SUMMARY
Mycobacterium tuberculosis is an important bacterial pathogen with an extremely slow growth rate, an unusual outer membrane of very low permeability and a cunning ability to survive inside the human host despite a potent immune response. A key trait of M. tuberculosis is to acquire essential nutrients while still preserving its natural resistance to toxic compounds. In this regard, copper homeostasis mechanisms are particularly interesting, because copper is an important element for bacterial growth, but copper overload is toxic. In M. tuberculosis at least two enzymes require copper as a cofactor: the Cu/Zn-superoxide dismutase SodC and the cytochrome c oxidase which is essential for growth in vitro. Mutants of M. tuberculosis lacking the copper metallothionein MymT, the efflux pump CtpV and the membrane protein MctB are more susceptible to copper indicating that these proteins are part of a multipronged system to balance intracellular copper levels. Recent evidence showed that part of copper toxicity is a reversible damage of accessible Fe-S clusters of dehydratases and the displacement of other divalent cations such as zinc and manganese as cofactors in proteins. There is accumulating evidence that macrophages use copper to poison bacteria trapped inside phagosomes.
Here, we review the rapidly increasing knowledge about copper homeostasis mechanisms in M. tuberculosis and contrast those with similar mechanisms in E. coli. These findings reveal an intricate interplay between the host which aims to overload the phagosome with copper and M. tuberculosis which utilizes several mechanisms to reduce the toxic effects of excess copper.
Keywords: nutrient, uptake, bactericidal, efflux, homeostasis, immune defence
Copper is an important element for bacterial growth
The ability of copper ions to undergo reversible oxidation from Cu(I) to Cu(II) combined with its high redox potential makes copper an important cofactor in enzymes used for electron transfer reactions in the presence of oxygen1. Thus, it is widely believed that copper is an essential trace element required for survival by all organisms from bacteria to humans2. While copper homeostasis in E. coli is well studied, the corresponding proteins and mechanisms in mycobacteria remain largely uncharacterized. Because the environment in which M. tuberculosis spends most of its life cycle is the macrophage phagosome, a compartment of oxidative and nitrosative stress, understanding the homeostasis of redox active metals such as copper is of critical importance.
In M. tuberculosis at least two enzymes require copper as a cofactor: the Cu/Zn-superoxide dismutase SodC and the cytochrome c oxidase. Since superoxide anions cannot cross lipid membranes, bacteria use two superoxide dismutases to protect both the periplasm and the cytoplasm from oxidative stress: The periplasmic Cu/Zn-containing superoxide dismutase SodC protects against extracellular superoxide3 or superoxide generated in the periplasm4, while the Mn/Fe-containing superoxide dismutase SodA detoxifies superoxide in the cytoplasm5. The sodC mutant of M. tuberculosis is more susceptible to superoxide in vitro and to killing by activated macrophages6 establishing that the Cu/Zn superoxide dismutase contributes to the resistance of M. tuberculosis against oxidative stress. However, sodC expression is reduced when M. tuberculosis switches from replicative to persistent infection states, suggesting that its activity is required only during the early stages of infection7
The cytochrome c oxidase is the most energy-efficient terminal oxidase and is used during exponential growth of M. tuberculosis in the presence of oxygen8. The genes encoding the three subunits of the cytochrome c oxidase CtaC, CtaD, CtaE (Rv2200c, Rv3043c, Rv2193) are essential for growth of M. tuberculosis in vitro9. The fact that copper is required for the function of the cytochrome c oxidase indicates that copper is an essential nutrient, at least in the exponential growth phase of M. tuberculosis.
Mechanism(s) of copper toxicity
Iron-sulfur cluster degradation
It has long been thought that copper toxicity is due to a Fenton-like reaction between Cu(I) and hydrogen peroxide, producing hydroxyl radicals which then induce oxidative stress inside cells and cause DNA damage10. Indeed, DNA damage has been observed in eukaryotic cells upon copper exposure11 and prokaryotic cells respond to copper stress by inducing the reactive oxygen species (ROS) stress response12, 13. However, recent work in E. coli and Bacillus subtilis suggests an alternate mechanism of copper toxicity. Macomber et al. showed that a copper accumulating E. coli mutant is more sensitive to copper stress during anaerobic growth than aerobic growth, that excess copper actually protects the mutant from hydrogen peroxide-mediated cellular damage and that copper accumulation does not increase the rate of hydroxyl radical production, leading the authors to conclude that Fenton-like chemistry does not occur upon copper accumulation in E. coli cells14. Indeed, even wild-type E. coli is more sensitive to copper under anaerobic conditions than when grown in the presence of oxygen15. It also has been shown that copper reversibly damages accessible Fe-S clusters of dehydratases in E. coli, while proteins with buried Fe-S clusters are not subject to copper damage10. It has been proposed that copper converts [4Fe-4S]2+ to [3Fe-4S]0 clusters which are then further degraded by an unknown mechanism10. In B. subtilis, copper-stress induces expression of genes involved in iron acquisition and Fe-S cluster biogenesis, but only a few genes responding to reactive oxygen intermediates are induced16. The role of dehydratases in metabolism may explain why excess copper reduces the metabolic capacity of cells10, 16.
Metal cofactor replacement
Copper also is able to replace other metals which act as cofactors in proteins. For example, Cu(II) can replace Zn(II) in the active site of human glyoxalase I17 and in zinc-finger domains of human estrogen receptor protein18, thereby inactivating these proteins. In M. tuberculosis, copper stress greatly induces the expression of cadI, encoding a putative glyoxalase I, which likely contains zinc12. The authors hypothesized that replacement of zinc by copper resulted in inactive enzyme and a feedback loop that upregulates cadI expression12. Deletion of the gene encoding the multicopper oxidase CueO from E. coli results in copper accumulation and growth defects under copper stress, both of which can be reduced by the addition of other transition metals (Zn(II), Mn(II), Fe(III))19. These results suggest that metal cofactor replacement is also a mechanism of copper toxicity.
No free copper in the cytoplasm
Bacteria respond quickly to increased cellular copper by increasing expression of efflux pumps as well as copper chaperones and multicopper oxidases15, 20, 21. Therefore, it is unlikely that there is free copper in bacterial cells which would be available to create reactive oxygen species under standard growth conditions. In E. coli, under standard conditions, the cellular copper concentration is approximately 10 μM21. However, the copper sensing transcriptional regulator CueR responds to zeptomolar concentrations of Cu(I), leading authors to conclude there is no free copper in the cytosol of prokaryotic cells22, 23. In E. coli, mutation of dsbC, a periplasmic disulfide bond isomerase, which resolves incorrect disulfide bonds, results in copper sensitivity24. A possible explanation for this observation is that excess copper in the periplasm leads to incorrect disulfide bond formation. Thus, loss of functional DsbC may result in increased copper sensitivity24. However, direct experimental evidence for free copper in the periplasm of E. coli is lacking.
Role of copper in the host immune response
Copper is required for the development and maintenance of the mammalian immune system
Several lines of evidence indicate that copper is required for both innate and adaptive immune responses. Human infants with Menkes disease, which causes copper deficiency, are more susceptible to infection25. Increasing the amount of copper in the diets of malnourished children reduces the incidence of infectious diseases26. Mice raised on a copper-depleted diet produce fewer antibody-producing cells than those fed a normal diet and show increased morbidity upon infection27, 28. Copper-deficient diets reduce the respiratory burst and subsequent microbicidal activities of neutrophils and macrophages as well as T-cell dependent antibody production29 and references therein. We have shown that increasing the dietary copper of M. tuberculosis-infected mice reduces the number of lymphocytic infiltrates and lung lesions30. Additionally, copper accumulates in granulomas isolated from M. tuberculosis-infected guinea pigs compared to un-affected lung tissue indicating that copper is utilized by the immune system to fight the infection30. These results show that copper is required not only for the functional development of the animal as a whole, but also for the multiple cell types that make up the host immune system and that copper may be used directly as a host defense mechanism.
Macrophages take up copper in response to mycobacterial infection
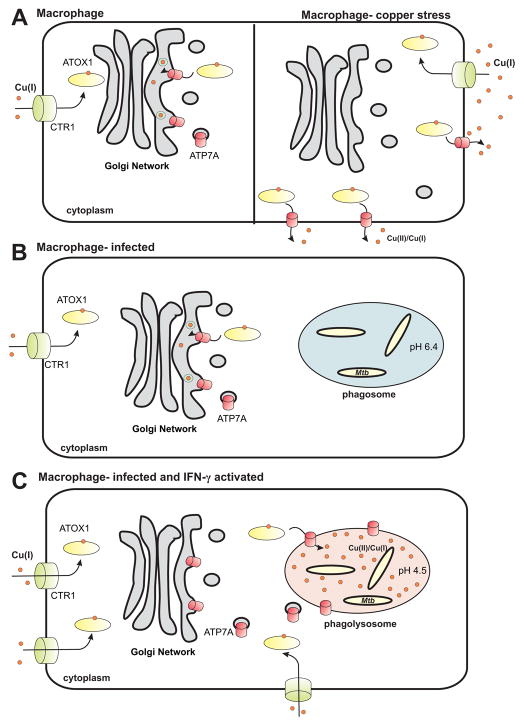
M. tuberculosis elicits multiple immunological responses upon infection of the human host. Macrophages are recruited to the infection site, and the bacteria are phagocytosed. M. tuberculosis interrupts the normal phagocytosis program by blocking phagosome-lysosome fusion, without which the phagosome cannot acidify. Hence, the pH within the M. tuberculosis-containing phagosome remains near 6.4 (Fig. 1C) (reviewed in31). When the macrophage is activated by interferon-γ (IFN-γ) phagosome maturation proceeds as normal and the vacuole acidifies to a pH of 4.5 – 5.0 (Fig. 1CD)32, 33. When infected with Mycobacterium avium, macrophage phagosomes significantly accumulate copper in an IFN-γ dependent manner34 (Fig. 1D). Infected macrophages left unactivated or activated with TNF-α do not significantly accumulate copper in bacteria-containing phagosomes34. Whether the increased copper acts directly or indirectly to kill M. tuberculosis is unclear. Copper could be used to generate oxidative stress within the phagolysosome, or it could be taken up by bacteria directly and exert toxic effects as described above. Additionally, it was recently shown that release of zinc into phagolysosomes increases bactericidal activity of macrophages35, indicating that other heavy metals may be used in a manner similar to copper as antibacterial agents.
Fig. 1. Copper homeostasis in macrophages.
A. In resting macrophages CTR1 imports Cu(I) which is bound by the chaperone ATOX1. ATP7A is localized to the Golgi Network to deliver Cu to copper-requiring proteins. B. Under copper-stress ATP7A is trafficked to the membrane to export excess copper, but its transcription and translation are not increased. C. Initially upon phagocytosis into macrophages, M. tuberculosis suppresses phagolysosome formation. D. Once infected macrophages are activated with IFN-γ, phagolysosome fusion proceeds, the pH of the M. tuberculosis containing vacuole is reduced to pH4.5, CTR1 and ATP7A expression is increased and ATP7A is trafficked to the phagolysosome.
ATP-driven copper-transport is required for bactericidal activity of macrophages
The role of copper in aiding macrophage bactericidal activity was further investigated by Petris and co-workers. Addition of copper to IFN-γ activated macrophages increases their bactericidal activity against E. coli36. Activation of human macrophages with IFN-γ or LPS results in a dose-dependent increase in copper uptake and increased expression of the copper transport protein, CTR1, as well as the copper-transporting ATPase, ATP7A36. Under normal growth conditions CTR1 is mainly localized on the cytoplasmic membranes of eukaryotic cells, while ATP7A is associated with the trans-Golgi network (TGN)37. ATP7A remains associated with the TGN under low copper conditions where it transports copper into the TGN lumen for incorporation into copper-requiring proteins (Fig. 1A), but is trafficked to the cytoplasmic membrane under high copper to excrete excess copper (Fig. 1B)38. However, protein and mRNA levels remain unchanged in response to copper, showing that the mechanism of ATP7A response to copper stress is post-translational39. Trafficking of ATP7A from the TGN to post-Golgi vesicles is dependent on the monomeric copper chaperone ATOX1, which binds a single copper atom38, 40, 41 (Fig. 1A). Additionally, vesicles containing ATP7A are trafficked to the phagosome when macrophages are stimulated with IFN-γ and exposed to excess copper36. ATP7A also is required for killing of E. coli in infected macrophages36. E. coli lacking the copper efflux pump, CopA, is more susceptible to macrophage killing than wild-type E. coli. This phenotype is rescued by RNAi-mediated knockdown of the ATP7A gene36. From these data, a model for the role of copper in controlling M. tuberculosis infection of macrophages can be proposed (Fig. 1CD). Upon infection, M. tuberculosis prevents phagolysosome fusion and therefore acidification of the bacteria-containing compartment. Once the macrophages are activated by IFN-γ phagolysosome fusion proceeds, expression of CTR1 and ATP7A is increased, ATP7A protein is trafficked to the phagolysosome, and the copper concentration of the compartment increases. Taken together, these results reveal an important role for copper in macrophage-mediated killing of bacteria.
Copper resistance and virulence of pathogenic bacteria
Considering the importance of copper for the immune system of mammals it is not surprising that copper resistance is required for full virulence of pathogenic bacteria. For example, E. coli mutants deficient in copper efflux are more susceptible to killing by macrophages36. The copper-transporting P-type ATPases of Salmonella enterica sv. Typhimurium (S. Typhimurium) are required for survival in macrophages42, and in Listeria monocytogenes for persistence in the liver during murine infection43. The Cu-efflux pumps CopA1 and CopA2 of Pseudomonas aeruginosa are required to establish infection in an Arabidopsis thaliana model system44. Mutation of the multi-copper oxidase gene cueO of S. Typhimurium results in attenuated mouse colonization, but does not increase susceptibility to macrophage killing45. It appears that copper efflux alone is not sufficient to prevent copper toxicity in bacteria; multicopper oxidases and perhaps other copper binding proteins may also be required.
Copper homeostasis in M. tuberculosis
In the last few years several mechanisms of copper homeostasis have been identified in M. tuberculosis30, 46–49. Here, we will discuss these mechanisms of M. tuberculosis in detail (Fig. 2A).
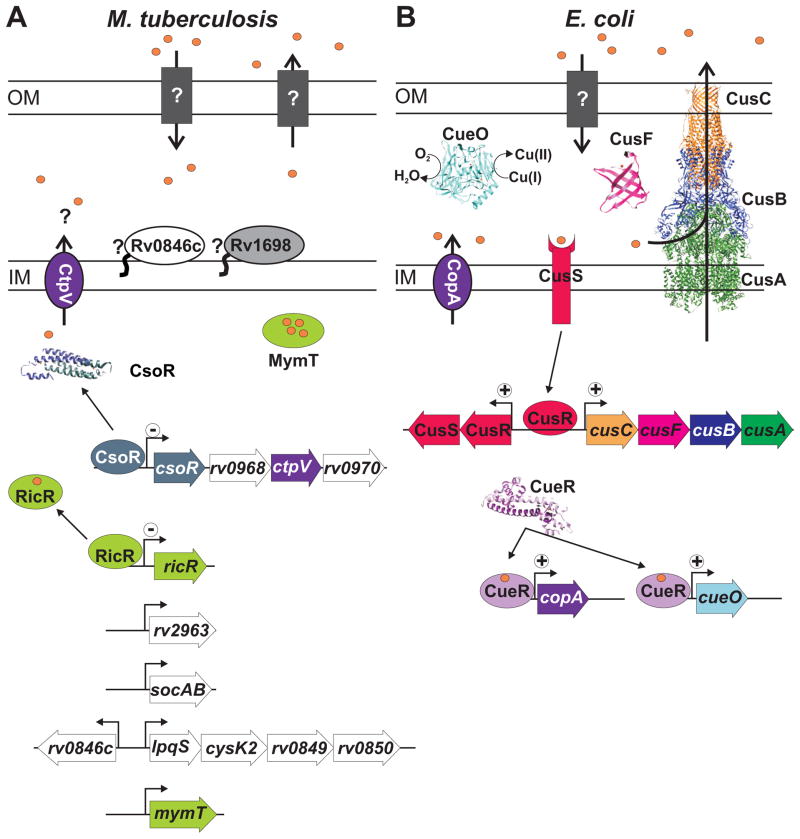
Fig. 2. Copper homeostasis in M. tuberculosis and E. coli.
The thicknesses of the membranes were drawn according to measurements derived from cryo-electron microscopy. The size of the periplasmic space in mycobacteria is larger compared to E. coli, but is not shown proportionally in this figure.
A. In M. tuberculosis outer membrane proteins involved in copper uptake or efflux are unknown. CtpV is an inner membrane (IM) transporter which likely functions as a copper efflux pump and whose expression is regulated by CsoR (PDB: 2HH7). Rv0846c is a putative multi-copper oxidase. The exact localization of the membrane protein Rv1698 (MctB) which reduces intracellular copper levels is unknown. MymT encodes a cytoplasmic copper metallothionein whose expression is regulated by RicR. Other genes in the RicR regulon (rv2963, socAB and lpqS) have undetermined functions. RicR and CsoR are both repressors which are induced by copper binding. B. In E. coli the tripartite efflux pump CusABC transports copper from the cytoplasm to the extracellular space (CusAB, PDB: 3NE5; CusC, PDB: 3PIK). CusF (PDB: 2VB2) may act as a periplasmic copper chaperone. CueO (PDB 1KV7) is a multi-copper oxidase and CopA is an IM copper transporting P-type ATPase. The two-component regulator CusSR activates transcription of cusCFBA, but does not regulate its own transcription. CueR (PDB 1Q05) binds copper to activate transcription of copA and cueO. Molecular structures were prepared using the UCSF Chimera program91.
Copper import
The mechanism of copper import awaits characterization in M. tuberculosis; indeed copper influx has not been described even for the most-studied organisms such as E. coli. It is unknown if there are dedicated copper import proteins in M. tuberculosis. Considering the very low permeability of cations across any lipid membrane50, direct diffusion of copper ions across the mycobacterial outer and inner membranes is unlikely. However, heavy metal import proteins have been characterized in other bacteria. For example, overexpression of hmtA of Pseudomonas aeruginosa results in copper hypersensitivity and copper accumulation in E. coli51. Putative copper import proteins have been proposed in Enterococcus hirae and B. subtilis, but copper import has not been shown directly20. In E. hirae copA is induced under copper-limiting conditions and the deletion mutant is unable to grow on copper limiting media indicating that this gene encodes a copper uptake protein52. Similarly, in B. subtilis, ycnJ is induced under low copper conditions and the knock-out strain has a growth defect on copper-deficient media53.
Copper efflux - CtpV and its regulator CsoR
The proteins involved in copper efflux are well characterized in E. coli54(see below and Fig. 2B) and E. hirae20, but have not been studied in M. tuberculosis until recently. The first hints to the M. tuberculosis copper-stress response came from mouse infection studies. Expression of an operon comprising genes rv0967-rv0970 was induced during infection of mice compared to growth of M. tuberculosis in vitro55. The operon contains ctpV (rv0969), a putative cation transport P-type ATPase predicted to transport copper56. Rv0968 and rv0970 encode proteins of unknown function (Fig. 2A). The operon was induced under copper stress in vitro and was named copper-sensitive operon (cso)47. Copper highly induced expression from the cso promoter compared to other metals indicating that the operon is copper specific and not part of a general metal-ion response pathway47. Because the structure of cso is reminiscent of the cmt (cadmium/lead-responsive) operon, in which the first gene encodes a regulator for the operon, the authors hypothesized the protein product of rv0967 might also be a transcriptional regulator. Indeed, purified Rv0967 protein (renamed CsoR, copper sensitive operon regulator) specifically binds a pseudopalindromic sequence overlapping the cso promoter. Addition of copper to the CsoR-DNA complex releases the regulator from its cognate DNA sequence47. Furthermore, apo-CsoR binds equimolar amounts of Cu(I) which is coordinated by two cysteines and a histidine47.
The characterization and structure of CsoR revealed that this protein represents a previously unknown class of copper-responsive regulators, which is much more widely distributed among prokaryotes than the well-characterized examples from E. coli (CueR) or E. hirae (CopY)47. Apo-CsoR represses transcription of cso and loses affinity to its operator upon copper binding, allowing transcription of the operon (Fig. 2A). In B. subtilis, CsoR negatively regulates expression of copZ and copA encoding a copper chaperone and a copper efflux pump, respectively, in a manner similar to M. tuberculosis. However unlike M. tuberculosis, csoR is transcribed separately from its target genes in B. subtilis57. Global transcriptional analysis by microarray experiments showed that CsoR regulates only its own promoter and hence expression of cso in M. tuberculosis49.
The role of CtpV in the M. tuberculosis copper-stress response was further studied because CtpV is predicted to be a copper-transporting ATPase and ctpV expression is induced by elevated levels of copper12. M. tuberculosis CtpV has significant sequence similarity to CopA of both E. coli and E. hirae. Importantly, motifs conserved among metal-transport P-type ATPases are found in M. tuberculosis CtpV12. However, unlike the CopA proteins, CtpV lacks an apparent metal binding domain. Whether and how this alteration affects the activity of CtpV or its interactions with other proteins, such as copper chaperones, is unknown12. Deletion of ctpV from M. tuberculosis H37Rv does not give a general growth defect, but results in increased copper sensitivity compared to wild-type46. Moreover, expression of the cso in M. smegmatis decreases copper accumulation compared to wild-type M. smegmatis, indicating that cso mediates copper resistance by efflux46. Taken together, the copper-induced expression of ctpV, the similarity of CtpV to known copper efflux pumps, and its ability to reduce the copper burden of M. smegmatis, suggest that CtpV indeed functions as a copper efflux pump in M. tuberculosis (Fig. 2A). However, transport of copper by CtpV has yet to be demonstrated directly.
The M. tuberculosis genome encodes 11 putative cation transport P-type ATPases, three of which are predicted to transport Cu (CtpV, CtpA and CtpB)12, 56. Only expression of ctpv and ctpG is induced by copper. CtpG expression is further upregulated by copper in the absence of ctpV, suggesting CtpG may functionally compensate for the loss of CtpV46. Additionally, two predicted permease genes of unknown function, rv0849 and rv2963, are induced by copper12; the role of these proteins in the copper response remains uncharacterized.
Expression of ctpV is induced during infection of mice compared to in vitro growth55 and copper resistance is a known virulence determinant in several pathogenic bacteria (reviewed above) suggesting CtpV plays a role in M. tuberculosis pathogenesis. In one guinea pig infection experiment deletion of ctpV resulted in decreased colony forming units (CFUs) in the lung compared to wild-type M. tuberculosis 21 days after infection, but this difference was resolved later46. Additionally, the ctpV deletion mutant did not show any virulence defect in mice. Mouse survival was greatly increased compared to wild-type infection, but was not complemented by expression of ctpV, raising doubts whether this phenotype resulted from deletion of ctpV46. Taken together, these experiments suggested that CtpV is not required for colonization or proliferation46.
Copper efflux - MctB (Rv1698)
Initial experiments showed that Rv1698 is a membrane protein, is surface-accessible, increases uptake of glycerol in a M. smegmatis porin mutant, and forms pores in artificial lipid bilayers58, 59. Additionally we found that mutation of rv1698 in M. tuberculosis or its homolog in M. smegmatis, ms3747, resulted in increase of total cellular copperand increased the copper sensitivity of these mutants. These findings led us to suggest that Rv1698 was a pore-forming outer membrane protein involved in copper efflux30. However, additional experiments did not confirm surface accessibility of Rv1698 and indicated that it might be anchored in the inner membrane (manuscript in preparation, Fig. 2A). Copper accumulation by M. smegmatis lacking ms3747 has been verified by inductively coupled plasma mass spectrometry (ICP-MS) (manuscript in preparation). It was confirmed that Ms3747 and Rv1698 are required for copper resistance, but it is unclear whether these proteins play a direct role in copper efflux. Alternatively, loss of Rv1698 may change the outer membrane so that more copper enters the periplasm and/or the cytoplasm of M. tuberculosis. We are currently performing experiments to identify the exact role of Rv1698 in copper resistance. The observation that rv1698 expression is not induced by copper stress12, but is induced during starvation60 is consistent with a more pleiotropic function of Rv1698. Rv1698 is required for full virulence in mouse and guinea pigs30, however, it is unclear whether the virulence defect of the rv1698 mutant of M. tuberculosis is directly caused by its increased copper susceptibility.
The copper metallothionein MymT of M. tuberculosis
Metallothioneins (MTs) are characterized by their small size (<10kD) and cysteine-rich sequences with few hydrophobic residues. To date, only a few MTs have been described in bacteria, but many examples are present in eukaryotes61, 62. Metallothioneins typically bind 4–12 heavy metal ions in a solvent-shielded thiolate core61. The precise biological functions of MTs have yet to be defined; they certainly serve as metal ion sinks, but may have other functions as well. It has been proposed that MTs may function as metal chaperones, delivering metal ions to proteins that require them as cofactors63. Furthermore, it has been shown in S. cerevisiae that a copper loaded MT can be transported out of the cell to aid in copper-detoxification64. MTs, therefore, play an important role in copper homeostasis and detoxification in many cell types.
The mycobacterial metallothionein MymT was first identified by Nathan and co-workers through a chemical-genetic screen. Homologs of MymT are present in all pathogenic mycobacteria. Expression of mymT (mt0196 in CDC1551 or rv0186A in H37Rv) is upregulated by multiple transition metals: copper, cadmium, cobalt, nickel and zinc, with the strongest induction by copper. Expression of mymT also is increased under conditions mimicking the host macrophage, including nitric oxide and superoxide stress and cell wall disruption. Because mymT encodes multiple cysteines organized in typical metal-binding C-X-C or C-X-H motifs, the authors suspected MymT might be a metallothionein. Mass-spectroscopy (MS) analysis showed that MymT binds increasing amounts of copper as the molar ratio of Cu(I) to MymT is increased with the major species containing 4–6 copper ions. Furthermore, degradation of the Cu(I) core of MymT by nitric oxide resulted in the release of copper inside M. tuberculosis, suggesting another mechanism for the toxicity of reactive nitrogen species48. Importantly, MymT is required for robust growth in excess copper in vitro, but is not required for resistance to other divalent metals, indicating that the protein is a copper-specific MT48. Additionally, MymT is not required for virulence in mice48; this may be due to redundancy in the copper homeostasis mechanisms of M. tuberculosis that protect the bacteria even when one copper-handling protein is missing or mutated (Fig. 2A).
The best-characterized bacterial metallothionein is SmtA from Synechococcus PCC 7942, which contains a cluster of four zinc ions as well as a zinc-finger domain65. Zinc-MTs have been biochemically characterized in Anabaena and Pseudomonas species but their requirement for zinc tolerance has not been shown66. Although a zinc-metallothionein was identified bioinformatically in E. coli, biochemical analysis showed the protein bound only a single zinc ion, likely in a zinc-finger domain66. It is likely that other bacteria encode MTs, but they may be difficult to identify bioinformatically because of the lack of nucleotide sequence conservation48, 66, their small size, and by their poorly defined amino acid sequence and structural requirements61. Furthermore, bacterial MTs are different from the canonical eukaryotic MTs, in that they can utilize histidine to coordinate metals in the solvent-shielded core65. MymT remains a unique example as a prokaryotic copper metallothionein. It will be interesting to see if other bacterial copper MTs can be identified or if this is truly a novel adaptation of M. tuberculosis to its host.
The RicR (Rv0190) regulon
Mutations in either the mpa (mycobacterium proteasomal ATPase) gene or pafA (proteasome accessory factor A), both of which are required for proteasome function, results in the significant repression of expression of almost 40 genes during growth of M. tuberculosis in vitro49. A conserved palindromic sequence was found in the putative promoter regions of lpqS, rv2963, rv0190, and mymT, as well as two previously unannotated open reading frames, small ORF induced by copper A and B (socAB), suggesting that these genes were regulated by the same transcription factor49. Growth experiments confirmed that expression of lpqS and socAB also are induced by copper49 as suspected from the previously identified regulation of rv0190, rv2963 and mymT by copper12, 48. Although the functions of these proteins are unknown except for MymT, it is reasonable to assume that they are also involved in the copper homeostasis mechanisms of M. tuberculosis. LpqS, rv2963, mymT and socAB are found only in pathogenic mycobacteria, suggesting the proteins encoded by these genes may also be important for virulence.
Rv0190 is similar to M. tuberculosis CsoR and has homologs in all mycobacteria and as well as in other bacteria, including B. subtilis CsoR49. The Cu(I) coordinating amino acids Cys38, His63 and Cys67 (residue numbers based on M. tuberculosis CsoR) are conserved in Rv0190 and its homologs49. The lpqS, rv2963, mymT, and socAB genes are constitutively expressed in the absence of Rv019049. Several other genes, most of which encode hypothetical proteins, were found to be upregulated in the rv0190 mutant strain compared to wild-type M. tuberculosis, including the other genes in the predicted lpqS operon49. Rv0190 specifically binds to the palindromic sequence in the promoter DNA of at least one of its targets, lpqS49. The interaction of Rv0190 and the lpqS promoter is lost in a dose dependent manner when copper is added49. Finally, the rv0190 mutant is more resistant to copper-stress than wild-type M. tuberculosis, likely because the increased expression of genes in the regulon alleviated copper toxicity49. Together, the location of the regulatory sequence overlapping with promoters, the increased expression of target genes when Rv0190 is missing, and the loss of promoter-Rv0190 interaction upon addition of copper, indicate that Rv0190 is a copper-responsive repressor (Fig. 2A). Rv0190 was therefore renamed RicR (regulated in copper repressor).
The putative multicopper oxidase Rv0846c
Multicopper oxidases couple the reduction of molecular oxygen to water with the oxidation of a substrate in a copper-dependent manner67. Substrates for oxidation include phenolic compounds, amines and other metals, such as iron67. MCOs are found throughout eukaryotes, prokaryotes and archaea68. Rv0846c of M. tuberculosis has amino acid similarity to CueO, a multicopper oxidase of E. coli, but has not been characterized yet. Expression of rv0846c is upregulated by excess copper12 and is also upregulated in a ricR mutant strain49, supporting the hypothesis that Rv0846c is involved in copper homeostasis of M. tuberculosis. RicR regulates lpqS which is located directly downstream of rv0846c and, because rv0846c is transcribed in the opposite direction of lpqS, it is possible that a single RicR binding site regulates expression of both genes (Fig. 2A)49. Bioinformatic analysis suggests that Rv0846c is translocated to the periplasm by the Twin-Arginine Translocation (TAT) system and that the amino-terminus is lipidated indicating that, unlike CueO of E. coli, Rv0846c may be membrane-associated (Fig. 2AB). The mechanism of regulation, localization, its role in virulence and in detoxifying the periplasm have yet to be determined experimentally for Rv0846c. The fact that rv0846c is deleted from a clinical isolate of virulent M. tuberculosis69 indicates that this gene is not required for virulence, probably due to redundant copper detoxification mechanisms.
Copper homeostasis in E. coli
The copper homeostasis mechanisms of E. coli and E. hirae are both well characterized20, 54. Because the cell envelope of mycobacteria contains an outer membrane and is more similar to a gram-negative than a gram-positive cell envelope70, we will use E. coli as a comparison to the copper homeostasis mechanisms of mycobacteria. Two primary copper resistance systems are encoded in the genome of E. coli: the inner membrane copper-efflux pump CopA and the multicopper oxidase CueO regulated by CueR, and the CusRS regulated cell envelope-spanning copper-efflux system CusCFBA. Some E. coli strains also carry plasmid-encoded copper homeostasis elements, which are reviewed elsewhere54.
CueR/CopA/CueO
The cue (Cu-efflux) regulon comprises the house-keeping copper resistance system of E. coli. It is expressed at a basal level at all times and is induced by increasing copper concentrations15. CueR (Cu-efflux regulator) is a Cu(I)-sensing transcriptional activator, which binds the copA and cueO promoters when Cu(I) levels are increased (Fig. 2B)15, 71. CueR is a member of the MerR family of transcriptional regulators, many of which respond to heavy metal stress72. Promoters regulated by MerR-like proteins are characterized by an elongated spacer between the −10 and −35 promoter elements72. MerR-like proteins bind operators between the −10 and −35 regions, and twist the DNA to better align the elements, increasing promoter strength drastically and thus activating transcription72. Like other MerR-regulated promoters, the CueR binding site lies between the −10 and −35 elements of its target promoters at cueO and copA71.
CueR binds copper and responds to changes in copper concentration with exquisite sensitivity: CueR-mediated induction of copA expression is half-maximal at a copper concentration in the zeptomolar range (2x10−21 M Cu)22. CueR can sense even one “free” copper ion in the cytoplasm and responds to Cu(I), Ag(I) and Au(I) but not to divalent cations such as Zn(II) or Hg(II)22. The crystal structure reveals that CueR forms a dimer and each monomer contains a DNA-binding domain, dimerization helix and metal-binding loop (Fig. 2B, PDB 1Q05)22. Two cysteines in the metal-binding domain are required for binding of monovalent cations22. Although CueR can bind multiple monovalent metal cations, E. coli is unlikely to encounter Ag(I) and Au(I) in its natural environments, making the CueR response to Cu(I) the only biologically relevant interaction.
CopA is a metal efflux pump that specifically exports Cu(I) in an ATP-dependent manner (Fig. 2B)73. CopA is required for copper resistance of E. coli in vitro and during model infection of macrophages36, 73. Deletion of copA results in intracellular accumulation of copper74. Because copA is regulated by CueR, its expression is induced by increasing concentrations of copper15, 71, 73.
CueO (Cu-efflux Oxidase) is a multicopper oxidase required for copper resistance of E. coli75, 76. Typically multicopper oxidases have at least two copper clusters containing four copper atoms, with additional clusters possible; the type 1 (T1) blue cluster contains one copper atom and is the site of substrate oxidation (or cluster reduction), the combined T2/T3 cluster contains three copper atoms and is the site of oxygen reduction67, 68. CueO, like the prototypical multicopper oxidases, contains four copper atoms arranged in the T1 and T2/T3 catalytic sites75, 77. A methionine rich helix in CueO binds two additional copper atoms, which increase the catalytic activity of the protein, and a third Cu(I) which is the substrate of oxidation78. CueO is likely translocated to the periplasm by the twin arginine protein export system and CueO protects the periplasm from copper-stress (Fig. 2B)75. Like other multicopper oxidases, CueO is able to oxidise multiple substrates in vitro including Fe(II) and its oxidase activity is copper-dependent75. The crystal structure of CueO (PDB 1KV7) confirms that is contains four copper atoms in an organization characteristic of multicopper oxidases77. The in vivo oxidation substrates of E. coli CueO are not known, but it is hypothesized that CueO may oxidize Cu(I) to Cu(II) to protect the periplasm from the more toxic reduced form of copper15, 75.
Together, the cue regulon forms the basis of aerobic copper resistance in E. coli. CopA transports excess copper from the cytoplasm to the periplasm and CueO protects the periplasm from toxic copper, probably by oxidizing Cu(I) to the less toxic Cu(II)77. The regulator CueR is itself highly effective at preventing free copper in the cytoplasm, based on its extreme affinity for Cu(I)22.
CusRS and the tripartite efflux pump
The second chromosomally encoded copper response system in E. coli is the cus (Cu-sensing) locus, and is regulated by a two-component regulatory system: CusR (Cu-sensing regulator) and CusS (Cu-sensing rensor)79. CusS senses increases in copper in the periplasm and activates CusR which induces expression of the cusCFBA operon, which is divergently transcribed from the cusRS operon; CusR likely regulates both promoters (Fig. 2B)79. Unlike the cue system, cus is not expressed at basal levels at all times, it is only induced under extreme copper stress, presumably when the cue system is overloaded, and during anaerobic growth15, 54. Indeed, during aerobic growth, deletions of cus only increase copper sensitivity when cueO also is deleted75. In a ΔcueO mutant, each of the cusCFBA genes is required for full copper resistance of E. coli during aerobic growth80.
CusCBA forms a tripartite copper efflux pump and is part of the resistance-nodulation-cell division (RND) family of efflux pumps. RND-type pumps consist of three components, an inner membrane efflux pump, which provides the energy and specificity for the transported substrate, an outer membrane channel and a periplasmic membrane fusion protein which connects both membrane components81. It is assumed that the tripartite Cus efflux system exports cytoplasmic copper in one step across both membranes (Fig. 2B). The inner membrane component CusA forms a homotrimer and is likely able to efflux metal ions from both the cytoplasm and periplasm82. The movement of ions is dependent on methionine residues lining the channel of the pump82, 83. The co-crystal of CusA with the membrane fusion protein CusB shows that six CusB molecules form a complex with three molecules of CusA (PDB 3NE5)84. Specifically, two molecules of CusB interact with each other and one molecule of CusA84. The crystal structure shows that the trimeric CusC forms a β-barrel in the outer membrane and a barrel composed of α-helices in periplasm(PDB 3PIK)85. Together, CusCBA form a complete heavy metal efflux pump, which translocates copper in one step across two membranes out of the cell (Fig. 2B).
The remaining component of the cus operon, CusF, is located in the periplasm80. CusF binds a single Cu(I) atom, which is likely coordinated by two methionines and one histidine residue86, 87. CusF acts as a periplasmic copper chaperone and can directly transfer copper to CusB, thus protecting cells from copper-mediated damage88–90. In Fig. 2B we show the Cu(I) bound form of CusF (PDB 2VB2)87.
The two chromosomally encoded copper-handling systems of E. coli have overlapping but distinct functions. CueR regulates the housekeeping copper resistance mechanism, mediated by the inner membrane efflux ATPase, CopA, and the periplasmic multicopper oxidase CueO15, 54. Under extreme copper stress or during anaerobic growth, the cus system takes on greater importance, removing copper completely from the cell15, 54.
Conclusions
Several of the M. tuberculosis proteins discussed in this review were discovered through experiments initially focused on describing other areas of M. tuberculosis’s physiology. The copper sensitive operon, cso, was first discovered as part of a genomic island induced during infection55, MymT was discovered while investigating targets of potential anti-tuberculosis drugs48, and RicR and it’s regulon were found during investigation of the proteasome49. These fortuitous discoveries suggest that copper homeostasis mechanisms overlap with other physiological aspects of M. tuberculosis.
The variety and increasing numbers of copper-responsive proteins and their mild individual contributions to virulence suggest that there are overlapping mechanisms for copper resistance in M. tuberculosis. In E. coli, no single protein or complex is entirely responsible for copper resistance, and mutations in multiple systems must be made to confer a strong copper sensitivity phenotype. It has been suggested by several groups that multiple mutations in copper-responsive genes of M. tuberculosis must be created in a single strain in order to fully deplete the copper resistance mechanisms and induce a more robust virulence defect48, 49. Given the broad effects of copper toxicity, it is also likely that many proteins that are neither copper binding nor copper translocating, may be important in combating copper toxicity; the loss of these seemingly irrelevant proteins could result in copper-sensitivity and virulence defects. By characterizing the many unknown hypothetical proteins and the apparent redundancies in copper resistance, we will develop a much clearer picture of copper homeostasis in M. tuberculosis and the role of copper in the host immune response.
Acknowledgments
We thank all members of the Mycolab for valuable discussions and contributions. This work was funded by NIH grants T32 GM08111-23 to JLR and AI083632 to MN.
Abbreviations
- wt
wild-type
- IM
inner membrane
- OM
outer membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crichton RR, Pierre JL. Old iron, young copper: from Mars to Venus. Biometals. 2001;14:99–112. doi: 10.1023/a:1016710810701. [DOI] [PubMed] [Google Scholar]
- 2.Pena MM, Lee J, Thiele DJ. A delicate balance: homeostatic control of copper uptake and distribution. J Nutr. 1999;129:1251–60. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- 3.Battistoni A, Donnarumma G, Greco R, Valenti P, Rotilio G. Overexpression of a hydrogen peroxide-resistant periplasmic Cu,Zn superoxide dismutase protects Escherichia coli from macrophage killing. Biochem Biophys Res Commun. 1998;243:804–7. doi: 10.1006/bbrc.1998.8182. [DOI] [PubMed] [Google Scholar]
- 4.Korshunov S, Imlay JA. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J Bacteriol. 2006;188:6326–34. doi: 10.1128/JB.00554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battistoni A, Mazzetti AP, Rotilio G. In vivo formation of Cu,Zn superoxide dismutase disulfide bond in Escherichia coli. FEBS Lett. 1999;443:313–6. doi: 10.1016/s0014-5793(98)01725-6. [DOI] [PubMed] [Google Scholar]
- 6.Piddington DL, Fang FC, Laessig T, Cooper AM, Orme IM, Buchmeier NA. Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect Immun. 2001;69:4980–7. doi: 10.1128/IAI.69.8.4980-4987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci USA. 2003;100:241–6. doi: 10.1073/pnas.0136863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A. 2005;102:15629–34. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 10.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106:8344–9. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleha Banu B, Ishaq M, Danadevi K, Padmavathi P, Ahuja YR. DNA damage in leukocytes of mice treated with copper sulfate. Food Chem Toxicol. 2004;42:1931–6. doi: 10.1016/j.fct.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Ward SK, Hoye EA, Talaat AM. The global responses of Mycobacterium tuberculosis to physiological levels of copper. J Bacteriol. 2008;190:2939–46. doi: 10.1128/JB.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kershaw CJ, Brown NL, Constantinidou C, Patel MD, Hobman JL. The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology. 2005;151:1187–98. doi: 10.1099/mic.0.27650-0. [DOI] [PubMed] [Google Scholar]
- 14.Macomber L, Rensing C, Imlay JA. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol. 2007;189:1616–26. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Outten FW, Huffman DL, Hale JA, O’Halloran TV. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem. 2001;276:30670–7. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- 16.Chillappagari S, Seubert A, Trip H, Kuipers OP, Marahiel MA, Miethke M. Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis. J Bacteriol. 2010;192:2512–24. doi: 10.1128/JB.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellin S, Eriksson LE, Mannervik B. Electron paramagnetic resonance study of the active site of copper-substituted human glyoxalase I. Biochemistry. 1987;26:6779–84. doi: 10.1021/bi00395a030. [DOI] [PubMed] [Google Scholar]
- 18.Predki PF, Sarkar B. Effect of replacement of “zinc finger” zinc on estrogen receptor DNA interactions. J Biol Chem. 1992;267:5842–6. [PubMed] [Google Scholar]
- 19.Tree JJ, Kidd SP, Jennings MP, McEwan AG. Copper sensitivity of cueO mutants of Escherichia coli K-12 and the biochemical suppression of this phenotype. Biochem Biophys Res Commun. 2005;328:1205–10. doi: 10.1016/j.bbrc.2005.01.084. [DOI] [PubMed] [Google Scholar]
- 20.Solioz M, Abicht HK, Mermod M, Mancini S. Response of gram-positive bacteria to copper stress. J Biol Inorg Chem. 2010;15:3–14. doi: 10.1007/s00775-009-0588-3. [DOI] [PubMed] [Google Scholar]
- 21.Finney LA, O’Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–6. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 22.Changela A, Chen K, Xue Y, Holschen J, Outten CE, O’Halloran TV, Mondragon A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301:1383–7. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- 23.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 24.Hiniker A, Collet JF, Bardwell JC. Copper stress causes an in vivo requirement for the Escherichia coli disulfide isomerase DsbC. J Biol Chem. 2005;280:33785–91. doi: 10.1074/jbc.M505742200. [DOI] [PubMed] [Google Scholar]
- 25.Prohaska JR, Lukasewycz OA. Effects of copper deficiency on the immune system. Adv Exp Med Biol. 1990;262:123–43. doi: 10.1007/978-1-4613-0553-8_11. [DOI] [PubMed] [Google Scholar]
- 26.Castillo-Duran C, Fisberg M, Valenzuela A, Egana JI, Uauy R. Controlled trial of copper supplementation during the recovery from marasmus. Am J Clin Nutr. 1983;37:898–903. doi: 10.1093/ajcn/37.6.898. [DOI] [PubMed] [Google Scholar]
- 27.Prohaska JR, Lukasewycz OA. Copper deficiency suppresses the immune response of mice. Science. 1981;213:559–61. doi: 10.1126/science.7244654. [DOI] [PubMed] [Google Scholar]
- 28.Jones DG, Suttle NF. The effect of copper deficiency on the resistance of mice to infection with Pasteurella haemolytica. J Comp Pathol. 1983;93:143–9. doi: 10.1016/0021-9975(83)90052-x. [DOI] [PubMed] [Google Scholar]
- 29.Failla ML, Hopkins RG. Is low copper status immunosuppressive? Nutr Rev. 1998;56:S59–64. doi: 10.1111/j.1753-4887.1998.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 30.Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, Wang Y, Bossmann SH, Basaraba RJ, Niederweis M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2011;108:1621–6. doi: 10.1073/pnas.1009261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 32.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–6. [PubMed] [Google Scholar]
- 33.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–9. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 34.Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Honer Zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol. 2005;174:1491–500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 35.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–59. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–56. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Lutsenko S. Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med Chem. 2009;1:1125–42. doi: 10.4155/fmc.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci U S A. 2003;100:1215–20. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. Embo J. 1996;15:6084–95. [PMC free article] [PubMed] [Google Scholar]
- 40.Hamza I, Schaefer M, Klomp LW, Gitlin JD. Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc Natl Acad Sci U S A. 1999;96:13363–8. doi: 10.1073/pnas.96.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singleton C, Le Brun NE. Atx1-like chaperones and their cognate P-type ATPases: copper-binding and transfer. Biometals. 2007;20:275–89. doi: 10.1007/s10534-006-9068-1. [DOI] [PubMed] [Google Scholar]
- 42.Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem. 2010;285:25259–68. doi: 10.1074/jbc.M110.145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francis MS, Thomas CJ. The Listeria monocytogenes gene ctpA encodes a putative P-type ATPase involved in copper transport. Mol Gen Genet. 1997;253:484–91. doi: 10.1007/s004380050347. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Guerrero M, Raimunda D, Cheng X, Arguello JM. Distinct functional roles of homologous Cu+ efflux ATPases in Pseudomonas aeruginosa. Mol Microbiol. 78:1246–58. doi: 10.1111/j.1365-2958.2010.07402.x. [DOI] [PubMed] [Google Scholar]
- 45.Achard ME, Tree JJ, Holden JA, Simpfendorfer KR, Wijburg OL, Strugnell RA, Schembri MA, Sweet MJ, Jennings MP, McEwan AG. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun. 2011;78:2312–9. doi: 10.1128/IAI.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–8. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 48.Gold B, Deng H, Bryk R, Vargas D, Eliezer D, Roberts J, Jiang X, Nathan C. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat Chem Biol. 2008;4:609–16. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Festa RA, Jones MB, Butler-Wu S, Sinsimer D, Gerads R, Bishai WR, Peterson SN, Darwin KH. A novel copper-responsive regulon in Mycobacterium tuberculosis. Mol Microbiol. 2011;79:133–48. doi: 10.1111/j.1365-2958.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuks B, Homble F. Permeability and electrical properties of planar lipid membranes from thylakoid lipids. Biophys J. 1994;66:1404–14. doi: 10.1016/S0006-3495(94)80931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewinson O, Lee AT, Rees DC. A P-type ATPase importer that discriminates between essential and toxic transition metals. Proc Natl Acad Sci U S A. 2009;106:4677–82. doi: 10.1073/pnas.0900666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odermatt A, Krapf R, Solioz M. Induction of the putative copper ATPases, CopA and CopB, of Enterococcus hirae by Ag+ and Cu2+, and Ag+ extrusion by CopB. Biochem Biophys Res Commun. 1994;202:44–8. doi: 10.1006/bbrc.1994.1891. [DOI] [PubMed] [Google Scholar]
- 53.Chillappagari S, Miethke M, Trip H, Kuipers OP, Marahiel MA. Copper acquisition is mediated by YcnJ and regulated by YcnK and CsoR in Bacillus subtilis. J Bacteriol. 2009;191:2362–70. doi: 10.1128/JB.01616-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rensing C, Grass G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev. 2003;27:197–213. doi: 10.1016/S0168-6445(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 55.Talaat AM, Lyons R, Howard ST, Johnston SA. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc Natl Acad Sci U S A. 2004;101:4602–7. doi: 10.1073/pnas.0306023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmgren MG, Axelsen KB. Evolution of P-type ATPases. Biochim Biophys Acta. 1998;1365:37–45. doi: 10.1016/s0005-2728(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 57.Smaldone GT, Helmann JD. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology. 2007;153:4123–8. doi: 10.1099/mic.0.2007/011742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song H, Sandie R, Wang Y, Andrade-Navarro MA, Niederweis M. Identification of outer membrane proteins of Mycobacterium tuberculosis. Tuberculosis. 2008;88:526–44. doi: 10.1016/j.tube.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siroy A, Mailaender C, Harder D, Koerber S, Wolschendorf F, Danilchanka O, Wang Y, Heinz C, Niederweis M. Rv1698 of Mycobacterium tuberculosis represents a new class of channel-forming outer membrane proteins. J Biol Chem. 2008;283:17827–37. doi: 10.1074/jbc.M800866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–31. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 61.Hamer DH. Metallothionein. Annu Rev Biochem. 1986;55:913–51. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 62.Dameron CT, Harrison MD. Mechanisms for protection against copper toxicity. Am J Clin Nutr. 1998;67:1091S–7S. doi: 10.1093/ajcn/67.5.1091S. [DOI] [PubMed] [Google Scholar]
- 63.Calderone V, Dolderer B, Hartmann HJ, Echner H, Luchinat C, Del Bianco C, Mangani S, Weser U. The crystal structure of yeast copper thionein: the solution of a long-lasting enigma. Proc Natl Acad Sci U S A. 2005;102:51–6. doi: 10.1073/pnas.0408254101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felix K, Hartmann HJ, Weser U. Cu(I)-thionein release from copper-loaded yeast cells. Biol Met. 1989;2:50–4. doi: 10.1007/BF01116202. [DOI] [PubMed] [Google Scholar]
- 65.Blindauer CA, Harrison MD, Parkinson JA, Robinson AK, Cavet JS, Robinson NJ, Sadler PJ. A metallothionein containing a zinc finger within a four-metal cluster protects a bacterium from zinc toxicity. Proc Natl Acad Sci U S A. 2001;98:9593–8. doi: 10.1073/pnas.171120098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blindauer CA, Harrison MD, Robinson AK, Parkinson JA, Bowness PW, Sadler PJ, Robinson NJ. Multiple bacteria encode metallothioneins and SmtA-like zinc fingers. Mol Microbiol. 2002;45:1421–32. doi: 10.1046/j.1365-2958.2002.03109.x. [DOI] [PubMed] [Google Scholar]
- 67.Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases. Chem Rev. 1996;96:2563–606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 68.Quintanar L, Stoj C, Taylor AB, Hart PJ, Kosman DJ, Solomon EI. Shall we dance? How a multicopper oxidase chooses its electron transfer partner. Acc Chem Res. 2007;40:445–52. doi: 10.1021/ar600051a. [DOI] [PubMed] [Google Scholar]
- 69.Tsolaki AG, Hirsh AE, DeRiemer K, Enciso JA, Wong MZ, Hannan M, Goguet de la Salmoniere YO, Aman K, Kato-Maeda M, Small PM. Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc Natl Acad Sci U S A. 2004;101:4865–70. doi: 10.1073/pnas.0305634101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: Cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci U S A. 2008;105:3963–7. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Outten FW, Outten CE, Hale J, O’Halloran TV. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, CueR. J Biol Chem. 2000;275:31024–9. doi: 10.1074/jbc.M006508200. [DOI] [PubMed] [Google Scholar]
- 72.Brown NL, Stoyanov JV, Kidd SP, Hobman JL. The MerR family of transcriptional regulators. FEMS Microbiol Rev. 2003;27:145–63. doi: 10.1016/S0168-6445(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 73.Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. CopA: An Escherichia coli Cu(I)-translocating P-type ATPase. Proc Natl Acad Sci USA. 2000;97:652–6. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petersen C, Moller LB. Control of copper homeostasis in Escherichia coli by a P-type ATPase, CopA, and a MerR-like transcriptional activator, CopR. Gene. 2000;261:289–98. doi: 10.1016/s0378-1119(00)00509-6. [DOI] [PubMed] [Google Scholar]
- 75.Grass G, Rensing C. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem Biophys Res Commun. 2001;286:902–8. doi: 10.1006/bbrc.2001.5474. [DOI] [PubMed] [Google Scholar]
- 76.Grass G, Rensing C. Genes involved in copper homeostasis in Escherichia coli. J Bacteriol. 2001;183:2145–7. doi: 10.1128/JB.183.6.2145-2147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roberts SA, Weichsel A, Grass G, Thakali K, Hazzard JT, Tollin G, Rensing C, Montfort WR. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc Natl Acad Sci USA. 2002;99:2766–71. doi: 10.1073/pnas.052710499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh SK, Roberts SA, McDevitt SF, Weichsel A, Wildner GF, Grass GB, Rensing C, Montfort WR. Crystal Structures of Multicopper Oxidase CueO Bound to Copper(I) and Silver(I) Journal of Biological Chemistry. 2011;286:37849–57. doi: 10.1074/jbc.M111.293589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munson GP, Lam DL, Outten FW, O’Halloran TV. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J Bacteriol. 2000;182:5864–71. doi: 10.1128/jb.182.20.5864-5871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Franke S, Grass G, Rensing C, Nies DH. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J Bacteriol. 2003;185:3804–12. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev. 2003;27:313–39. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 82.Long F, Su CC, Zimmermann MT, Boyken SE, Rajashankar KR, Jernigan RL, Yu EW. Crystal structures of the CusA efflux pump suggest methionine-mediated metal transport. Nature. 467:484–8. doi: 10.1038/nature09395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su CC, Long F, Yu EW. The Cus efflux system removes toxic ions via a methionine shuttle. Protein Sci. 20:6–18. doi: 10.1002/pro.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su CC, Long F, Zimmermann MT, Rajashankar KR, Jernigan RL, Yu EW. Crystal structure of the CusBA heavy-metal efflux complex of Escherichia coli. Nature. 470:558–62. doi: 10.1038/nature09743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kulathila R, Kulathila R, Indic M, van den Berg B. Crystal structure of Escherichia coli CusC, the outer membrane component of a heavy metal efflux pump. PLoS One. 6:e15610. doi: 10.1371/journal.pone.0015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loftin IR, Franke S, Roberts SA, Weichsel A, Heroux A, Montfort WR, Rensing C, McEvoy MM. A novel copper-binding fold for the periplasmic copper resistance protein CusF. Biochemistry. 2005;44:10533–40. doi: 10.1021/bi050827b. [DOI] [PubMed] [Google Scholar]
- 87.Xue Y, Davis AV, Balakrishnan G, Stasser JP, Staehlin BM, Focia P, Spiro TG, Penner-Hahn JE, O’Halloran TV. Cu(I) recognition via cation-pi and methionine interactions in CusF. Nat Chem Biol. 2008;4:107–9. doi: 10.1038/nchembio.2007.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim EH, Rensing C, McEvoy MM. Chaperone-mediated copper handling in the periplasm. Nat Prod Rep. 2010;27:711–9. doi: 10.1039/b906681k. [DOI] [PubMed] [Google Scholar]
- 89.Bagai I, Rensing C, Blackburn NJ, McEvoy MM. Direct metal transfer between periplasmic proteins identifies a bacterial copper chaperone. Biochemistry. 2008;47:11408–14. doi: 10.1021/bi801638m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mealman TD, Bagai I, Singh P, Goodlett DR, Rensing C, Zhou H, Wysocki VH, McEvoy MM. Interactions between CusF and CusB Identified by NMR Spectroscopy and Chemical Cross-Linking Coupled to Mass Spectrometry. Biochemistry. 50:2559–66. doi: 10.1021/bi102012j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]