Abstract
The first differentiation event in mammalian development gives rise to the blastocyst, consisting of two cell lineages that have also segregated in how the cell cycle is structured. Pluripotent cells of the inner cell mass divide mitotically to retain a diploid DNA content, but the outer trophoblast cells can amplify their genomes more than 500-fold by undergoing multiple rounds of DNA replication, completely bypassing mitosis. Central to this striking divergence in cell cycle control is the E3 ubiquitin-ligase activity of the anaphase-promoting complex or cyclosome (APC/C). Extended suppression of APC/C activity during interphase of mouse pluripotent cells promotes rapid cell cycle progression by allowing stabilization of cyclins, whereas unopposed APC/C activity during S phase of mouse trophoblast cells triggers proteasomal-mediated degradation of geminin and giant cell formation. While differential APC/C activity might govern the atypical cell cycles observed in pre-implantation mouse embryos, geminin is a critical APC/C substrate that: (1) escapes degradation in pluripotent cells to maintain expression of Oct4, Sox2 and Nanog and (2) mediates specification and endoreduplication when targeted for ectopic destruction in trophoblast. Thus, in contrast to trophoblast giant cells that lack geminin, geminin is preserved in both mouse pluripotent cells and non-endoreduplicating human cytotrophoblast cells.
Key words: APC/C, geminin, Emi1, cell cycle, pluripotency, trophoblast, endoreduplication, DNA damage
Introduction
Cell cycle progression in somatic cells is driven by mirrored oscillations in the activity of cyclin-dependent kinases (Cdks) and the anaphase-promoting complex or cyclosome (APC/C). These enzymatic protein complexes coordinate DNA replication with cell division as sequential steps, with periodic states of low and high Cdk activity complemented by converse activities of the APC/C. Cdks are protein kinases activated by the cyclical binding of cyclins,1 whereas the APC/C is a multisubunit cullin-RING finger E3 ubiquitin ligase that associates with specific co-activators to polyubiquitinate key cell cycle proteins.2 Polyubiquitination of APC/C substrates, such as cyclins, geminin, Cdk inhibitors and securin, targets them for 26S-proteasomal degradation during mitosis and G1 phase, when Cdk activity is low. Two co-activator proteins of the APC/C are Cdc20 and Cdh1. APC/CCdc20 is active from prophase to the metaphase-anaphase transition, when APC/CCdh1 activity increases and then persists throughout G1 until the initiation of S phase. During DNA replication and G2 phase, when Cdk activity is high, Early mitotic inhibitor 1 (Emi1) prevents APC/CCdh1 from targeting its substrates for destruction until prophase, when APC/CCdc20 activity is restored.3–6 Hence, alternating activation and inactivation of cyclin-Cdk complexes and the APC/C are crucial for ordered progression through the somatic cell cycle.
Mammalian embryonic cells, however, are not subject to the same cell cycle controls that have been studied more extensively in somatic cells. This is exemplified by the earliest recognized cell types that emerge during embryogenesis, namely pluripotent cells and trophoblast cells, both of which can be observed at the blastocyst stage of pre-implantation mouse development on approximately embryonic day 3.5. Pluripotent cells of the inner cell mass give rise to the embryo proper, and embryonic stem (ES) cells derived from this niche transit rapidly through the cell cycle due to a shortened G1 phase. Mouse ES cells maintain high levels of cyclins during interphase,7–9 which presumably mediate the accelerated cell cycle progression. However, although suppression of APC/C activity might account for the elevated cyclin levels in pluripotent cells,9 how cell cycle progression is possible without the canonical oscillations observed in somatic cells is difficult to reconcile, although temporal activity of cyclin B1-Cdk1 might remain conserved.10 Similarly, the alternative controls that allow endoreduplication in mouse trophoblast cells remain poorly understood. Uncoupling DNA replication from mitosis in endoreduplicating trophoblast cells requires a reduction in mitotic Cdk activity,11 but this alone cannot account for progress through endocycle-specific events, such as the repeated recruitment of DNA replication proteins to chromatin.
We have previously shown that the APC/CCdh1 substrates geminin and cyclin A2 are actively degraded in endoreduplicating mouse trophoblast giant cells,12 while genetic ablation of geminin in the mouse results in pre-implantation embryonic lethality due to loss of pluripotency and premature endoreduplication at the eight-cell stage.12,13 We have also recently described a molecular pathway that focuses on geminin as a critical cell cycle protein that is required for sustained expression of core pluripotency factors in mouse embryonic stem (ES) cells.9 Collectively, these findings suggest that the APC/C itself is an important regulator of the atypical cell cycles observed in both pluripotent cells and trophoblast cells of the pre-implantation mouse embryo. In this article, we integrate these findings and propose a model for cell cycle control in mouse embryonic cells. We further report that in both mouse embryonal carcinoma (EC) and ES cells, depletion of Emi1 or the APC/CCdh1 substrate geminin not only induces trophoblast differentiation and giant cell formation, but that despite extensive DNA damage, there is failure to trigger apoptotic cell death, suggestive of an attenuated DNA damage response in these differentiated cells. We also review the important species-specific differences between mouse and human trophoblast cells that add a further layer of complexity to the study of cell cycle regulation in mammalian embryonic cells.
Results
A model for APC/C activity in mouse embryonic cells.
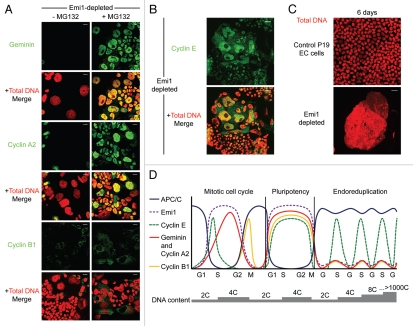
Emi1 inhibits APC/CCdh1 activity in both somatic cells3,4 and pluripotent cells.9 Hence, depletion of Emi1 in P19 EC cells results in unopposed APC/CCdh1 activity and degradation of the APC/CCdh1 substrates geminin, cyclin A2 and cyclin B1 (Fig. 1A). Figure 1A further illustrates the dramatic stabilization of these APC/CCdh1 substrates by immunoflurorescence following treatment of Emi1-depleted pluripotent cells with the proteasome inhibitor, MG132. Geminin and cyclin A2-Cdk2 (and Cdk1) are essential proteins that prevent illegitimate assembly of pre-replication complexes on chromatin when APC/C activity is low in somatic cells during S phase and G2,12,16–19 whereas cyclin B1-Cdk1 initiates mitosis and is required for its progression.20 Interestingly, cyclin E, which is not an APC/C substrate in somatic cells and is targeted for proteasomal degradation by the SCFSkp2 ubiquitin ligase,21 is upregulated in enlarged nuclei of pluripotent cells depleted of Emi1 (Fig. 1B).
Figure 1.
Emi1 depletion in pluripotent cells results in destabilization of APC/CCdh1 substrates and gross nuclear enlargement. (A) Emi1 depletion in P19 EC cells results in downregulation of geminin, cyclin A2 and cyclin B1 (green) at 48 h, with stabilization of each of these substrates following treatment with the proteasome inhibitor, MG132. Total DNA, red. Scale bar = 20 µm. (B) Cyclin E (green), which is not a target of the APC/C, is upregulated by immunofluorescence in enlarged nuclei following depletion of Emi1 in pluripotent cells. Total DNA, red. Scale bar = 20 µm. (C) Giant nuclei are evident at six days in B6/Blu-1 mouse embryonic stem cells depleted of Emi1. Total DNA, red. Scale bar = 20 µm. (D) Model of how APC/C activity distinguishes somatic, pluripotent and endoreduplicating cell cycles. Alternating oscillations in Cdk activity and APC/C activity (blue) drive the mitotic cell cycle. APC/C targets cyclin A2 (red), geminin (also red) and cyclin B1 (yellow) but not cyclin E (green) for degradation during mitosis and G1 phase. Emi1 (purple) inhibits APC/C activity during S phase, G2 and early mitosis. In pluripotent cell cycles, however, Emi1 suppresses APC/C activity for an extended duration, resulting in high levels of cyclins that drive rapid cell cycle progression. Periodicity in Cdk1 activity is likely to be conserved.10 In contrast, high APC/C activity (due to low levels of Emi1) targets geminin, cyclin A2 and cyclin B1 for degradation during endoreduplication. Cyclin E remains as the only S phase-promoting Cdk partner in endoreduplicating cells. This model would explain why cyclin E is essential for endoreduplication in mouse trophoblast giant cells.14,15
That APC/C substrates are present during interphase of pluripotent cell cycles and Emi1 depletion results in giant cell formation (Fig. 1C) leads us to propose a model that focuses on APC/C activity to distinguish between somatic cell cycles, pluripotent cell cycles and endoreduplication in mouse trophoblast cells (Fig. 1D). During a somatic cell cycle, high APC/C activity promotes pre-replication complex assembly by targeting key inhibitory proteins for degradation in late mitosis and G1 phase, such as geminin and cyclin A2. Conversely, cyclin A2-Cdk2 provides the main S phase-promoting Cdk activity during periods of low APC/C activity, while geminin cooperates with Cdk activity to prevent recurrent pre-replication complex assembly on chromatin. Emi1 plays a crucial role in coupling DNA replication to mitosis in somatic cells by inhibiting APC/CCdh1 and stabilizing cyclin A2, cyclin B1 and geminin.3,4,22
As illustrated schematically in Figure 1D, we9 and others8,10 have shown that levels of APC/CCdh1 substrates remain elevated throughout the cell cycle in mouse ES cells, leading us to conclude that APC/C activity is inhibited during pluripotent cell cycles,9 possibly due to interphase suppression by Emi1. The extremely high levels of cyclins in pluripotent cells is unprecedented, far exceeding the cyclin levels normally present in proliferating somatic cells.8–10 In mouse ES cells released from chemically induced blocks, Cdk2 activity appears to be cell cycle-independent but responsible for the accelerated rate of cell cycle progression in pluripotent cells, where cyclins E and A are highly abundant.8,10 Given that Cdk1 is the only essential Cdk in mammalian cells,23 conserved oscillations of cyclin B1 levels and inhibitory phosphorylations of Cdk1 on tyrosine (Y) 15 would fully explain how pluripotent cells undergo cell division.10 In our experience, however, it is not possible to release ES cells from chemically induced cell cycle blocks, and thus, although post-translational modifications of Cdks are likely to be fundamental for ordered progression through pluripotent cell cycles, any results obtained from such experiments should be interpreted with caution. Hence, the precise mechanisms of cell cycle control in mammalian pluripotent cells still need to be evaluated in detail. Interestingly, the ectopic presence of geminin during the rapid cell cycles of early Xenopus embryos is fully compatible with structured cell cycle progression.24 Dynamic interactions between geminin and Cdt1, rather than proteolysis, dictate its functionality in amphibian embryonic cells.
We further postulate that in contrast to pluripotent cell cycles in which the APC/C is suppressed, APC/C activity is high in endoreduplicating mouse trophoblast cells, possibly mediated by downregulation of Emi1, which simplifies the trophoblast cell cycle to alternate S and “gap-like” phases (Fig. 1D). Endoreduplicating trophoblast cells fail to enter mitosis due to absence of mitotic Cdk partners, since cyclin B1 and cyclin A2 are both degraded.12 Translational inhibition of cyclin B1 has also been observed in mouse trophoblast giant cells.11 In particular, high APC/C activity removes an important block to pre-replication complex assembly by targeting geminin for degradation. Since cyclin E is not an APC/C target but is instead ubiquitinated by the SCFSkp2 ubiquitin ligase,21 activation of the APC/C would render cyclin E as the surviving S phase-promoting Cdk partner in endoreduplicating trophoblast cells. Hence, oscillations in cyclin E alone would sustain endocycles in mouse trophoblast giant cells. The proposed model explains why loss of APC/CCdh1 in the mouse leads to impaired placental development25,26 and why cyclin E1/E2 double-knockout mice show a specific defect in trophoblast endoreduplication.14,15
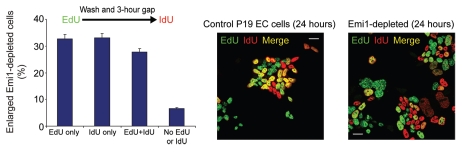
We also performed pulse-chase experiments to determine whether or not S phases are continuous in Emi1-depleted cells using a 30 min pulse with 5-ethynyl-2′-deoxyuridine (EdU), followed by a second 30 min pulse with iodouridine (IdU) after a three hour gap. Interestingly, Figure 2 shows that a significant proportion of P19 EC cells depleted of Emi1 incorporate the second pulse only, suggesting that DNA replication is discontinuous in these cells and might be interspersed with “gap-like” phases. Although the evidence for this is light at present, these experiments support the notion that reduction of geminin levels not only represses the expression of core pluripotency factors, but that geminin depletion might also be the trigger for inducing endocycles during Brg-1-dependent trophectoderm differentiation of mouse pluripotent cells.9
Figure 2.
Acquisition of a high DNA content in pluripotent cells depleted of Emi1. Control and Emi1-depleted cells were pulsed for 30 min with 5-ethynyl-2′-deoxyuridine (EdU, green) followed by a second 30 min pulse with iodouridine (IdU, red) after a three hour gap (merge, yellow). Total DNA, blue. Scale bar = 20 µm. The bar chart shows the proportion of enlarged Emi1-depleted cells labeled with EdU or IdU. At least 1,000 cells were scored for each time point. Bars represent 5% standard error.
DNA damage response in pluripotent cells depleted of geminin and Emi1.
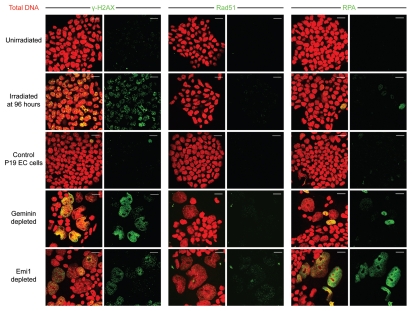
Geminin depletion in somatic cells induces genomic over-replication due to aberrant pre-replication complex assembly;18,19 a similar phenotype is also observed when geminin is degraded by Emi1 depletion, resulting in unopposed APC/C activity.3,4 DNA over-replication from repeated firing of replication origins within the same replicon causes both single- and double-strand DNA breaks in somatic cells, with induction of the DNA damage response and apoptosis occurring at 3–8 d.4,18,19 In contrast to somatic cells, however, pluripotent cells depleted of geminin or Emi1 do not apoptose despite sustaining extensive DNA damage (Figs. 3 and 4). To first determine whether DNA damage-induced nuclear foci accumulate, we analyzed geminin and Emi1-depleted cells for increased foci of γ-H2AX, Rad51 and replication protein A (RPA). γ-H2AX refers to H2AX phosphorylated on serine 139, an early mark of double-strand DNA breaks, whereas Rad51 is required for homologous recombination, and RPA binds to single strands of DNA that arise because of genomic damage. Depletion of geminin or Emi1 in mouse pluripotent cells results in significant upregulation of γ-H2AX, Rad51 (to a lesser extent) and RPA foci relative to unirradiated controls at 48 h post-transfection (Fig. 3). Figure 3 further illustrates that the upregulation of each marker is not simply a consequence of the gross nuclear enlargement acquired, but that more foci indicative of DNA strand breaks develop in both geminin and Emi1-depleted pluripotent cells. Preliminary results also suggest that despite the extensive DNA damage that probably arises from replication fork collisions, the ATR/ATM-dependent checkpoint that is normally activated in somatic cells4,18,19 is not induced in pluripotent cells, with failure to induce the inhibitory phosphorylation of Cdk1 on Y15 and failure to phosphorylate p53 on serine 15 (data not shown).
Figure 3.
Geminin and Emi1 depletion in pluripotent cells results in induction of DNA damage proteins. Geminin and Emi1 depletion in P19 EC cells induces upregulation of γ-H2AX, Rad51 and replication protein A (RPA) foci (green) relative to unirradiated controls at 48 h post-transfection. Irradiated positive controls were treated with 2 Gy. Total DNA, red. Scale bar = 20 µm.
Figure 4.
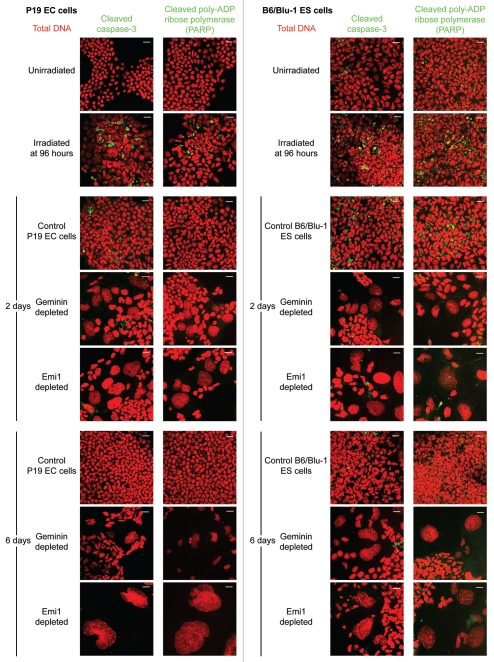
Emi1 depletion in pluripotent cells does not induce apoptotic markers. Geminin and Emi1 depletion in P19 EC cells does not elevate levels of cleaved caspase 3 or cleaved poly-ADP ribose polymerase (PARP) (green) relative to unirradiated controls at 48 h post-transfection. Total DNA, red. Scale bar = 20 µm.
We further asked whether DNA damage induced by geminin and Emi1 depletion in pluripotent cells would trigger cell death by apoptosis, as previously reported in some somatic cells18,19,27 (but possibly not all primary cell types28), particularly when the G2/M checkpoint is abrogated. However, no increase in apoptotic markers was observed above background levels for control cells, at neither two days nor six days post-transfection for cleaved caspase 3 (Fig. 4), cleaved poly-ADP ribose polymerase (PARP, Fig. 4) or Annexin V labeling (data not shown). Hence, in contrast to somatic cells,9 geminin or Emi1-depleted pluripotent cells would appear to be tolerant of the extensive DNA damage induced by genomic over-replication, a property that might explain the extreme nuclear enlargement that can be observed as they differentiate to trophoblast cells (Fig. 1C). This observation needs to be further tested in vivo in mouse trophoblast giant cells that potentially acquire significant DNA damage during endoreduplication to achieve the extremely high DNA contents of > 1,000C.29 Interestingly, rat trophoblast giant cells are resistant to DNA damage-inducing agents, whereas proliferating trophoblast stem cells remain sensitive to this genotoxic stress and rapidly undergo apoptosis.30 Differentiation of rat trophoblast stem cells to trophoblast giant cells requires downregulation of p53 and Rb at the protein level,30 which explains the acquired ability to evade programmed cell death. A similar suppression of apoptosis to genotoxic stress is characteristic of endoreduplicating cells in Drosophila melanogaster.31 DNA over-replication in ovarian follicle, salivary gland and fat body cells of Drosophila induces γ-H2AV repair foci in response to DNA damage (particularly at fragile heterochromatic sites), but these cells remain viable, with cleavage of caspase 3 or induction of other pro-apoptotic mediators not observed, possibly due to epigenetic silencing at their promoters.
Geminin in normal and malignant human trophoblast cells.
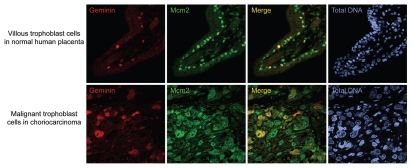
Although the consequences of geminin depletion in mouse pluripotent cells have now been described in reference 9, the effects of depleting geminin in human ES cells remain to be elucidated. Here, it is important to acknowledge the species-specific differences in cell cycle regulation between mouse and human embryonic cells. Unlike mouse trophoblast cells that can endoreduplicate, human trophoblast cells invariably divide mitotically to retain a diploid DNA content of 2C, with only 0.3% of extravillous cytotrophoblast cells in normal placenta acquiring a DNA content of 16C by unknown means.32 Indeed, when we examined human tissue sections of normal placenta, the premalignant conditions of complete and partial hydatidiform mole and the malignant diseases of choriocarcinoma and placental site trophoblastic tumor (Fig. 5), the APC/C substrate geminin was not downregulated by immunohistochemistry in normal or diseased trophoblast cells. Since cyclin A2 and cyclin B1 are also abundant in human trophoblast cells,33 these observations indicate that APC/C substrates are regulated differently during the cell cycles of mouse and human trophoblast cells, with human trophoblast cells undergoing the canonical cell cycles characteristic of somatic cells. Given its strength as a prognostic biomarker,34 the clinico-pathological value of geminin and other APC/C substrates requires further evaluation for the management of women with trophoblastic diseases, using a larger series of cases and long-term follow-up data.
Figure 5.
Geminin is present at high levels in normal and malignant human trophoblast cells. In trophoblast cells of normal human placenta, geminin (red) is present in proliferating cells that co-label with cell cycle markers Mcm2 (green) and Ki67 (not shown). Geminin is only present in a subset of cytotrophoblast cells immunolabelled with Mcm2, accounting for the expected proportion of actively cycling cells in S phase, G2 and early mitosis. Geminin is also present at high levels in both the nucleus and cytoplasm of malignant trophoblast cells of choriocarcinomas with enlarged nuclei. Total DNA, blue. Scale bar = 20 µm.
It is also important to note that different phenotypes arise when Oct4 is depleted in mouse and human ES cells. Depletion of Oct4 in mouse ES cells induces trophectoderm differentiation,7 in contrast to human ES cells, where loss of Oct4 induces differentiation toward mesoderm and endoderm.35 Analogously, geminin-deficient mouse embryos, EC and ES cells result in trophoblast cell differentiation. Formation of a blastocoelic cavity in mouse embryos that lack geminin can only result from the functional Na+/K+ pumps of trophoblast cells, which pump fluid toward the center of the hitherto embryonic mass known as the morula. Moreover, if depletion of geminin in human ES cells also mimics the phenotype observed following loss of Oct4 in inducing mesoderm or endoderm differentiation, this would reinforce the role of geminin as an integral component of a pluripotency network. Geminin might therefore induce pluripotency when overexpressed in somatic cells, either alone or in combination with established transcription factors that are known to induce pluripotency. In a small study, geminin was found to be a factor present in oocytes that promotes chromatin remodelling.36 Geminin directly interacts with the chromatin remodeler Brg1,37 an association that has clear implications for terminal differentiation.9,38 By maintaining chromatin in an accessible hyperacetylated state,39 the abundance of geminin in Xenopus egg extracts might possibly account for the efficient nuclear reprogramming observed in the generation of “M-phase egg extract-induced pluripotent stem (M-iPS) cells.”40,41 This further emphasizes the potential role of geminin for inducing pluripotency in mammalian somatic cells.
Discussion
In contrast to the relatively well-characterized somatic cell cycle, the cell cycle of embryonic cells remains poorly understood and warrants urgent attention given the major barrier that it is likely to pose for efficient formation of induced pluripotent stem cells. Using mouse EC and ES cells, we have been able to resolve important questions in cell cycle control that would have been otherwise difficult to address using pre-implantation mouse embryos, where downregulation of geminin and cyclin A2 in endoreduplicating trophoblast giant cells was initially reported in reference 12. In our recent experiments,9 further described here, both mouse EC and ES cells behave like developing mouse embryos in which geminin has been genetically ablated. Silencing the expression of Emi1 in mouse pluripotent cells induces giant cell differentiation and upregulation of trophectoderm markers by targeting geminin for degradation. These observations shed light into how endoreduplication is regulated in mouse trophoblast cells, suggesting that high APC/C activity targets specific proteins for destruction to prevent mitosis and promote S-phase re-entry, allowing oscillations in cyclin E to sustain progress through endocycles. The similarity between the consequences of Emi1 or geminin depletion and loss of Oct4 in pluripotent cells is also striking.9 Disrupting the expression of specific cell cycle regulators might not only be required for the switch from mitotic to endoreduplicative cycles but is also associated with concomitant loss of pluripotency. By sustaining expression of the core pluripotency factors Oct4, Sox2 and Nanog, geminin represents an important link between the embryonic cell cycle and pluripotency. In early mammalian embryogenesis, its known role in inhibiting aberrant prereplication complex assembly during the cell cycle further ensures the transmission of uncommitted progenitors with stably diploid genomes.
But if APC/C activity is suppressed during interphase of pluripotent cells, how is cell cycle progression possible in pluripotent cells when oscillations in the important APC/C substrates are lost? As with the elevated levels of cyclins E1 and A2, if geminin remains present during G1 of pluripotent cells, when do pre-replication complexes assemble on chromatin in pluripotent cells to allow S-phase entry and the firing of replication origins? A recent report using Xenopus embryonic cells24 suggests a possible solution: that geminin and cyclins undergo posttranslational modifications that inactivate them during pluripotent cell cycles without the need for proteolysis. This was first observed (also by the same authors) in Xenopus egg extracts for geminin, where geminin is polyubiquitinated during exit from mitosis to prevent its binding to Cdt1, thereby inactivating it effectively.42 Although another recent publication suggests that APC/C substrates do fluctuate during the ES cell cycle, but to a lesser extent than observed in somatic cells,43 all experiments were based on synchronized ES cells, which, in our experience, fail to exit a cell cycle block and will apoptose or differentiate instead (unpublished observations). We therefore believe that conserved post-translational modifications analogous to those observed in amphibian embryonic cells are more likely to regulate the activity of geminin and other APC/C substrates during mitosis and G1 phase of the ES cell cycle.
Our findings9 and those presented here also allow an alternative interpretation of the studies on Cdh1-deficient mice.25,26 Consistent with our results, both studies have shown that genetic ablation of APC/CCdh1 in the mouse results in post-implantation embryonic lethality due to defective endoreduplication in trophoblast cells and impaired placental development. In both of these studies, the authors suggest that this is a consequence of failure to destroy cyclins, which, like geminin, are substrates of the APC/C. However, our results suggest instead that geminin is the APC/C target affected in Cdh1-deficient mice, rather than cyclins as both studies propose. Depletion of cyclins A2 and B1 in pluripotent cells does not induce either giant cell differentiation or upregulation of trophectoderm markers.9 Since genetic ablation of cyclin E in mouse ES cells does not impair their pluripotency,14,15 this leads us to conclude that disrupting the expression of cyclins in mouse pluripotent cells is not sufficient to induce a “default” trophectoderm fate.
A recent proposal2 suggests that as for Drosophila salivary glands, cyclin E-Cdk-dependent phosphorylation of Cdh1 imposes oscillations in APC/C activity to generate cycles of geminin stability and destruction44 that would be more physiologically compatible for pre-replication complex assembly during trophoblast endocycles. Our results presented here, however, also suggest that endoreduplication in trophoblast cells would be permissive to an element of genomic over-replication and DNA damage that appears to be tolerated in this specialized cell type, as might occur to a lesser extent in endoreduplicating cell types of Drosophila that better exemplify polyploidization.31,44 Both processes might not be mutually exclusive and could occur as a part of normal extra-embryonic development in trophoblast, rather than being an experimentally induced artifact caused by either geminin or Emi1 depletion. This interpretation is supported by the observation that geminin is notably absent or downregulated during S phase of trophoblast giant cells in mouse blastocysts.12 Although it is difficult to distinguish genomic over-replication from endoreduplication in mouse embryos, ultra-structural karyotypic analysis would ultimately determine whether trophoblast endoreduplication differs substantially from the specialized endocycles described in other systems.
In conclusion, transcriptional regulation is crucial for maintaining pluripotency and determining cell fate during early mammalian development, but precise control of the unique cell cycles of embryonic cells is also likely to play a key role in both preserving the identity of pluripotent cells and in lineage commitment. Since these cellular processes must be tightly linked, strategies in regenerative medicine that involve transdifferentiation and nuclear reprogramming will benefit from an improved understanding of the distinct cell cycles of mammalian embryonic cells, in which critical cell cycle regulators such as Emi1 and geminin might be important for specifying stem cell identity.
Experimental Procedures
Cell lines.
P19 EC cells (ATCC) were grown in Alpha Minimum Essential Medium (α-MEM, Sigma-Aldrich) supplemented with 7.5% donor bovine serum, 2.5% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/ml penicillin and 0.1 mg/ml streptomycin (all from Gibco). B6/Blu-1 ES cells were maintained in KnockOut DMEM (Gibco), supplemented with 15% FBS, 10 pM β-mercaptoethanol (Sigma-Aldrich), 1,000 U/ml leukemia inhibitory factor (LIF; Millipore), 2 mM L-glutamine (Amresco), 0.012 g/ml penicillin and 0.02 g/ml streptomycin and were cultured feeder-free on 0.1% gelatin-coated flasks.
RNA interference.
siRNA duplexes were synthesized by Qiagen and designed to target the following sequences:
Emi1: TAC AAA GAT TGT GAT AGA TCA, ACC GTG GAC GGT TGT AAA GAA, CAG CGG CAT GGA CTT AGT AAA and GTG GGA CAT GAT AAC AAG GAA.
Geminin: CAG GAA TTT GAT TCT GAA GAA, CAG GAA GCC TTT GAT CTT ATA, CCG CCT GAG AAA GGA GAA TAA and ATC GAG AGG CTG AGT AAT GAA.
Control siRNAs were designed by substitution of two base pairs for each sequence. Sequences did not correspond to any known cDNAs on the Ensembl database. P19 EC cells were transfected using Oligofectamine (Invitrogen), whereas B6/Blu-1 ES cells were transfected using Lipofectamine RNAiMax (Invitrogen).
Antibodies, immunoblotting, immunofluorescence and immunohistochemistry.
Antibodies used include rabbit anti-cyclin A2 (a kind gift from Dr. Mark Carrington, Department of Biochemistry), mouse anti-cyclin B1 (GNS1, Santa Cruz Biotechnology), rabbit anti-cyclin E (M-20, Santa Cruz Biotechnology), mouse anti-Mcm-2 (generated in our laboratory), rabbit anti-phospho-histone H2AX (Ser139, #2577, Cell Signaling) and rabbit anticleaved caspase 3 (Asp175, #9661, Cell Signaling). Rabbit polyclonal antibodies against geminin were generated in our laboratory, as previously described in reference 9.
For immunofluorescent staining of cyclin E, cells were permeabilised with 0.1% Triton X-100 on ice prior to fixation in 4% paraformaldehyde. For all other antibodies, fixed cells were permeabilised with 0.1% Triton X-100 and 0.02% SDS, blocked with 1% bovine serum albumin (Sigma-Aldrich) and incubated with primary antibody. For immunofluorescent staining of formalin-fixed, paraffinembedded (FFPE) tissue sections (see below), microwave antigen-retrieval in citrate buffer was required prior to blocking and incubation with primary antibodies. Coverslips and tissue sections were incubated with Alexa Fluor-conjugated secondary antibodies (Molecular Probes) and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), mounted with fluorescence mounting medium (Dako) and sealed with nail varnish. Confocal images were collected on a Zeiss LSM 510 META confocal microscope.
For immunohistochemistry, FFPE tissue sections of normal human placenta were provided by the MRC/Wellcome Trust-funded Human Developmental Biology Resource. FFPE sections from hydatidiform moles, choriocarcinoma and placental site trophoblastic tumors were obtained from the National Gestational Trophoblastic Disease Unit at Charing Cross Hospital with local research ethics committee approval. Tissue sections were blocked in 1% bovine serum albumin and 10% goat serum prior to staining with the appropriate antibodies using a Dakoautostainer Universal Staining System and DAKOChemMate (Dako).
Acknowledgements
The authors thank members of the Laskey laboratory and Dr. W. Kaiba for helpful discussions. V.Y. is supported by the Agency for Science, Technology and Research (A*STAR, Singapore). The Medical Research Council and Cancer Research UK have funded this work.
Abbreviations
- APC/C
anaphase promoting complex/cyclosome
- Cdk
cyclin-dependent kinase
- Emi1
early mitotic inhibitor 1
- EC
embryonal carcinoma cells
- ES
embryonic stem cells
- TGCs
trophoblast giant cells
References
- 1.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell cycle transitions: does one fit all? Nat Rev Mol Cell Biol. 2008;9:910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- 2.Pines J. Cubism and the cell cycle: the many faces of the APC/C. Nat Rev Mol Cell Biol. 2011;12:427–438. doi: 10.1038/nrm3132. [DOI] [PubMed] [Google Scholar]
- 3.Di Fiore B, Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J Cell Biol. 2007;177:425–437. doi: 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machida YJ, Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/S0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 6.Hsu JY, Reimann JD, Sørensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- 7.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.016/S0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 8.Fujii-Yamamoto H, Kim JM, Arai K, Masai H. Cell cycle and developmental regulations of replication factors in mouse embryonic stem cells. J Biol Chem. 2005;280:12976–12987. doi: 10.1074/jbc.M412224200. [DOI] [PubMed] [Google Scholar]
- 9.Yang VS, Carter SA, Hyland SJ, Tachibana-Konwalski K, Laskey RA, Gonzalez MA. Geminin escapes degradation in G1 of mouse pluripotent cells and mediates the expression of Oct 4, Sox2 and Nanog. Current biology: CB. 2011;21:692–699. doi: 10.1016/j.cub.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stead E, White J, Faast R, Conn S, Goldstone S, Rathjen J, et al. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002;21:8320–8333. doi: 10.1038/sj.onc.1206015. [DOI] [PubMed] [Google Scholar]
- 11.Palazón LS, Davies TJ, Gardner RL. Translational inhibition of cyclin B1 and appearance of cyclin D1 very early in the differentiation of mouse trophoblast giant cells. Mol Hum Reprod. 1998;4:1013–1020. doi: 10.1093/molehr/4.11.1013. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez MA, Tachibana KE, Adams DJ, van der Weyden L, Hemberger M, Coleman N, et al. Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes Dev. 2006;20:1880–1884. doi: 10.1101/gad.379706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara K, Nakayama KI, Nakayama K. Geminin is essential for the development of preimplantation mouse embryos. Genes to cells: devoted to molecular & cellular mechanisms. 2006;11:1281–1293. doi: 10.1111/j.1365-2443.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- 14.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/S0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 15.Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, et al. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003;22:4794–803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGarry TJ, Kirschner MW. Geminin an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/S0092-8674(00)81209-X. [DOI] [PubMed] [Google Scholar]
- 17.Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 18.Melixetian M, Ballabeni A, Masiero L, Gasparini P, Zamponi R, Bartek J, et al. Loss of Geminin induces rereplication in the presence of functional p53. J Cell Biol. 2004;165:473–482. doi: 10.1083/jcb.200403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu W, Chen Y, Dutta A. Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol Cell Biol. 2004;24:7140–7150. doi: 10.1128/MCB.24.16.7140-50.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riabowol K, Draetta G, Brizuela L, Vandre D, Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989;57:393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuno N, den Elzen N, Pines J. Human cyclin A is required for mitosis until mid prophase. J Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santamaría D, Barrière C, Cerqueira A, Hunt S, Tardy C, Newton K, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 24.Kisielewska J, Blow JJ. Dynamic interactions of high Cdt1 and geminin levels regulate S phase in early Xenopus embryos. Development. 2012;139:63–74. doi: 10.1242/dev.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Higuera I, Manchado E, Dubus P, Cañamero M, Méndez J, Moreno S, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10:802–811. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, et al. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol. 2008;10:1083–1089. doi: 10.1038/ncb1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tachibana KE, Gonzalez MA, Guarguaglini G, Nigg EA, Laskey RA. Depletion of licensing inhibitor geminin causes centrosome overduplication and mitotic defects. EMBO Rep. 2005;6:1052–1057. doi: 10.1038/sj.embor.7400527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu W, Depamphilis ML. Selective killing of cancer cells by suppression of geminin activity. Cancer Res. 2009;69:4870–4877. doi: 10.1158/0008-5472.CAN-08-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zybina EV, Zybina TG. Polytene chromosomes in mammalian cells. Int Rev Cytol. 1996;165:53–119. doi: 10.1016/S0074-7696(08)62220-2. [DOI] [PubMed] [Google Scholar]
- 30.Soloveva V, Linzer DI. Differentiation of placental trophoblast giant cells requires downregulation of p53 and Rb. Placenta. 2004;25:29–36. doi: 10.1016/S0143-4004(03)00215-7. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra S, Maqbool SB, Kolpakas A, Murnen K, Calvi BR. Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev. 2008;22:3158–3171. doi: 10.1101/gad.1710208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zybina TG, Kaufmann P, Frank HG, Freed J, Kadyrov M, Biesterfeld S. Genome multiplication of extravillous trophoblast cells in human placenta in the course of differentiation and invasion into endometrium and myometrium. I. Dynamics of polyploidization. Tsitologiia. 2002;44:1058–1067. [PubMed] [Google Scholar]
- 33.Ichikawa N, Zhai YL, Shiozawa T, Toki T, Noguchi H, Nikaido T, et al. Immunohistochemical analysis of cell cycle regulatory gene products in normal trophoblast and placental site trophoblastic tumor. International journal of gynecological pathology: official journal of the International Society of Gynecological Pathologists. 1998;17:235–240. doi: 10.1097/00004347-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez MA, Tachibana KE, Chin SF, Callagy G, Madine MA, Vowler SL, et al. Geminin predicts adverse clinical outcome in breast cancer by reflecting cell cycle progression. J Pathol. 2004;204:121–130. doi: 10.1002/path.1625. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez RT, Velkey JM, Lutzko C, Seerke R, Kohn DB, O'Shea KS, et al. Manipulation of OCT4 levels in human embryonic stem cells results in induction of differential cell types. Exp Biol Med (Maywood) 2007;232:1368–1380. doi: 10.3181/0703-RM-63. [DOI] [PubMed] [Google Scholar]
- 36.Sylvestre EL, Pennetier S, Bureau M, Robert C, Sirard MA. Investigating the potential of genes preferentially expressed in oocyte to induce chromatin remodeling in somatic cells. Cellular reprogramming. 2010;12:519–528. doi: 10.1089/cell.2010.0012. [DOI] [PubMed] [Google Scholar]
- 37.Seo S, Herr A, Lim JW, Richardson GA, Richardson H, Kroll KL. Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev. 2005;19:1723–1734. doi: 10.1101/gad.1319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim JW, Hummert P, Mills JC, Kroll KL. Geminin cooperates with Polycomb to restrain multi-lineage commitment in the early embryo. Development. 2011;138:33–44. doi: 10.1242/dev.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yellajoshyula D, Patterson ES, Elitt MS, Kroll KL. Geminin promotes neural fate acquisition of embryonic stem cells by maintaining chromatin in an accessible and hyperacetylated state. Proc Natl Acad Sci USA. 2011;108:3294–3299. doi: 10.1073/pnas.1012053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganier O, Bocquet S, Peiffer I, Brochard V, Arnaud P, Puy A, et al. Synergic reprogramming of mammalian cells by combined exposure to mitotic Xenopus egg extracts and transcription factors. Proc Natl Acad Sci USA. 2011;108:17331–17336. doi: 10.1073/pnas.1100733108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez MA. Induced pluripotency leapfrogs ahead. Proc Natl Acad Sci USA. 2011;108:17245–17246. doi: 10.1073/pnas.1114517108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li A, Blow JJ. Non-proteolytic inactivation of geminin requires CDK-dependent ubiquitination. Nat Cell Biol. 2004;6:260–267. doi: 10.1038/ncb1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballabeni A, Park IH, Zhao R, Wang W, Lerou PH, Daley GQ, et al. Cell cycle adaptations of embryonic stem cells. Proc Natl Acad Sci USA. 2011;108:19252–19257. doi: 10.1073/pnas.1116794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zielke N, Querings S, Rottig C, Lehner C, Sprenger F. The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles. Genes Dev. 2008;22:1690–1703. doi: 10.1101/gad.469108. [DOI] [PMC free article] [PubMed] [Google Scholar]