Abstract
Amyloid β-protein (Aβ) deposits in brains of Alzheimer’s disease (AD) patients generate proinflammatory cytokines and chemokines that recruit microglial cells to phagocytose Aβ. Nucleotides released from apoptotic cells activate P2Y2 receptors (P2Y2Rs) in macrophages to promote clearance of dead cells. In this study, we investigated the role of P2Y2Rs in the phagocytosis and clearance of Aβ. Treatment of mouse primary microglial cells with fibrillar (fAβ1–42) and oligomeric (oAβ1–42)Aβ1–42 aggregation solutions caused a rapid release of ATP (maximum after 10 min). Furthermore, fAβ1–42 and oAβ1–42 treatment for 24 h caused an increase in P2Y2R gene expression. Treatment with fAβ1–42 and oAβ1–42 aggregation solutions increased the motility of neighboring microglial cells, a response inhibited by pre-treatment with apyrase, an enzyme that hydrolyzes nucleotides. The P2Y2R agonists ATP and UTP caused significant uptake of Aβ1–42 by microglial cells within 30 min, which reached a maximum within 1 h, but did not increase Aβ1–42 uptake by primary microglial cells isolated from P2Y2R−/− mice. Inhibitors of αv integrins, Src and Rac decreased UTP-induced Aβ1–42 uptake, suggesting that these previously identified components of the P2Y2R signaling pathway play a role in Aβ phagocytosis by microglial cells. Finally, we found that UTP treatment enhances Aβ1–42 degradation by microglial cells, but not in cells isolated from P2Y2R−/− mice. Taken together, our findings suggest that P2Y2Rs can activate microglial cells to enhance Aβ clearance and highlight the P2Y2R as a therapeutic target in AD.
Keywords: Alzheimer’s disease, beta-amyloid phagocytosis, microglia, migration, nucleotide, P2Y2 receptor
Alzheimer’s disease (AD) is characterized by the progressive accumulation of amyloid β-protein (Aβ)-containing plaques in the brain that may be related to alterations in the Aβ clearance pathway (Tanzi & Bertram 2005; Wang et al. 2006). Aβ peptides (39 to 42 amino acids) are produced from proteolysis of the amyloid precursor protein. Under normal conditions, Aβ peptides are produced and cleared at equivalent rates in both human and mouse brains (Bateman et al, 2006). Thus, a moderate decrease in the rate of clearance could lead to an increase in Aβ plaque deposition in the brain of AD patients.
Microglial cells are resident macrophages in the brain and the primary immune effector cells in the CNS. In AD brain, microglia play a major role in the internalization and degradation of Aβ (Frackowiak et al. 1992; Bolmont et al. 2008; Bergfeld & Forrester 1992). Microglia are closely associated with Aβ plaques and exhibit an activated proinflammatory phenotype (Perlmutter et al. 1990; Frautschy, 1998; Zheng, 2010 ). In addition, the number and size of microglia increase in proportion to the size of plaques (Wegiel et al. 2004; Wegiel et al. 2003; Wegiel et al. 2001). Recent in vivo imaging studies demonstrate that local resident microglia rapidly migrate toward new plaques within 1–2 days of their appearance (Bolmont et al. 2008; Meyer-Luehmann, 2008 ). Other studies suggest that Aβ deposits in AD brain generate proinflammatory cytokines, e.g., interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α), and chemokines, e.g., IL-8, macrophage inflammatory protein-1β (MIP-1β), and monocyte chemoattractant protein-1 (MCP-1), that recruit microglial cells to phagocytose Aβ (Walker et al. 1995; Fiala et al. 1998, Akiyama et al. 2000).
Extracellular nucleotides are released from injured or stressed cells and tissues (Bergfeld & Forrester 1992; Ciccarelli et al. 1999; Bodin & Burnstock 2001; Pedersen et al. 1999) whereupon they activate cell surface P2 receptors belonging to two structurally distinct families, the G protein-coupled P2Y receptors (P2YRs) and the ion channel P2X receptors (P2XRs). P2Y2R expression is upregulated in response to stress and injury in a variety of tissues (Koshiba et al. 1997; Seye et al. 1997; Turner et al. 1998; Seye et al. 2002) and P2Y2R activation increases migration of microglial cells, primary rat cortical astrocytes, arterial smooth muscle cells and endothelial cells (Blondel et al. 2000; Honda et al. 2001; Chaulet et al. 2001; Pillois et al. 2002; Kaczmarek et al. 2005; Wang et al. 2005; Bagchi et al. 2005). Recent studies have shown that nucleotides released from apoptotic thymocytes act as “a find-me signal” and enhance phagocytosis of dead cells by macrophages through activation of P2Y2Rs (Elliott et al. 2009). Thus, it is plausible that P2Y2R activation by nucleotides, such as ATP or UTP, can increase Aβ phagocytosis by microglial cells in AD brain. In this study, we present results indicating that fibrillar Aβ1–42 (fAβ1–42) or oligomeric Aβ1–42 (oAβ1–42) aggregates promote the release of nucleotides from primary mouse microglial cells, which enhances cell migration and promotes Aβ1–42 phagocytosis through activation of the P2Y2R.
Methods
Materials
Fetal bovine serum (FBS) was obtained from Hyclone (Logan, UT). Dulbecco’s modified Eagle’s medium (DMEM), penicillin (100 units/ml), and streptomycin (100 units/ml) were obtained from Gibco-BRL (Carlsbad, CA). Anti-integrin αvβ5 (clone P1F6) antibody was purchased from Millipore (Billerica, MA). Pyrazole pyrimidine-type 2 (PP2), NSC23766, LY294002, RO 31–8220, and AG1478 were from Calbiochem (Gibbstown, NJ). TNF-α protease inhibitor-2 (TAPI-2) was from Peptides International (Louisville, KY) and C3 (1 μg/ml) was from Cytoskeleton (Denver, CO). Aβ1–42 or scrambled Aβ1–42 lyophilized powder was from American Peptide Company (Sunnyvale, CA). Nucleotides and all other biochemical reagents, including Y27632 and anti-IgG antibody were obtained from Sigma Chemical Co. (St. Louis, MO).
Primary microglial cell preparation
Primary microglial cells were isolated from inbred neonatal C57BL/6 mice (wild type) and mice deleted of the P2Y2 receptor on a C57BL/6 background (P2Y2R−/− mice, strain B6.129P2-P2ry2tm1Bhk/J; Jackson Laboratory, Bar Harbor, Maine). The homozygous P2Y2R−/− mice are viable, fertile, normal in size and do not manifest any physical abnormalities or behavioral deficits. All animals were handled using protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Missouri (protocol # 6728). Briefly, age matched litters of both sexes (postnatal day 2–5) were killed, and brains were collected under aseptic conditions and carefully freed of blood vessels and meninges. Tissues were minced, suspended in trypsin-EDTA (Hyclone) and incubated at 37°C for 20 min. Subsequently, cells were resuspended in Complete Medium comprised of DMEM, 10% FBS, 4.5 g/L glucose, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 0.25 μg/ml fungizone, OPI supplement (i.e., oxaloacetic acid, pyruvate, insulin; Sigma), and 0.5 ng/ml recombinant mouse GM-CSF (Invitrogen, Carlsbad, CA, Cat # PMC2015). The cell suspension was filtered through a 70 μm cell strainer (Fisher Scientific), and cells were pelleted by centrifugation, resuspended in Complete Medium, and seeded into 75 cm2 flasks. After 7–10 days, the confluent microglial cell layer was separated from the underlying astrocytic monolayer by gently shaking the flasks overnight at 200 rpm at 37°C in a humidified 5% CO2 atmosphere. Microglia were collected by resuspending the pellet, plated in Complete Medium without GM-CSF, and used for experiments. Microglial cell cultures were routinely greater than 95% pure, as determined by immunohistochemical staining with anti-CD11b (Abcam, Cambridge, CA) and anti-GFAP antibodies (BD Transduction Laboratories, Franklin Lakes, NJ) to identify microglia and astrocytes, respectively.
Preparation of fibrillar Aβ1–42 (fAβ1–42) and oligomeric Aβ1–42 (oAβ1–42) aggregation solutions
Aβ1–42 lyophilized powder was dissolved to 1 mg/200 μl in 100% hexafluoroisopropanol and aliquoted into 10 μl portions, dried in a vacuum centrifuge, and stored at −20°C. The fAβ1–42 and oAβ1–42 were prepared as described (Dahlgren et al. 2002; Stine et al. 2003) with slight modification for fAβ1–42. For fAβ1–42, lyophilized peptides were resuspended in 2 μl DMSO followed by 98 μl PBS and incubated at 37°C for 24 h. A solution of monomeric Aβ1–42 was prepared by resuspending lyophilized peptide in 2 μl DMSO followed by 98 μl PBS, and the freshly reconstituted Aβ1–42 solution was used for cell treatment. Western blot analysis indicated that more than 70% of the fAβ1–42 preparation was converted into large aggregates (Fig. 1A). Dot blot analysis of fAβ1–42 and oAβ1–42 suspensions indicated that both preparations reacted with 6E10 (Covance, Princeton, NJ), a monoclonal antibody that recognizes residues 1–16 of Aβ, whereas only the oAβ1–42 preparation reacted with A11 (Millipore, Billerica, MA), a monoclonal antibody that specifically recognizes oligomeric Aβ (Fig. 1B).
Fig. 1. fAβ1–42 and oAβ1–42 aggregates induce extracellular ATP release in primary microglial cells.
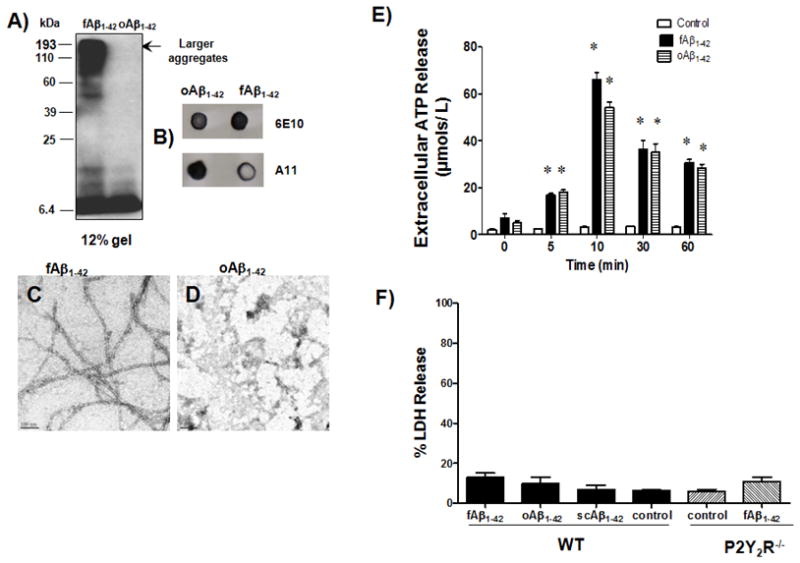
Solutions of fAβ1–42 and oAβ1–42 aggregates were prepared as described in Methods. A) Twenty μg of fAβ1–42 was subjected to 12% SDS-PAGE and Western blot analysis was performed using 6E10 antibody that recognizes amino acid residues 1–16 of the Aβ peptide. B) Five μl of fAβ1–42 or oAβ1–42 were spotted on nitrocellulose membrane as described in Methods and probed using 6E10 antibody that recognizes amino acid residues 1–16 of the Aβ peptide and A11 antibody that is specific for oligomer Aβ. C and D) Two μM fAβ1–42 aggregation solution (C) or oAβ1–42 aggregation solution (D) was applied on separate carbon-coated copper mesh grids, as described in Methods, and Aβ morphology was analyzed by transmission electron microscopy (TEM); scale bars: 100 nm for fAβ1–42 and 200 nm for oAβ1–42. E) Primary microglial cells were plated at a concentration of 5 × 105 cells/well in DMEM and incubated at 37°C in an atmosphere of 5% CO2. Cells were washed and incubated for 15 min with HEPES buffer containing AOPCP, a selective inhibitor of ecto-5′-nucleotidase, followed by treatment with or without 1μM fAβ1–42 or oAβ1–42 for 5, 10, 30, or 60 min, whereupon ATP release was measured as described in Methods. ATP release with 1 μM monomeric Aβ1–42 or scAβ1–42 was the same as control (data not shown). Data from 3 experiments represent means± SEM (n = 6), where *P < 0.05 indicates a significant difference from untreated controls. F) Primary microglial cells from wild type (WT) or P2Y2R−/− mice were plated at a concentration of 1 × 105 cells/well in DMEM and incubated at 37°C in an atmosphere of 5% CO2 for 24 h with or without 0.5 μM fAβ1–42, oAβ1–42 or scAβ1–42. Supernatants were collected and LDH release was measured, as described in Methods. Data are averages of triplicate determinations from 3 experiments and differences between each condition were not significant. Statistical analysis (Kruskal-Wallis test) indicated no significant differences between any treatment group (p = 0.4060).
Dot blot analysis
Five μl of 100 μM fAβ1–42 and oAβ1–42 were each spotted on two separate nitrocellulose membranes and allowed to stand for 20 min, followed byblocking with 10% milk in PBS-0.2% Tween 20 (PBST) for 1 h at 4°C. One membrane was incubated with 6E10 antibody (1:5000 dilution), and the other with A11 antibody(1:2000 dilution) for 2 h with gentle shaking at room temperature. Membranes were washed 3X (5 min each wash) with PBST, followed by incubation for 1 h with horseradish peroxidase-conjugated, anti-mouse IgG (Santa Cruz Biotechnology Inc; 1:1000 dilution). The membranes were washed 3X (5 min each wash) with PBST and then developed using the LumiGlo Chemiluminescence System (New England BioLabs, Ipswich, MA), according to the manufacturer’s instructions.
Transmission electron microscopy (TEM)
The fAβ1–42 and oAβ1–42 (1μM) were applied to grids containing carbon film on 200-square mesh copper grids (Electron Microscopy Sciences, Hatfield, PA). Samples were applied on the carbon side and allowed to adsorb for 10 min at room temperature, excess sample was wicked away with tissue wipe, washed 3X by placing grids sample side down on a droplet of water. Then, heavy metal staining of the samples was done by incubation on a droplet of 2% uranyl acetate for 5–10 min. The excess stain was wicked away with tissue paper and the grids were air dried and visualized with a JEOL 1400 transmission electron microscope operated at 100 kV. The width of Aβ aggregates was measured manually.
Western blot analysis
Twenty μg of fAβ1–42 or oAβ1–42 aggregates were subjected to 12% (w/v) SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes for protein immunoblotting. The membranes were blocked with 5% nonfat milk in Tris-buffered saline (TBS) containing 0.05% Tween 20 (TBS-T) for 1 h at room temperature. Then, the membranes were incubated overnight at 4°C with 6E10 antibody that recognizes amino acid residues 1–16 of the Aβ peptide, and the bound antibody was detected with horseradish peroxidase-conjugated rabbit anti-mouse IgG. The membranes were washed and then developed using the LumiGlo Chemiluminescence System (New England BioLabs), according to the manufacturer’s instructions.
Real-time and reverse transcription-PCR analysis of P2Y2R mRNA expression
Primary microglial cells were treated with or without 1 μM fAβ1–42 or oAβ1–42 aggregates for 24 h and total RNA was isolated and purified using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized from 500 ng RNA using the First Strand cDNA Synthesis Kit for RT-PCR (AMV; Roche, Indianapolis, IN). For TaqMan quantitative real-time PCR analysis, the probes (P2Y2R and GAPDH; Applied Biosystems, Carlsbad, California) were labeled at the 5′-end with 6-carboxy-fluorescein phosphoramidite (FAM; for P2Y2R) or VICR dye (for GAPDH; stable endogenous control) and at the 3′-end with minor groove binder (MGB) dye as the quencher. The samples were run in quadruplicate for the P2Y2R target and the endogenous GAPDH control. After computing the relative amounts of P2Y2R and GAPDH for each sample, the data were expressed as a ratio of the amounts of P2Y2R to GAPDH, using Applied Biosystems software. Relative mRNA levels for the control were normalized to 1.
Extracellular ATP release measurements
Primary microglial cells were incubated for 15 min at 37°C with HEPES buffer (132 mM NaCl, 4 mM KCl, 1.4 mM MgCl2, 1.2 mM H3PO4, 1 mM CaCl2, 6 mM glucose, 10 mM HEPES, pH 7.4) containing AOPCP, a selective inhibitor of ecto-5′-nucleotidase. Cells were washed 3X, treated with or without 1μM fAβ1–42 or oAβ1–42 aggregates and incubated for different time periods at 37°C. Supernatants were collected at specific time points, and released ATP was measured with the ATP Bioluminescence Assay kit HSII (Roche). ATP levels were calculated based on an ATP standard curve. The results are expressed as ATP released (μmols/L).
Transwell cell migration assay
Cell migration assays were performed with Transwell cell culture inserts comprised of two chambers separated by an 8-μm pore size membrane (Becton Dickinson, Franklin Lakes, NJ). Primary microglial cells (1 × 105) suspended in serum-free DMEM were added to the bottom chamber of the inserts placed in a 6-well plate and then treated with solutions of 0.5 μM fAβ1–42 or oAβ1–42 aggregates. After 30 min incubation at 37°C, microglial cells (1 × 105) were added to the upper chamber of the inserts. As a positive control, 100 μM ATP or UTP was added to the bottom chamber of two other wells without cells for 30 min followed by addition of cells to the upper chamber of inserts. After 6 h at 37°C, cells remaining on the upper surface of the membrane were removed by scraping with a cotton swab. Cells that migrated through the membrane were fixed with paraformaldehyde, stained with DAPI and counted under an Olympus IX70 inverted microscope at 20X magnification.
Aβ1–42 ELISA
Cells were incubated with solutions of 0.5 μM fAβ1–42 or oAβ1–42 aggregates in serum-free DMEM for the indicated time, and then washed extensively with PBS and cold acidic buffer (100 mM glycine, 20 mM magnesium acetate, 50 mM KCl, pH 2.2). Washed cells were lysed with Triple Detergent Lysis Buffer (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate) containing 5M guanidine hydrochloride. Aβ1–42 levels in lysates were quantitated using an ELISA (Invitrogen), according to the manufacturer’s instructions. All assays were performed in triplicate.
Lactate dehydrogenase (LDH) cytotoxicity assay
Primary microglial cells (1 × 105 suspended in serum-free DMEM) were plated in 12-well plates and 0.5 μM fAβ1–42 was added except for control cells. After 24 h, supernatants were collected to measure LDH release from cells and cells were lysed to measure total LDH. The supernatant and cell lysate (50 μl) were transferred to 96-well plates, followed by addition of 50 μl of CytoTox 96R Substrate Mix (Promega, Fitchburg, WI) for 30 min at room temperature in the absence of light followed by addition of Stop Solution (50 μl), and sample absorbance was measured at 490 nm. The data are expressed as the amount of LDH released by cells divided by total LDH in cell lysates X 100.
Statistical analysis
Results are expressed as means ± SEM of data obtained from at least 3 experiments. Treatment groups were compared using the t-test. *P < 0.05 or **p < 0.01 was accepted as significant.
Results
Fibrillar (fAβ1–42) and oligomeric (oAβ1–42) Aβ aggregates induceATP release in primary microglial cells
Transmission electron microscopy images showed that the fAβ1–42 preparations were comprised of fibrils with an average width of 95 +/− 18 nm (Fig. 1C). Images for oAβ1–42 (Fig. 1D) showed very few globular structures. It is possible that small Aβ aggregates in the solution failed to be trapped by the 200-square mesh grids used for TEM imaging. Incubation of primary microglial cells with solutions of fAβ1–42 or oAβ1–42 aggregates (1μM) increased the release of ATP into the extracellular medium in a time-dependent manner, as compared to the untreated control (Fig. 1E). A peak response was obtained after a 10 min incubation with fAβ1–42 (66 μMATP) or oAβ1–42 (54 μM ATP), aggregation solutions whereupon the levels of extracellular ATP decreased by 1 h. However, ATP levels were detectable up to 2.5 h (data not shown). Solutions of scrambled Aβ1–42 (scAβ1–42; 1 μM) and monomeric Aβ1–42 (1 μM) had no effect on ATP release relative to the untreated control (data not shown). To rule out the possibility that Aβ1–42 treatment causes extracellular ATP release due to microglial cell lysis, cell supernatants were analyzed for LDH release after fAβ1–42 or oAβ1–42 treatment, as compared to untreated control. The results showed that a 24 h treatment of wild type and P2Y2R−/− microglial cells with fAβ1–42 or oAβ1–42 did not significantly increase the level of LDH release, as compared to cells incubated without Aβ. (Fig. 1F).
Incubation with fAβ1–42 or oAβ1–42 aggregates increases P2Y2R mRNA expression
Treatment of primary microglial cells with fAβ1–42 or oAβ1–42 aggregates for 24 h caused a 6.5-fold or a 5.2-fold increase in P2Y2R mRNA expression, respectively, as compared to untreated control (Fig. 2). Treatment with scAβ1–42 under the same conditions had no effect on P2Y2R expression.
Fig. 2. fAβ1–42 and oAβ1–42 aggregates increase P2Y2R mRNA expression.
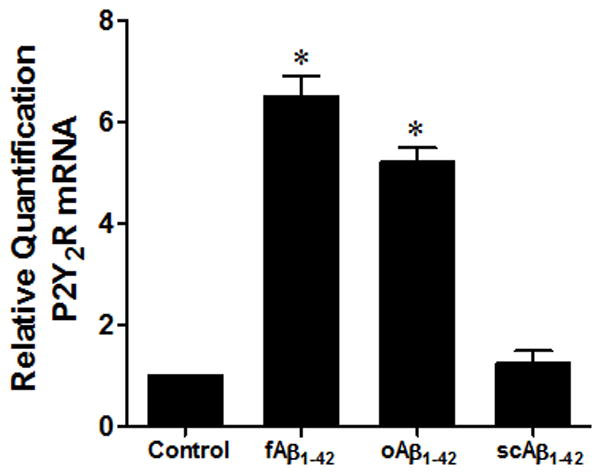
Primary mouse microglial cells (5 × 105 cells/well) were incubated with or without 1 μM fAβ1– 42, oAβ1–42 or scAβ1–42 aggregation solutions for 24 h at 37°C in a humidified atmosphere of 5% CO2. Total RNA was isolated from cells using the RNeasy Plus Mini Kit and RT-PCR was performed, as described in Methods. Data from 4 experiments represent means± SEM (n = 4), where *P < 0.05 indicates a significant difference from untreated controls.
fAβ1–42 or oAβ1–42 aggregates causeATPrelease, which increases cell migration in primary microglial cells
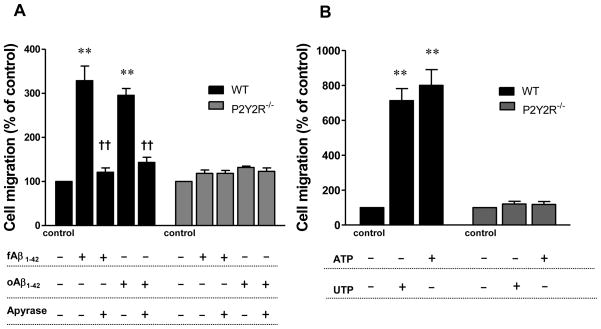
Previous studies have shown that ATP and UTP can induce cell migration by activating the P2Y2R (Seye et al., 2002, Bagchi et al., 2005). Since fAβ1–42 or oAβ1–42 aggregate treatment induces the release of ATP from primary microglial cells, we tested whether nucleotide release in fAβ1–42 or oAβ1–42-treated microglia can initiate the migration of neighboring microglial cells. A confluent layer of primary microglial cells were seeded on the bottom of a 6-well plate and treated with fAβ1–42 or oAβ1–42 aggregates alone or with apyrase, an enzyme that rapidly hydrolyzes extracellular nucleoside 5′-diphosphates and triphosphates. Additional cells were added to the upper chamber of the Transwell insert and cell migration across the microporous Transwell membrane was measured as described in Methods. We observed that fAβ1–42 or oAβ1–42 aggregates (0.5μM) cause about a 3-fold increase in cell migration across the Transwell membrane, as compared to untreated control (Fig. 3A). Addition of apyrase significantly inhibited the increase in cell migration caused by fAβ1–42 or oAβ1–42 aggregates (Fig. 3A). To verify that nucleotides were capable of inducing migration of microglial cells, ATP or UTP (100 μM) was added to the bottom chamber and migration of cells added to the upper chamber was measured. ATP or UTP increased cell migration by ~ 8- or 7-fold, respectively (Fig. 3B). Primary microglial cells isolated from P2Y2R−/− mice did not migrate in response to fAβ1–42, oAβ1–42 or nucleotide treatment (Figs. 3A and 3B), indicating a critical role for the P2Y2R and nucleotide release in fAβ1–42-induced cell migration.
Fig. 3. ATPreleased from Aβ1–42-treated primary microglial cells induces cell migration.
A) Cells (1 × 105 cells/well) from wild type (WT) or P2Y2R−/− mice on 6-well plates were pretreated with or without apyrase (5 U/ml) for 30 min and then fAβ1–42 or oAβ1–42 (0.5 μM) was added for 30 min. Transwell inserts were placed into the wells and an additional 1 × 105 cells/1 ml/well were seeded in the upper chamber of the Transwell insert. B) ATP or UTP (100 μM) was added to the bottom chamber of the Transwell insert in the absence of cells and fAβ1–42 or oAβ1–42, and cells were seeded in the upper chamber. For A) and B) after 6 h, cells on the upper surface of the Transwell membrane were removed and cells that migrated through the membrane were fixed with 5.5% paraformaldehyde and stained with DAPI. The number of cells migrating across the membrane was counted under an Olympus XI70 widefield microscope at 20X magnification (micrographs not shown). Control wells were treated with fAβ1–42 or oAβ1–42 and plot represents average of two controls. Data from 3 experiments performed in triplicate represent means± SEM (n = 6), where **P < 0.01 indicates a significant difference from untreated controls and ††P < 0.01 indicates a significant difference from fAβ1–42-treated cells.
P2Y2R activation by UTPor ATP increases fAβ1–42 or oAβ1–42 uptake by microglial cells
UTP induces Aβ1–42 uptake with a maximal response obtained at 100 μM UTP with an EC50 of 2.95 μM (Fig. 4A). P2Y2R activation by UTP or ATP (100 μM) increased fAβ1–42 uptake in primary microglial cells isolated from wild type mice within 30 min with a peak response occurring within 1 h, as compared to the control (Fig. 4B). UTP or ATP treatment causes significant fAβ1–42 or oAβ1–42 uptake after 1 h in primary microglial cells, but not in cells isolated from P2Y2R−/− mice (Fig. 4C), highlighting a role for the P2Y2R in Aβ1–42 uptake by microglial cells.
Fig. 4. UTP or ATP increases Aβ1–42 uptake by microglial cells from wild type but not P2Y2R−/− mice.
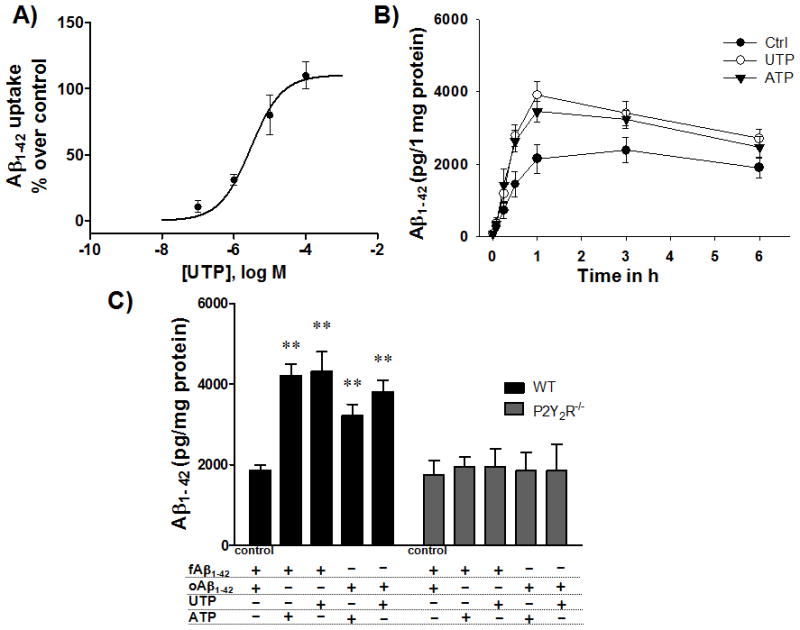
A) Primary microglial cells (5 × 105 cells/well in DMEM) from wild type (WT) mice were incubated in an atmosphere of 5% CO2 at 37°C and treated with 0.1, 1, 10, or 100 μM UTP and fAβ1–42 aggregation solution (0.5 μM) for 1 h. Cells were collected and intracellular Aβ1–42 levels were determined by ELISA. Data represent means ± SEM (n = 3). B) Primary microglial cells (5 × 105 cells/well in DMEM) from WT mice were incubated at 37°C in an atmosphere of 5% CO2 and treated with or without 100 μM UTP or ATP and fAβ1–42 aggregation solution (0.5 μM) for up to 6 h. Cells were collected 15 min, 1 h, 3 h and 6 h after addition of fAβ1–42 aggregation solution and intracellular Aβ1–42 levels were determined by ELISA. C) Primary microglial cells (5 × 105 cells/well in DMEM) from WT or P2Y2R−/− mice were incubated at 37°C in an atmosphere of 5% CO2 and treated with UTP or ATP and fAβ1–42 or oAβ1–42 for 1 h. Control cells were treated with fAβ1–42 or oAβ1–42 and the graph represents the average value. Data from 3 experiments represent means ± SEM (n = 3), where **P < 0.01 indicates a significant difference from control.
The αv integrin, Rac and Src pathways mediate UTP-induced fAβ1–42 uptake
The P2Y2R contains an integrin-binding Arg-Gly-Asp (RGD) motif in its first extracellular loop that enables nucleotides to activate integrin signaling pathways (Erb et al. 2001). The RGD sequence in the P2Y2R promotes its direct interaction with αvβ3/5 integrins (Erb et al. 2001) that, following P2Y2R activation by UTP, mediates an increase in the activation of monomeric Go and G12 proteins and the subsequent stimulation of the small GTPases Rho and Rac (Schenk et al. 1999; Bagchi et al. 2005; Liao et al. 2007). To determine the P2Y2R signaling pathways that mediate Aβ1–42 uptake, we used UTP, a relatively selective ligand for the P2Y2R. When primary microglial cells were treated with αv integrin neutralizing antibody, UTP-induced Aβ1–42 uptake was abrogated (Fig. 5A). The selective Src inhibitor, PP2 (1 μM), and the selective Rac 1 inhibitor, NSC23766 (50 μM) significantly inhibited UTP-induced Aβ1–42 uptake (Fig. 5B), whereas C3, an inhibitor of RhoA, Y27632, an inhibitor of ROCK, and specific inhibitors of PI3-kinase, PKC, EGFR tyrosine kinase and matrix metalloproteases (MMPs) failed to inhibit UTP-induced fAβ1–42 uptake (Figs. 5B and 5C). These results suggest that UTP-induced P2Y2R activation requires αv integrins, Src, and Rac to promote Aβ1–42 uptake.
Fig. 5. αv Integrin, Src and Rac mediate UTP-induced fAβ1–42 uptake.
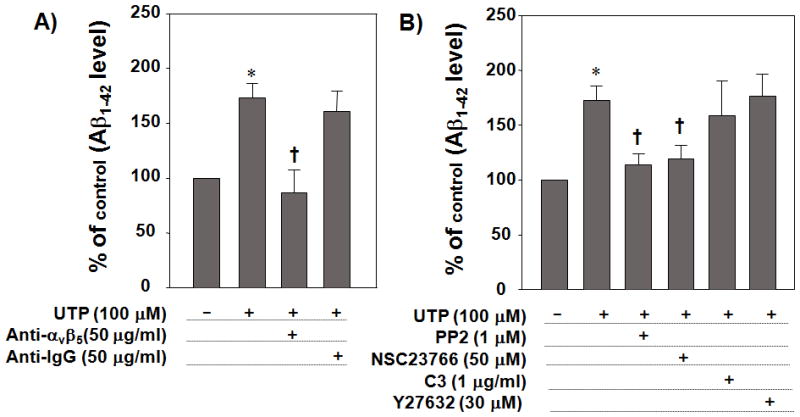
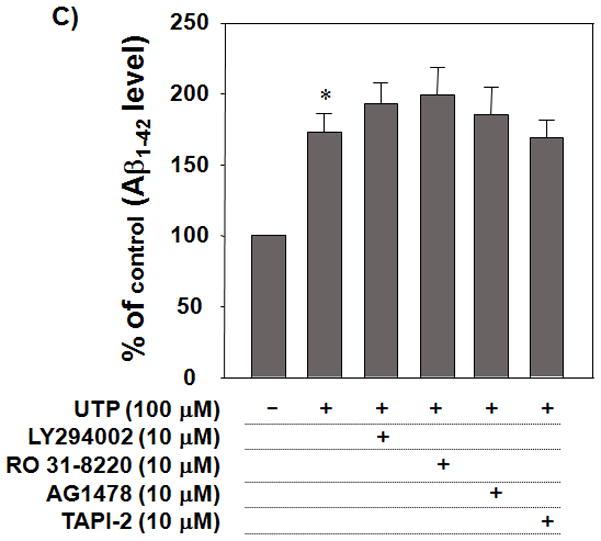
Primary microglial cells (5 × 105 cells/well in DMEM) were incubated at 37°C in an atmosphere of 5% CO2 and pretreated with (A) anti-αv neutralizing antibody (50 μg/ml) or anti-IgG1 antibody (50 μg/ml), as a negative control, (B) PP2 (a Src inhibitor; 10μM), NSC23766 (a Rac1 inhibitor; 50 μM), C3 (a cell permeable Rho inhibitor; 1μg/ml), Y27632 (a ROCK inhibitor; 30 μM), or (C) LY294002 (a PI-3 kinase inhibitor; 10 μM), RO 31–8220 (a PKC inhibitor; 10 μM), AG1478 (an EGFR tyrosine kinase inhibitor; 10 μM) or TAPI-2 (a MMP inhibitor; 10 μM) for 30 min and then stimulated with 100 μM UTP in the presence of fAβ1–42 (0.5μM). After 1 h, cells were lysed and intracellular Aβ1–42 levels were determined by ELISA. Data from 3 experiments performed in triplicate represent means± SEM (n = 6), where *P < 0.05 indicates a significant difference from untreated control and †P < 0.05 indicates a significant difference from UTP treatment alone.
P2Y2R activation by UTP enhances Aβ1–42 degradation in microglial cells
To examine the effect of UTP on Aβ1–42 degradation by microglial cells, we exposed primary microglial cells to fAβ1–42 or oAβ1–42 aggregates (0.5 μM) for 1 h to induce uptake and then cells were washed and incubated with or without UTP (100 μM) for 24 h. UTP-treated wild type cells showed a significant decrease in the amount of intracellular Aβ1–42 remaining after 24 h, as compared to cells treated without UTP, in contrast to primary microglial cells from P2Y2R−/− mice that showed no difference in Aβ1–42 degradation with or without UTP (Fig. 6). There was virtually no Aβ1–42 detectable in the cell supernatant over 24 h, indicating that the decrease in intracellular Aβ1–42 levels was not due to release from cells (data not shown).
Fig. 6. UTP enhances Aβ1–42 degradation by microglial cells from wild type but not P2Y2R−/− mice.
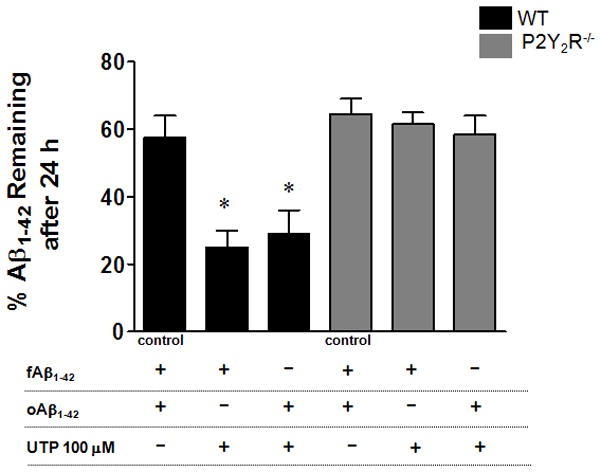
Primary microglial cells (5 × 105 cells/well in DMEM) from wild type (WT) or P2Y2R−/− mice were treated with fAβ1–42 or oAβ1–42 (0.5 μM) for 1 h, and then the medium was removed and cells were washed with PBS and cold acidic buffer, as described in the Methods. Then, fresh medium was added and cells were treated with or without UTP (100 μM). After 24 h, levels of Aβ1–42 in cell lysates and secreted Aβ1–42 in the media were analyzed by ELISA. Control cells were treated with fAβ1–42 or oAβ1–42 and data represent the average value. Data represent means +/− SEM (n = 4), where *p < 0.05 indicates a significant difference from UTP-untreated control.
Discussion
Nucleotides, such as ATP and UTP, act as extracellular signaling molecules by activating plasma membrane P2 receptors, including the P2Y2R subtype. In this study, we demonstrate the role of the P2Y2R in the migration of mouse primary microglial cells and their uptake of fAβ1–42 and oAβ1–42. Previous studies (Kim et al. 2007; Sanz et al. 2009) and data presented here (Fig. 1E) indicate that Aβ treatment stimulates ATP release in microglial cells. Aβ-induced ATP release has been shown to occur via activation of an ATP-gated ion channel, the P2X7 receptor, through interactions with a pannexin hemi-channel (Kim et al. 2007; Sanz et al. 2009). In this study, we show that fAβ1–42 and oAβ1–42 aggregates induce significant release of extracellular ATP (Fig. 1E) and an upregulation of P2Y2R mRNA (Fig. 2) in mouse primary microglial cells.
The P2Y2R has been shown to be upregulated in response to stress and injury in a variety of tissues (Koshiba et al. 1997; Seye et al. 1997; Turner et al. 1998). The P2Y2R can be activated equipotently by ATP and UTP and mediates proinflammatory responses, such as the increased cell migration (Chaulet et al. 2001; Pillois et al. 2002; Bagchi et al. 2005), including in microglial cells (Blondel et al., 2000, Honda et al., 2001), and enhanced phagocytic clearance (Elliott et al. 2009). In this study, we found that release of > 50 μM ATP is induced by fAβ1–42 or oAβ1–42 in primarymicroglial cells (Fig. 1E) and, therefore, we routinely used 100 μM ATP or UTP for our experiments, since this concentration maximally activates the P2Y2R (Bagchi et al. 2005; Camden et al. 2005; Kong et al. 2009). In particular, the use of UTP avoids complications involved with the co-activation of P2X and other P2Y receptors for which ATP, but not UTP, are agonists. Moreover, ATP degradation by cells can generate adenosine and, therefore, the use of UTP also can prevent potential contributions from P1 adenosine receptors.
Chronic inflammation contributes to neurodegeneration in AD (Akiyama et al. 2000; Ho et al. 2005; Griffin 2006), however, acute inflammation is important for tissue repair and can limit brain damage (Monsonego & Weiner 2003), in part by promoting the clearance of Aβ. In the AD brain, microglial cells are involved in the inflammatory process through their role in internalization and degradation of Aβ (Frackowiak et al. 1992; Bolmont et al. 2008). In addition, microglial cells present at Aβ lesions produce several chemotactic agents, including MCP-1 and IL-1β (Walker et al. 1995; Fiala et al. 1998; Akiyama et al. 2000), which promote further microglial cell recruitment. We have shown that microglial cells treated with fAβ1–42 or oAβ1–42 aggregates exhibited increased P2Y2R gene expression within 24 h (Fig. 2). In addition, fAβ1–42 or oAβ1–42 treatment of wild type mouse primary microglial cells, but not primary microglial cells from P2Y2R−/− mice, induced an increase in cell migration, which was inhibited by apyrase treatment (Fig. 3A), indicating that nucleotide release activates P2Y2Rs in microglial cells to facilitate their accumulation around Aβ aggregates. The effect on cell migration due to nucleotides released from microglial cells treated with solutions of fAβ1–42 or oAβ1–42 preparations (Fig. 3A) was somewhat weaker than the effect of direct addition of ATP or UTP (Fig. 3B), a difference that was likely due to increased cell-associated nucleotide hydrolysis when ATP was released from cells, although antagonistic contributions of P1 or other P2 receptors cannot be excluded. In this study, ATP release from Aβ1–42-treated cells was detectable after 2.5 h (data not shown). In vivo, ATP released from microglia likely causes further release of ATP and other factors from surrounding astrocytes that increase migration of microglia (Davalos et al. 2005). Although a variety of factors affect the migration of microglial cells (Zola et al. 1990; Samuels et al. 2010; Fleisher-Berkovich et al. 2010; Damani et al. 2011), our data indicate that nucleotides increase microglial cell migration through P2Y2R activation (Fig. 3). Furthermore, treatment with nucleotides (ATP or UTP) enhanced fAβ1–42 or oAβ1–42 uptake by primary microglial cells from wild type, but not P2Y2R−/−, mice (Fig 4C). These results support our hypothesis that P2Y2R activation is a probable mechanism whereby brain microglial cells are recruited to phagocytose Aβ deposits.
Our findings indicate that fAβ1–42 or oAβ1–42 induces P2Y2R upregulation (Fig. 2), that likely occurs through P2X7R-mediated IL-1β release (Takenouchi et al. 2009; Choi et al. 2007; Chakfe et al. 2002; Suzuki et al. 2004). It also has been reported that P2X7R activation increases P2Y2R expression in rat astrocytes (D’Alimonte et al. 2007). Our previous studies have shown that IL-1β upregulates P2Y2R expression in neurons, whereupon P2Y2R activation promotes non-amyloidogenic APP processing (Kong et al. 2009). Therefore, it is plausible that activation of the P2Y2R counteracts neurodegenerative aspects of IL-1β by limiting Aβ generation by neurons and by increasing Aβ uptake and degradation by microglia. We postulate that a major effect of P2Y2R upregulation in the CNS is to delay the progression of neurodegeneration that occurs with chronic inflammation. A better understanding of the mechanisms responsible for microglial cell recruitment may provide insight into the regulation of the transition from acute to chronic inflammation and neurodegeneration. Our recent data show a 3–4 fold increase in levels of TNF-α and IL-1β release when primary microglial cells were exposed to oAβ1–42 for 24 h as compared to control cells without Aβ1–42 treatment (data not shown). In addition, there was a significant reduction in cytokine release in response to Aβ1–42 in microglial cells from P2Y2R−/− mice (data not shown). Further investigations are needed to evaluate the coordinated regulation of nucleotide and cytokine release by microglia in response to Aβ1–42.
We reported previously that the Gq-coupled P2Y2R can activate several intracellular signal transduction pathways independent of Gqα-dependent stimulation of PLC that results in intracellular calcium mobilization and PKC activation (Lustig et al. 1992; Weisman et al. 1999). We also found that an Arg-Gly-Asp (RGD) motif in the first extracellular loop of the P2Y2R interacts with αvβ3/5 integrins and the integrin-associated protein CD47 to mediate nucleotide-induced cytoskeletal rearrangements and cell migration by enabling the P2Y2R to access pools of Go and G12 proteins associated with these integrins, thereby activating Rac1 and RhoA GTPases that regulate actin polymerization (Erb et al. 2001; Bagchi et al. 2005; Liao et al. 2007). Our results indicate that P2Y2R-mediated uptake of fAβ1–42 requires αv integrins, Src and Rac (Fig. 5), suggesting a direct role for P2Y2R/αvβ3/5 integrin interactions in Aβ uptake. Aβ interacts with a multi-component cell surface receptor complex, including the B-class scavenger receptor CD36, the α6β1 integrin and the integrin-associated protein CD47, to stimulate the phagocytic activity of microglial cells (Knauer et al. 1992; Bamberger et al. 2003; Koenigsknecht & Landreth 2004). Since the P2Y2R and CD36 both interact with CD47 and its associated integrins, it is possible that all of these proteins form a complex that together regulates phagocytosis of Aβ by microglia. Similar to the signaling cascade activated by the P2Y2R (Bagchi et al. 2005), the interaction of Aβ with CD36 has been shown to initiate a tyrosine kinase-Vav-Rac1-based signaling cascade, resulting in phagosome formation through Rac-dependent actin cytoskeleton reorganization (Wilkinson et al. 2006). Taken together, our results suggest that P2Y2R activation plays a role in Aβ1–42 uptake by microglial cells through P2Y2R interaction with αv integrins that results in activation of Rac1, a signaling protein known to regulate phagocytosis (Majeed et al. 2001; Cougoule et al. 2006; Wilkinson et al. 2006). Finally, we have shown that P2Y2R may play a role in degradation of fAβ1–42 or oAβ1–42 aggregates (Fig. 6) although further experiments are needed to determine the mechanism involved in this process.
An interesting finding is that P2Y2R activation promotes the uptake of both fAβ1–42 and oAβ1–42 aggregates. Previous studies have demonstrated that Aβ exists in several conformations, including monomeric, oligomeric, protofibrillar, fibrillar and soluble fibrillar forms that possess distinct toxic and biological activities (Dahlgren et al. 2002; Walsh et al. 2002; Deshpande et al. 2006; Ajit et al. 2009). Our results show that fAβ1–42 of predominantly fibrillar form (Fig. 1C) and oAβ1–42 aggregates containing low levels of dimers and trimers upregulate P2Y2R mRNA expression (Fig. 2) and enhance Aβ1–42 uptake (Fig. 4) in primary microglial cells. Further studies are needed to isolate and identify the specific Aβ conformations that activate the P2Y2R to promote Aβ uptake and degradation.
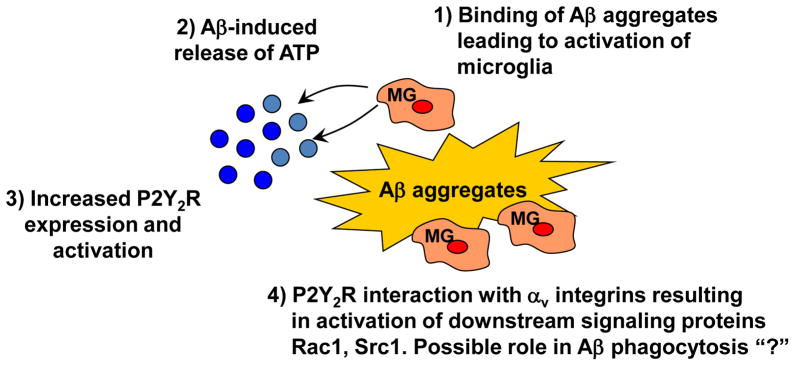
In conclusion, our results suggest that microglial cells exposed to fAβ1–42 or oAβ1–42 aggregates release ATP which stimulates their migration and uptake of Aβ1–42 through a pathway involving P2Y2R-mediated activation of αv integrin, enabling the downstream stimulation of Src and Rac (see Fig. 7). It has been shown that microglia from the APPsw transgenic mouse model of AD are unable to eliminate β-amyloid deposits (Wegiel et al. 2001; Wegiel et al. 2003; Wegiel et al. 2004), suggesting that failure to clear Aβ from the AD brain contributes to neurodegeneration. Thus, the P2Y2R may represent a promising target for preventing Aβ plaque accumulation in the AD brain that leads to neurodegeneration.
Fig. 7. Schematic representation of the proposed pathway for P2Y2R-mediated Aβ1–42 uptake.
Binding of Aβ1–42 to microglial cells (1) causes ATP release (2) and Aβ-induced upregulation of P2Y2R expression and ATP-induced P2Y2R activation (3). P2Y2R activation regulatesAβ1–42 uptake by microglial cells through P2Y2R interaction with αv integrins and perhaps other proteins that results in activation of the downstream signaling molecules Rac1 and Src1 (4).
Acknowledgments
This work was supported by NIH grant AG018357 and a National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010-013-E00001).
Abbreviations used
- Aβ
β-amyloid protein
- AD
Alzheimer’s disease
- AOPCP
α,βmethylene-adenosine diphosphate
- CNS
central nervous system
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- EGFR
epidermal growth factor receptor
- fAβ
fibrillar Aβ
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- GM-CSF
granulocyte macrophage colony stimulating factor
- IL-1β
interleukin-1β
- LDH
lactate dehydrogenase
- MCP-1
monocyte chemoattractant protein-1
- MIP-1β
macrophage inflammatory protein-1β
- MMP
matrix metalloprotease
- PBS
phosphate buffered saline
- PI-3 kinase
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- PKC
protein kinase C
- PP2
pyrazole pyrimidine-type 2
- P2Y2R
P2Y2 receptor
- RGD
Arg-Gly-Asp
- ROCK
Rho-associated protein kinase
- scAβ
scrambled Aβ
- SH3
Src-homology-3
- TAPI-2
TNF-α protease inhibitor-2
- TNF-α
tumor necrosis factor-α
Footnotes
The authors declare no conflict of interest.
References
- Ajit D, Udan ML, Paranjape G, Nichols MR. Amyloid-beta(1–42) fibrillar precursors are optimal for inducing tumor necrosis factor-alpha production in the THP-1 human monocytic cell line. Biochemistry. 2009;48:9011–9021. doi: 10.1021/bi9003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with αv integrins to activate Go and induce cell migration. J Biol Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Blondel O, Collin C, McCarran WJ, Zhu S, Zamostiano R, Gozes I, Brenneman DE, McKay RD. A glia-derived signal regulating neuronal differentiation. J Neurosci. 2000;20:8012–8020. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22:3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau AP. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Giuliani P, D’Alimonte I, Ballerini P, Caciagli F, Rathbone MP. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25:93–98. [PubMed] [Google Scholar]
- Cougoule C, Hoshino S, Dart A, Lim J, Caron E. Dissociation of recruitment and activation of the small G-protein Rac during Fcgamma receptor-mediated phagocytosis. J Biol Chem. 2006;281:8756–8764. doi: 10.1074/jbc.M513731200. [DOI] [PubMed] [Google Scholar]
- D’Alimonte I, Ciccarelli R, Di Iorio P, et al. Activation of P2X(7) receptors stimulates the expression of P2Y(2) receptor mRNA in astrocytes cultured from rat brain. Int J Immunopathol Pharmacol. 2007;20:301–316. doi: 10.1177/039463200702000210. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L, Liu J, Ockerhausen J, et al. An RGD sequence in the P2Y2 receptor interacts with αVβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol. 2001;153:491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Zhang L, Gan X, et al. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood--brain barrier model. Mol Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- Fleisher-Berkovich S, Filipovich-Rimon T, Ben-Shmuel S, Hulsmann C, Kummer MP, Heneka MT. Distinct modulation of microglial amyloid beta phagocytosis and migration by neuropeptides. J Neuro Inflamm. 2010;7:61–73. doi: 10.1186/1742-2094-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992;84:225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83:470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr Drug Targets Inflamm Allergy. 2005;4:247–256. doi: 10.2174/1568010053586237. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek E, Erb L, Koziak K, et al. Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb Haemost. 2005;93:735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Moon JH, Lee HG, Kim SU, Lee YB. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp Mol Med. 2007;39:820–827. doi: 10.1038/emm.2007.89. [DOI] [PubMed] [Google Scholar]
- Knauer MF, Soreghan B, Burdick D, Kosmoski J, Glabe CG. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci U S A. 1992;89:7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Peterson TS, Baker O, et al. Interleukin-1β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y2 receptor. J Neurochem. 2009;109:1300–1310. doi: 10.1111/j.1471-4159.2009.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc Natl Acad Sci U S A. 1997;94:831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with αv integrins to access and activate G12. J Cell Sci. 2007;120:1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig KD, Erb L, Landis DM, Hicks-Taylor CS, Zhang X, Sportiello MG, Weisman GA. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta. 1992;1134:61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- Majeed M, Caveggion E, Lowell CA, Berton G. Role of Src kinases and Syk in Fcgamma receptor-mediated phagocytosis and phagosome-lysosome fusion. J Leukoc Biol. 2001;70:801–811. [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer’s disease. Science. 2003;302:834–838. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- Pedersen S, Pedersen SF, Nilius B, Lambert IH, Hoffmann EK. Mechanical stress induces release of ATP from Ehrlich ascites tumor cells. Biochim Biophys Acta. 1999;1416:271–284. doi: 10.1016/s0005-2736(98)00228-4. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett. 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- Pillois X, Chaulet H, Belloc I, Dupuch F, Desgranges C, Gadeau AP. Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res. 2002;90:678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- Samuels SE, Lipitz JB, Dahl G, Muller KJ. Neuroglial ATP release through innexin channels controls microglial cell movement to a nerve injury. J Gen Physiol. 2010;136:425–442. doi: 10.1085/jgp.201010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Di Virgilio F. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J Immunol. 2009;182:4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- Seye CI, Kong Q, Erb L, Garrad RC, Krugh BW, Wang M, Turner JT, Sturek M, González FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells. Crit Rev Immunol. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Turner JT, Park M, Camden JM, Weisman GA. Salivary gland nucleotide receptors. Changes in expression and activity related to development and tissue damage. Ann N Y Acad Sci. 1998;842:70–75. doi: 10.1111/j.1749-6632.1998.tb09633.x. [DOI] [PubMed] [Google Scholar]
- Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglial derived from postmortem human brains. J Neurosci Res. 1995;40:478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y nucleotide receptor interaction with alpha integrin mediates astrocyte migration. J Neurochem. 2005;95:630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Zhou HD, Zhou XF. Clearance of amyloid-beta in Alzheimer’s disease: progress, problems and perspectives. Drug Discov Today. 2006;11:931–938. doi: 10.1016/j.drudis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Imaki H, Wang KC, Rubenstein R. Cells of monocyte/microglial lineage are involved in both microvessel amyloidosis and fibrillar plaque formation in APPsw tg mice. Brain Res. 2004;1022:19–29. doi: 10.1016/j.brainres.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Imaki H, Wang KC, Wronska A, Osuchowski M, Rubenstein R. Origin and turnover of microglial cells in fibrillar plaques of APPsw transgenic mice. Acta Neuropathol. 2003;105:393–402. doi: 10.1007/s00401-002-0660-3. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Wang KC, Imaki H, Rubenstein R, Wronska A, Osuchowski M, Lipinski WJ, Walker LC, LeVine H. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol Aging. 2001;22:49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Garrad RC, Erb LJ, Santos-Berrios C, Gonzalez FA. P2Y receptors in the nervous system: molecular studies of a P2Y2 receptor subtype from NG108-15 neuroblastoma x glioma hybrid cells. Prog Brain Res. 1999;120:33–43. doi: 10.1016/s0079-6123(08)63544-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- Zheng Z, White C, Lee J, Peterson TS, Bush AI, Sun GY, Weisman GA, Petris MJ. Altered microglial copper homeostasis in a mouse model of Alzheimer’s disease. J Neurochem. 2010;114:1630–1638. doi: 10.1111/j.1471-4159.2010.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola H, Melo JV, Zowtyj HN, Nikoloutsopoulos A, Skinner J. The leukocyte-common antigen (CD45) complex and B-lymphocyte activation. Hum Immunol. 1990;27:368–377. doi: 10.1016/0198-8859(90)90087-6. [DOI] [PubMed] [Google Scholar]