Abstract
Background
Induction with lymphocyte-depleting antibodies is routinely employed to prevent rejection but often skews T cells towards memory. It is not fully understood which memory and regulatory T cell subsets are most affected and how they relate to clinical outcomes.
Methods
We analyzed T cells from 57 living-donor renal transplant recipients (12 reactive and 45 quiescent) 2.8±1.4 years after Alemtuzumab induction. 34 healthy subjects and 9 patients with acute cellular rejection (ACR) were also studied.
Results
We found that Alemtuzumab caused protracted CD4>CD8 T lymphocyte deficiency, increased proportion of CD4+ memory T cells (TM), and decreased proportion of CD4+ regulatory T cells (TREG). Reactive patients exhibited higher proportions of CD4+ effector memory (TEM) and CD8+ terminally differentiated effector memory (TEMRA), with greater CD4+ TEM and CD8+ TEMRA to TREG ratios, than quiescent patients or healthy controls. Patients with ongoing ACR had profound reduction in circulating CD8+ TEMRA. Mixed lymphocyte assays showed significantly lower T cell proliferation to donor than third party antigens in the quiescent group, while reactive and ACR patients exhibited increased effector molecules in CD8+ T cells.
Conclusions
Our findings provide evidence that T cell skewing towards effector memory may be associated with anti-graft reactivity long after lymphodepletion. Further testing of TEM and TEMRA subsets as rejection predictors is warranted.
Keywords: kidney transplantation, memory T cells, regulatory T cells, alemtuzumab
Introduction
Approximately 80% of kidney transplant recipients currently receive antibody induction therapy at the time of transplantation to prevent acute rejection and decrease the burden of chronic immunosuppression (1–3). Although several clinical studies have shown that transient T cell depletion could afford patients steroid-free maintenance immunosuppression without jeopardizing graft survival in the short-term (4–7), some patients still develop acute or chronic rejection despite aggressive T cell depletion at the time of transplantation, and immunosuppression withdrawal is seldom achieved without significant risk of graft rejection (8, 9).
In an effort to better understand the effects of lymphodepleting induction therapy, several groups have performed immunological measurements on transplant recipients after Thymoglobulin or Alemtuzumab administration (10–18). The consensus from these studies is that transient T cell depletion leads to skewing of T cells that repopulate the host towards memory, but that donor-specific hyporesponsiveness could occur in some recipients (12, 18). Most memory T cells (TM) arise from either undepleted naïve T cells (TN) or TM undergoing homeostatic expansion, a phenomenon known as lymphopenia-induced proliferation (LIP) that is driven by IL-7 and IL-15 present in excess amounts in lymphopenic hosts (19, 20). Evidence in mice and humans indicates that TM, including those generated by LIP, carry alloreactive specificities, cause rejection, and prevent tolerance (21–24). In addition to alterations in TM and TN proportions, lymphodepletion influences regulatory T cell (TREG) populations. Some studies reported an increase in percentage of TREG, defined as CD4+CD25highFoxp3+ cells, after either induction therapy (11, 25, 26). These findings, however, are inconclusive, as Foxp3 expression in humans is not restricted to TREG but is also present in recently activated T cells (27–29).
Despite these important insights, several questions remain: does skewing of the T cell pool towards memory after transient lymphocyte depletion persist long-term; which TM subsets are most affected; what changes are observed in the TREG population (identified by stringent phenotypic criteria in addition to Foxp3); and are there immunological markers that could identify patients at risk of rejection or, conversely, those who are quiescent? In a prior study, we found that alloreactive TM cells in healthy human subjects consist of heterogeneous subpopulations characterized by differential proliferative capacity and variable expression of effector molecules (23). CD4+ and CD8+ central memory (TCM) cells proliferated robustly in response to alloantigen, while effector memory (TEM) and terminally differentiated effector memory (TEMRA) cells proliferated less but expressed higher levels of perforin and granzyme B. This led us to hypothesize that TEM and TEMRA present a threat to transplanted organs. To test this hypothesis and to further define the long-term immunological effects of transient lymphocyte-depletion, we performed a cross-sectional phenotypic and functional analysis of peripheral blood T cells from renal transplant recipients up to six years after receiving Alemtuzumab induction.
Results
Long-term effects of Alemtuzumab induction on CD4+ and CD8+ memory T cell subsets and their relationship to clinical status
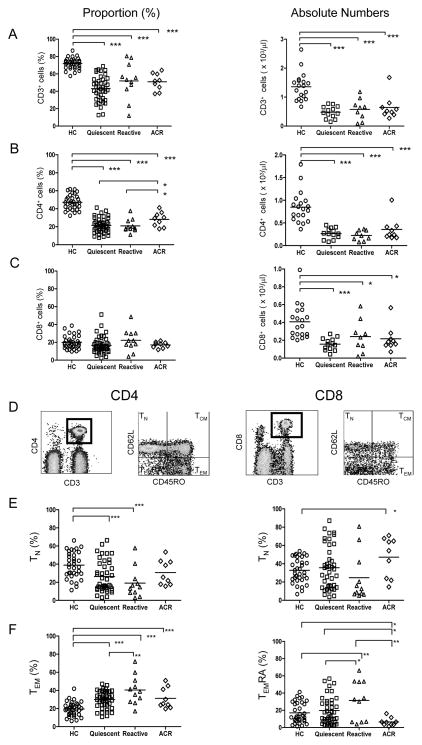
The number and proportion of lymphocyte subpopulations were measured in the peripheral blood of the patient groups outlined in Table 1 and compared to those of healthy controls (HC). As shown in Fig. 1A–C, a significant reduction in proportion and absolute number of total CD3+ and CD3+CD4+ T cells, and absolute number but not proportion (%) of CD3+CD8+ T cells was observed in transplant recipients compared to HC. The CD4+ T cell population contracted more than the CD8+ T cell population in all patient groups, leading to reduced CD4/CD8 ratio (data not shown). Neither B nor NK cells were diminished in absolute number (data not shown). These data indicate that Alemtuzumab induction is associated with protracted deficiency of CD4>CD8 T cells but not B or NK cells.
TABLE 1.
Demographics of patients and healthy controls
| Quiescent n=45 |
Reactive n=12 |
ACR n=9 |
HC n=34 |
ANOVA p-value |
|
|---|---|---|---|---|---|
| Time (yrs) from transplant to blood draw: mean ± SD (range) | 2.8 ± 1.2 (1.0–5.7) | 2.9 ± 1.6 (1.0–5.9) | 2.8 ± 0.6 (0.9–3.8) | N/A | NS |
| Age: mean ± SD (range) | 53.3 ± 15.4 (21.7–86.5) | 58.6 ± 18.1 (20.6–76.6) | 38.8 ± 16.7 (20.7–60.6) | 49.4 ± 11.3 (29.1–70.9) | 0.0091 |
| Female/Male gender* | 17/28 | 4/8 | 6/3 | 16/18 | N/A |
| African American race* | 4/45 | 1/12 | 1/9 | 1/34 | N/A |
| De novo DSA* | 8 (17%) | 4 (33%) | 3 (33%) | N/A | N/A |
| DSA during follow-up* | 2 (4%) | 2 (17%) | 2 (22%) | N/A | N/A |
| HLA mismatches class I: mean ± SD | 2.3 ± 1.0 | 2.3 ± 0.6 | 2.4 ± 0.7 | N/A | NS |
| HLA mismatches class II: mean ± SD | 1.1 ± 0.5 | 1.2 ± 0.4 | 1.1 ± 0.4 | N/A | NS |
| Estimated GFR at blood draw: mean ± SD (ml/min) | 50.9 ± 7.9 | 42.9 ± 8.9 | 35.6 ± 6.6 | N/A | <0.0012 |
| Tacrolimus level at blood draw: mean ± SD (ng/ml) | 4.6 ± 2.3 | 5.7 ± 1.8 | 8.5 ± 2.3 | N/A | 0.0013 |
| Daily MMF usage | 7/45 | 2/12 | 5/9 | NA | N/A |
| Daily steroid usage | 1/45 | 0/12 | 0/9 | N/A | N/A |
| Living donor | 45/45 | 12/12 | 5/9 | N/A | N/A |
NS= non-significant; N/A= not applicable
Not significant by Fisher’s exact test, pairwise comparisons.
Any DSA occurring post-transplant.
Any DSA present with ±3 months of blood sampling.
Student t Test: HC vs. Reactive: p=0.02; HC vs. ACR: p=0.02; ACR vs. Reactive: p=0.02; ACR vs. Quiescent: p=0.01
Wilcoxon’s rank test: ACR vs. Quiescent: p=0.02; Reactive vs. Quiescent: p=0.03
Student t Test: ACR vs. Quiescent: p<0.001; ACR vs. Reactive: p=0.03
Figure 1. Identification and quantitation of peripheral blood T cell subsets.
Proportion (%) and absolute numbers of CD3+ (A), CD3+CD4+ (B), and CD3+CD8+ (C) T cells in each study group. (D) Gating strategy for identifying TN (CD45RO−CD62L+), TCM (CD45RO+CD62L+), TEM (CD45RO+CD62L−), and TEMRA (CD45RO−CD62L−) CD4 and CD8 T cells. (E–F) Proportion (%) of CD4+ TN, TEM, and CD8+ TN, TEMRA cells for each study group. Each symbol represents a single subject, while the horizontal line reprsents the mean value. *p<0.05; ** p<0.01; *** p<0.005.
We next investigated the relative distribution of TN and TM subsets among CD4+ and CD8+ T cells (Fig. 1D–F). The gating strategy used to identify TN (CD45RO−CD62L+), TCM (CD45RO+CD62L+), TEM (CD45RO+CD62L−), and TEMRA (CD45RO−CD62L−) is shown in Fig. 1D (23). Among CD4+ T cells, we found a significant decrease in % TN accompanied by a reciprocal increase in % TEM in all patient groups (except ACR) compared to HC (Fig. 1E–F). Importantly, % TEM was significantly higher in the reactive than the quiescent patient group (Fig. 1F). Receiver operating characteristic (ROC) curves to assess accuracy of % CD4+ TEM in discriminating between reactive and either quiescent or HC yielded an area under ROC (AUROC) of 0.79 and 0.92, respectively (Supplemental Fig. 1A). CD4 TCM and TEMRA subsets were similar among patient groups and between patients and HC (data not shown). Among CD8+ T cells, we observed a significant increase in % TEMRA in the reactive group compared to quiescent patients (AUROC = 0.68) and HC (AUROC = 0.70) (Fig. 1F & Supplemental Fig. 1B). A striking decline in CD8+ TEMRA, however, was observed in ACR patients compared to all other groups (Fig. 1F), most significantly when compared to reactive or quiescent patients (AUROC 0.88 and 0.79, respectively) (Supplemental Fig.1C), suggesting that this cell population may have exited the circulation or differentiated into a different phenotype during rejection. These results indicate that T cells are skewed towards memory (TEM and TEMRA) even several years after Alemtuzumab induction and that increased proportions of CD4+ TEM and CD8+ TEMRA may be associated with clinical reactivity.
Long-term effects of Alemtuzumab on regulatory and effector T cell subsets among CD4+CD25high T cells
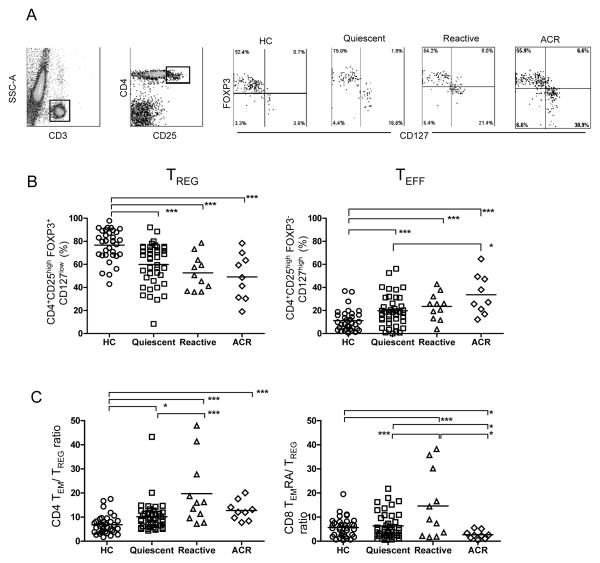
Previous studies provided evidence that TREG, defined as CD4+CD25high or CD4+CD25highFoxp3+ cells, increase after Alemtuzumab induction (11, 25, 26). This evidence is inconclusive because in humans the CD4+CD25highFoxp3+ phenotype also encompass recently activated or effector T cells (TEFF) (27–29). We therefore quantified % and absolute number of TREG based on low IL-7Rα (CD127) expression (TREG = CD3+CD4+CD25highFoxp3+CD127low) (30–33) (Fig. 2A). We found that while absolute counts of Tregs were significantly reduced in all patient groups compared to HC, % TREG of all CD4+ T cells was not altered (data not shown). However, the % TREG among CD4+CD25high T cells was significantly decreased in all patient groups compared to HC (Fig. 2B), with AUROC values of 0.88, 0.88, and 0.75 for the ACR, reactive, and quiescent patients, respectively (Supplemental Fig. 2A). Importantly, we observed a reciprocal increase in % CD4+CD25highFoxp3−CD127high (Fig. 2B), which in humans represents T effector (TEFF) that are precursors of TM (33). This TEFF population was increased in all patients compared to HC and over-represented in the ACR group compared to quiescent patients. The data therefore suggest that Alemtuzumab induction does not favor long-term immune regulation but instead effector and memory generation.
Figure 2. Identification and quantitation of peripheral blood CD4 TREG and TEFF cells.
(A) Gating strategy and representative flow cytometry of TREG (CD3+CD4+CD25highFOXP3+CD127low) and TEFF (CD3+CD4+CD25highFOXP3−CD127high) in the CD3+CD4+CD25high T cell gate in each of the study groups. (B) Proportion (%) of TREG and of TEFF within the CD3+CD4+CD25high T cell gate. (C) CD4 TEM/TREG and CD8 TEMRA/TREG ratios in each of the study groups. Each symbol represents a single individual, and the horizontal line represents the mean value. *p<0.05; ** p<0.01; *** p<0.005.
Increased ratios of CD4+ TEM/TREG and CD8+ TEMRA/TREG are associated with reactive status
Kreijveld et al. reported that increased TM/TREG ratio is associated with acute rejection upon tacrolimus reduction in kidney transplant recipients who had not received induction therapy (34). Given that we observed skewing of T cells towards higher TM and lower TREG proportions after Alemtuzumab, we asked whether the TM/TREG ratio correlates with clinical status in our patient cohort. We found that all patient groups had higher CD4+ TEM/TREG ratio than HC (Fig. 2C) and this ratio was predictive for reactive patients when compared to either quiescent patients or HC (AUROC = 0.76 and 0.88, respectively) (Supplemental Fig. 2B). A similar pattern was observed for the CD8+ TEMRA/TREG ratio except that it was significantly diminished in the ACR group (Fig. 2C & supplemental Fig. 2C). These results suggest that the ratio of effector memory to regulatory T cells after Alemtuzumab induction could potentially distinguish reactive patients at risk of rejection from those who are quiescent.
Long-term effects of Alemtuzumab induction on CD4+ and CD8+ T cell function
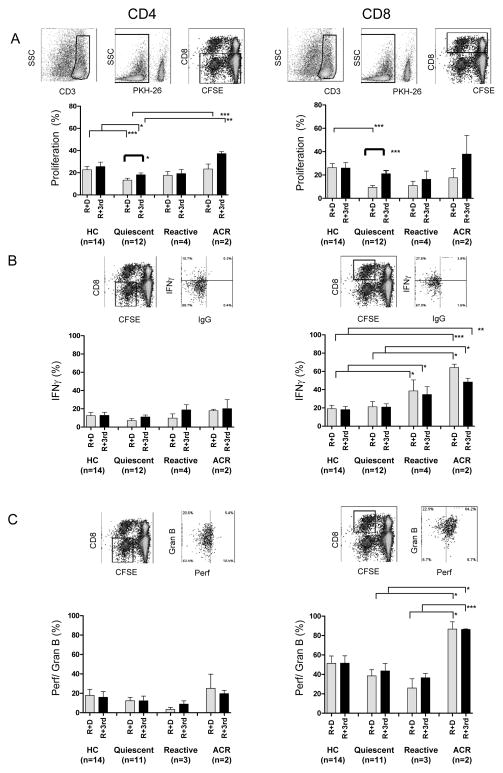
We next investigated whether alterations in naïve, memory, and regulatory T cell proportions after Alemtuzumab induction translate to differences in T cell alloreactivity. CD4+ and CD8+ T cells from quiescent patients proliferated significantly less in response to donor than third party cells, and less than HC cells (Fig. 3A). On the other hand, CD8+ T cells from reactive and ACR patients expressed more IFNγ than those from HC (Fig. 3B). T cells from ACR patients showed heightened CD8+ T cell perforin/granzyme B compared to reactive patients, and of both perforin/granzyme B and IFNγ compared to quiescent patients (Fig. 3B,C). These studies suggest that increased effector molecule expression may mark CD8+ T cells involved in ACR and identify reactive patients.
Figure 3. T cell proliferation and IFNγ and peforin/granzyme B expression in one-way CFSE-MLR.
Analyses were performed after excluding PKH-26+ stimulator cells and gating on CD3+CD8− (CD4+) and CD3+CD8+ T cells at the end of the 5-day MLR. (A) Proliferation of responder CD4+ and CD8+ T cells, presented as proportion (%) of cells that diluted CFSE, after stimulation with allogeneic donor (R+D) or third party PBMC (R+3rd). (B) IFNγ production by proliferated CD4+ and CD8+ T cells 4 hrs after re-stimulation with donor or third party CD3-depleted allogeneic PBMC. (C) Perforin/granzyme B expression by proliferated responder CD4+ and CD8+ T cells. HC (n=14), quiescent (n=12), reactive (n=4), and ACR (n=2). *p<0.05; ** p<0.01; ***p<0.005.
Identification of T cell subsets in renal allograft biopsies
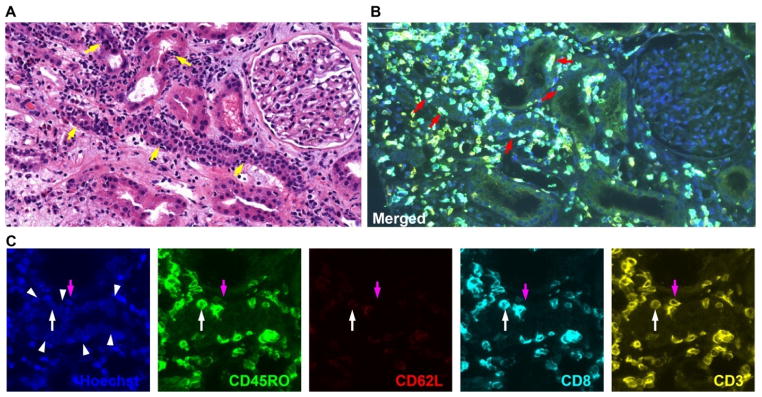
We analyzed kidney biopsies from patients undergoing ACR (n=4), borderline rejection (n=3), or drug toxicity (n=3) (Fig. 4). Enumeration of T cells in areas of tubulitis identified 9.3±5.8 T (CD3+) cells/mm2 in ACR patients versus 2.7±1.3 and 2.6±1.7 in borderline rejection and drug toxicity patients, respectively (p=0.1). Similar numbers of CD3+CD8+ and CD3+CD8− cells were identified in all groups, but infiltrating CD3+CD8+ T cells were significantly greater in ACR (4.7±3.0 vs. 1.4±0.8 and 0.7±0.2 cells/mm2, respectively, p=0.03). Among CD8+ T cells, 93% had a CD45RO+CD62L− phenotype, consistent with effector or effector memory T cells. CD8− T cells were evenly divided between CD45RO+CD62L− (TEM) and CD45RO−CD62L− (TEMRA). These results support the possibility that effector memory T cell subsets are preferentially recruited to the graft during acute rejection.
Figure 4. Identification of T cell subsets in renal allograft tissue of a patient undergoing ACR.
(A) H&E staining shows irregularly shaped tubules and mononuclear cell infiltrates (tubulitis) (yellow arrows). (B and C) Multiplex quantum dot staining of CD45RO (green), CD62L (red), CD8 (cyan), and CD3 (yellow) in the same tissue section as A. Panel B depicts merged image, red arrows indicate infiltrating cells which are mostly CD8+CD3+CD45RO+CD62L− T cells (TE or TEM). Panel C depicts cropped images from Panel B. White arrow heads indicate tubules. Purple arrows identify CD3+CD8−CD45RO−CD62L− (TEMRA) while white arrows identify CD3+CD8+CD45RO+CD62L− (TEM) infiltrating T cells.
Discussion
We investigated the phenotype of regulatory and memory T cell subsets after induction in a cohort of patients with diverse clinical outcomes and at a later time point after transplantation than previous studies (10–18). First, we found that Alemtuzumab-induced patients have long-term skewing of CD4+ T cells towards TEM and a reduction in TREG with reciprocal increase in TEFF among CD4+CD25high T cells. Since serial blood samples were not obtained in our study, the mechanisms by which this skewing occurred (differential depletion of T cell subsets vs differential repopulation) were not determined. Second, we observed a correlation between increased CD4+ TEM/TREG and CD8+ TEMRA/TREG ratios and clinical reactivity. Third, the CD8+ TEMRA population, which is characterized by high cytolytic activity (23), was consistently and profoundly diminished in the circulation of patients with ongoing ACR. Fourth, despite skewing towards memory and increased CD8 effector molecule expression in some recipients, other patients achieved clinical quiescence associated with lower donor-specific T cell proliferation.
Our finding that T cell repopulation after Alemtuzumab does not favor TREG but that they are reduced as a proportion of CD4+CD25high T cells is at odds with previous studies (11, 25). Gurkan et al found that administration of Thymoglobulin in adult renal transplant recipients induced peripheral expansion and new thymic emigration of CD4+Foxp3+ Treg in the first 6 months post-depletion (17). Ciancio et al found that Foxp3 mRNA expression and the proportion of CD4+CD25+ T cells among circulating T lymphocytes were significantly higher between two and eight months after transplantation in patients that received Alemtuzumab than those induced with either Thymoglobulin or Daclizumab (11). We believe that the discrepancy can be explained by the fact that we defined TREG as CD4+CD25highFoxp3+CD127low T cells. In humans, Foxp3 is expressed on recently activated CD4+ T cells in addition to TREG (27–29), and among CD4+CD25high lymphocytes only the CD127low are suppressive (27, 30–32). Another potential reason for the discrepancy is that we analyzed peripheral blood T cells at late time points (average 2.8 years) after Alemtuzumab induction. It is possible that increased % TREG observed early after lymphodepletion does not persist long-term. In addition, certain immunosuppressive drugs used in other studies, such as rapamycin, could favor TREG expansion (25).
Previous studies have found preferential expansion of CD4+ TEM after lymphodepletion in kidney and pancreatic islet transplant recipients (10, 15), but no clear correlation with clinical outcomes or ACR was established. Our results showed that Alemtuzumab is associated with long-term increase in the proportion of CD4+ TEM and identified reactive patients as having the highest proportion of this memory subpopulation. These data indicate a correlation between CD4+ TEM and risk of immune reactivity or rejection after T cell repopulation, but other factors likely contribute, as not all patients with increased CD4+ TEM proportion develop rejection and vice versa. Two other cell populations that participate in determining patient risk are CD4+ TREG and CD8+ TEMRA. This is borne out by our finding that a high proportion of CD8+ TEMRA and a high ratio of CD4+ TEM or CD8+ TEMRA to TREG correlate with reactive status. Finally, in the absence of a comparison group receiving a different depleting or non-depleting induction agent, we could not definitively determine that alterations in T cell subsets were indeed due to Alemtuzumab. Reports in which such groups were studied suggest that skewing towards memory is a consequence of lymphopenia, irrespective of depleting agent used (15, 17, 18).
A striking finding in our study is the profound and consistent decline in proportion of circulating CD8+ TEMRA in patients with ongoing ACR. It is possible that CD8+ TEMRA migrate to the graft and differentiate into effectors or enter other non-lymphoid tissues during rejection. These cells are rich in perforin/granzyme B and could be pathogenic (35). We found in biopsy analyses that the majority of tubule-infiltrating T cells during ACR have an effector phenotype. An alternative explanation is that CD8+ TEMRA are regulatory lymphocytes whose decline heralds acute rejection. Trzonkowski et al. have shown that most repopulating CD8+ T cells after Alemtuzumab induction display a senescent (CD28−) phenotype (36), and others suggested that CD8+CD28− T cells may be suppressive (37, 38). CD8+CD28− suppressor T cells however had a TCM and not TEMRA phenotype (38). While it is recognized that CD8+ TEMRA are mostly CD28− (39), our results and those of others demonstrate that this subpopulation is rich in perforin/granzyme B and IFNγ (23, 40, 41), indicating that it has effector rather than regulatory functions. Finally, it is possible that higher tacrolimus levels at the time of blood draw in the ACR group (Table 1) could alter memory T cell distribution, preferentially reducing number of CD8+ TEMRA cells.
In conclusion, our results show that Alemtuzumab induction in kidney transplant recipients is associated with long-term phenotypic and functional T cell alterations. We found significant differences in relative proportion of memory and regulatory T cell subsets among quiescent, reactive, and ACR patients. Although promising, the prognostic value of peripheral blood T cell profiling in our patient cohort should be interpreted with caution because of the cross-sectional nature of the study, heterogeneity and small size of the reactive group, and lack of control patients not receiving lymphodepleting induction therapy. Therefore, future prospective randomized studies comparing larger cohorts of patients receiving different induction therapies and employing serial monitoring, similar to that recently performed by Cherkassky et al (18), are needed to determine whether T cell profiling would accurately identify patients experiencing ongoing acute rejection, patients at risk of rejection, or patients who are quiescent. Such studies would also address whether observations made after Alemtuzumab induction can be generalized to kidney transplant recipients managed by different immunosuppression protocols.
Materials and Methods
Human subjects
Sixty-three first time living-donor renal transplant recipients (66 samples), 34 healthy control (HC) volunteers, and 30 living kidney donors at the University of Pittsburgh Medical Center (UPMC) were consented over a period of three years (2007–2010) under IRB-approved protocols to participate in this cross-sectional, observational study. Demographic and clinical characteristics of recipients and HC are shown in Table 1. Patients were induced with Alemtuzumab (30mg) plus methyl-prednisolone (2×1000mg) i.v. at the time of transplantation. Maintenance immunosuppression consisted of tacrolimus ± mycophenolate mofetil (MMF) (Table 1). Recipients were classified as reactive (n=12) or quiescent (n=45) based on clinical status ±3 months from the time of sample collection. Specifically, reactive patients were those who within this 6 month window (i) developed de novo donor specific antibodies (DSA) (n = 2); (ii) had biopsy-proven acute cellular or humoral rejection within the 6 month window but not on the day of blood sampling (n=2), or (iii) had a significant change in serum creatinine (> 20% of baseline without evidence of non-immunological causes of acute renal failure) and/or borderline ACR on biopsy, triggering pulse corticosteroids and/or increase in maintenance tacrolimus ± MMF based on the clinician’s judgment (n= 10, including 2 patients who also had either de novo DSA or biopsy-proven ACR). Quiescent patients were those who had none of the above. ACR (n=9) patients were those who had biopsy-proven ACR on the day of blood sample collection before any anti-rejection therapy was administered.
Isolation of peripheral blood mononuclear cells (PBMC)
One ml heparinized blood was used directly for lymphocyte phenotyping and the remainder to isolate PBMC by density gradient centrifugation. PBMC were frozen for functional assays.
Phenotyping of T cell subsets
Cells were surface stained with mAbs which included anti-CD62L-FITC (Beckman Coulter, Fullerton, CA), anti-CD45RO-PE, anti-CD8 or CD4-PerCpCy5.5, CD25-PECy7, anti-CD3-V450 (BD, San Jose, CA), anti-CD127-APC-eFluor-780, and intracellularly stained with anti-FoxP3-APC (eBioscience, San Diego, CA) prior to RBC lysis (23). Events were collected using a LSRII flow cytometer (BD) and analyzed with Diva (BD) or FlowJo software (Tree Star, Ashland, OR).
One-way CFSE-MLR
Responder PBMC were labeled with 2 μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Eugene, OR) and incubated with γ-irradiated allogeneic donor or third party PKH-26 (Sigma, St. Louis, MO)-labeled PBMC (1:1 ratio) for 5 days. Proliferation of CD3+CD8− (CD4+) or CD3+CD8+ T cells was measured by CFSE dilution by flow cytometry (23). Surface-stained, fixed cells were further permeabilized and incubated with perforin-APC (Biolegend, San Diego, CA) and granzyme B-Alexa700 (BD). For IFNγ intracellular staining, cultured cells were re-stimulated in vitro for 4hrs with PKH26-labeled, γ-irradiated allogeneic CD3-depleted (ratio 1:1) PBMC in the presence of Golgi-Plug (BD) prior to staining with anti-IFNγ-Alexa700 mAb (BD).
HLA typing and measurement of DSA
HLA typing was performed in the Tissue Typing Laboratory at the UPMC. Screening for anti-HLA antibodies was performed by ELISA (LAT-M kit, One Lambda, Canoga Park, CA) followed by Luminex single-antigen bead analysis (One Lambda). MFI values ≥1000 were considered positive.
Multiplex quantum dot staining and whole slide image
Formalin-fixed, paraffin-embedded renal allograft tissue was immunostained using multiplex quantum dot staining as reported (42). Antibodies used were anti-CD45RO (NeoMarkers, Fremont, CA), anti-CD62L (Novocastra, Newcastle Upon Tyne, UK), anti-CD8 and anti-CD3 (DAKOCytomation, Carpinteria, CA).
Statistical analysis
Data were reported as mean±standard deviation (SD). Significance was measured by one-way ANOVA and two-tail Student t tests for normally distributed values or Wilcoxon’s rank test for non-normally distributed data. Fisher’s test was used on qualitative data. Statistically significance was set at p≤0.05. ROC curves were plotted to assess sensitivity for ≥ 80% specificity over the entire range of cut-offs, and calculated the AUROC to measure how well the markers discriminated between groups.
Supplementary Material
Acknowledgments
This work was supported by NIH AI 049466 (FGL), AI 096553 (FGL, DM, GC), HL 094603 (DM) and 5UL1 RR024153-04 from the National Center for Research Resources (NCRR) (DL). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abbreviations
- ACR
Acute cellular rejection
- AUROC
Area under ROC curve
- DSA
Donor specific antibodies
- HC
Healthy controls
- ROC
Reporter operator characteristic
- TCM
Central memory T cell
- TEM
Effector memory T cell
- TEMRA
Terminally differentiated effector memory T cell
- TN
Naïve T cell
- TREG
Regulatory T cell
Footnotes
Camila Macedo1: participated in research design, performance, analysis, and writing of the manuscript
John T. Walters1: participated in research performance and data analysis
Elizabeth A. Orkis 1*: participated in research performance and data analysis
Kumiko Isse 2: performed immunostaining and biopsy analysis
Beth D. Elinoff 1: participated in recruiting subjects and coordinating samples
Sheila P. Fedorek1: participated in recruiting subjects
John M. McMichael 1*: designed database
Geetha Chalasani 3,4: provided scientific advice
Parmjeet Randhawa 1,2: performed biopsy analysis
Anthony J. Demetris 1,2: performed biopsy analysis
Adriana Zeevi 2,4: provided scientific advice
Henkie Tan1: performed donor nephrectomies
Ron Shapiro1: performed kidney transplantation
Doug Landsittel 3: performed statistical analysis
Fadi G. Lakkis1,3,4†: participated in research design, manuscript writing, and oversight of project
Diana Metes1,4†: participated in research design, manuscript writing and oversight of project
All authors declare no conflict of interest.
References
- 1.Cai J, Terasaki PI. Induction Immunosuppression Improves Long-Term Graft and Patient Outcome in Organ Transplantation: An Analysis of United Network for Organ Sharing Registry Data. Transplantation. 2010;90:1511. doi: 10.1097/TP.0b013e3181fecfcb. [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche H-U, Li S, Gruessner RWG, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6 (5 Pt 2):1111. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 3.Kirk AD. Induction immunosuppression. Transplantation. 2006;82 (5):593. doi: 10.1097/01.tp.0000234905.56926.7f. [DOI] [PubMed] [Google Scholar]
- 4.Margreiter R, Klempnauer J, Neuhaus P, Muehlbacher F, Boesmueller C, Calne RY. Alemtuzumab (Campath-1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. Am J Transplant. 2008;8 (7):1480. doi: 10.1111/j.1600-6143.2008.02273.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DB, Leventhal JR, Gallon LG, Parker MA. Alemtuzumab induction and prednisone-free maintenance immunotherapy in simultaneous pancreas-kidney transplantation comparison with rabbit antithymocyte globulin induction - long-term results. Am J Transplant. 2006;6 (2):331. doi: 10.1111/j.1600-6143.2005.01166.x. [DOI] [PubMed] [Google Scholar]
- 6.Tan HP, Donaldson J, Basu A, et al. Two hundred living donor kidney transplantations under alemtuzumab induction and tacrolimus monotherapy: 3-year follow-up. Am J Transplant. 2009;9 (2):355. doi: 10.1111/j.1600-6143.2008.02492.x. [DOI] [PubMed] [Google Scholar]
- 7.Farney AC, Doares W, Rogers J, et al. A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation. 2009;88 (6):810. doi: 10.1097/TP.0b013e3181b4acfb. [DOI] [PubMed] [Google Scholar]
- 8.Kirk A, Hale D, Mannon R, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 9.Orlando G, Hematti P, Stratta RJ, et al. Clinical operational tolerance after renal transplantation: current status and future challenges. Ann Surg. 2010;252 (6):915. doi: 10.1097/SLA.0b013e3181f3efb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearl J, Parris J, Hale D, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 11.Ciancio G, Burke GW, Gaynor JJ, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80 (4):457. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 12.Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81 (1):81. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- 13.Trzonkowski P, Zilvetti M, Friend P, Wood KJ. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006;82 (10):1342. doi: 10.1097/01.tp.0000239268.64408.84. [DOI] [PubMed] [Google Scholar]
- 14.Louis S, Audrain M, Cantarovich D, et al. Long-term cell monitoring of kidney recipients after an antilymphocyte globulin induction with and without steroids. Transplantation. 2007;83 (6):712. doi: 10.1097/01.tp.0000255683.66156.d3. [DOI] [PubMed] [Google Scholar]
- 15.Toso C, Edgar R, Pawlick R, et al. Effect of different induction strategies on effector, regulatory and memory lymphocyte sub-populations in clinical islet transplantation. Transpl Int. 2009;22 (2):182. doi: 10.1111/j.1432-2277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- 16.Serban G, Whittaker V, Fan J, et al. Significance of immune cell function monitoring in renal transplantation after Thymoglobulin induction therapy. Hum Immunol. 2009;70 (11):882. doi: 10.1016/j.humimm.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Gurkan S, Luan Y, Dhillon N, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10 (9):2132. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherkassky L, Lanning M, Lalli PN, et al. Evaluation of alloreactivity in kidney transplant recipients treated with antithymocyte globulin versus IL-2 receptor blocker. Am J Transplant. 2011;11 (7):1388. doi: 10.1111/j.1600-6143.2011.03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19 (5):318. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29 (6):848. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Chalasani G, Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci USA. 2002;99:6175. doi: 10.1073/pnas.092596999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10 (1):87. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macedo C, Orkis EA, Popescu I, et al. Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009;9 (9):2057. doi: 10.1111/j.1600-6143.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 24.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15 (4):405. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloom DD, Chang Z, Fechner JH, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant. 2008;8 (4):793. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 26.Morelon E, Lefrançois N, Besson C, et al. Preferential increase in memory and regulatory subsets during T-lymphocyte immune reconstitution after Thymoglobulin induction therapy with maintenance sirolimus vs cyclosporine. Transpl Immunol. 2010;23 (1–2):53. doi: 10.1016/j.trim.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38 (4):925. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37 (1):129. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 29.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110 (8):2983. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203 (7):1701. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203 (7):1693. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel L, Berthelot L, Pettre S, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118 (10):3411. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Codarri L, Vallotton L, Ciuffreda D, et al. Expansion and tissue infiltration of an allospecific CD4+CD25+CD45RO+IL-7Ralphahigh cell population in solid organ transplant recipients. J Exp Med. 2007;204 (7):1533. doi: 10.1084/jem.20062120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreijveld E, Koenen HJ, van Cranenbroek B, van Rijssen E, Joosten I, Hilbrands LB. Immunological monitoring of renal transplant recipients to predict acute allograft rejection following the discontinuation of tacrolimus. PLoS ONE. 2008;3 (7):e2711. doi: 10.1371/journal.pone.0002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Hartono C, Ding R, et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344 (13):947. doi: 10.1056/NEJM200103293441301. [DOI] [PubMed] [Google Scholar]
- 36.Trzonkowski P, Zilvetti M, Chapman S, et al. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8 (2):338. doi: 10.1111/j.1600-6143.2007.02078.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Tugulea S, Cortesini R, Sucia-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- T cells. Int Immunol. 1998;10:775. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 38.Colovai AI, Mirza M, Vlad G, et al. Regulatory CD8+CD28− T cells in heart transplant recipients. Hum Immunol. 2003;64:31. doi: 10.1016/s0198-8859(02)00742-5. [DOI] [PubMed] [Google Scholar]
- 39.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73 (11):975. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 40.Baeten D, Louis S, Braud C, et al. Phenotypically and functionally distinct CD8+ lymphocyte populations in long-term drug-free tolerance and chronic rejection in human kidney graft recipients. J Am Soc Nephrol. 2006;17 (1):294. doi: 10.1681/ASN.2005020178. [DOI] [PubMed] [Google Scholar]
- 41.Lo DJ, Weaver TA, Stempora L, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11 (1):22. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isse K, Grama K, Abbott IM, et al. Adding value to liver (and allograft) biopsy evaluation using a combination of multiplex quantum dot immunostaining, high-resolution whole-slide digital imaging, and automated image analysis. Clin Liver Dis. 2010;14 (4):669. doi: 10.1016/j.cld.2010.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.