SUMMARY
Tissue Factor (TF) initiates thrombin generation, and whole blood TF (WBTF) is elevated in sickle cell disease (SCD). We sought to identify the presence of TF-positive monocytes in SCD and their relationship with the other coagulation markers including WBTF, microparticle-associated TF, thrombin-antithrombin (TAT) complexes and D-dimer. Whether major SCD-related pathobiological processes, including haemolysis, inflammation and endothelial activation, contribute to the coagulation abnormalities was also studied. The cohort comprised children with SCD (18 HbSS, 12 HbSC, mean age 3.6 years). We demonstrated elevated levels of TF-positive monocytes in HbSS, which correlated with WBTF, TAT and D-Dimer (p=0.02 to p=0.0003). While TF-positive monocytes, WBTF, TAT and D-dimer correlated with several biomarkers of haemolysis, inflammation and endothelial activation in univariate analyses, in multiple regression models the haemolytic markers (reticulocytes and lactate dehydrogenase) contributed exclusively to the association with all four coagulant markers evaluated. The demonstration that haemolysis is the predominant operative pathology in the associated perturbations of coagulation in HbSS at a young age provides additional evidence for the early use of therapeutic agents, such as hydroxycarbamide to reduce the haemolytic component of this disease.
Keywords: Sickle cell disease, tissue factor-positive monocytes, coagulation abnormalities, biomarkers of haemolysis, biomarkers of inflammation
INTRODUCTION
Several previous studies have documented haemostatic abnormalities in sickle cell disease (SCD), including evidence for enhanced thrombin generation (Ataga et al, 2007; Stuart & Setty, 2001) as indicated by elevated levels of the prothrombin fragment F1+2 (a measure of conversion of prothrombin to thrombin); thrombin-antithrombin (TAT) complexes (a measure of thrombin formation); and D-dimer (a measure of formation and degradation of cross-linked fibrin). Depletion of circulating anticoagulants (protein C; free protein S; and heparin cofactor II) and the presence of anti-phospholipid antibodies have also been noted (Ataga et al, 2007; Stuart & Setty, 2001; Westerman et al, 1999). Numerous aspects of SCD-related pathobiology remain unclear, including whether the haemostatic perturbations identified in this disease translate into individual thrombogenic risk (Tomer et al, 2001), and/or contribute to sickle vasculopathy (Kato et al, 2009). Clinical evidence for a thrombophilic phenotype in patients with SCD includes the occurrence of infarctive strokes in children (Ohene-Frempong et al, 1998; Hillery & Panepinto, 2004), and the presence of thrombi in the pulmonary vasculature in adults (Adedeji et al, 2001; Dessap et al, 2011; Stein et al, 2006). Although laboratory and clinical evidence demonstrates haemostatic abnormalities in SCD, the mechanisms accounting for the initiation or modulation of this process have not been clarified.
Tissue factor (TF), a cell surface receptor for factor VII/VIIa, is the physiological initiator of blood coagulation (Camerer et al, 1996; Mackman, 2004). TF forms a complex with circulating factor VIIa, activating factor X with subsequent thrombin generation. Although TF is not constitutively expressed on endothelium or monocytes in vivo, its expression on these cells can be induced in vitro by a variety of pathological stimuli relevant to SCD, including ischaemia/reperfusion injury (Solovey et al, 2004), shear stress and agonists, such as the inflammatory cytokines, growth factors and CD40-ligand (Camerer et al, 1996; Lee et al, 2006). In fact, increased levels of TF in whole blood (Key et al, 1998) and plasma (Lee et al, 2006; Mohan et al, 2005), and the presence of TF-positive circulating endothelial cells (Solovey et al, 1998) and microparticles (Shet et al, 2003) have been demonstrated in SCD, suggesting a potential TF-mediated mechanism for the increased coagulation activation in this patient group. Although the documentation in the adult plasma of microparticles that are positive for both CD14 and TF suggests the existence of TF-positive monocytes, the presence of such cells in this patient group has not been reported to date. Our study sought to identify the presence of TF-positive monocytes in subjects with SCD, and evaluate their relationship with other markers of coagulation activation and markers of the various pathobiological processes known to play a role in SCD.
MATERIALS, PATIENTS and METHODS
Materials
For flow cytometric analyses, phycoerythrin (PE)-, and fluorescein isothiocyanate (FITC)- labelled mouse monoclonal antibodies against human antigens, and isotypic-negative control antibodies were obtained from Immunotec (Beckman-Coulter, Miami, FL), Caltag Laboratories (Burlingame, CA), or American Diagnostica (Stamford, CT). These antibodies included anti-glycophorin A-PE [anti-CD235a-PE, clone 11E4B7.6(KC16)], anti-CD14-PE (clone TüK4), anti-TF-FITC (CD142, product # 4507CJ), and isotypic control antibodies (clones 679.1Mc7 and U7.27). FITC-labelled annexin-V was obtained from R&D Systems (Minneapolis, MN). Glycophorin-A, CD14 and annexin-V were used as markers for red cells, monocytes and PS-positivity, respectively.
Study Population
The study population included 30 children with SCD in steady state, 18 with SS and 12 with the SC genotypes. Ages ranged from 2 to 10 years, with a mean age of 3.6 years. Blood samples were collected at least 10 weeks remote from a previous blood transfusion, and at least 2 weeks remote from any acute illness, hospitalization, or vaso-occlusive episode. No subject was on hydroxycarbamide. Blood samples were also obtained from 11 age- and race-matched paediatric controls (2 to 10 years, mean age 4.6 years). This study was approved by the Institutional Review Committee for Human Subjects Protection at St Christopher’s Hospital for Children/Drexel University and at Thomas Jefferson University. Blood samples were obtained following informed consent. For minors, patient’s assent was also obtained as appropriate.
Analysis of Biomarkers
Biomarkers that were evaluated in this study were grouped into four different SCD-related pathobiological processes based on their source and biological role. These included markers representing coagulation activation [TF-positive monocytes, whole blood TF (WBTF), microparticle-associated TF (MPTF), TAT and D-dimer]; haemolysis [percent reticulocyte count, lactate dehydrogenase (LDH), and type-2 phosphatidylserine (PS)-positive erythrocytes]; inflammation [white cells, high sensitivity C-reactive protein (hs-CRP), and tumour necrosis factor-α (TNFα)]; and endothelial activation [soluble vascular cell adhesion molecule-1 (soluble VCAM1), soluble E-selectin, soluble P-selectin and nitric oxide (NOx) metabolites]. These biomarkers in whole blood and plasma were quantitated as detailed below.
Analysis of TF-positive Monocytes
TF-positive monocytes in whole blood were analysed by flow cytometry as described previously (Brambilla et al, 2008). Briefly, 200 μl fresh whole blood collected in sodium heparin anticoagulant was incubated within 30 min after phlebotomy with 5 μl anti-CD14-PE and either 10 μl anti-TF-FITC or 20 μl FITC-labelled negative isotypic control antibody (Becton Dickinson, cat # 340755) for 30 min at room temperature in the dark. Samples were diluted with 2 ml of PharM Lyse (Becton Dickinson). Following a 15- min incubation at room temperature in the dark, cells were washed twice with 2 ml Dulbecco’s phosphate buffered saline (DPBS), suspended in 0.5 ml DPBS, and analysed immediately in a flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) formatted for two-colour analysis. 5000 events in the monocyte region were collected, and analysed using CellQuest software (Becton Dickinson). The percentage of TF-positive monocytes in the gated population were obtained after subtracting the non-specific immunofluorescence determined using blood stained with FITC-labelled isotypic negative control antibody.
Analysis of other Markers of Coagulation Activation
TF procoagulant activity in whole blood was assayed in cell lysates using a two-stage functional clotting assay (Key et al, 1998). This assay measures both cell-associated and microparticle-associated TF, as whole blood was processed without separating cellular elements from plasma. The assay also measures both encrypted and de-encrypted TF. TF procoagulant activity in the microparticle pellet, obtained following a high speed centrifugation of platelet-free citrated plasma at 21,000 × g for 30 min, was measured using a chromogenic assay as previously described (Ataga et al, 2012; Khorana et al, 2008; Manly et al, 2009; van Beers et al, 2009). Plasma samples for this assay and others detailed below were prepared as previously described (Krishnan et al, 2010). Levels of TAT and D-dimer in citrated plasma were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (TAT – Enzygnost, Dade Behring, Marburg, Germany; D-dimer - American Diagnostica, Stamford, CT).
Analysis of Markers of Haemolysis, Inflammation and Endothelial activation
LDH levels in citrated plasma were evaluated using an LDH assay kit (TOX-7, Sigma Aldrich, St Louis, MO). The enzyme activity was read from a calibration curve generated using an LDH standard, and expressed as iu/l. Levels of soluble VCAM1, soluble E-selectin, soluble P-selectin, hs-CRP, and TNFα were measured in citrated plasma using ELISA kits from R&D Systems (Minneapolis, MN) or ALPCO Immunoassays (Salem, NH). Levels of NOx metabolites (nitrates and nitrites) were measured fluorometrically in ultra-filtered plasma using 2,3-diaminonaphthalene following reduction of nitrates to nitrites (Stuart & Setty, 1999). Haematological parameters (haemoglobin levels, reticulocyte and white cell counts) were also obtained. Red cell PS was analysed by flow cytometry as previously described (Setty et al, 2001). These PS-positive erythrocytes were arbitrarily divided into types-1 and-2, depending on the intensity of their immunofluorescence, with type-2 values approximately 1 log higher than in type-1 (Yasin et al, 2003). Levels of type-2 PS-positive erythrocytes were obtained from the annexin-V-FITC fluorescence histograms (Yasin et al, 2003). Most type-1 cells are transferrin receptor-positive reticulocytes and are present in other haemolytic states, whereas type-2 PS-positive erythrocytes are more specific for SCD, contain low levels of fetal haemoglobin, and have been identified in the dense cell fraction (Yasin et al, 2003). A previous study in a murine model of sickle cell anaemia has also demonstrated that PS-positive erythrocytes are rapidly removed from the circulation, thereby contributing to the reduced erythrocyte survival noted in that model (de Jong et al, 2001).
Statistical analysis
Statistical evaluation was performed using SigmaStat Statistical Package (Version 3, SPSS Inc, Plover, WI). Biomarker levels are presented both as mean ± standard deviation (SD), and median and quartile values. Multiple group comparison was done using either one-way analysis of variance (ANOVA), for data with normal distribution) or the Kruskal-Wallis test (for data with non-normal distribution). If the P-value for this overall comparison was <0.05, group-wise comparisons were done with the Holm-Sidak test or the Dunn’s test. Appropriate corrections were made to account for multiple comparisons according to the method of Bonferroni, and differences at p-values ≤0.01 were considered statistically significant. Association between any two variables was tested with the Pearson Correlation test using either non-transformed data (for variables demonstrating normal distribution), or normalized data following logarithmic, or inverse transformation (for variables demonstrating non-normal distribution), and reconfirmed with Spearman rank correlation test. Both tests yielded similar results for the same pair of variables analysed. Subset regression analyses were performed to identify the best pathway-select biomarkers, which were found to be the variables with the best Spearman correlation (Table II) or Pearson correlation (data not shown). Multiple regression analyses were performed to identify SCD-related pathobiological processes that potentially modulated haemostatic activation. Both forward and backward regression models were tested using the rank-transformed data. In these models TF-positive monocytes, WBTF, TAT, or D-dimer was used as a dependent variable, and the best pathway-select biomarkers identified using subset regression analysis were included as representative independent variables.
Table II.
Correlations between Haemostatic Markers and Biomarkers representing Haemolysis, Inflammation or Endothelial Activation
| Marker | TF-positive Monocyte (%) | WBTF Procoagulant Activity (pg/ml) | MPTF Procoagulant Activity (pg/l) | TAT (ng/ml) | D-Dimer (ng/ml) | |
|---|---|---|---|---|---|---|
| Haemolytic Markers | Percent Reticulocytes | 0.67 (p<0.0001) | 0.55b (p=0.002) | 0.06 (p=0.769) | 0.56 (p=0.002) | 0.64 (p<0.0002) |
| LDH (iu/l) | 0.59 (p=0.0006) | 0.57 (p=0.0009) | 0.20 (p=0.282) | 0.40 (p=0.031) | 0.37 (p=0.043) | |
| percent Type-2 PS-positive RBCs | 0.40 (p=0.030) | 0.54 (p=0.002) | 0.06 (p=0.746) | 0.41 (p=0.025) | 0.30 (p=0.101) | |
| Inflammatory Markers | WBC count (× 109/l) | 0.43 (p=0.017) | 0.52 (p=0.003) | −0.06 (p=0.756) | 0.42 (p=0.023) | 0.38 (p=0.038) |
| hs-CRP (ng/ml) | 0.40 (p=0.029) | 0.28 (p=0.134) | −0.16 (p=0.402) | 0.45 (p=0.012) | 0.47 (p=0.008) | |
| TNF-α (pg/ml) | 0.02 (p=0.907) | 0.11 (p=0.579) | −0.28 (p=0.136) | −0.01 (p=0.95) | 0.39 (p=0.036) | |
| Endothelial Activation Markers | s-VCAM1 (ng/ml) | 0.50 (p=0.005) | 0.39 (p=0.033) | 0.00 (p=0.998) | 0.41 (p=0.024) | 0.38 (p=0.037) |
| s-E-Selectin (ng/ml) | 0.62 (p=0.0003) | 0.43 (p=0.018) | 0.14 (p=0.463) | 0.31 (p=0.094) | 0.46 (p=0.011) | |
| s-P-Selectin (ng/ml) | 0.54 (p=0.002) | 0.49 (p=0.006) | −0.16 (p=0.395) | 0.28 (p=0.137) | 0.10 (p=0.592) | |
| NOx Metabolites (nMol/ml) | 0.00 (p=0.991) | 0.11 (p=0.556) | 0.31 (p=0.097) | 0.18 (p=0.349) | 0.46 (p=0.012) |
Values presented are the Spearman r-value and (the p-value), respectively, from 30 patients with SCD (18 with HbSS and 12 with HbSC disease). Bold entries represent variables with the best correlation in each sub-group and that were used in multiple regression analyses. Microparticle-associated tissue factor (MPTF) activity did not correlate with any biomarker evaluated, and therefore multiple regression analyses were not done with MPTF activity. TF, tissue factor, WBTF, whole blood tissue factor; TAT, thrombin-antithrombin; LDH, lactate dehydrogenase; PS, phosphatidylserine; RBCs, red blood cells; WBC, white blood cell; hs-CRP, high sensitivity C-reactive protein; TNFα, tumour necrosis factor-α; s-VCAM1, soluble vascular cell adhesion molecule-1; NOx, nitric oxide.
RESULTS
Biomarkers and Haematological Parameters in SCD
Plasma biomarkers and haematological parameters assessed in children with SCD (HbSS and HbSC genotypes), and controls are shown in Table I. Given that published studies report either the mean or the median levels, for comparison with our data, we have presented both mean and median values. Results presented in Table I demonstrate that levels of biomarkers and haematological parameters were comparable to those reported by several investigators including our laboratory (Blann et al, 2008; Krishnan et al, 2010; Mohan et al, 2005; Setty et al, 2001; 2003; Westerman et al, 1999). Consistent with published reports, reticulocyte levels were higher, and haemoglobin levels were lower in HbSS compared to HbSC. As documented previously, levels of white blood cells (WBC), LDH, type-2 PS-positive erythrocytes, hs-CRP, TNFα, soluble VCAM1, soluble E-selectin, soluble P-selectin, WBTF, TAT complexes, and D-dimer were elevated in patients with the SS genotype when compared to HbSC disease, or controls. Levels of TNFα, and soluble VCAM1 were also higher in HbSC compared to controls. NOx metabolites and MPTF procoagulant activity were not significantly different among all three groups.
Table I.
Haematological Parameters and Biomarkers in Children with SCD and Controls
| Parameter/Biomarker | Control (n=11) | HbSS Disease (n=18) | HbSC Disease (n=12) | ANOVA p-value | Multiple Comparison p-values | ||
|---|---|---|---|---|---|---|---|
| HbSS vs Control | HbSS vs HbSC | HbSC vs Control | |||||
| Haemoglobin (g/l) | 121 ± 5 (121, 120, 124) | *84 ± 16 (82, 74, 86) | 111 ± 8 (108, 106, 114) | < 0.001 | <0.01 | <0.01 | NS |
| Reticulocyte count (%) | 0.43 ± 0.31 (0.30, 0.20, 0.70) | *11.90 ± 5.28 (11.1, 9.3, 14.7) | 2.33 ± 0.74 (2.25, 1.85, 2.55) | < 0.001 | <0.01 | <0.01 | <0.05 |
| LDH (iu/l) | 276 ± 56 (259, 239, 307) | *662 ± 221 (609, 465, 781) | 351 ± 96 (343, 288, 370) | < 0.001 | <0.01 | <0.01 | NS |
| Type-2 PS-positive red cells (%) | 0.13 ± 0.06 (0.13, 0.10, 0.15) | †1.14 ± 1.02 (0.76, 0.42, 1.71) | 0.26 ± 0.18 (0.17, 0.15, 0.30) | < 0.001 | <0.01 | <0.05 | NS |
| WBC count (× 109/l) | 6.24 ± 1.25 (6.50, 5.10, 6.93) | †14.31 ± 4.71 (15.2, 11.9, 17.2) | 8.40 ± 2.33 (7.60, 7.05, 10.2) | < 0.001 | <0.01 | <0.05 | NS |
| hs-CRP (ng/ml) | 255 ± 226 (146, 90, 465) | †4752 ± 4842 (2365, 491, 8124) | 1560 ± 1850 (711, 266, 2518) | < 0.001 | <0.01 | NS | NS |
| TNFα (pg/ml) | 0.87 ± 0.29 (0.90, 0.62, 1.16) | †1.93 ± 0.68 (1.83, 1.56, 2.21) | †1.92 ± 0.70 (2.06, 1.37, 2.38) | < 0.001 | <0.01 | NS | <0.01 |
| sVCAM1 (ng/ml) | 536 ± 105 (565, 457, 601) | †1112 ± 388 (957, 837, 1277) | †836 ± 189 (841, 675, 948) | < 0.001 | <0.01 | NS | <0.01 |
| s-E-Selectin (ng/ml) | 53.4 ± 12.6 (54.7, 44.4, 61.0) | *174.2 ± 45.0 (178, 141, 217) | 89.1 ± 37.8 (90.2, 65.8, 113.9) | < 0.001 | <0.01 | <0.01 | NS |
| s-P-Selectin (ng/ml) | 38.1 ± 8.5 (36.3, 33.2, 42.6) | *66.4 ± 17.3 (61.7, 55.1, 68.5) | 44.5 ± 14.0 (42.9, 31.4, 51.5) | < 0.001 | <0.01 | <0.01 | NS |
| NOx Metabolites (nMol/ml) | 19.45 ± 6.66 (17.53, 14.38, 24.22) | 16.56 ± 5.02 (15.69, 13.59, 18.41) | 16.26 ± 4.88 (14.75, 12.59, 19.49) | = 0.395 | NT | NT | NT |
| TF-positive Monocytes (%) | 2.17 ± 1.39 (1.99, 0.99, 3.48) | *7.41 ± 3.25 (6.31, 4.63, 10.36) | 1.95 ± 1.11 (1.66, 1.23, 2.36) | < 0.001 | <0.01 | <0.01 | NS |
| WBTF (pg/ml) | 3.39 ± 0.56 (3.22, 3.10, 3.92) | *6.13 ± 1.78 (5.98, 4.78, 7.29) | 3.87 ± 0.95 (4.04, 3.23, 4.43) | < 0.001 | <0.01 | <0.01 | NS |
| MPTF (pg/l) | 315 ± 50 (327, 290, 349) | 260 ± 283 (231, 0, 382) | 135 ± 215 (50, 0, 195) | = 0.052 | NT | NT | NT |
| TAT (ng/ml) | 2.06 ± 1.28 (1.61, 1.05, 2.59) | *4.85 ± 2.84 (5.18, 2.62, 6.18) | 2.03 ± 1.63 (1.63, 1.04, 2.38) | = 0.002 | <0.01 | =0.01 | NS |
| D-Dimer (ng/ml) | 78 ± 24 (84, 61, 89) | ‡243 ± 233 (158, 94, 358) | 134 ± 276 (40, 32, 63) | = 0.003 | NS | <0.01 | NS |
Results presented in each cell are the mean ± SD, and (the median and 25th and 75th quartile values). Levels of all haematalogical parameters and plasma biomarkers (except TNFα and soluble P-selectin) were analysed for significant differences using the Kruskal-Wallis test. TNF-α and soluble P-selectin were analysed using the parametric ANOVA test. P-values obtained for all pair-wise multiple comparisons using the Dunn’s test or the Holm-Sidak test are shown. Three comparisons were made for each biomarker or haematological parameter. Therefore, the p value required to declare statistically significant differences in means or medians was 0.017. This critical p was obtained according to the method of Bonferroni by dividing 0.05 by the number of comparisons made. Therefore, only differences with p≤0.01 were declared statistically significant. Values are significantly different from the respective controls†, SC group‡, or significantly different from both control and SC groups*. NS=not significant, NT=not tested since p>0.05 for the ANOVA test.
TF, tissue factor, WBTF, whole blood tissue factor; MPTF, microparticle-associated tissue factor; TAT, thrombin-antithrombin; LDH, lactate dehydrogenase; PS, phosphatidylserine; RBCs, red blood cells; WBC, white blood cell; hs-CRP, high sensitivity C-reactive protein; TNFα, tumour necrosis factor-α; s-VCAM1, soluble vascular cell adhesion molecule-1; NOx, nitric oxide.
TF-positive Monocytes: Relationship with Biomarkers of Haemolysis, Inflammation, and Endothelial Activation
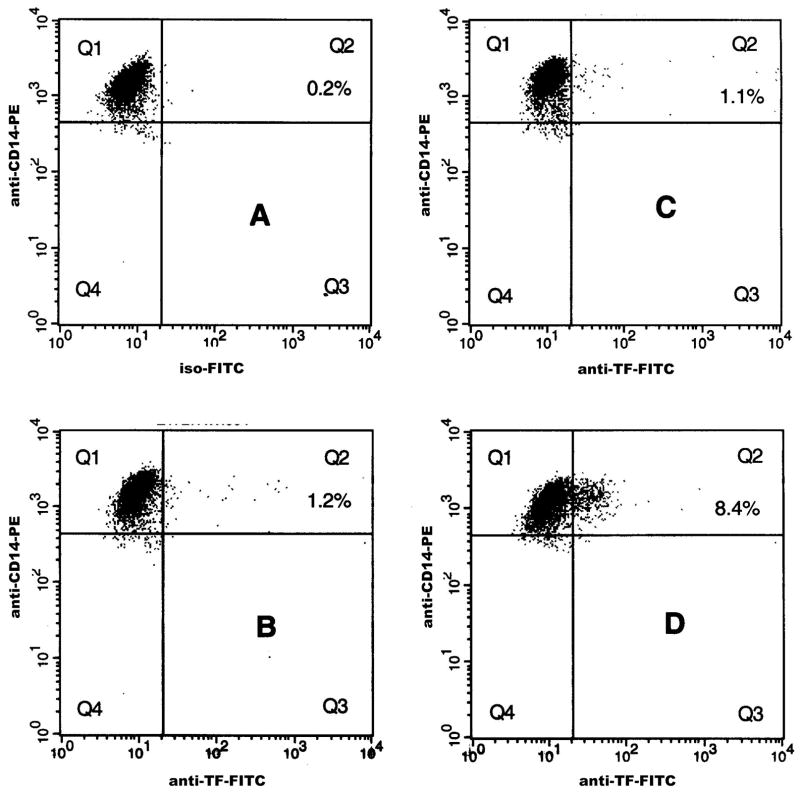
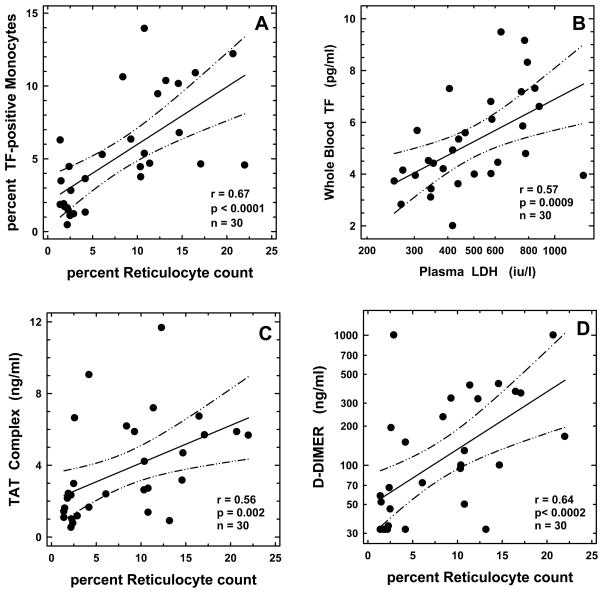
The median (25th and 75th percentile) percent TF-positive monocytes in controls, and individuals with HbSC and HbSS disease were 1.99% (0.99%, 3.48%), 1.66% (1.23%, 2.36%), and 6.31% (4.63%, 10.36%), respectively (Table I). While there were no significant differences between HbSC and controls, percent TF-positive monocytes was increased in HbSS compared to either controls (p<0.01) or children with HbSC disease (p<0.01). Fig 1 demonstrates representative flow cytometric profiles of TF-positive monocytes in HbSS (panel D), HbSC (panel C), and control groups (panel B). Levels of TF-positive monocytes from both HbSS and HbSC patient groups were next analysed for their association with biomarkers representing various SCD-relevant pathobiological processes. Significant associations were observed between monocyte TF and several independent variables from the haemolytic, inflammatory, and endothelial activation pathways (Table II) with percent reticulocyte count (r=0.67; p<0.0001), WBC count (r=0.43; p=0.017), and soluble E-selectin (r=0.62; p=0.0003) identified as the best independent pathway-specific variables using subset regression analyses. These biomarkers were also identified as the best pathway-specific independent variables in both Spearman (Table II) and Pearson correlation tests (data not shown). In multiple regression analyses using percentage of TF-positive monocyte as the dependent variable, and percent reticulocyte count, WBC count, and soluble E-selectin as independent variables, the percent reticulocyte count was the only biomarker that remained in the model, contributing 45% (p<0.001) to the overall association. Fig 2A shows the association of percent TF-positive monocyte with the best haemolytic variable in our study, the percent reticulocyte count. When absolute levels of TF-positive monocytes were used in the analyses, results similar to those noted with percent TF-positive monocytes were also observed (data not shown).
Figure 1. Flow Cytometric Analysis of tissue factor (TF)-positive Monocytes from a representative Control, and Patients with HbSC and HbSS Disease.
Following incubation of whole blood with desired antibodies, monocytes were analysed by flow cytometry as described. 5000 CD14-positive events were collected and analysed for percent TF-positive events. Representative dot plots from a control (Panel B), and patients with HbSC (Panel C) and HbSS disease (Panel D) are depicted. TF-negative (Quadrant Q1) and TF-positive (Quadrant Q2) regions were set up using a blood sample stained with anti-CD14-PE and FITC-labeled isotype-matched negative control antibody (Panel A).
Figure 2. Correlation between percent tissue factor (TF)-positive monocytes and percent reticulocyte count (panel A), whole blood TF procoagulant activity and plasma lactate dehydrogenase (LDH) levels (panel B), plasma levels of thrombin-antithrombin (TAT) complexes and percent reticulocyte count (panel C), and plasma levels of D-dimer and percent reticulocyte count (panel D).
The solid and dotted lines represent the regression fit to the data from all patients with SCD, and the 95% confidence interval curves, respectively. The r-, p-, and n-values for the correlation are shown in the respective panels.
Whole Blood TF (WBTF) Procoagulant Activity: Relationship with Biomarkers of Haemolysis, Inflammation, and Endothelial Activation
Mean (±SD) WBTF procoagulant activity in controls, and children with HbSC, and HbSS disease were 3.39 (±0.56), 3.87 (±0.95), and 6.13 (±1.78) pg/ml, respectively (Table I). While there were no significant differences between HbSC and controls, WBTF activity was elevated in HbSS compared to either controls (p<0.01) or children with HbSC disease (p<0.01). When WBTF levels from both HbSS and HbSC patient groups were analysed for association, significant correlations were observed between WBTF and several independent variables from the haemolytic, inflammatory, and endothelial activation pathways (Table II) with LDH (r=0.57; p=0.0009), WBC count (r=0.52; p=0.003), and soluble P-selectin (r=0.49; p=0.006) identified as the best independent pathway-specific variables using subset regression analyses. In multiple regression analyses using WBTF as the dependent variable, and LDH, WBC count, and soluble P-selectin as independent variables, LDH was the only marker that stayed in the model contributing 33% (p<0.001) to the overall association. Fig 2B depicts the association between WBTF and LDH levels (r=0.57; p=0.0009).
Microparticle-Associated TF (MPTF) Procoagulant Activity: Relationship with Biomarkers of Haemolysis, Inflammation, and Endothelial Activation
Median MPTF procoagulant activity in plasma from controls, and children with HbSC, and HbSS disease were 327, 50 and 231 pg/l, respectively (Table I). There were no significant differences between the patient and the control groups. When the combined MPTF data from both HbSC and HbSS patient groups were analysed for association with biomarkers representing haemolysis, inflammation, and endothelial activation, no significant correlations were found with any of the biomarkers evaluated (Table II).
TAT: Relationship with Biomarkers of Haemolysis, Inflammation, and Endothelial Activation
Median TAT levels in controls, and children with HbSC and HbSS disease were 1.61, 1.63 and 5.18 ng/ml, respectively (Table I). While there were no significant differences between HbSC and controls, TAT levels were increased in HbSS compared to either controls (p<0.01) or HbSC disease (p=0.01). When TAT levels from both HbSS and HbSC patient groups were analysed for associations, significant correlations were noted with several independent biomarkers related to haemolysis, inflammation, and endothelial activation (Table II), with percent reticulocyte count (r=0.56; p=0.002), hs-CRP (r=0.45; p=0.012), and soluble VCAM1 (r=0.41, p=0.024) identified as the best pathway-specific independent variables using subset regression analyses. In multiple regression analysis using TAT as the dependent variable, and percent reticulocyte count, hs-CRP, and soluble VCAM1 as independent variables, reticulocyte count was the only variable that stayed in the model, contributing 31% (p=0.001) to the overall association. The scatter plot in Fig 2C shows the association of TAT with the best haemolytic variable in our study, the percent reticulocyte count.
D-Dimer: Relationship with Biomarkers of Haemolysis, Inflammation, and Endothelial Activation
Median D-dimer levels in controls, and children with HbSC and HbSS disease were 84, 40 and 158 ng/ml, respectively (Table I). While there were no significant differences between HbSC and controls, D-dimer levels were increased in HbSS compared to HbSC disease (p<0.01). When D-dimer levels from both HbSS and HbSC patient groups were analysed for associations, significant correlations were noted with several independent biomarkers related to haemolysis, inflammation, and endothelial activation (Table II). Subset regression analyses identified percent reticulocyte count (r=0.64; p<0.0002), hs-CRP (r=0.47; p=0.008), and soluble E-selectin (r=0.46, p=0.011) as the best independent variables representing the processes of haemolysis, inflammation, and endothelial activation, respectively (Table II). In multiple regression analysis using D-dimer as the dependent variable, and percent reticulocyte count, hs-CRP, and soluble E-selectin as independent variables, reticulocyte count was the only variable that stayed in the model contributing 41% (p<0.001) to the overall association. The scatter plot in Fig 2D shows the association of D-dimer with the haemolytic marker the percent reticulocyte count.
Associations among Haemostatic Markers
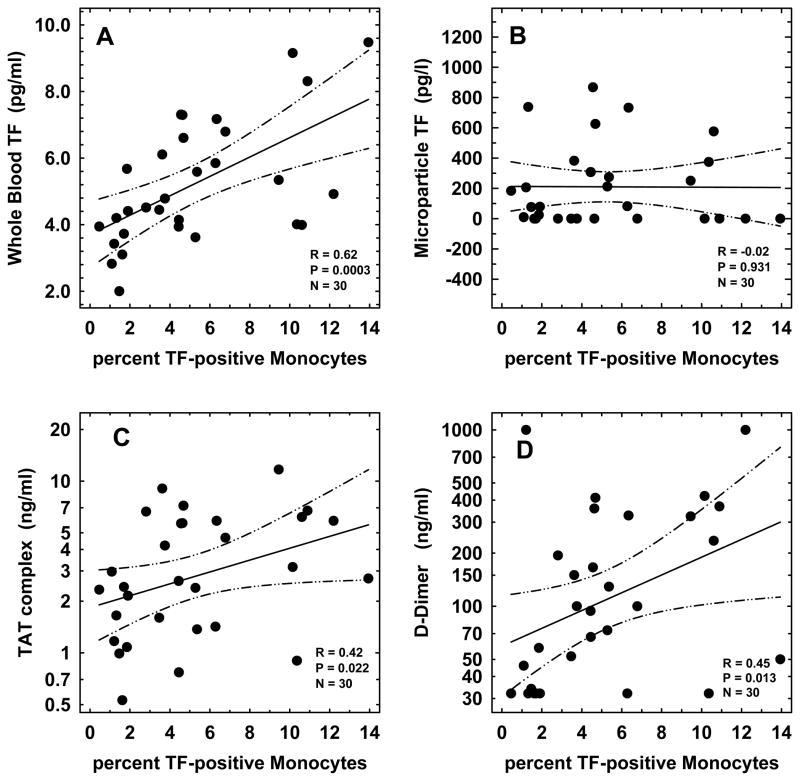
We examined the relationships among the haemostatic markers percent TF-positive monocytes, WBTF, MPTF, TAT and D-dimer. As shown in Table III, significant correlations were noted among these biomarkers with the exception of MPTF, which did not correlate with any of the haemostatic markers evaluated. Greater correlations were observed between the markers of initiation of thrombin generation (percent TF-positive monocytes and WBTF, r=0.62, p=0.0003); and also between the later stage markers of coagulation activation (TAT and D-dimer, r=0.64, p=0.0001). The scatter plots in Fig 3 depict the correlation between percent TF-positive monocytes and WBTF (r=0.62, p=0.0003, panel A), percent TF-positive monocytes and MPTF (r=−0.02, p=0.931, panel B), percent TF-positive monocytes and TAT (r=0.42, p=0.022, panel C), and between percent TF-positive monocytes and D-dimer (r=0.45, p=0.013, panel D).
Table III.
Correlation among Prothrombotic Markers
| Marker | TF-positive Monocyte (%) | WBTF Procoagulant Activity (pg/ml) | MPTF Procoagulant Activity (pg/l) | TAT (ng/ml) | D-Dimer (ng/ml) |
|---|---|---|---|---|---|
| TF-positive Monocyte (%) | 0.62 (p=0.0003) | −0.02 (p=0.931) | 0.42 (p=0.022) | 0.45 (p=0.013) | |
| WBTF Procoagulant Activity (pg/ml) | 0.62 (p=0.0003) | −0.07 (p=0.698) | 0.51 (p=0.004) | 0.47 (p=0.010) | |
| MPTF Procoagulant Activity (pg/l) | −0.02 (p=0.931) | −0.07 (p=0.698) | 0.02 (p=0.897) | 0.02 (p=0.933) | |
| TAT (ng/ml) | 0.42 (p=0.022) | 0.51 (p=0.004) | 0.02 (p=0.897) | 0.64 (p=0.0001) | |
| D-dimer (ng/ml) | 0.45 (p=0.013) | 0.47 (p=0.010) | 0.02 (p=0.933) | 0.64 (p=0.0001) |
Values represent the Spearman r-value and (the p-value), respectively, from 30 patients with SCD (18 with HbSS and 12 with HbSC disease). TF, tissue factor; WBTF, whole blood tissue factor; MPTF, microparticle-associated tissue factor; TAT, thrombin-antithrombin.
Figure 3. Correlation between percent tissue factor (TF)-positive Monocytes and either Whole Blood TF Procoagulant Activity (panel A), Microparticle-associated TF (panel B), Plasma Levels of thrombin-antithrombin (TAT) Complexes (panel C), or Plasma Levels of D-Dimer (panel D).
The solid and dotted lines represent the regression fit to the data from all patients with SCD, and the 95% confidence interval curves, respectively. The r-, p-, and n-values for the correlation are shown in the respective panels.
DISCUSSION
This study presents novel data demonstrating the presence of increased numbers of circulating TF-positive monocytes in children with HbSS (Fig 1 and Table I). In addition, significant correlations (Fig 3 and Table III) were shown between TF-positive circulating monocytes and both the early (WBTF) and late stages (TAT and D-dimer) of coagulation activation. While previous studies from our laboratory and others have demonstrated coagulation activation in children and adults with SCD (Ataga et al, 2007; Stuart & Setty, 2001), our present report documents the presence of these abnormalities very early in the child with homozygous SS disease, and provides evidence that one of the cellular sources of the coagulation changes is the activated circulating monocyte. We also demonstrated that microparticle-associated TF (MPTF) procoagulant activity was not elevated in the plasma of young children with SCD, and did not correlate with markers of coagulation activation (Table III). A similar lack of correlation between MPTF and TAT or D-dimer was noted in adults with SCD (Ataga et al, 2012). In aggregate, these results suggest that cell-associated TF, rather than microparticle-associated TF, plays an important role in haemostatic activation in SCD.
Previous studies have identified circulating pools of TF associated with intact cells, microparticles, and plasma (reviewed in Key & Mackman, 2010). Studies by Key et al (1998) have shown that the WBTF procoagulant activity is elevated in patients with SCD, with evidence suggesting that monocytes may be the dominant source. The current study, as well two additional preliminary reports (Colella et al, 2011; Vasse et al, 2003) confirm this impression. Identification of TF-positive circulating endothelial cells in SCD, and endothelial expression of TF in murine model of SCD has demonstrated that endothelial cells may also be a source of circulating cell-associated TF activity (Solovey et al, 1998, 2004). However, given that monocytes outnumber circulating endothelial cells (CECs) in the peripheral blood by approximately 5 orders of magnitude, it seems unlikely that CECs contribute significantly to net TF procoagulant activity. While a previous documentation of the presence in plasma of microparticles that are positive for both CD14 (a monocyte marker) and TF in adults with SCD suggested the existence of TF-positive monocytes in SCD, a more recent study in adults with HbSS and SC disease found no evidence for the presence of TF bearing microparticles (Shet et al, 2003; van Beers et al, 2009). Although the latter report documented the presence of both RBC and platelet microparticles (with factor XI-dependent procoagulant properties), it did not identify any CD14-positive or TF-positive microparticles in patient samples. The authors speculated that differences in sample centrifugation for microparticle assessment potentially contributed to the divergent results. The present report demonstrated the presence of microparticle-associated TF procoagulant activity in SCD plasma, although it was not elevated compared to controls, and was undetectable in approximately one third of our patient cohort (Table I). Finally, several studies have demonstrated elevated plasma TF antigen levels in SCD (Lee et al, 2006; Mohan et al, 2005). However, concerns have been raised regarding the precise nature of the TF antigen detected in plasma using commercial ELISA kits, and the frequent lack of correlation with MPTF (Johnson et al, 2009; Lee et al, 2012; Parhami-Seren et al, 2006).
The role of humoral factors in SCD-related haemostatic perturbations has not been extensively evaluated, although evidence suggests that cytokines released during the co-incubation of sickle monocytes with endothelial cells can induce TF expression (Belcher et al, 2000). Haemolysis and inflammation, critical players in SCD-related pathology, are also involved in TF expression and coagulation activation. A previous study from our laboratory has demonstrated that haem, a product of intravascular haemolysis, induces the expression of functionally active TF on both micro- and macro-vascular endothelial cells in vitro (Setty et al, 2008). In vivo animal studies have also demonstrated that the hypoxia and re-oxygenation cycles of ischaemia-reperfusion injury can cause enhanced leucocyte trafficking, inflammation (Kaul & Hebbel, 2000; Turhan et al, 2002) and endothelial TF expression (Solovey et al, 2004). To further assess the in vivo relevance of the various major pathobiological pathways to coagulation activation in SCD we have evaluated the relationship between the levels of TF-positive monocytes to relevant biomarkers of haemolysis, inflammation and endothelial activation. While TF-positive monocytes demonstrated multiple associations in univariate analyses (Table II), multiple regression analyses identified an association that was confined to percent reticulocytes (45% contribution P<0.001). In similar univariate followed by multiple regression statistical analyses, we showed that another marker of haemolysis, LDH, was the sole contributor to the association with WBTF (33%, p<0.001). Percent reticulocyte count also was the only variable that remained in the models with TAT (31% contribution, p=0.001) and D-dimer (41%; P<0.001). A recent review categorized the relative hierarchy of haemolytic markers, labelling the percent reticulocyte count and LDH as secondary and quaternary biomarkers, respectively, based on the strength of their association with red cell lifespan (Hebbel, 2011). Our study demonstrates predominant associations between both the early and late coagulation assays with these secondary and quaternary markers of the haemolytic process, to the exclusion, in multiple regression models, of biomarkers of inflammation and endothelial activation. It must be noted, however, that this is an early childhood cohort, such that whether a predominance of haemolytic-related associations with coagulation activation will occur in a population of older children or the adult is unknown.
Our findings provide evidence for perturbations in haemostasis very early in the life of the child with HbSS disease. Our report also provides supportive evidence that future investigations may bridge the gap between associative findings and anticipated cause and effect relationships. For instance, hydroxycarbamide is a therapeutic agent that prevents haemoglobin S polymerization by increasing fetal haemoglobin levels with a concomitant reduction in the haemolytic rate (Ware, 2010), and according to a preliminary report, coagulation activation (Colella et al, 2011). A cause and effect relationship between red cell haemolysis and haemostatic perturbations in early childhood could be potentially demonstrated in children in whom hydroxyurea therapy is begun in the early postnatal months. Such studies would provide definitive evidence as to the ongoing cross-talk and profound effects of the central haemolytic paradigm on haemostasis and the other secondary pathological processes, including inflammation and endothelial activation, leading to the protean manifestations of SCD. We hope that such laboratory investigations will provide a second tier of critical analyses as part of the recently completed double-blind, placebo-controlled Baby Hug Clinical Trial (Wang et al, 2011).
Acknowledgments
We thank the staff members of the division of Hematology, St Christopher’s Hospital for Children, Drexel University for their efforts at patient recruitment and blood sample collection. We also thank Mrs. Dorothy Shields, Mr. Robert Bradford and Mrs. Surekha Kulkarni for their technical assistance, Miss Priyanka Setty for preparing illustrations, and Mr. Lorenzo Thomas for providing secretarial assistance. This work was supported by grants P60HL62148 (MJS, BNYS, CDD, and AKR), and U54 HL70585 (MJS, BNYS, CCD and SK) from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD.
Footnotes
AUTHOR CONTRIBUTION
BNYS – collaborated in study design, supervised and performed select experiments, collaborated in critical evaluation of the data, performed statistical analyses, and wrote the manuscript. NSK – performed WBTF and MPTF measurements and reviewed the manuscript. AKR - performed TAT and D-dimer measurements and reviewed the manuscript. SGB - performed hs-CRP measurements. SK - collaborated in study design and reviewed the manuscript. CDD – provided patient samples and reviewed the manuscript. MJS – collaborated in study design, critical evaluation of the data, and facilitated manuscript revisions.
DISCLOSURE OF CONFLICT OF INTEREST
We declare that we have no conflict of interest.
References
- Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Archives of Pathology and Laboratory Medicine. 2001;125:1436–1441. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Cappellini MD, Rachmilewitz EA. β-Thalassaemia and sickle cell anaemia as paradigms of hypercoagulabilty. British Journal of Haematology. 2007;139:3–13. doi: 10.1111/j.1365-2141.2007.06740.x. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Brittain JE, Desai P, May R, Jones S, Delaney J, Strayhorn D, Hinderliter A, Key NS. Association of coagulation activation with clinical complications in sickle cell disease. PLoS One. 2012;7:e2978. doi: 10.1371/journal.pone.0029786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocyes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlution. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]
- Blann AD, Mohan JS, Bareford D, Lip GY. Soluble P-selectin and vascular endothelial growth factor in steady state sickle cell disease: relationship to genotype. Journal of Thrombosis and Thrombolysis. 2008;25:185–189. doi: 10.1007/s11239-007-0177-7. [DOI] [PubMed] [Google Scholar]
- Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes. Expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arteriosclerosis Thrombosis and Vascular Biology. 2008;28:947–953. doi: 10.1161/ATVBAHA.107.161471. [DOI] [PubMed] [Google Scholar]
- Camerer E, Kolstø AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thrombosis Research. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Colella MP, Machado-Neto J, Quaino SKP, Conran N, Annichino-Bizzacchi JM, Costa FF, Saad SO, de Paula EV, Traina F. Hydroxyurea Reduces the Hypercoagulable State In Sickle Cell Anemia. Blood. 2011;118:abstract 1068. [Google Scholar]
- De Jong K, Emerson RK, Butler J, Bastacky J, Mohandas N, Kuypers FA. Short survival of phosphatidylserine-exposing red blood cells in murine sickle cell anemia. Blood. 2001;98:1577–1584. doi: 10.1182/blood.v98.5.1577. [DOI] [PubMed] [Google Scholar]
- Dessap AM, Deux JF, Abidi N, Lavenu-Bombled C, Melica G, Renaud B, Godeau B, Adnot S, Brochard L, Brun-Buisson C, Galacteros F, Rahmouni A, Habibi A, Maitre B. Pulmonary arterythrombosis during acute chest syndrome in sickle cell disease. American Journal of Respiratory and Critical Care Medicine. 2011;184:1022–1029. doi: 10.1164/rccm.201105-0783OC. [DOI] [PubMed] [Google Scholar]
- Hebbel RP. Reconstructing sickle cell disease: A data-based anslysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence-based medicine. American Journal of Hematology. 2011;86:123–154. doi: 10.1002/ajh.21952. [DOI] [PubMed] [Google Scholar]
- Hillery CA, Panepinto JA. Pathophysiology of stroke in sickle cell disease. Microcirculation. 2004;11:195–208. doi: 10.1080/10739680490278600. [DOI] [PubMed] [Google Scholar]
- Johnson GJ, Leis LA, Bach RR. Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty. Thrombosis and Haemostasis. 2009;102:728–734. doi: 10.1160/TH09-04-0261. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. American Journal of Hematology. 2009;84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. Journal of Clinical Investigation. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Seminars in Thrombosis and Hemostasis. 2010;36:865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, Kuypers FA, Bach RR. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998;91:4216–4223. [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. Journal of Thrombosis and Haemostasis. 2008;6:1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Setty Y, Gayen-Betal S, Vijender V, Rao K, Dampier C, Stuart M. Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vasocclusive crises. British Journal of Haematology. 2010;148:797–804. doi: 10.1111/j.1365-2141.2009.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Ataga KI, Orringer EP, Phillips DR, Parise LV. Biologically active CD40 ligand is elevated in sickle cell anemia: potential role for platelet-mediated inflammation. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26:1626–1631. doi: 10.1161/01.ATV.0000220374.00602.a2. [DOI] [PubMed] [Google Scholar]
- Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, Key NS, Mackman N. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thrmbosis Research. 2012;129:80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N. Role of tissue factor in hemostasis, thrombosis and vascular development. Arteriosclerosis Thrombosis and Vascular Biology. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- Manly DA, Wang J, Glover SL, Kasthuri R, Leibman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thrmbosis Research. 2009;125:511–512. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan JS, Lip GY, Wright J, Bareford D, Blann AD. Plasma levels of tissue factor and soluble E-selectin in sickle cell disease: relationship to genotype and to inflammation. Blood Coagulation and Fibrinolysis. 2005;16:209–214. doi: 10.1097/01.mbc.0000164431.98169.8f. [DOI] [PubMed] [Google Scholar]
- Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, Wethers DL, Pegelow CH, Gill FM the cooperative study of sickle cell disease. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–94. [PubMed] [Google Scholar]
- Parhami-Seren B, Butenas S, Krudysz-Amblo J, Mann KG. Immunologic quantification of tissue factors. Journal of thrombosis and Haemostasis. 2006;4:1745–1755. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- Setty BNY, Rao AK, Stuart MJ. Thrombophilia in sickle cell disease: the red cell connection. Blood. 2001;98:3228–3233. doi: 10.1182/blood.v98.12.3228. [DOI] [PubMed] [Google Scholar]
- Setty BNY, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- Setty BNY, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. Journal of Thrombosis and Haemostasis. 2008;6:2202–2209. doi: 10.1111/j.1538-7836.2008.03177.x. [DOI] [PubMed] [Google Scholar]
- Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- Solovey A, Gui L, Key NS, Hebbel RP. Tissue factor expression by endothelial cells in sickle cell anemia. Journal of Clinical Investigation. 1998;101:1899–1904. doi: 10.1172/JCI1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovey A, Kollander R, Shet A, Milbauer LC, Choong S, Panoskaltsis-Mortari A, Blazar BR, Kelm RJ, Jr, Hebbel RP. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104:840–846. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- Stein PA, Beemath A, Meyers FA, Skaf E, Olson RE. Deep venous thrombosis and pulmonary embolism in hospitalized patients with SCD. American Journal of Medicine. 2006;119:897.e7–897.e11. doi: 10.1016/j.amjmed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Setty BNY. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood. 1999;94:1555–1560. [PubMed] [Google Scholar]
- Stuart MJ, Setty BNY. Hemostatic alterations in sickle cell disease: relationships to disease pathophysiology. Pediatric Pathology and Molecular Medicine. 2001;20:27–46. [PubMed] [Google Scholar]
- Tomer A, Harker LA, Kasey S, Eckman JR. Thrombogenesis in sickle cell disease. Journal of Laboratory and Clinical Medicine. 2001;137:398–407. doi: 10.1067/mlc.2001.115450. [DOI] [PubMed] [Google Scholar]
- Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlution: a new paradigm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers EJ, Marianne CL, Schaap RJ, Berckmans RJ, Nieuwland R, Sturk A, van Doormaal FF, Meijers JCM, Biemond BJ on behalf of the CURAMA study group. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–1519. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse M, Lahary A, Chevallier C, Blois S, Levesque H, Vannier JP, Lenormand B. Hypercoagulability in sickle cell disease: increased monocyte tissue factor expression and increased erythrocyte microparticles expressing phosphatidylserine. Journal of Thrombosis and Haemostasis. 2003;1(supplement 1):abstract P0927. [Google Scholar]
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik SA, Howard TH, Wynn LW, Kutlar A, Armstrong FD, Files BA, Goldsmith JC, Waclawiw MA, Huang X, Thompson BW BABY HUG investigators. Hydroxycarbamide in very young children with sickle-cell anaemia multicentre, randomized, controlled trail (BABY HUG) Lancet. 2011;377 (9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MP, Green D, Gilman-Sachs A, Beaman K, Freels S, Boggio L, Allen S, Zuckerman L, Schlegel R, Williamson P. Antiphospholipid antibodies, proteins C and S, and coagulation changes in sickle cell disease. Journal of Laboratory and Clinical Medicine. 1999;134:352–362. doi: 10.1016/s0022-2143(99)90149-x. [DOI] [PubMed] [Google Scholar]
- Yasin Z, Witting S, Palascak MB, Joiner CH, Rucknagel DL, Franco RS. Phosphatidylserine externalization in sickle red blood cells: associations with cell age, density and hemoglobin F. Blood. 2003;102:365–370. doi: 10.1182/blood-2002-11-3416. [DOI] [PubMed] [Google Scholar]