. 2011;108:8408–8413. doi: 10.1073/pnas.1019209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28α; overexpression protects against oxidative stress. FASEB J. 2011;25:883–893. doi: 10.1096/fj.10-160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Wong J, Wong B. Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy. Cardiovasc Res. 2010;85:347–356. doi: 10.1093/cvr/cvp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcnaught K, Jnobaptiste R, Jackson T, Jengelley T. The pattern of neuronal loss and survival may reflect differential expression of proteasome activators in Parkinson’s disease. Synapse. 2010;64:241–250. doi: 10.1002/syn.20719. [DOI] [PubMed] [Google Scholar]
- Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M, Licastro F, Sorbi S, Pession A, Ohm T, Grune T, Franceschi C. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol Aging. 2006;27:54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raijmakers R, Berkers CR, De Jong A, Ovaa H, Heck AJ, Mohammed S. Automated online sequential isotope labeling for protein quantitation applied to proteasome tissue-specific diversity. Mol Cell Proteomics. 2008;7:1755–1762. doi: 10.1074/mcp.M800093-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Rivett AJ, Hearn AR. Proteasome function in antigen presentation: immunoproteasome complexes, peptide production and interactions with viral proteins. Curr Protein Pept Sci. 2004;5:153–161. doi: 10.2174/1389203043379774. [DOI] [PubMed] [Google Scholar]
- Rodgers KJ, Dean RT. Assessment of proteasome activity in cell lysates and tissue homogenates using peptide substrates. Int J Biochem Cell Biol. 2003;35:716–727. doi: 10.1016/s1357-2725(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Seifert U, Lukasz P, Ebstein F, Bech-Otschir D, Voigt A, Schröter F, Prozorovski T, Lange N, Steffen J, Rieger M, Kuckelkorn U, Aktas O, Kloetzel P, Krüger E. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Zaiss DM, Standera S, Holzhütter H, Kloetzel PM, Sijts AJ. The proteasome inhibitor PI31 competes with PA28 for binding to 20S proteasomes. FEBS Lett. 1999;457:333–338. doi: 10.1016/s0014-5793(99)01072-8. [DOI] [PubMed] [Google Scholar]
- Zaiss DM, Standera S, Kloetzel PM, Sijts AJ. PI31 is a modulator of proteasome formation and antigen processing. Proc Natl Acad Sci. 2002;99:14344–14349. doi: 10.1073/pnas.212257299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Bizzozero OA. Accumulation of protein carbonyls within cerebellar astrocytes in murine experimental autoimmune encephalomyelitis. J Neurosci Res. 2010a;88:3376–3385. doi: 10.1002/jnr.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Bizzozero OA. Reduced proteasomal activity contributes to the accumulation of carbonylated proteins in chronic experimental autoimmune encephalomyelitis. J Neurochem. 2010b;115:1556–1567. doi: 10.1111/j.1471-4159.2010.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Bizzozero OA. Decreased activity of the 20S proteasome in the brain white matter and gray matter of patients with multiple sclerosis. J Neurochem. 2011;117:143–153. doi: 10.1111/j.1471-4159.2011.07182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.